Abstract
There is a recognizable and urgent need to speed the development and application of novel, more efficacious anti-cancer vaccine therapies that inhibit tumor progression and prevent acquisition of tumor resistance. We have created and established a portfolio of validated peptide epitopes against multiple receptor tyrosine kinases and we have identified the most biologically effective combinations of EGFR (HER-1), HER-2, HER-3, VEGF and IGF-1R peptide vaccines/mimics to selectively inhibit multiple receptors and signaling pathways. The strategy is based on the use of chimeric conformational B-cell epitope peptides incorporating “promiscuous” T-cell epitopes that afford the possibility of generating an enduring immune response, eliciting protein-reactive high-affinity anti-peptide antibodies as potential vaccines and peptide mimics that act as antagonists to receptor signaling that drive cancer metastasis. In this review we will summarize our ongoing studies based on the development of combinatorial immunotherapeutic strategies that act synergistically to enhance immune-mediated tumor killing aimed at addressing mechanisms of tumor resistance for several tumor types.
Keywords: antibodies, epitopes, immunogenicity, peptide mimics, resistance, vaccine candidates
Abbreviations
- IGF-1R
Insulin-like growth factor-1 receptor
- MVF
measles virus fusion protein
- HER
human epidermal growth factor receptor
Introduction
Cancer represents an enormous burden on patients, their families and society. Despite the significant advancement in treating patients with solid tumors, the majority of those patients still die from their disease. The American Cancer Society (http://www.cancer.org) estimates that 40,000 people will die from breast cancer in 2014 and 235,030 women will be newly diagnosed with it, whereas for colon cancer there are an estimated 96,830 new cases with 50,310 deaths for men and women. An estimated 46,220 new cases of pancreatic cancer (PC) are expected to occur in the US during 2014 with an estimated 39,590 PC deaths for both men and women. An estimated 18,170 new cases of esophagus are expected to occur in the US during 2014 with an estimated 15,450 deaths for both men and women. Together, those cancers account for more than 50% of all cancer deaths. According to the World Health Organization (WHO), cancer is a global pandemic with 14 million cases diagnosed in 2012 and 22 million cases expected by 2032. These dire numbers have barely changed within the past 5 years urgently warranting innovative strategies to put a dent in these numbers.
In this review I address the challenge of developing an immunotherapeutic strategy based on the use of chimeric peptides as immunogenic epitopes that drive immune responses and peptide mimics that can interfere with cell signaling that drives cancer metastasis. We have established a strong and solid foundation for these strategies over 2 decades of incremental translational research in our laboratory and validated in both pre-clinical and clinical studies,1–4 The idea of using a chimeric B and T-cell peptide vaccine to engage the immune system to elicit memory-like antibodies represents a therapy with great potential which has merit over the infusion of humanized monoclonal antibodies (hmAbs) to treat cancer without the significant toxicities associated with the latter. In this review article, I will restrict the discussions to our own experience with synthetic peptide vaccines that stimulate tumor-specific B-cell responses eliciting high affinity anti-peptide antibodies and peptide mimics aimed at interfering with cellular signaling. In both cases the importance of peptide structure as it relates to the 3-dimensional space is critically important to modulate these peptide:protein interactions. The vaccine strategies are 2-fold: (i) engineering conformational B-cell epitopes and (ii) synthesizing chimeric peptides with “promiscuous” T cell-epitopes and delivering these vaccines emulsified with an adjuvant nor-muramyl-dipeptide (nor-MDP, N-acetyl-glucosamine-3yl-acetyl-L-alanyl-D-isoglutamine) and ISA 720 (squalene/arlacel) vehicle (SEPPIC, Paris, France). Similarly, peptide mimic strategies are also 2-fold: (i) the peptide mimics are initially designed to improve their stability by various chemical means and (ii) further engineered as retro-inverso D-analogs to circumvent proteolysis. We will discuss the development of combinatorial immunotherapeutic strategies that act synergistically to enhance immune-mediated tumor killing aimed at addressing mechanisms of tumor resistance. Finally, we will demonstrate that combinatorial approaches have the best potency to accomplish the most significant tumor destruction. Further research utilizing humanized mouse models in which human cells and tissues have been engrafted will be required to optimize such approaches. 5 The use of synthetic peptide vaccines that stimulate tumor-specific T-cell responses in particular CD8+ cytotoxic T-cells (CTLs) has resulted in some but limited clinical benefit. The importance of CD4+ T helper cells in the anti-tumor immune is now generally well appreciated. However, no T-cell peptide vaccine has been approved to date, likely due to the fact that tumors can evade immune responses by exploiting immune checkpoint pathways and rendering the vaccine ineffective. The ability of cancer cells to evade anti-tumor T--cell activity in the microenvironment has recently been accepted as a hallmark of cancer progression. Recognition of these evasive processes led to development of immunotherapeutic antibodies targeting the co-stimulatory and co-inhibitory receptors on T-cells and are beyond the scope of this article. 6
Current treatments for solid tumors involve a series of first line therapies that include surgery, radiation, hormone therapy, and chemotherapy. Despite clinical success in treatment, traditional chemotherapy and radiotherapy regimens are often associated with severe debilitating side effects and toxicity. 7–9 During the past decade we have seen significant advances in our understanding of the signaling networks that drive cancer progression. This progress has ushered in a new era of cancer therapeutics with agents that inhibit specific growth stimulatory pathways, most of which are directed against oncogenic receptor tyrosine kinases (RTKs) or their downstream signaling components. RTKs have established roles in the development and progression of many types of cancer due their dysregulation and their overexpression that is associated with poor prognosis in several tumor types. 10 Agents targeting RTKs in oncology include therapeutic antibodies to RTK ligands or the receptors themselves, and small-molecule inhibitors that target the intracellular kinase domains of the RTKs. 11–13
Although development of targeted therapeutics (hmAbs and TKIs) has improved cancer treatment significantly, these current treatments are not completely curative. Intrinsic or acquired resistance to these targeted therapies is also a major problem. Many of the FDA approved therapies targeting HER-2 (Trastuzumab, Pertuzumab, Lapatanib), HER-1 (Cetuximab, Gefitinib, Erlotonib) and VEGF (Bevacizumab, Sunitinib) have significant toxicities and many of the patients demonstrate disease progression and show problems of selectivity and efficacy, development of resistance and unacceptable safety profiles that continues to hamper their clinical acceptance. 14–16 Clinical applications of hmAb therapy in general is limited by a number of concerns such as the frequency of treatments, associated costs, limited duration of action, undesired immunogenicity, and significant risks of cardiotoxicities. 17 Small molecules RTK inhibitors lack specificity, have short half-lives, are limited to proteins with well-defined small binding pockets, are prone to development of drug resistance and exhibit severe and unacceptable toxicities ranging from rash, diarrhea, nausea, vomiting to elevated liver function tests (LFT's) and cardiac toxicities. Novel immune therapies targeting these aberrant molecular pathways are urgently needed that can offer hope that the effectiveness and duration of response can be greatly improved.
Innovative vaccine targeted strategies
Immune-based therapies that target receptor kinases have the potential to achieve long-term control in several tumor types without causing the numerous toxicities associated with several approved FDA-approved regimens. The greatest potential significance of vaccine immunotherapy is 2-fold. (i) It combines multiple mechanisms of action by activating both B and T cell functions and promoting immunological clearance. (ii) It is also a targeted approach aimed at inhibiting molecular signaling pathways that are crucial for tumor growth and maintenance. The major attraction of the peptide vaccine approach is the potential for a sustained antitumor effect related to immunological memory that protects against relapsing tumors, obviating the requirement for multiple infusions of humanized mAbs and overcoming escape mechanisms. Active immunotherapy offers multitude advantages, including tumor specificity and the activation of immune responses against antigens that are selectively expressed by tumor cells. As a result, non-specific toxicity to normal tissues is reduced and anti-tumor responses are more durable and adaptable to cancer control at different stages of disease, including those with early-stage disease and low tumor burdens. Additional benefits of the peptide vaccine approach include the ease of rapid synthesis, safety, lack of toxicity, and cost-effectiveness.
Unique peptide B-cell epitope vaccines
Many of the hurdles facing the development of peptide vaccines have been previously discussed. 18 The main hurdle was addressed by the publication of the first antigen:antibody complex between an antigen (lysozyme) and the Fab fragment from a monoclonal antibody against lysozyme in 1986. 19 It showed the complementarity between the interfaces to be over a large area (750 Å2) involving discontinuous segments of the polypeptide chain. Thus, it became clear that contiguous or linear peptides often fail as a vaccine because they do not adequately represent the complexity of the B-cell epitopes or mimic the epitope structure. Identification and characterization of B-cell epitopes in target antigens is one of the key steps in epitope-driven vaccine design and antibody production. Among the 3 most significant hurdles of developing efficacious B-cell epitope vaccines is how: (i) to identify immunogenic B-cell epitopes; (ii) to design conformational B-cell epitopes; (iii) to overcome MHC restriction and vaccinate an outbred population. The first hurdle can be partially overcome by prediction of continuous B-cell antigenic determinants using informatics approaches.20,21 Sela-Culang has recently discussed the structural basis of antigen:antibody recognition and describes attempts to predict B-cell epitopes by incorporating Ab information into B-cell epitope prediction schemes. 22 The second hurdle is more complex in that most protein epitopes are actually discontinuous (i.e., such epitopes are comprised of amino acids belonging to residues located far apart in the primary structure, but brought close together by the overall folding of the peptide chain and whose antigenicity depends upon the protein conformation). We have developed a rational structure-based design of complex peptides to mimic the secondary and tertiary structure of protein antigens and we used similar principles to design HER-2 specific epitopes. 23,24 25–27 Other methods include the new phage-display based approach, and the Large Fragment Phage Display (LFPD) has been shown to be effective for mapping conformational epitopes on HER-2. The results obtained in this study demonstrated the utility of LFPD and its potential application to the detection of conformational epitopes on many other molecules of interest, as well as, the development of new and potentially more effective B-cell conformational epitopes based vaccines. 28 We addressed the third hurdle by engineering chimeric peptide constructs by incorporating a ‘promiscuous’ T-cell epitope, which can bind multiple haplotypes with the appropriate B-cell epitope. 29 We surveyed several “promiscuous” T cell-epitope and found that the measle virus fusion (MVF) protein (epitope sequence 298–302) to be the most efficacious. Another T-helper epitope peptide P30 from tetanus toxoid was used as the immunostimulant in MUC1 glycopeptide antitumor vaccines and apparently it also acts as a built-in adjuvant P30-conjugated glycopeptide vaccines. 30
There are several groups that have proposed and tested other strategies of developing effective HER-2 B-cell vaccines. Wiedermann and co authors have shown that vaccination with 3 HER-2 peptides representing B-cell epitopes of the extracellular domain of HER-2 induces specific IgG antibodies in mice with strong anti-tumor activity in vitro and in vivo. 31 The virosomal formulated HER-2 multipeptide vaccine was safe, well tolerated and effective in overcoming immunological tolerance resulting in clinical benefit comparable to passive anti-HER-2 antibody therapy. Delivery systems for HER-2 antigen with special focus on T and B cell peptide vaccines for breast cancer improving anti-cancer responses have been reviewed. 32 Phage display technique has been used to generate epitope mimics “mimotopes” by screening trastuzumab. Five candidate mimotopes were isolated from a constrained 10 mer library. These peptides were specifically recognized by trastuzumab, and showed distinctive mimicry with HER-2 in 2 experimental groups. These results indicate that the selected mimotopes are suitable for formulation of a breast cancer vaccine because the resulting Abs show similar biological features as trastuzumab. Miyako and co-workers identified a new epitope of Mab CH401 against HER-2 ECD (N: 167–175), and evaluated the effect of active immunization of the 20mer peptide containing the epitope (CH401 peptide). The new HER-2 peptide contained epitopes for CD4(+) and CD8(+) T-cells, which contributes to the suppressive effect on HER-2/neu-expressing tumor cell growth. 33 Epitope-specific immunization for active anti-EGFR immunotherapy has been shown to induce “cetuximab-like” antibodies in vivo using the mimotope approach. The in vitro biologic features of mimotope-induced antibodies are similar to those of the monoclonal antibody cetuximab. 34 Similar to our strategies, Zhu and co-workers were able to evoke high titers of antibodies targeting the dimer interface of EGFR in patients, using a chimeric peptide, comprising a linear B-cell epitope peptide from the highly conservative β-hairpin loop of dimer interface of human EGFR (EGFR237–267) and a ‘promiscuous’ Th-cell epitope from the measles fusion protein (MVF) was constructed. 35 The chimeric peptide immunization was able to significantly inhibit the growth of subcutaneously transplanted LLC cells in C57BL6 mice. Therefore, the MVF-EGFR(237–267) construct represents a promising candidate for active anti-EGFR immunotherapy and provides a novel targeting strategy for the anti-EGFR therapy.
Enabling chimeric peptide B-Cell vaccine strategy
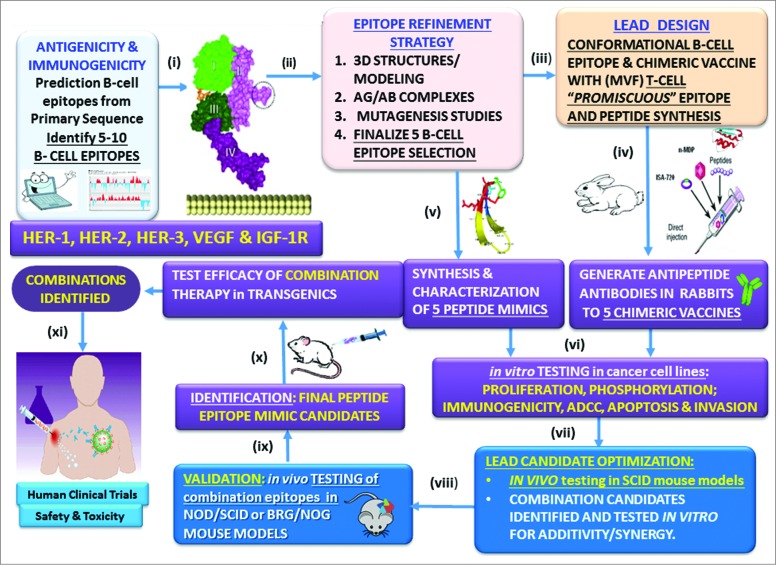
We have tested our model outlined in Figure 1 successfully in multiple different disease types which has evolved over a period of time. We have addressed several crucial issues in developing epitope-driven peptide vaccines over the past 2 decades by developing innovative anti-cancer strategies. 36–41 We begin by predicting B-cell epitopes followed by molecular modeling to recapitulate the native structure of the tumor antigen. 23,29,42 This is followed by the design of the chimeric vaccine by incorporating a “promiscuous” T cell epitope for the production of antipeptide-antibodies in animals. 25–27,43 Stable peptide mimics are designed, synthesized and tested in a series of in vitro assays in different human cancer cell lines to corroborate efficacy with antipeptide antibodies. 25,44,45 Epitope combinations that offer synergy/additivity are identified and tested in SCID mouse models to simulate human cancers to support conducting human clinical trials to assess safety and toxicity. 46
Figure 1.
Peptide overall strategy. (i) Prediction of B-cell epitopes based on multiple computerized antigenicity/immunogenicity algorithms. This represents a critical component in the overall process; (ii) B-cell linear epitopes are refined through mutagenesis studies, and epitopes are modeled based on the 3-dimensional native antigen structure and antigen:antibody complex if available. The unique B cell epitope peptides are engineered to recapitulate the exquisite native structure of the tumor antigen. This is a crucial prerequisite to developing high affinity antibodies given that B-cell epitopes can be both linear (contiguous amino acid sequence) and conformational (discontinuous in the primary sequence but close in proximity of the folded 3D structure) play an important role; (iii) The chimeric vaccine is designed to include a “promiscuous” T cell epitope as a vital component in the development of a universal vaccine for an outbred population that can bypass haplotype restricted immune responses; (iv) Highly efficacious native-like antipeptide-antibodies are then elicited in animals when the vaccine is emulsified with an adjuvant (nor-MDP) in a squalene-arlacel oil-water ISA720 vehicle; (v) Stable peptide mimics are designed as retro-inverso analogs that can block receptor:ligand interactions; (vi) Both antibodies and peptide mimics are used in a series of in vitro assays in different human cell lines to evaluate their efficacy. By corroborating the data we are able to reduce the numbers of lead candidates to a minimum; (vii) Combinations of the best performing epitopes are assessed in combination in human cell lines to determine synergy/additivity; (viii) In vitro testing of potential combinations in different tumor cell lines using NOD/SCID mice as a surrogate animal model to simulate human cancers and evaluate the effects of peptide antibodies elicited in response to the vaccine epitopes in different tumor cell lines; (ix) the combinations are then tested using SCID mouse models as a surrogate animal model to simulate human cancers and evaluate the effects of peptide antibodies elicited in response to the vaccine epitopes to finalize the potential candidates; (x) Test efficacy in transgenics or appropriate animal model; (xi) Finally, we conduct human clinical trials to assess safety and toxicity.
Road map for the design of an effective peptide vaccine versus a peptide mimic strategy
The novelty of our incremental approach resides in a hypothesis-driven basic research in various aspects of oncoimmunology involving the elucidation of several basic immunological and structural concepts that eventually can be translated to the clinic. Given the lack of preclinical models (transgenic or syngeneic animal models) to reliably predict clinical activity of vaccine constructs (active immunization) that target multiple tumor types concurrently, we have instead utilized a surrogate and indirect model in which rabbit antibodies elicited by the vaccine are used to test the efficacy of the vaccine in transplantable mouse models challenged with appropriate and specific human tumor cell lines. Similarly, our peptide mimics serves a similar purpose: to corroborate the effects of the antibodies in the transplantable mouse models as their mode of action is similar in preventing the heterodimerization of the RTKs. This allows us to have more confidence in validating the vaccine epitopes.
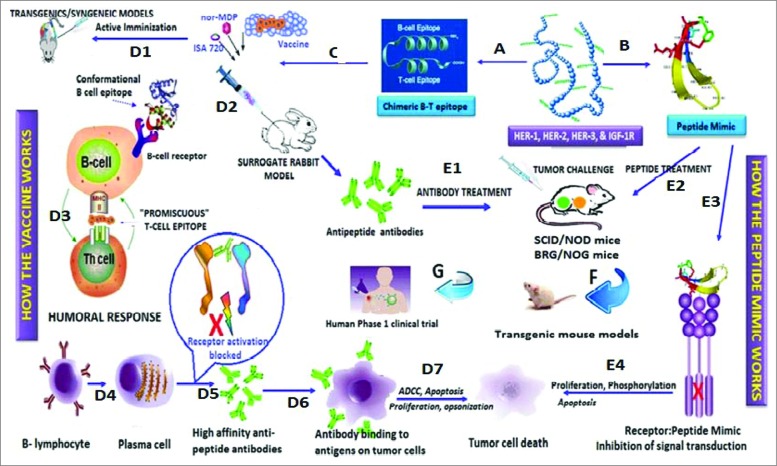
Figure 2 outlines our road map for designing chimeric B cell-peptide vaccines with “promiscuous” T-cell epitope (Pathway A). 23,29,47 Mice (transgenic/syngeneic) are immunized with the vaccines and tumor growths/reductions are monitored over time. 26,27,43,48 Rabbits are used as a surrogate model to develop high affinity antipeptide antibodies which are used to treat transplantable mouse models (SCID) after tumor challenge. 1,49 Pathway B involves the design of proteolytically stable B cell-peptide mimics that lacks a T-cell epitope. The peptide mimic is not immunogenic and treatment of mice does not elicit antibody production. 4,44,49 This strategy allows us to corroborate effectiveness of antibody treatment vs. peptide treatment permitting us to prioritize peptide constructs for testing in transgenic mice models that can translate to human clinical trials. 46 Either pathway results in potential tumor cell death. The mode of action for active immunization: The conformational peptide epitope binds the B-cell receptor, and the measles virus MVF “promiscuous” T-cell epitope binds MHC class II without antigen processing, thus preserving the native structure of the vaccine construct. 47,50 The uniqueness of our strategy is that we do not use carrier proteins to generate T cell help for the B cells to make high affinity Abs. The mechanism of action for the peptide mimic is direct binding of the B-cell peptide mimic to the receptor preventing dimerization/heterodimerization. The overall mechanism of anti-tumor action involves the binding of the antibody or the peptide mimic to the receptor target on the tumor cell, preventing signal transduction and eliciting a variety of anti-tumor cellular mechanisms.
Figure 2.
Roadmap of peptide vaccine versus peptide mimic strategies. Pathway A involves the design of chimeric peptides comprising the conformational B-cell epitope and incorporating a “promiscuous” T-cell epitope. The vaccine is emulsified with an adjuvant (nor-MDP) in a vehicle (ISA 720; squalene/arlacel) (C), and used for vaccination. When relevant transgenic mice (MMTV/neu) or a syngeneic model) (D1) are available for assessing vaccine efficacy, these mice are immunized sc with the chimeric peptide vaccine and tumor growths/reductions are monitored over time. Rabbits are used as a surrogate model (D2) to develop high affinity antipeptide antibodies which can be used to treat transplantable mouse models (SCID) (E1) after tumor challenge. Tumor growth is then monitored over time as we have described. The humoral response (D3) results in activated B-lymphocyte (D4) and the plasma cell give rise to high affinity antibodies (D5) that recognizes the antigen (D6) on tumor cells causing cell death through several different mechanisms (D7) (ADCC, Apoptosis, etc). On the other hand, Pathway B involves the design of conformational B cell-peptide mimics with enhanced proteolytic stability. Because a T-cell epitope is not included, the peptide lacks immunogenicity, thus the peptide mimic treatment does not elicit antibody production. The peptide mimics are dissolved in PBS and are injected in mice by iv treatment (E2) at the time of tumor challenge, and treated repeatedly over several weeks until control mice develop large tumors. This strategy allows us to corroborate effectiveness of antibody treatment vs. peptide treatment, permitting us to prioritize peptide constructs for testing in (i) transgenic mouse models (F); and (ii) human clinical trials (G). The peptide mimics bind the receptor (E3) and inhibits signal transduction (E4) resulting in tumot cell death.
Peptide mimics as safe therapeutics
Peptides are small protein-like chains of amino acids and a peptide mimic is a peptide or a peptide-like molecule that intends to mimic one portion of the entire protein which is usually a binding or active site of an enzyme. 51 Peptides are short sequences of amino acids that can be assembled chemically by solution–based or solid-phase peptide synthesis. New synthetic strategies to improve productivity and reduce metabolism of peptides, along with alternative routes of administration have been developed in recent years, and a large number of peptide-based drugs are now being marketed. 52 There are approximately 60 therapeutic peptides on the market, 150 in clinical phases and 400 in advance pre-clinical stages. Of the products now approved, about 30 peptide drugs are currently marketed in the US, and 17% are used in the cancer clinical setting. 53
One of the major drawbacks of using peptides as therapy is their stability, low bioavailability and high susceptibility to proteosomal degradation. 54 These obstacles can be overcome with the use of non–natural amino acids (D-amino acids), pseudo and modified peptides such as cyclisation, glycosylation, pegylation, amidation and acetylation that can be implemented to increase the usability of synthetic peptides. Peptides that block receptor-ligand interaction can be obtained by the use of structure-based design. The main goal is to maintain the conformational space and orientation of the bioactive surface while retaining sufficient flexibility to bind cooperatively with a given receptor. 55 We have successfully demonstrated the viability of the use of peptide mimics on the blockade of receptor:ligand interactions such as B7:CD28 in an autoimmune disease (EAE) model. 25,44,45 The retro-inverso modification is characterized by a reversal of the peptide backbone by inverting the amino acid sequence, as well as reversal of the amino acid chirality by utilizing D-amino acids. The resulting product is a topographical equivalent of the parent peptide with the amino side chain in similar orientation (Fig. 3). Because retro-inverso peptides are synthesized with D-amino acids and proteases usually recognize L-amino acids, they should be resistant to proteosomal degradation, and therefore will increase the bioavailability of the peptidomimetic therapeutic in vivo. 25,54,56 Synthetic peptides offer the benefits of being water soluble, non-immunogenic, and have the ability to easily cross tissue barriers. 57 Peptide mimics represent a safe and viable therapeutic strategy for blocking aberrant signaling pathways with high affinity and strong potency. There are many authoritative reviews of peptides as vaccines and peptidomimetics as immunomodulators. 58–61 Other advantages of peptide therapeutics include: low cost, higher specificity and potency; due to their superior compatibility with targeted proteins, ability to penetrate the cell membrane, lack of immunogenicity and improved safety profiles.
Figure 3.
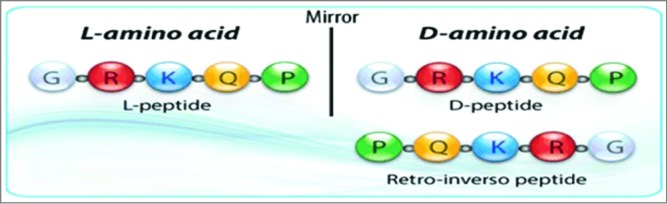
Schematics of retro-inverso peptides. (A) Parent peptide, synthesized with L-amino acids. (B) Parent peptide, synthesized with D-amino acids; side chains in opposite orientation. (C) Retro-inverso peptide (amino acid sequence reversed in relation to the parent peptide) synthesized with D-amino acids in reverse order (RI); Reversal of the peptide backbone direction will result in the mirroring of protein structure while inversion of amino acid chirality (L to D) achieves a mirroring of side chain positions (exact mirror image of parent peptide).
Receptor tyrosine kinases (RTKs) drives cancer metastasis
Receptor tyrosine kinases (RTKs) are an important family of signaling proteins involved in many protein-protein interactions that form the basis of many cellular processes such as proliferation, differentiation, migration, apoptosis and survival. 10,62,63 Upon ligand binding, the RTKs form homodimers or heterodimers and receptor-dimerization has been shown to be an essential requirement for HER function and for the signaling activity of these receptors. 64 Overexpression of the RTK's is associated with poor prognosis in several tumor types, including breast, pancreatic and colon cancers. Among the most aggressive RTKs are the epidermal growth factor receptors (ErbB) as represented by HER-1, HER-2, HER-3 and HER-4, vascular endothelial growth factor receptor (VEGFR) and insulin growth factor receptor (IGFR). 65–77 Tumors induce blood vessel growth (angiogenesis) by secreting various growth factors which play a critical role in the growth and spread of cancer. Vascular endothelial growth factor (VEGF) has been shown to stimulate tumor growth at both primary and metastatic sites. Ligand binding promotes receptor dimerization, autophosphorylation, and activation of multiple downstream signaling cascades that stimulate vascular permeability, cell survival, proliferation, migration, or adhesion. 78,79 IGFR has also been implicated in the growth and development of several malignancies. 80 The IGF pathway plays a major role in cancer cell proliferation, survival and resistance to antineoplastic therapies in many human malignancies many different types of cancer. 76,77 As with the HER and VEGF families, IGF-1R undergoes conformational changes and autophosphorylation upon ligand binding followed by phosphorylation of intracellular substrates, the MAPK and PI3K/Akt pathways are activated which has been shown to lead to cell proliferation and resistance to apoptosis. 81–83
Targeted therapy inhibits signal transduction pathways
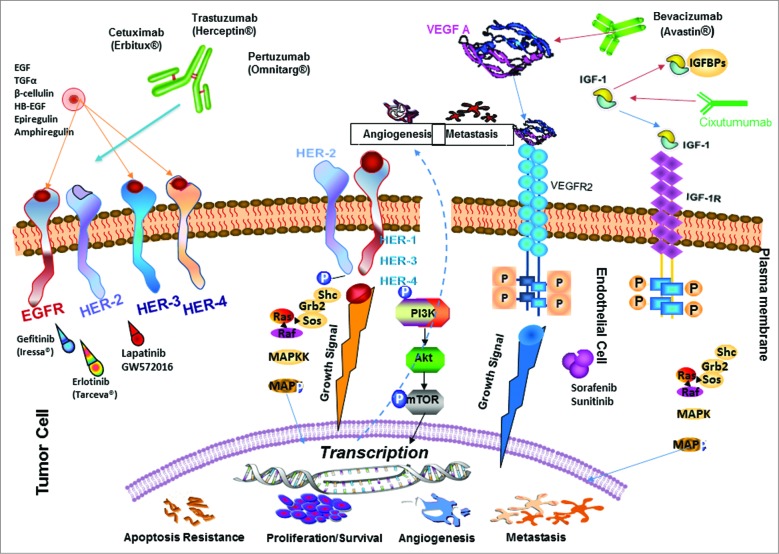
A plethora of FDA approved drugs (Fig. 4) that target HER, VEGF and IGF-1R receptors consists of 2 types of antagonists: hmAbs and small molecule TKIs. hmAbs have been developed against the extracellular domains (ECD) of the HER family members, and they exert their anti-tumor effect via several different mechanisms, including: inhibition of ligand binding or dimerization, induction of apoptosis, inhibition of tumor cell signaling and activation of complement dependent cytotoxicity or antibody dependent cellular cytotoxicity. 84–89 Trastuzumab (Herceptin®, Genentech Inc..,) is a recombinant hmAb directed against the ECD of HER-2 protein that works by blocking intracellular signaling. 17,90 Another recombinant humanized HER-2 mAb pertuzumab (Perjeta) sterically blocks dimerization of HER-2 with EGFR and HER-3 and thus can inhibit signaling from HER-2/HER-3 and HER-2/EGFR heterodimers. 91 Combination of trastuzumab and pertuzumab was shown to synergistically inhibited the survival of BT474 breast cancer cell line with increased apoptosis and was the rationale to combine these HER-2-targeting agents as a more effective therapeutic strategy to overcome trastuzumab resistance. 92–94 Other approved humanized mAbs developed against HER-2 include ado-trastuzumab emtansine (Kadcyla) which has a different mechanism of action and was developed with the intention to avoid the toxicities of targeted therapies. Cetuximab, a chimeric anti-HER-1 antibody was approved for treatment of patients with advanced colorectal cancer in 2003. Other HER-1 approved agents are panitumumab (Vectbix) and matuzumab. Specialized cancer treatments with anti-angiogenic agents approved by the FDA include mAb bevacizumab (Avastin) or small molecules TKIs SU11248 (sunitinib) and BAY 43–9006 (sorafenib). 95–97 Anti-angiogenic therapies include blocking VEGF:VEGFR-2 interactions using antibodies that bind VEGF as well as the extra-cellular domain of VEGFR-2. 98–106 Currently, there are several anti-IGF-1R monoclonal antibodies that have been developed to block IGF-mediated proliferation. One such agent is cixutumumab (CIX), or IMC-A12. CIX is a fully human IgG1/λ monoclonal antibody directed at the type I IGF receptor (IGF-1R). CIX binds IGF-1R with high affinity and blocks interaction between IGF-1R and its ligands, IGF-1 and -II, and induces internalization and degradation of IGF-1R. CIX has demonstrated tumor growth inhibition in both in vitro and in vivo studies. Currently, there are at least 58 active clinical trials evaluating these agents alone or in various combinations, and the most clinically viable options include usage of antagonistic mAbs and small molecule TKIs. 107
Figure 4.
Signal transduction pathways drive cancer metastasis. The signaling pathways of HER family members, VEGF and IGF-1R and the current drugs that target these oncoproteins in cancer are shown. HER-2 can heterodimerize with any of the ligand-activated HER receptors (HER-1, HER-3 or HER-4) and this association leads to intracellular signaling via 2 major pathways, the MAPK pathway and the PI3K pathway, leading to proliferation, cell survival, metastasis and angiogenesis. On the other hand, VEGF can bind to its main receptor, VEGFR-2 (KDR), and this binding causes intracellular phosphorylation of the receptor, thereby stimulating the PI3K pathway and stimulating angiogenesis. The signaling pathways can be targeted extracellularly using humanized monoclonal antibodies, such as trastuzumab and pertuzumab (HER-2), cetuximab (EGF receptor), bevacizumab (Avastin) (VEGF), and cixutumumab (IGF-1R) which can prevent ligand binding and activation of the receptors or can directly block binding of an activated receptor to another. At the intracellular level, small-molecule inhibitors, such as sunitinib (VEGF), lapatinib (HER-1 and HER-2) and erlotinib (HER-2), can disrupt the phosphorylation sites and directly prevent activation of the PI3K or MAPK pathways. P: Phosphate; VEGFR: VEGF receptor.
Cross-talk among RTKs to overcome acquired resistance
Overwhelming data exists on the extensive cross-talk between the RTK multiple receptors: vertical signaling (HER-1, HER-2, HER-3) and horizontal signaling via VEGF and IGF-1R (reviewed in Kaumaya & Foy). 1 Several molecular mechanisms are operative within the HER family of receptors and compensatory signaling from outside the HER family such as VEGF, (IGF-1R) and other intracellular downstream signaling pathway. There is a wealth of evidence that implicates the IGF-1R as a major target in cancer drug discovery with emphasis on resistance mechanisms.
Targeting two or more receptors has the potentials to inhibit cross talk and development of resistance that occurs in single treatment. 108 Novel combination therapies targeting these multiple signaling pathways are urgently needed that can offer hope, circumvent resistance mechanisms, provide synergy and enhance anti-tumor effects. For these reasons, numerous new agents and therapeutic strategies are under intense investigation. Innovative immune-based therapies that target these receptors are particularly intriguing, as they have the potential to achieve long-term control in several tumor types without causing the numerous toxicities associated with present FDA-approved regimens. For example, resistance to trastuzumab is poorly understood and is thought to be mediated by a number of different mechanisms such as overexpression of HER-1 or HER-3, activation of the PI3K/Akt pathway by upregulation of IGF-1R and cleavage of ECD.
HER-2 B-cell epitope peptide vaccines
HER-2/neu is an oncoprotein that is overexpressed in many types of tumors and is associated with highly aggressive forms of cancers. 65,66 HER-2 is an attractive therapeutic target in cancers for several reasons. The amount of HER-2 expressed on cancer cells is much higher than in normal adult tissues potentially reducing the toxicity of HER-2 targeting drugs. 109 Tumors with a high expression of HER-2 often show homogenous, intense IHC staining, signifying that HER-2 targeted therapy would target most cancer cells in a given patient. 110 Moreover, HER-2 overexpression is found in both the primary and metastatic sites, suggesting that HER-2 targeted therapy may be effective in all disease sites. 111 HER-2 is thus an important therapeutic target and therapeutic modalities have been devised that target the receptor and downstream molecular pathways. We have advanced innovative platforms to stimulate the immune system by constructing novel chimeric peptides able to elicit high affinity vaccine antibodies. We have developed novel HER-2 vaccines that have the potential to act as highly effective immunotherapies against a wide variety of tumor types. 25,43,48,112
First generation HER-2 vaccine
I identified by using antigenicity algorithms (reviewed by Kaumaya and co-workers) several B-cell epitopes of HER-2 ECD using computer-aided analysis. 43,48 These peptide B-cell sequences were assembled by solid phase peptide synthesis together with a measles virus fusion protein (MVF, amino acid sequence 288–302) “promiscuous” T cell epitope. The vaccination protocol consisted of emulsifying the peptide with nor-MDP adjuvant in ISA-720. Extensive in vivo studies in neu transgenic mice and mice challenged with syngeneic tumor cells transfected with human HER-2 (RENCA/lacZ/HER-2) demonstrated efficacy in prevention of mammary tumors as well as reduction in pulmonary metastases in the syngeneic model. The most effective combination vaccine was found to be HER-2 sequences 316–339 and 628–647 identified through our peptide strategy. 43 It turns out that these 2 sequences overlapped the pertuzumab and trastuzumab binding sites of HER-2. Our first generation HER-2 peptide vaccines were identified prior to the publications of the crystal structure of the HER-2 ECD. 113–115 The efficacy of the combination vaccines was ascertained by numerous in vitro studies such as receptor down-modulation, flow cytometry, immunoprecipitation, ADCC and phosphorylation. We initiated a Phase 1 clinical trial with the combination vaccines in 2004.
Phase I active immunotherapy with combination of 2 chimeric HER-2 B-Cell epitopes with metastatic and/or recurrent solid tumors
An NCI funded (CA84356), OSU Cancer IRB approved (2001C0108) and FDA approved (BB-IND-9803) Phase 1 clinical trial with a “first” generation combination of 2 HER-2 Chimeric B-cell MVF 316–339 epitopes and MVF 628–647 emulsified with nor-MDP as adjuvant and ISA 720 vehicle was successfully completed at the OSU James Cancer Hospital. The purpose of this phase I trial was to evaluate the maximum-tolerated dose (MTD), safety profile, and immunogenicity of 2 chimeric, B-cell epitopes derived from the HER-2 extracellular domain in a combination vaccine with a promiscuous T-cell epitope (i.e., MVF) and n-MDP adjuvant emulsified in ISA 720. Eligible patients with metastatic and/or recurrent solid tumors received 3 inoculations on days 1, 22, and 43 at doses of total peptide that ranged from 0.5 to 3.0 mg. Immunogenicity was evaluated by enzyme-linked immunosorbent assay, flow cytometry, and HER-2 signaling assays. Twenty-four patients received 3 inoculations at the intended dose levels, which elicited antibodies able to recognize native HER-2 receptor and inhibited both the proliferation of HER-2-expressing cell lines and phosphorylation of the HER-2 protein. The MTD was determined to be the highest dose level of 3.0 mg of the combination vaccine. There was a significant increase from dose level 1 (0.5 mg) to dose level 4 (3.0 mg) in HER-2-specific antibodies. Four patients (one each with adrenal, colon, ovarian, and squamous cell carcinoma of unknown primary) were judged to have stable disease; 2 patients (one each with endometrial and ovarian cancer) had partial responses; and 11 patients had progressive disease. Patients with stable disease received 6-month boosts, and one patient received a 20-month boost. There was preliminary evidence of clinical activity in several patients. In conclusion the combination vaccines were safe and effective in eliciting antibody responses in a subset of patients (62.5%) and were associated with no serious adverse events, autoimmune disease, or cardiotoxicity. 46
Second generation HER-2 vaccines
The structures of the HER-2-trastuzumab and pertuzumab complexes have led to new insights into the mechanistic and biological activities of HER-2 antibodies as well as the process of ligand-induced receptor dimerization. 115 which in turn has empowered us to rationally design more effective HER-2 conformational epitopes including the trastuzumab-binding epitope (597–626) and the pertuzumab-binding epitope (266–296).
Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu
The three-dimensional structure of the HER-2 complex with trastuzumab revealed that the antigen binding region of HER-2 spans residues 563–626 that comprises an extensive disulfide bonding pattern. 113 In order to minimally dissect the interacting binding region of HER-2, we designed 4 synthetic peptides with different levels of conformational flexibility. The interacting loops in subdomain IV comprises residues in loop 1: 579–582 (2 disulfide pairings between C563-C576 and between C567-C584), loop 2: 592–595 (cysteine disulfide pairings between C587-C596), and loop 3: 615–625 (cysteine disulfide between C600-C623). Chimeric peptides incorporating the measles virus fusion “promiscuous” T cell epitope via a 4 residue linker sequence were successfully synthesized, purified and characterized. All conformationally restricted peptides were recognized by trastuzumab with 563–598 and 597–626 showing higher reactivity. All peptide sequences prevented the function of trastuzumab inhibiting tumor cell proliferation. All epitopes were immunogenic in FVB/n mice with antibodies against the 597–626 and 613–626 epitopes recognizing HER-2. The 597–626 epitope was immunogenic in outbred rabbits eliciting antibodies which recognized HER-2 at the HER-2/trastuzumab interface, inhibited proliferation of HER-2 expressing breast cancer cells in vitro and caused their ADCC. Moreover, immunization with the 597–626 epitope significantly reduced tumor growth in transgenic BALB-neuT mice. These results suggest the peptide B-cell immunogen is appropriate as a vaccine for HER-2 overexpressing cancers since the resulting antibodies show analogous biological properties to trastuzumab. We concluded that the HER-2 sequences 597–626 and the 266–296 could be used as a combination vaccine and translated to the clinic as detailed in the next section. 26
Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo
The crystal structure of pertuzumab bound to the ECD of HER-2/neu shows that the details of interaction lie in residues 266–333. 115 We examined 3 different sequences, 266–296, 298–333 and 315–333 to determine the best minimal conformational epitope for effective B cell immunization. We reported on the activity of several constructs containing complex differential disulfide pairings as well as their linear counterparts in order to mimic the pertuzumab-binding conformational region. The immunogenicity of each cyclized and noncyclized constructs were determined in both mice and rabbits, eliciting a high affinity high titer antibody responses. The antibodies recognized the native HER-2/neu receptor. Additionally, only 3 of the 6 putative constructs 266–296(CYC), 266–296(NC) and 315–333(CYC) were able to mediate ADCC and reduce phosphorylation of the HER-2/neu tyrosine kinase domain. Epitope 266–296 was able to suppress cellular proliferation in HRG-stimulated MCF-7 cells. Our results show that the pertuzumab-like epitope constructs had statistically reduced tumor onset in both transplantable tumor models (FVB/n and Balb/c) and significant reduction in tumor development in 2 transgenic mouse models (Balb-neuT and VEGF+/− Neu2.5+/−). These studies demonstrate that both linear (NC) and conformational (CYC) peptide vaccines corresponding to residues 266–296 of the dimerization region of HER-2/neu are able to elicit an immune response with antitumor capabilities, resulting in a peptide vaccine that will be able to mimic the effects of pertuzumab in vivo without the harmful side effects associated with the mAb therapy. 27
2nd generation HER-2 vaccine phase 1 clinical trial
We are currently in the process of completing a new FDA approved, NCI funded Phase 1/11b trial (NCT01376505) with an improved vaccine combination that targets HER-1:HER-2, HER-2:HER-2, and HER-2-HER3, thus overcoming mechanisms of resistance that are common to HER-targeted therapies at the OSUCCC. http://clinicaltrials.gov/ct2/show/NCT01376505?term=Kaumayaandrank=1. The main objective of this study is to determine the optimal biological dose of the human epidermal growth factor receptor 2 (HER-2/neu) multivalent vaccines in patients with advanced solid tumors comprising of a trastuzumab-like and pertuzumab-like antigenic epitope. The clinical trial is being conducted under an IND application (IND#14633) approved by the FDA and the Ohio State University human IRB. The combination vaccine is a mixture of 2 synthetic peptides that consists of B-cell epitopes of HER-2 that corresponds to amino acids 597–626 and 266–296. These peptides are fused via a 4-residue linker sequence (GPSL) to the T helper (MVF) epitope that corresponds to amino acids 288 to 302. The GMP peptide and n-MDP met the all FDA and US Pharmacopeia requirements for sterility (ie, bacterial/fungal), endotoxins, and potency. The saline-oil phase vehicle (Montanide ISA 720) was used as an emulsifying agent with approved certificate of analyses for toxicity, emulsifying property, and sterility. Patients receive 3 vaccinations, each given 21 days apart. Following the 3 doses, they can receive booster shots every 3 months if they continue to have stable or responding disease. To date, a total of 8, 9, 15 and 4 patients were enrolled in dose levels 1–4 respectively. Nineteen of 36 patients completed at least 3 vaccinations (N=5, 5, 6 and 3 in cohorts 1–4 respectively). Of those, 6 patients (N=3 in cohorts 1 and 2 each) were able to receive at least 1 booster due to stable disease and good tolerance of therapy. The vaccine was very well tolerated with minimal or no toxicities in most patients. No dose limiting toxicities were seen in the study patients. The most common toxicities in 36 patients that were judged by the investigators to be at least possibly related to the study treatment included grade 3 injection site reactions (1 patient in cohort 1 and 2 patients in cohort 3; all grade 3) and grade 2 buttock hematoma that developed at the site of the injection (1 patient in cohort 2). One additional patient in cohort 1 developed grade 2 systemic allergic reaction (mild hypotension and diaphoresis). None of the injection site reactions were considered serious because they were mainly associated with self-limiting swelling and pain at the site of the injection and none of them resulted in adverse outcome or hospitalization. 10 patients had stable disease at day 71 with 5 patients receiving a 6 months boost and one patient received 4 6 months boost (2.5 years). The OBD/MTD is yet to be determined.
Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways
Angiogenesis is the formation of new blood vessels from pre-existing ones and is crucial to cancer tumor growth. Tumor growth, progression and metastasis are critically influenced by the production of pro-angiogenic VEGF. 71 VEGF expression is increased in many different types of cancer and most tumor cells secrete VEGF. 116 It is also thought that VEGF is a key promoter of metastasis. 117 VEGF is a 34–42kDa, homodimeric, heparin binding, disulfide-bonded glycoprotein that has several isoforms arising from splice variants. 68,71,116,118,119 VEGF has 3 known tyrosine kinase receptors: Flt-1 (VEGF-R1), KDR (VEGF-R2, Flk-1), and Flt-4 (VEGF-R3). VEGF-R1 has a higher affinity for VEGF, but it has been shown that VEGF-R2 is the biologically relevant receptor. Therefore, VEGF and its receptors VEGFR-1 and VEGFR-2 are prime targets for anti-angiogenic intervention. 72–74 Most importantly, overexpression of HER-2 is associated with increases expression of VEGF at both the RNA and protein level in breast cancer cells and a positive association between HER-2 and VEGF expression in breast cancer patients has been identified. 73 Targeting these 2 receptors using a combination strategy can therefore interact in a synergistic/additive manner killing tumor cells and retarding tumor development. 120–122 Promising anti-angiogenic drugs are currently available; however, their susceptibilities to drug resistance and long term toxicity are serious impediments to their use, thus requiring the development of new therapeutic approaches for safe and effective angiogenic inhibitors.
We have designed several peptides to mimic the VEGF-binding site to its receptor VEGFR-2.4 The VEGF conformational peptide mimic VEGF-P3(CYC) included 2 artificial cysteine residues which upon cyclization constrained the peptide in a loop native-like conformation to better mimic the anti-parallel structure of VEGF. The engineered cyclic VEGF mimic peptide demonstrated the highest affinity to VEGFR-2 by surface plasmon resonance assay. The VEGF peptide mimics were evaluated as inhibitors in several in vitro assays in which VEGF-dependent signaling pathways were observed. All VEGF mimics inhibited VEGFR-2 phosphorylation with VEGF-P3(CYC) showing the highest inhibitory effects when compared with unstructured peptides. Additionally, we show in several angiogenic in vitro assays that all the VEGF mimics inhibited endothelial cell proliferation, migration, and network formation with the conformational VEGF-P3 (CYC) being the best. The VEGF-P3(CYC) also caused a significant delay in tumor development in a transgenic model of VEGF(+/−)Neu2–5(+/−). These results indicate that the structure-based design is important for the development of this peptidomimetic and for its anti-angiogenic effects.
Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo
HER-2 overexpression causes increased expression of VEGF at both the RNA and protein levels. HER-2 and VEGF are therefore considered good targets for combination immunotherapy. We designed and synthesized peptides based on the binding of HER-2 with pertuzumab and VEGF with VEGFR2. We demonstrated that treatment with the HER-2 peptide mimics induces potent anti-tumor responses in vitro as determined by cell viability, proliferation, and HER-2 phosphorylation assays. We also demonstrate in a transplantable BALB/c mouse tumor model that treatment with the peptide mimics resulted in a greater delay in tumor growth and development. Similarly, treatment with the VEGF peptide mimics inhibited angiogenesis in vivo as assessed by a Matrigel plug assay. Our study shows that combination treatment with HER-2 and VEGF peptide mimics produces superior anti-tumor effects in vitro and in vivo compared to targeting single pathway. 123
Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer
HER-2 and VEGF represent validated targets for several tumor types and inhibitors of these growth factors have become key therapies to improve antitumor efficacy. Novel biologics that might result in significantly better therapeutic outcomes and improved safety profiles are urgently required. Specially engineered HER-2 and VEGF peptides in combination with low-dose chemotherapy regimen could provide a substantial direct impact on tumor metastasis and cancer progression. The antitumor effects of HER-2 and VEGF peptide mimics in combination with metronomic paclitaxel were tested in both transgenic and transplantable mouse models of human breast cancer. The combined treatment with low dose Paclitaxel and HER-2 or VEGF peptide mimics had greater inhibitory effects than either agent alone. Peptide treatment caused no cardiotoxic effects when compared to paclitaxel and trastuzumab (Herceptin) which showed elevated cardiotoxic effects. Combination treatment demonstrated significant reduction in tumor burden and prolonged survival rates in both transgenic and transplantable tumor models. Tumor weights were reduced significantly in mice treated with HER-2 peptides alone (P<0.005) with a more significant value in the case of combination treatment (P<0.001). Low dose paclitaxel alone had no significant effect on tumor growth in the transgenic model (P=0.999). Combined therapy with specially engineered native peptide sequences of HER-2 and VEGF used in combination with metronomic paclitaxel demonstrated enhanced efficacy and acceptable safety profiles. 49 This novel approach to targeted therapy may offer new avenues for the treatment of breast cancers and other solid tumors with HER-2 and VEGF overexpression.
EGFR (HER-1) peptide vaccines and peptide mimics
HER-1 is a validated target for several cancers including lung, colorectal and breast. We have developed several HER-1 vaccine epitopes as well as peptide mimics. In this study, we selected 3 critical epitopes of HER-1 (347–374, 382–410 and 418–435) as potential candidates for both the vaccine and therapeutic approach. The choice was based on (i) the crystal structure of the complex between EGF and EGFR and analysis of the contact residues on both sides of the interface that are all involved in ligand binding, and (ii) computer aided analysis of various algorithms of immunogenicity and antigenicity. The chimeric B-cell epitopes were synthesized incorporating the MVF “promiscuous” T-cell epitope and sequence specific antipeptide antibodies to the HER-1 ligand binding domain was elicited by immunization of rabbits and mice. The peptide vaccines were highly immunogenic in both mice and rabbits and the antibodies elicited were able to specifically recognize the native HER-1 receptor as shown by their reactivity in MDA-MB-468 triple negative breast cancer (TNBC; estrogen receptor-negative, progesterone receptor-negative and HER-2-negative) cells and A549 lung cancer cells in flow cytometry and their binding to recombinant human HER-1 in ELISA. The peptide mimics and antipeptide antibodies to all 3 epitopes inhibited cancer cell growth, prevented HER-1 specific phosphorylation, downregulated HER-1R signaling pathways, caused increased apoptosis and ADCC in HER-1 expressing cells. In mouse model studies, 2 of the vaccine epitope were able to delay tumor growth in a met-1 transplantable breast cancer model and in SCID mice that were inoculated with A549 lung cancer cells. The combined in vitro and in vivo results obtained showed that the HER-1 (382–410) and HER-1 (418–435) sequences were the best candidates for the production of a vaccine and a therapeutic peptide mimic. 124
HER-3 peptide vaccine and peptide mimics
Several recent studies have shown that HER-3 is frequently up-regulated in cancers with HER-1 or HER-2 over-expression. HER-3 synergistically increases the transforming potential of HER-2. 125, 126 HER-3 also has the highest binding affinity for phosphoinositol 3-kinase (PI3K) when compared to that of other HER family members, because phosphorylation of the HER-3 cytoplasmic tail creates 6 docking sites for the PI3K p85 subunit. As a result, HER-3 serves as a key activator of PI3K signaling and subsequent resistance to apoptotic signals in a wide range of cancers. 127–130 HER-3 expression or signaling is associated with resistance to: HER-2 inhibitors in HER-2-amplified breast cancers, HER-1 inhibitors in lung cancers, pertuzumab resistance in ovarian cancers, anti-estrogen therapies in ER-positive breast cancers, EGFR inhibitors in head and neck cancer, and hormone resistance in prostate cancers. 131–137 We designed, synthesized and evaluated 4 novel HER-3 peptide peptides encompassing residues 99–122, 140–162, 237–269 and 461–479 of the HER-3 extracellular domain as B cell epitopes for active immunotherapy. The vaccine antibodies and peptide mimics induced anti-tumor responses, such as: inhibition of cancer cell proliferation, inhibition of receptor phosphorylation, induction of apoptosis and ADCC. The peptide mimics and vaccine antibodies also significantly inhibited growth of xenografts originating from both pancreatic and breast cancers. We also showed synergistic effects of combination treatment with the HER-3 461 epitope and HER-2 and IGF-1R vaccine antibodies in vitro. 138
IGF-1R peptide vaccine and peptide mimics
IGF-1R has been implicated in the growth and development of multiple tumor types.80 The IGF-1R:IGF-1 pathway is also implicated in the development of resistance to anti-cancer drugs 76,139 in lung, 140 breast, 141 pancreas, 142 colorectal, 143 prostate,144 and head and neck 145 cancers. Increased expression of IGF-1R increases expression of HER-1 and results in the formation of IGF-1R/HER-1 dimers. 146,147 We have identified, synthesized and characterized 4 sequences that are derived from the IGF-IR ligand binding domain. These epitopes were designed based on the ligand binding site of the receptor using crystallographic structures, mutagenesis studies and models of the complex between the receptor ligand interactions. 148 The 4 peptides are IGF-IR-6–33, IGF-IR-56–81, IGF-IR-234–251 and IGF-IR-246–273. We showed that, the peptide vaccines were immunogenic in outbred rabbits and the vaccine antibodies and peptide mimics were able to block cancer dependent pathways like cell proliferation, receptor phosphorylation, and induction of apoptosis and cause ADCC. We used both pancreatic cancer cells and Trastuzumab resistant breast cancer cells in all these assays and also in establishing tumors in SCID mice. The peptide mimics significantly inhibited tumor growth in the BxPC-3 transplantable model and also in the JIMT-1 model. Overall, our results showed that the 56–81 and the 234–252 epitopes could be considered potential therapeutic and vaccine candidates for the treatment of Trastuzumab resistant cancers and other IGF-IR dependent cancers. 149
Expression of HER and IGF family of receptors in human cancer cells govern the choice of combination of tumor types
Table 1 shows expression levels of HER family receptors and IGF-1R in different cancer cells used in our studies. Expression levels were evaluated either through simple ELISA or western blotting and cell lines used to target the combination approach: (i) For HER-2 and IGF-1R: BT-474 and JIMT-1 cells was used as they express medium to high levels of both receptors. (ii) For HER-2 and HER-3: BT-474 and JIMT-1 was used; (iii) For HER-1 and IGF-1R: BxPC-3, MDA-231, Caco-2 and BT-549 was used since all these cell lines express significant levels of the target receptors. (iv) For HER-3 and IGF-1R: BT-474, BxPC-3 Caco-2 and JIMT-1 was used due to their medium to high expression levels of the receptors.
Table 2:
HER-2 & IGF-1R
| Peptide Abs | Proliferation % inhibition BT474/ JIMT-1 | Phosphorylation % Inhibition BT474/ JIMT-1 | ADCC % Lysis (100:1) JIMT-1 | Apoptosis Luminescense x106 BT474/ JIMT-1 | Invasion Assay JIMT-1 cells invaded |
|---|---|---|---|---|---|
| α-HER-2 (597) | 35/8 | pHER-2; HER-2: 10/5 IGF-1R:10/15 | 12 | 14/3 | 580 |
| α-IGF-1R (56) | 12/25 | p-IGF-1R; HER-2: 7/7 IGF-1R: 20/45 | 40 | 4/14 | 550 |
| α-HER-2 597 + α-IGF-1R(56) | 40/45;P<0.003 | pHER-2; 10/25; P<0.05; p-IGF-1R; 25/55; P<0.005 | 70; P<0.001 | 15/17; P<0.001 | 400; P<0.05 |
Table 3:
HER-1 & IGF-1R
| Peptide mimics or Peptide Abs | Proliferation % inhibition JIMT-1 /BxPC3 Peptide Mimics | Phosphorylation % Inhibition JIMT-1 /BxPC3 Peptide Mimics | ADCC %Lysis(100:1) JIMT-1 Peptide Abs | Apoptosis Luminescense x106 JIMT-1 /BxPC3 Peptide Abs | Invasion Assay JIMT-1 cells invaded Peptide Abs |
|---|---|---|---|---|---|
| HER-1 (418) | 25/50 P<0.005 | p-HER-1; HER-1: 20/18 IGF-1R: 18/20 | 20 P<0.001 | 4/4 P<0.001 | 800 |
| IGF-1R(56) | 50/25 P<0.005 | p-IGF-1R; HER-1:30/25 IGF-1R: 30/30 | 50 P<0.001 | 8/8 P<0.001 | 800 |
| HER-1(418) + IGF-1R (56) | 75/75 P<0.001 | p-HER-1; HER-1: 50/30; P<001 p-IGF-1R: IGF-1R: 40/55; P<005 | 70 P<0.001 | 12/15 P<0.001 | 500; P<0.05 |
Table 4:
HER-3 & IGF-1R
| Peptide mimics /peptide Abs | Proliferation % inhibition JIMT-1 /BxPC3 peptide mimics | Phosphorylation % Inhibition JIMT-1 /BxPC3 peptide mimics | ADCC %Lysis(100:1) JIMT-1 peptide Abs | Apoptosis Luminescense x106 JIMT-1 peptide Abs |
|---|---|---|---|---|
| HER-3 | 10/10 | p-HER3 HER-3: 40/42; IGF-1R: 17/18 | 10 | 10 |
| IGF-1R | 40/20 | p-IGF-1R: HER-3: 22/22 IGF-1R: 20/22 | 40 | 12 |
| HER-3 + IGF-1R | 55/30; P<0.04 | p-HER-3; 55/65; P<0.01 p-IGF-1R; 45/ 30; P<0.02 | 70; P<0.037 | 15; P<0.002 |
Table 5:
HER-3 & HER-2
| peptide Abs | Proliferation % inhibition BT474 /JIMT-1 | Phosphorylation %Inhibition BT474 /JIMT-1 | ADCC % Lysis BT474 | Apoptosis Luminescense x104 BT474 |
|---|---|---|---|---|
| HER-2-(597) | 20/10 | p-HER-3 HER-3: 25/15; HER-2: 22/12 | 12 | 12 |
| HER-3 (461) | 35/10 | p-HER-2 HER-3: 20/5; HER-2: 40/10 | 10 | 4 |
| HER-2 + HER-3 | 50/18; P<0.02 | pHER-3; 35/17; P<0.05 pHER-2; 65/18; P<0.02 | 20; P<0.05 | 16 |
Table 1:
Expression of HER and IGF-1R in human cell lines
| Cancer Cell Lines | HER-1 | HER-2 | HER-3 | IGF-1R |
|---|---|---|---|---|
| BT-474 (breast) | low | high | medium | medium |
| BxPC-3 (pancreatic) | high | low | medium | high |
| OE-19 (esophageal) | high | high | low | high |
| JIMT-1 (breast) | medium | medium | medium | high |
| MDA-231 (TNBC) | high | low | low | high |
| BT-549 (TNBC) | high | low | low | high |
Novel combination approaches targeting HER-1, HER-2, HER-3 and IGF-1R
Novel combination therapies targeting these multiple signaling pathways are urgently needed that can offer hope, circumvent resistance mechanisms, provide synergy and enhance antitumor effects. Overwhelming data exists on the extensive cross-talk between the RTK multiple receptors (HER-1, HER-2, and VEGF) systems for cancer therapy 76,77 including many breast cancers 150–152 and to stimulate angiogenesis by up regulating VEGF expression.153 There is a wealth of evidence that implicates the insulin-like growth factor-1 receptor (IGF-1R) as a major target in cancer drug discovery with emphasis on resistance mechanisms. The receptor is a promising therapeutic target for several solid tumors and evidence of cross-talk between IGF-IR and other HER family receptors makes it even more important in a potential combination approach with other inhibitors. Recent evidence also suggests that HER-3 plays a central role and contributes to escape from therapeutic suppression by several tyrosine kinase inhibitors in breast cancer.131,154,155 We have delineated novel HER-3 and IGF-1R epitopes that will allow us to combine many different epitopes in the same vaccine or peptide mimics directed to multiple targets.
We have evaluated the potency and mechanism of new HER-3 and IGF-1R epitopes individually and in combination with HER-2 and HER-1 peptide vaccines/mimics. 138,149 Having established a portfolio of validated peptide epitopes as either vaccine candidates or peptide mimics, we have initiated a series of combination therapies to determine in vitro antitumor effects prior to verifying their efficacy in vivo in animal models. Ultimately, our goal is to identify the most biologically effective combinations of EGFR (HER-1), HER-2, HER-3, and/or IGF-1R peptide vaccines to selectively inhibit multiple receptors and signaling pathways to overcome the extensive receptor cross-talk that drives the biology and resistance of HER-overexpressing cancers. Optimal combinations of anti-HER-2 agents delivered with anyone of the following other growth factors such as HER-1, HER-3, VEGF and IGF-1R may provide the best therapy for breast cancers and other solid tumors including pancreatic, colon, GIST, and lung. This strategy holds the promise of achieving durable cures for multiple types of cancers that can be translated to human clinical trials.
-
(i)
HER-2 and IGF-1R. Resistance toward anti HER-2 antibodies trastuzumab has been shown to be mediated by increased IGF-1R signaling. 156 157 There is also significant evidence that shows cross-talk occurs between IGF-1R and HER-2 and both receptors form heterodimers in many breast cancers. 150–152 Preclinical and clinical studies from our laboratory have previously established 2 novel HER-2 B cell epitopes that can be used to target overexpression of the receptor in several cancers. 26,27 The IGF-1R-56–81 sequence was the most potent epitope using in vitro signaling assays and in vivo transplantable mouse models of cancer. Combination treatment with α-HER-2-(597–626) and α-IGF-1R-(56–81) peptide antibodies in trastuzumab-resistant (JIMT-1) and trastuzumab-sensitive (BT-474) human breast cancer cells inhibits proliferation, receptor phosphorylation, and significantly induces apoptosis, ADCC and cellular invasion. These results (Table 2) indicate that co-targeting HER-2 and IGF-1R produces significant anti-tumor effects, synergistically blocks tumor growth of breast cancers and can be used to overcome trastuzumab resistance in breast cancer and points to the potential benefits of dual targeting. 149 Additionally, in a recent study we found that the major biological effect promoted by IGF-1R was cellular invasion, which was mediated by both Src-Fak signaling and FoxM1. Our results strongly indicate that therapeutic combinations with IGF-1R-56 peptide antibody plus trastuzumab suppress invasion and induce ADCC of trastuzumab-resistant cells. 158
-
(ii)
HER-1 and IGF-1R. Crosstalk between IGF-1R and EGFR (HER-1) has also been extensively described in TNBC and human pancreatic cancer cells. 159 HER-1 is implicated in aggressive pancreatic cancers with poor patient outcome and decrease overall survival. 160,161 162–164 IGF-1R is expressed in 50–60% of pancreatic cancers. 165,166 This shows that HER-1 and IGF-IR are key players in many types of cancers. Co-targeting of HER-1 and IGF-IR have also shown great anti-tumor responses in breast cancers using MDA-MB-468 cells (Table 3).167 We have tested the effects of combination treatment with HER-1 and IGF-IR peptides on 2 cell lines that have relatively high IGF-IR and HER-1 expression. Our results validate the hypothesis that combined treatment with peptide mimics of HER-1 and IGF-1R are far superior to individual treatment in inhibition of proliferation and HER-1 and/or IGF-1R phosphorylation. These preliminary results indicate that targeting 2 relevant signaling pathways may be beneficial in future therapies of breast and pancreatic cancers which need further invitro and invivo studies. 149
-
(iii)
HER-3 & IGF-IR. IGF-IR and HER-3 directly bind and activate PI3K, Erk1/2, Src, and FAK signaling. 128,168–173 Increased PI3K, Src, and FAK signaling have each been independently associated with resistance to currently approved HER-2 targeted therapies and resistance to apoptotic signals in a wide range of cancers. 127–130,173–177 The IGF-1R:IGF-1 pathway is implicated in the development of resistance to anti-cancer drugs,76,139 in breast,141 pancreatic,142 and colorectal cancers.143 HER-3 signaling has been shown to be a major driver of resistance to IGF-1R-targeted agents. Many studies implicate extensive cross-talk between IGF-1R and HER-3 178 through the formation of heterodimers in breast cancers. 150–152 We evaluated the effects of peptide mimics and antipeptide antibodies on IGF-1 and HRG signaling in 2 breast (MCF-7, JIMT-1) and one pancreatic BxPC-3 cells. Our results showed that combination treatment in 3 different cell lines caused an increase in inhibition of proliferation (<60%) to either single treatment (35%). The effects of combination treatment on receptor phosphorylation were markedly increased compared to individual treatments. Overall, the results (Table 4) point to the potential benefits of targeting HER-3 and IGF-IR combinations in cancers that express these receptors. 138
-
(iv)
HER-2 and HER-3. HER-3 may also provide a route for resistance to agents that target HER-2. In fact, HER-3 expression or signaling is associated with resistance to: HER-2 inhibitors in HER-2-amplified breast cancers, EGFR inhibitors in lung cancers, pertuzumab resistance in ovarian cancers, anti-estrogen therapies in ER-positive breast cancers, EGFR inhibitors in head and neck cancer, and hormone resistance in prostate cancers. 131–137 HER-3 antibodies have been shown to act synergistically with EGFR/HER-2 inhibitors, suggesting that combination treatment may be essential to completely shut-down HER signaling. 127,179 Dual-specific antibodies against HER-2:HER-3 or EGFR:HER-3 heterodimers are being evaluated but none have yet to be useful. 180,181 Our results shows that combination treatment with HER-2 and HER-3 peptide vaccine antibodies in 3 different cell lines, BT-474 breast cancer cell line, Capan-2 pancreatic and HT-29 colon cells caused an increased rate of inhibition of proliferation versus single treatments. Phosphorylated levels of HER-2 and HER-3 following combined treatment with both HER-2 and HER-3 peptide antibodies caused enhanced inhibition of phosphorylation as compared to individual treatment. Significant inhibition was achieved in the BT-474 breast cancer cell which has high HER-2 and HER-3 over- expression. Overall, the results points to the potential benefits of a combination approach targeting HER-3 and HER-2 in breast, pancreatic and colon cancers. The combination of HER-2 and HER-3 receptors may be critical in breast cancer growth and progression, and HER-3 may be a necessary partner for the oncogenic activity of HER-2. 131,154,155,182,183 HER-3 may also provide a route for resistance to agents that target EGFR or HER-2. In fact, HER-3 antibodies have been shown to act synergistically with EGFR/HER-2 inhibitors, suggesting that combination treatment may be essential to completely shut-down Erbb signaling. 127,179 Dual-specific antibodies against HER-2:HER-3 or EGFR:HER-3 heterodimers are being evaluated but none have yet proven to be useful. 180,181 Overall, the results (Table 5) point to the potential benefits of targeting HER-3 and HER-2 combinations in cancers that express these receptors.138
Future Prospects
New opportunities have arisen to potentiate peptide-based B-cell vaccines with combinatorial approaches which elicit effective anti-tumor immunity with demonstrated synergistic inhibition of tumor growth and metastasis. To advance a rational, systematic development of combination therapies, next generation combination therapies must be designed with a thorough understanding of how mechanisms of resistance to multi-drug regimens differ from single agent resistance. We need to establish the underlying mechanisms that drive the advantages of combination therapies with independent mechanisms of action, and design methods to determine drug targets for combination regimen that effectuates long-lived tumor destruction. Our work has focused on the signaling cross-talk at the receptor level using peptide vaccine antibodies or peptide mimics to inhibit dimerization/heterodimerization of HER-1, HER-2, HER-3, VEGF and IGF-1R. We are not able to cover all of the potential signaling pathways that are associated with the resistance to RTKs inhibitors. Emerging evidence indicates the important roles of HGF/c-MET, and hedgehog signaling in mediating primary and secondary resistance to cancer therapies with the development of more inhibitors and more mechanistic studies must be conducted.
Optimal combinations of anti-HER-2 agents delivered with HER-1, HER-3, VEGF and IGF-1R may provide the best therapy for breast cancers and other solid tumors including pancreatic, colon, esophageal (GIST), and lung cancers. Although we have evaluated effects of combination HER-2 peptide vaccines in several different transgenic mouse model of human breast cancer, there are no effective transgenic mouse models for the new indications under study. Therefore we have recently evaluated the in vivo effects of combination treatment with vaccine antibodies and peptide mimics in transplantable (NOD/SCID mice) mouse models of human breast, colon and pancreatic cancers as surrogate models for active immunization. Although these mice models are valid, more stringent mouse models such as the C.Cg-Rag2tmFwaIl2rgt1lSug mouse model (CIEA-BRG) which is a highly immunodeficient model, with T, B and NK cells deficiencies. 184,185 These mouse models have significant advantages over tose we have previously used. 3 The NOG mouse model NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac (CIEA-NOG) is also similar to the BRG mouse in that they also lack mature T, B and NK cells and are excellent models for a variety of xenografts and human cell engraftment studies. 186 More importantly, the use of a human immune system (HIS)-engrafted mouse model may be more appropriate and desirable which involves the reconstitution of the human immune system into a BRG or NOG immunodeficient mouse. 187 The human immune system can be generated by transferring human cord blood and other immune cells into CIEA-BRG. 188 Such a model would be extremely important to test many of these combinations to demonstrate synergistic anticancer effects for clinical implementation in human clinical trials.
We strongly advocate interventions at the receptor level which must be prioritized in developing RTK inhibitors as opposed to creating inhibitors that prevent the activators of key downstream signaling, such as RAS/RAF/MEK/ERK and PI3K/AKT pathways. Inhibition of the downstream signaling events are more difficult as only small molecule inhibitors are able to cross the plasma membrane to compete with adenosine triphosphate (ATP) binding and inhibit kinase activity. Recently the total number of small molecule-based drugs in the clinical trials and approved drugs for the market is decreasing rapidly because of the toxicities associated with treatments that are non-specific. As a result of this, both pharma companies along with academia are increasingly paying more attention to peptides because of their better safety levels and lack of toxicities as compared to small molecules. The emphasis on the need to develop peptide inhibitors cannot be overstated given their potential impact as being more effective, less toxic and safe to overcome signaling cross-talk mediated drug resistance. The simultaneous targeting of multiple receptors with peptide vaccines/ therapeutics can abrogate cross-talk and mitigate drug resistance due to activation of alternative signaling pathways.
Conclusions
The studies described in this review with peptide-based vaccines and peptide mimics highlight the importance of developing RTK inhibitors and how combinatorial approaches may offer synergistic and co-operative inhibition that are safe with little toxicity. The greatest potential significance of our studies is 2-fold: (i) It combines multiple mechanisms of action by activating both B and T cell functions and promoting immunological clearance, (ii) It is also a targeted approach aimed at inhibiting molecular signaling pathways that are crucial for tumor growth and maintenance. The major attraction of the peptide vaccine approach is the potential for a sustained anti-tumor effect related to immunological memory that protects against relapsing tumors, obviating the requirement for multiple infusions of humanized mAbs and overcoming escape mechanisms. Active immunotherapy offers multitude advantages, including tumor specificity and the activation of immune responses against antigens that are selectively expressed by tumor cells. As a result, non-specific toxicity to normal tissues is reduced and anti-tumor responses are more durable and adaptable to cancer control at different stages of disease, including those with early-stage disease and low tumor burdens. Additional benefits of the peptide vaccine approach include the ease of rapid synthesis, safety, lack of toxicity, and cost-effectiveness. With the newly identified HER-3 and IGF-1R epitope constructs, we now have extended our portfolio of validated peptide vaccine/ peptide mimic candidates against HER-1, HER-2, and VEGF that can be used in combination to target multiple tumor types (breast, TNBC, pancreatic, colon and esophageal cancers). We have shown that various combinations can have synergistic antitumor effects in vitro in several disease types. Unique combinations of HER-3 and IGF-1R, HER-1 and IGF-1R and HER-2 and HER-3 peptide vaccines are being tested in animal models of breast, pancreatic, GIST and colon cancers, including those that are resistant to currently available HER-2-targeted agents. These studies have the potential to make major contributions to the existing fields of cancer immunology, drug development, and mechanisms of cancer pharmacology by (i) developing new immune-based approaches to combat receptor kinase-driven cancers, (ii) understanding and overcoming clinically relevant mechanisms of resistance to currently used RTK directed agents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol 2012; 8:961–87; PMID:22894670; http://dx.doi.org/ 10.2217/fon.12.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaumaya PT. Bridging oncology and immunology: expanding horizons with innovative peptide vaccines and peptidomimetics. Immunotherapy 2013; 5:1159–63; PMID:24188668; http://dx.doi.org/ 10.2217/imt.13.128 [DOI] [PubMed] [Google Scholar]
- 3.Miller MJ, Foy KC, Kaumaya PT. Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 2013; 15:166–76; PMID:23545045 [PubMed] [Google Scholar]
- 4.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem 2011; 286:13612–25; PMID:21321115; http://dx.doi.org/ 10.1074/jbc.M110.216812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol 2012; 9:208–14; PMID:22327211; http://dx.doi.org/ 10.1038/cmi.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamarin D, Postow MA. Immune checkpoint modulation: Rational design of combination strategies. Pharmacol Ther 2015; PMID:25583297 [DOI] [PubMed] [Google Scholar]
- 7.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11:239–53; PMID:21430696; http://dx.doi.org/ 10.1038/nrc3007 [DOI] [PubMed] [Google Scholar]
- 8.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237–51; PMID:22437869; http://dx.doi.org/ 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galmarini D, Galmarini CM, Galmarini FC. Cancer chemotherapy: a critical analysis of its 60 years of history. Crit Rev Oncol Hematol 2012; 84:181–99; PMID:22542531; http://dx.doi.org/ 10.1016/j.critrevonc.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127–37; PMID:11252954; http://dx.doi.org/ 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 11.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol 1989; 9:1165–72; PMID:2566907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, Figari I, Kotts CE, Palladino MA Jr., Ullrich A, et al.. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol 1991; 11:117–27; PMID:1679763; http://dx.doi.org/ 10.1007/BF00918679 [DOI] [PubMed] [Google Scholar]
- 13.Ryan AJ, Wedge SR. ZD6474–a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br J Cancer 2005; 92 Suppl 1:S6–13; PMID:15928657; http://dx.doi.org/ 10.1038/sj.bjc.6602603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, Bunting S, de Vos AM, Jin H. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension 2002; 39:1095–100; PMID:12052848; http://dx.doi.org/ 10.1161/01.HYP.0000018588.56950.7A [DOI] [PubMed] [Google Scholar]
- 15.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer 2006; 42:3127–39; PMID:17098419; http://dx.doi.org/ 10.1016/j.ejca.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 16.Grothey A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology (Williston Park) 2006; 20:21–8; PMID:17354514 [PubMed] [Google Scholar]
- 17.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992; 89:4285–9; PMID:1350088; http://dx.doi.org/ 10.1073/pnas.89.10.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaumaya P. Her-2/neu Cancer Vaccines: Present Status and Future Prospects. International journal of peptide research and therapeutics 2006; 12:65–77; http://dx.doi.org/ 10.1007/s10989-005-9000-5 [DOI] [Google Scholar]
- 19.Amit AG, Mariuzza RA, Phillips SE, Poljak RJ. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science 1986; 233:747–53; PMID:2426778; http://dx.doi.org/ 10.1126/science.2426778 [DOI] [PubMed] [Google Scholar]
- 20.Kaumaya P, Kobs-Conrad S, Digeorge AM, Stevens V. Denovo Engineering of Protein Immunogenic & Antigenic Determinants. 1994:133–64 [Google Scholar]
- 21.Gao J, Kurgan L. Computational prediction of B cell epitopes from antigen sequences. Methods Mol Biol 2014; 1184:197–215; PMID:25048126; http://dx.doi.org/ 10.1007/978-1-4939-1115-8_11 [DOI] [PubMed] [Google Scholar]
- 22.Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol 2013; 4:302; PMID:24115948; http://dx.doi.org/ 10.3389/fimmu.2013.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaumaya PT, VanBuskirk AM, Goldberg E, Pierce SK. Design and immunological properties of topographic immunogenic determinants of a protein antigen (LDH-C4) as vaccines. J Biol Chem 1992; 267:6338–46; PMID:1372905 [PubMed] [Google Scholar]
- 24.Kobs-Conrad S, Lee H, DiGeorge AM, Kaumaya PT. Engineered topographic determinants with α β, β α β, and β α β α topologies show high affinity binding to native protein antigen (lactate dehydrogenase-C4). J Biol Chem 1993; 268:25285–95; PMID:8244959 [PubMed] [Google Scholar]
- 25.Dakappagari NK, Lute KD, Rawale S, Steele JT, Allen SD, Phillips G, Reilly RT, Kaumaya PT. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. J Biol Chem 2005; 280:54–63; PMID:15507452; http://dx.doi.org/ 10.1074/jbc.M411020200 [DOI] [PubMed] [Google Scholar]
- 26.Garrett JT, Rawale S, Allen SD, Phillips G, Forni G, Morris JC, Kaumaya PT. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. Journal of immunology 2007; 178:7120–31; http://dx.doi.org/ 10.4049/jimmunol.178.11.7120 [DOI] [PubMed] [Google Scholar]
- 27.Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, Morris JC, Oshima RG, Kaumaya PT. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol 2007; 179:472–82; http://dx.doi.org/ 10.4049/jimmunol.179.1.472 [DOI] [PubMed] [Google Scholar]
- 28.Gabrielli F, Salvi R, Garulli C, Kalogris C, Arima S, Tardella L, Monaci P, Pupa SM, Tagliabue E, Montani M, et al.. Identification of relevant conformational epitopes on the HER2 oncoprotein by using Large Fragment Phage Display (LFPD). PLoS One 2013; 8:e58358; PMID:23555577; http://dx.doi.org/ 10.1371/journal.pone.0058358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaumaya PT, Kobs-Conrad S, Seo YH, Lee H, VanBuskirk AM, Feng N, Sheridan JF, Stevens V. Peptide vaccines incorporating a ‘promiscuous’ T-cell epitope bypass certain haplotype restricted immune responses and provide broad spectrum immunogenicity. J Mol Recognit 1993; 6:81–94; PMID:7508238; http://dx.doi.org/ 10.1002/jmr.300060206 [DOI] [PubMed] [Google Scholar]
- 30.Cai H, Chen MS, Sun ZY, Zhao YF, Kunz H, Li YM. Self-adjuvanting synthetic antitumor vaccines from MUC1 glycopeptides conjugated to T-cell epitopes from tetanus toxoid. Angew Chem Int Ed Engl 2013; 52:6106–10; PMID:23616304; http://dx.doi.org/ 10.1002/anie.201300390 [DOI] [PubMed] [Google Scholar]
- 31.Wiedermann U, Wiltschke C, Jasinska J, Kundi M, Zurbriggen R, Garner-Spitzer E, Bartsch R, Steger G, Pehamberger H, Scheiner O, et al.. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat 2010; 119:673–83; PMID:20092022; http://dx.doi.org/ 10.1007/s10549-009-0666-9 [DOI] [PubMed] [Google Scholar]
- 32.Wiedermann U, Davis AB, Zielinski CC. Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines. Breast Cancer Res Treat 2013; 138:1–12; PMID:23340862; http://dx.doi.org/ 10.1007/s10549-013-2410-8 [DOI] [PubMed] [Google Scholar]
- 33.Miyako H, Kametani Y, Katano I, Ito R, Tsuda B, Furukawa A, Saito Y, Ishikawa D, Ogino K, Sasaki S, et al.. Antitumor effect of new HER2 peptide vaccination based on B cell epitope. Anticancer Res 2011; 31:3361–8; PMID:21965747 [PubMed] [Google Scholar]
- 34.Riemer AB, Kurz H, Klinger M, Scheiner O, Zielinski CC, Jensen-Jarolim E. Vaccination with cetuximab mimotopes and biological properties of induced anti-epidermal growth factor receptor antibodies. J Natl Cancer Inst 2005; 97:1663–70; PMID:16288119; http://dx.doi.org/ 10.1093/jnci/dji373 [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Zhao L, Wu M, Chen Z, Li H. B-cell epitope peptide vaccination targeting dimer interface of epidermal growth factor receptor (EGFR). Immunol Lett 2013; 153:33–40; PMID:23871733; http://dx.doi.org/ 10.1016/j.imlet.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 36.Sundaram R, Dakappagari NK, Kaumaya PTP. Synthetic peptides as cancer vaccines. Biopolymers - Peptide Science Section 2002; 66:200–16; http://dx.doi.org/ 10.1002/bip.10258 [DOI] [PubMed] [Google Scholar]
- 37.Kaumaya PTP. HER-2/neu cancer vaccines: Present status and future prospects. International Journal of Peptide Research and Therapeutics 2006; 12:65–77; http://dx.doi.org/ 10.1007/s10989-005-9000-5 [DOI] [Google Scholar]
- 38.Lynch MP, Kaumaya PTP. Advances in HTLV-1 peptide vaccines and therapeutics. Current Protein and Peptide Science 2006; 7:137–45; PMID:16611139; http://dx.doi.org/ 10.2174/138920306776359803 [DOI] [PubMed] [Google Scholar]
- 39.Steele JT, Allen SD, Kaumaya PTP. Cancer Immunotherapy with Rationally Designed Synthetic Peptides. Handbook of Biologically Active Peptides, 2006:491–8; http://dx.doi.org/ 10.1016/B978-012369442-3/50074-X [DOI] [Google Scholar]
- 40.Foy KC, Vicari D, Kaumaya PTP. Therapeutic Peptides Targeting HER-2/neu and VEGF Signaling Pathways in Breast Cancer. Handbook of Biologically Active Peptides, 2013:612–6; http://dx.doi.org/ 10.1016/B978-0-12-385095-9.00083-X [DOI] [Google Scholar]
- 41.Miller MJ, Foy KC, Kaumaya PTP. Cancer immunotherapy: Present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 2013; 15:166–76; PMID:23545045 [PubMed] [Google Scholar]
- 42.Kaumaya PT, Berndt KD, Heidorn DB, Trewhella J, Kezdy FJ, Goldberg E. Synthesis and biophysical characterization of engineered topographic immunogenic determinants with α α topology. Biochemistry 1990; 29:13–23; PMID:1691014; http://dx.doi.org/ 10.1021/bi00453a002 [DOI] [PubMed] [Google Scholar]
- 43.Dakappagari NK, Pyles J, Parihar R, Carson WE, Young DC, Kaumaya PT. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. JImmunol 2003; 170:4242–53; http://dx.doi.org/ 10.4049/jimmunol.170.8.4242 [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan M, Wardrop RM, Gienapp IE, Stuckman SS, Whitacre CC, Kaumaya PT. A retro-inverso peptide mimic of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J Immunol 2001; 167:578–85; http://dx.doi.org/ 10.4049/jimmunol.167.1.578 [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol 2002; 169:2180–8; http://dx.doi.org/ 10.4049/jimmunol.169.4.2180 [DOI] [PubMed] [Google Scholar]
- 46.Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, Lamb T, Mani A, Kane Y, Balint CR, et al.. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol 2009; 27:5270–7; PMID:19752336; http://dx.doi.org/ 10.1200/JCO.2009.22.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan M, Domanico SZ, Kaumaya PT, Pierce SK. Peptides of 23 residues or greater are required to stimulate a high affinity class II-restricted T cell response. Eur J Immunol 1993; 23:1011–6; PMID:8386663; http://dx.doi.org/ 10.1002/eji.1830230504 [DOI] [PubMed] [Google Scholar]
- 48.Dakappagari NK, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res 2000; 60:3782–9; PMID:10919651 [PubMed] [Google Scholar]
- 49.Foy KC, Miller MJ, Moldovan N, Bozanovic T, Carson WE Iii, Kaumaya PT. Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer. Oncoimmunology 2012; 1:1004–16; PMID:23170249; http://dx.doi.org/ 10.4161/onci.21057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smolenski LA, Kaumaya P, Atassi MZ, Pierce SK. Characteristics of peptides which compete for presented antigen-binding sites on antigen-presenting cells. Eur J Immunol 1990; 20:953–60; PMID:2162778; http://dx.doi.org/ 10.1002/eji.1830200502 [DOI] [PubMed] [Google Scholar]
- 51.Mizejewski GJ. Peptides as receptor ligand drugs and their relationship to G-coupled signal transduction. Exp Opin Investig Drugs 2001; 10:1063–73; PMID:11772235; http://dx.doi.org/ 10.1517/13543784.10.6.1063 [DOI] [PubMed] [Google Scholar]
- 52.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today 2010; 15:40–56; PMID:19879957; http://dx.doi.org/ 10.1016/j.drudis.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 53.Reichert J. development trends for peptide therapeutics. Tufts Center for the Study of Drug Development, Tufts University, 2008 [Google Scholar]
- 54.Fischer PM. The design, synthesis and application of stereochemical and directional peptide isomers: a critical review. Curr Protein Pept Sci 2003; 4:339–56; PMID:14529528; http://dx.doi.org/ 10.2174/1389203033487054 [DOI] [PubMed] [Google Scholar]
- 55.Hruby VJ. Conformational and topographical considerations in the design of biologically active peptides. Biopolymers 1993; 33:1073–82; PMID:8102072; http://dx.doi.org/ 10.1002/bip.360330709 [DOI] [PubMed] [Google Scholar]
- 56.Fletcher MD, Campbell MM. Partially Modified Retro-Inverso Peptides: Development, Synthesis, and Conformational Behavior. Chem Rev 1998; 98:763–96; PMID:11848914; http://dx.doi.org/ 10.1021/cr970468t [DOI] [PubMed] [Google Scholar]
- 57.Taylor EM, Otero DA, Banks WA, O'Brien JS. Retro-inverso prosaptide peptides retain bioactivity, are stable In vivo, and are blood-brain barrier permeable. J Pharmacol Exp Ther 2000; 295:190–4; PMID:10991978 [PubMed] [Google Scholar]
- 58.Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin Immunol 2013; 25:182–90; PMID:23706598; http://dx.doi.org/ 10.1016/j.smim.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 59.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 2007; 6:404–14; PMID:17473845; http://dx.doi.org/ 10.1038/nrd2224 [DOI] [PubMed] [Google Scholar]
- 60.Croft NP, Purcell AW. Peptidomimetics: modifying peptides in the pursuit of better vaccines. Exp Rev Vaccines 2011; 10:211–26; PMID:21332270; http://dx.doi.org/ 10.1586/erv.10.161 [DOI] [PubMed] [Google Scholar]
- 61.Gokhale AS, Satyanarayanajois S. Peptides and peptidomimetics as immunomodulators. Immunotherapy 2014; 6:755–74; PMID:25186605; http://dx.doi.org/ 10.2217/imt.14.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12:553–63; PMID:22785351; http://dx.doi.org/ 10.1038/nrc3309 [DOI] [PubMed] [Google Scholar]
- 63.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103:211–25; PMID:11057895; http://dx.doi.org/ 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- 64.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 2010; 107:7692–7; PMID:20351256; http://dx.doi.org/ 10.1073/pnas.1002753107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 2005; 23:2445–59; PMID:15753456; http://dx.doi.org/ 10.1200/JCO.2005.11.890 [DOI] [PubMed] [Google Scholar]
- 66.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science 2006; 312:1175–8; PMID:16728632; http://dx.doi.org/ 10.1126/science.1125951 [DOI] [PubMed] [Google Scholar]
- 67.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 2006; 3:24–40; PMID:16407877; http://dx.doi.org/ 10.1038/ncponc0403 [DOI] [PubMed] [Google Scholar]
- 68.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol 1991; 5:1806–14; PMID:1791831; http://dx.doi.org/ 10.1210/mend-5-12-1806 [DOI] [PubMed] [Google Scholar]
- 69.Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 2003; 30:117–24; PMID:14613032; http://dx.doi.org/ 10.1053/j.seminoncol.2003.08.013 [DOI] [PubMed] [Google Scholar]
- 70.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005; 5:341–54; PMID:15864276; http://dx.doi.org/ 10.1038/nrc1609 [DOI] [PubMed] [Google Scholar]
- 71.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56:549–80; PMID:15602010; http://dx.doi.org/ 10.1124/pr.56.4.3 [DOI] [PubMed] [Google Scholar]
- 72.Zhu Z, Witte L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest New Drugs 1999; 17:195–212; PMID:10665474; http://dx.doi.org/ 10.1023/A:1006314501634 [DOI] [PubMed] [Google Scholar]
- 73.Oshima RG, Lesperance J, Munoz V, Hebbard L, Ranscht B, Sharan N, Muller WJ, Hauser CA, Cardiff RD. Angiogenic acceleration of Neu induced mammary tumor progression and metastasis. Cancer Res 2004; 64:169–79; PMID:14729621; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-1944 [DOI] [PubMed] [Google Scholar]
- 74.Folkman J. Tumor angiogenesis: therapeutic implications. NEng J Med 1971; 285:1182–6; PMID:4938153; http://dx.doi.org/ 10.1056/NEJM197108122850711 [DOI] [PubMed] [Google Scholar]
- 75.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009; 9:463–75; PMID:19536107; http://dx.doi.org/ 10.1038/nrc2656 [DOI] [PubMed] [Google Scholar]
- 76.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004; 4:505–18; PMID:15229476; http://dx.doi.org/ 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 77.Pollak MN. Insulin-like growth factors and neoplasia. Novartis Found Symp 2004; 262:84–98; discussion -107, 265–8 [PubMed] [Google Scholar]
- 78.Folkman J. Fighting cancer by attacking its blood supply. Scientific American 1996; 275:150–4; PMID:8701285; http://dx.doi.org/ 10.1038/scientificamerican0996-150 [DOI] [PubMed] [Google Scholar]
- 79.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438:967–74; PMID:16355214; http://dx.doi.org/ 10.1038/nature04483 [DOI] [PubMed] [Google Scholar]
- 80.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev 2005; 16:407–20; PMID:15886048; http://dx.doi.org/ 10.1016/j.cytogfr.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 81.Resnicoff M, Burgaud JL, Rotman HL, Abraham D, Baserga R. Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res 1995; 55:3739–41; PMID:7641185 [PubMed] [Google Scholar]
- 82.Resnicoff M, Abraham D, Yutanawiboonchai W, Rotman HL, Kajstura J, Rubin R, Zoltick P, Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res 1995; 55:2463–9; PMID:7758000 [PubMed] [Google Scholar]
- 83.Baserga R, Resnicoff M, D'Ambrosio C, Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam Horm 1997; 53:65–98; PMID:9197178; http://dx.doi.org/ 10.1016/S0083-6729(08)60704-9 [DOI] [PubMed] [Google Scholar]
- 84.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278–87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 85.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317–27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med 2003; 54:343–69; PMID:12525678; http://dx.doi.org/ 10.1146/annurev.med.54.101601.152442 [DOI] [PubMed] [Google Scholar]
- 87.Shuptrine CW, Surana R, Weiner LM. Monoclonal antibodies for the treatment of cancer. Semin Cancer Biol 2012; 22:3–13; PMID:22245472; http://dx.doi.org/ 10.1016/j.semcancer.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell 2012; 148:1081–4; PMID:22424219; http://dx.doi.org/ 10.1016/j.cell.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci 2009; 100:1566–72; PMID:19538497; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01222.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006; 3:269–80; PMID:16683005; http://dx.doi.org/ 10.1038/ncponc0509 [DOI] [PubMed] [Google Scholar]
- 91.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al.. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2:127–37; PMID:12204533; http://dx.doi.org/ 10.1016/S1535-6108(02)00097-1 [DOI] [PubMed] [Google Scholar]
- 92.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004; 64:2343–6; PMID:15059883; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-3856 [DOI] [PubMed] [Google Scholar]
- 93.Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, et al.. Phase I Clinical Study of Pertuzumab, a Novel HER Dimerization Inhibitor, in Patients With Advanced Cancer. J Clin Oncol 2005; 23:2534–43; PMID:15699478; http://dx.doi.org/ 10.1200/JCO.2005.03.184 [DOI] [PubMed] [Google Scholar]
- 94.Walshe JM, Denduluri N, Berman AW, Rosing DR, Swain SM. A phase II trial with trastuzumab and pertuzumab in patients with HER2-overexpressed locally advanced and metastatic breast cancer. Clin Breast Cancer 2006; 6:535–9; PMID:16595039; http://dx.doi.org/ 10.3816/CBC.2006.n.009 [DOI] [PubMed] [Google Scholar]
- 95.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005; 333:328–35; PMID:15961063; http://dx.doi.org/ 10.1016/j.bbrc.2005.05.132 [DOI] [PubMed] [Google Scholar]
- 96.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature 2006; 441:457–62; PMID:16724058; http://dx.doi.org/ 10.1038/nature04874 [DOI] [PubMed] [Google Scholar]
- 97.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug discov 2006; 5:649–59; PMID:16883303; http://dx.doi.org/ 10.1038/nrd2089 [DOI] [PubMed] [Google Scholar]
- 98.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669–76; PMID:12778165; http://dx.doi.org/ 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- 99.Herley MT, Yu Y, Whitney RG, Sato JD. Characterization of the VEGF binding site on the Flt-1 receptor. Biochem Biophys Res Commun 1999; 262:731–8; PMID:10471394; http://dx.doi.org/ 10.1006/bbrc.1999.1282 [DOI] [PubMed] [Google Scholar]
- 100.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 2000; 19:2138–46; PMID:10815805; http://dx.doi.org/ 10.1038/sj.onc.1203533 [DOI] [PubMed] [Google Scholar]
- 101.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996; 271:5638–46; PMID:8621427; http://dx.doi.org/ 10.1074/jbc.271.10.5638 [DOI] [PubMed] [Google Scholar]
- 102.Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis 2002; 5:35–44; PMID:12549858; http://dx.doi.org/ 10.1023/A:1021540120521 [DOI] [PubMed] [Google Scholar]
- 103.Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, Witte L, Zhu Z. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem 2003; 278:43496–507; PMID:12917408; http://dx.doi.org/ 10.1074/jbc.M307742200 [DOI] [PubMed] [Google Scholar]
- 104.Boldicke T, Tesar M, Griesel C, Rohde M, Grone HJ, Waltenberger J, Kollet O, Lapidot T, Yayon A, Weich H. Anti-VEGFR-2 scFvs for cell isolation. Single-chain antibodies recognizing the human vascular endothelial growth factor receptor-2 (VEGFR-2/flk-1) on the surface of primary endothelial cells and preselected CD34+ cells from cord blood. Stem cells 2001; 19:24–36; PMID:11209088; http://dx.doi.org/ 10.1634/stemcells.19-1-24 [DOI] [PubMed] [Google Scholar]
- 105.Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouet J, Derbin C, Perret G, Mazie JC. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. The EMBO journal 2000; 19:1525–33; PMID:10747021; http://dx.doi.org/ 10.1093/emboj/19.7.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verhoef C, de Wilt JH, Verheul HM. Angiogenesis inhibitors: perspectives for medical, surgical and radiation oncology. Current pharmaceutical design 2006; 12:2623–30; PMID:16842162; http://dx.doi.org/ 10.2174/138161206777698756 [DOI] [PubMed] [Google Scholar]
- 107.Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res 2010; 16:2512–7; PMID:20388853; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2232 [DOI] [PubMed] [Google Scholar]
- 108.Foy KC, Miller MJ, Moldovan N, Carson Iii WE, Kaumaya PT. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. Oncoimmunology 2012; 1:1048–60; PMID:23170253; http://dx.doi.org/ 10.4161/onci.20708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990; 5:953–62; PMID:1973830 [PubMed] [Google Scholar]
- 110.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol 1990; 8:103–12; PMID:1967301 [DOI] [PubMed] [Google Scholar]
- 111.Niehans GA, Singleton TP, Dykoski D, Kiang DT. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst 1993; 85:1230–5; PMID:8101229; http://dx.doi.org/ 10.1093/jnci/85.15.1230 [DOI] [PubMed] [Google Scholar]
- 112.Dakappagari NK, Sundaram R, Rawale S, Liner A, Galloway DR, Kaumaya PT. Intracellular delivery of a novel multiepitope peptide vaccine by an amphipathic peptide carrier enhances cytotoxic T-cell responses in HLA-A*201 mice. J Pept Res 2005; 65:189–99; PMID:15705163; http://dx.doi.org/ 10.1111/j.1399-3011.2005.00212.x [DOI] [PubMed] [Google Scholar]
- 113.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr., Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003; 421:756–60; PMID:12610629; http://dx.doi.org/ 10.1038/nature01392 [DOI] [PubMed] [Google Scholar]
- 114.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW, et al.. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003; 11:495–505; PMID:12620236; http://dx.doi.org/ 10.1016/S1097-2765(03)00048-0 [DOI] [PubMed] [Google Scholar]
- 115.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer cell 2004; 5:317–28; PMID:15093539; http://dx.doi.org/ 10.1016/S1535-6108(04)00083-2 [DOI] [PubMed] [Google Scholar]
- 116.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. The oncologist 2004; 9 Suppl 1:2–10; PMID:15178810; http://dx.doi.org/ 10.1634/theoncologist.9-suppl_1-2 [DOI] [PubMed] [Google Scholar]
- 117.Saito H, Tsujitani S, Ikeguchi M, Maeta M, Kaibara N. Relationship between the expression of vascular endothelial growth factor and the density of dendritic cells in gastric adenocarcinoma tissue. British journal of cancer 1998; 78:1573–7; PMID:9862566; http://dx.doi.org/ 10.1038/bjc.1998.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266:11947–54; PMID:1711045 [PubMed] [Google Scholar]
- 119.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989; 161:851–8; PMID:2735925; http://dx.doi.org/ 10.1016/0006-291X(89)92678-8 [DOI] [PubMed] [Google Scholar]
- 120.Casella I, Feccia T, Chelucci C, Samoggia P, Castelli G, Guerriero R, Parolini I, Petrucci E, Pelosi E, Morsilli O, et al.. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood 2003; 101:1316–23; PMID:12406876; http://dx.doi.org/ 10.1182/blood-2002-07-2184 [DOI] [PubMed] [Google Scholar]
- 121.Carpenito C, Davis PD, Dougherty ST, Dougherty GJ. Exploiting the differential production of angiogenic factors within the tumor microenvironment in the design of a novel vascular-targeted gene therapy-based approach to the treatment of cancer. Int J Radiat Oncol, Biol, Phys 2002; 54:1473–8; PMID:12459373; http://dx.doi.org/ 10.1016/S0360-3016(02)03921-4 [DOI] [PubMed] [Google Scholar]
- 122.Monsky WL, Mouta Carreira C, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res 2002; 8:1008–13; PMID:11948107 [PubMed] [Google Scholar]
- 123.Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination Treatment with HER-2 and VEGF Peptide Mimics Induces Potent Anti-tumor and Anti-angiogenic Responses in Vitro and in Vivo. J Biol Chem 2011; 286:13626–37; PMID:21325276; http://dx.doi.org/ 10.1074/jbc.M110.216820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PTP. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J Immunol 2013; 191:217–27; http://dx.doi.org/ 10.4049/jimmunol.1300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68:5878–87; PMID:18632642; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0380 [DOI] [PubMed] [Google Scholar]
- 126.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, Di Fiore PP, Kraus MH. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995; 10:1813–21; PMID:7538656 [PubMed] [Google Scholar]
- 127.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al.. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 2010; 70:2485–94; PMID:20215504; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 2003; 100:8933–8; PMID:12853564; http://dx.doi.org/ 10.1073/pnas.1537685100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther 2008; 15:413–48; PMID:18404164; http://dx.doi.org/ 10.1038/cgt.2008.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996; 16:5276–87; PMID:8816440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sergina NV, Moasser MM. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends in molecular medicine 2007; 13:527–34; PMID:17981505; http://dx.doi.org/ 10.1016/j.molmed.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al.. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316:1039–43; PMID:17463250; http://dx.doi.org/ 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 133.Amler LC. HER3 mRNA as a predictive biomarker in anticancer therapy. Exp Opin Biol Ther 2010; 10:1343–55; PMID:20695834; http://dx.doi.org/ 10.1517/14712598.2010.512003 [DOI] [PubMed] [Google Scholar]
- 134.Osipo C, Meeke K, Cheng D, Weichel A, Bertucci A, Liu H, Jordan VC. Role for HER2/neu and HER3 in fulvestrant-resistant breast cancer. Int J Oncol 2007; 30:509–20; PMID:17203234 [PubMed] [Google Scholar]
- 135.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ 2nd, de Cremoux P, Stenvang J, et al.. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 2009; 114:263–75; PMID:18409071; http://dx.doi.org/ 10.1007/s10549-008-0011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, Gonzalez-Angulo AM, Hennessy BT, Mills GB, Kennedy JP, et al.. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res 2009; 69:4192–201; PMID:19435893; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hamburger AW. The role of ErbB3 and its binding partners in breast cancer progression and resistance to hormone and tyrosine kinase directed therapies. J Mammary Gland Biol Neoplasia 2008; 13:225–33; PMID:18425425; http://dx.doi.org/ 10.1007/s10911-008-9077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller MJ, Foy KC, Overholser JP, Nahta R, Kaumaya PTP. HER-3 peptide vaccines/mimics: Combined therapy with IGF-1R, HER-2, and HER-1 peptides induces synergistic antitumor effects against breast and pancreatic cancer cells. OncoImmunology 2014; 3:e956012; http://dx.doi.org/ 10.4161/21624011.2014.956012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 2000; 57:1050–93; PMID:10961344; http://dx.doi.org/ 10.1007/PL00000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 1999; 91:151–6; PMID:9923856; http://dx.doi.org/ 10.1093/jnci/91.2.151 [DOI] [PubMed] [Google Scholar]
- 141.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998; 351:1393–6; PMID:9593409; http://dx.doi.org/ 10.1016/S0140-6736(97)10384-1 [DOI] [PubMed] [Google Scholar]
- 142.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res 1995; 55:2007–11; PMID:7743492 [PubMed] [Google Scholar]
- 143.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 1999; 91:620–5; PMID:10203281; http://dx.doi.org/ 10.1093/jnci/91.7.620 [DOI] [PubMed] [Google Scholar]
- 144.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 1998; 279:563–6; PMID:9438850; http://dx.doi.org/ 10.1126/science.279.5350.563 [DOI] [PubMed] [Google Scholar]
- 145.Wu X, Zhao H, Do KA, Johnson MM, Dong Q, Hong WK, Spitz MR. Serum levels of insulin growth factor (IGF-I) and IGF-binding protein predict risk of second primary tumors in patients with head and neck cancer. Clin Cancer Res 2004; 10:3988–95; PMID:15217929; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0762 [DOI] [PubMed] [Google Scholar]
- 146.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer research 2006; 66:10100–11; PMID:17047074; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1684 [DOI] [PubMed] [Google Scholar]
- 147.Kuribayashi A, Kataoka K, Kurabayashi T, Miura M. Evidence that basal activity, but not transactivation, of the epidermal growth factor receptor tyrosine kinase is required for insulin-like growth factor I-induced activation of extracellular signal-regulated kinase in oral carcinoma cells. Endocrinology 2004; 145:4976–84; PMID:15271882; http://dx.doi.org/ 10.1210/en.2004-0713 [DOI] [PubMed] [Google Scholar]
- 148.Epa VC, Ward CW. Model for the complex between the insulin-like growth factor I and its receptor: towards designing antagonists for the IGF-1 receptor. Protein Eng Des Sel 2006; 19:377–84; PMID:16772308; http://dx.doi.org/ 10.1093/protein/gzl022 [DOI] [PubMed] [Google Scholar]
- 149.Foy KC, Miller MJ, Overholser J, Donnelly SM, Nahta R, Kaumaya PTP. IGF-1R peptide vaccines/mimics inhibit the growth of BxPC3 and JIMT-1 cancer cells and exhibit synergistic antitumor effects with HER-1 and HER-2 peptides. OncoImmunology 2014; 3:e956005; http://dx.doi.org/ 10.4161/21624011.2014.956005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balana ME, Labriola L, Salatino M, Movsichoff F, Peters G, Charreau EH, Elizalde PV. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 2001; 20:34–47; PMID:11244498; http://dx.doi.org/ 10.1038/sj.onc.1204050 [DOI] [PubMed] [Google Scholar]
- 151.Camirand A, Lu Y, Pollak M. Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit 2002; 8:BR521–6 [PubMed] [Google Scholar]
- 152.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 2005; 65:11118–28; PMID:16322262; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3841 [DOI] [PubMed] [Google Scholar]
- 153.Slomiany MG, Black LA, Kibbey MM, Day TA, Rosenzweig SA. IGF-1 induced vascular endothelial growth factor secretion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun 2006; 342:851–8; PMID:16499871; http://dx.doi.org/ 10.1016/j.bbrc.2006.02.043 [DOI] [PubMed] [Google Scholar]
- 154.Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer 2007; 120:1874–82; PMID:17266042; http://dx.doi.org/ 10.1002/ijc.22423 [DOI] [PubMed] [Google Scholar]
- 155.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007; 445:437–41; PMID:17206155; http://dx.doi.org/ 10.1038/nature05474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer 2004; 108:334–41; PMID:14648698; http://dx.doi.org/ 10.1002/ijc.11445 [DOI] [PubMed] [Google Scholar]
- 157.Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, Sagar M, Wong TW, Gottardis MM, Erlichman C. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther 2008; 7:2589–98; PMID:18765823; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sanabria-Figueroa E, Donnelly S, Foy K, Buss M, Castellino RC, Paplomata E, Taliaferro-Smith L, Kaumaya P, Nahta R. Insulin-like growth factor-1 receptor signaling increases the invasive potential of HER2-overexpressing breast cancer cells via Src-FAK and FoxM1. Mol Pharmacol 2014; 87(2):150-61; PMID:25391374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL, et al.. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008; 118:2609–19; PMID:18568074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277–300; PMID:20610543; http://dx.doi.org/ 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 161.Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control 2008; 15:308–13; PMID:18813198 [DOI] [PubMed] [Google Scholar]
- 162.Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, Solomides C, Peiper SC, Witkiewicz AK. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer 2012; 118:3484–93; PMID:22086503; http://dx.doi.org/ 10.1002/cncr.26661 [DOI] [PubMed] [Google Scholar]
- 163.Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res 1998; 18:4613–9; PMID:9891528 [PubMed] [Google Scholar]
- 164.Sherwood ER, Van Dongen JL, Wood CG, Liao S, Kozlowski JM, Lee C. Epidermal growth factor receptor activation in androgen-independent but not androgen-stimulated growth of human prostatic carcinoma cells. Br J Cancer 1998; 77:855–61; PMID:9528825; http://dx.doi.org/ 10.1038/bjc.1998.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. A J Pathol 2003; 163:1001–11; PMID:12937141; http://dx.doi.org/ 10.1016/S0002-9440(10)63460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ishiwata T, Bergmann U, Kornmann M, Lopez M, Beger HG, Korc M. Altered expression of insulin-like growth factor II receptor in human pancreatic cancer. Pancreas 1997; 15:367–73; PMID:9361090; http://dx.doi.org/ 10.1097/00006676-199711000-00006 [DOI] [PubMed] [Google Scholar]
- 167.Li P, Veldwijk MR, Zhang Q, Li ZB, Xu WC, Fu S. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer 2013; 13:297; PMID:23777562; http://dx.doi.org/ 10.1186/1471-2407-13-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 2010; 16:1373–83; PMID:20179223; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol 2005; 1:2005 0008; PMID:16729043; http://dx.doi.org/ 10.1038/msb4100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tai YT, Podar K, Catley L, Tseng YH, Akiyama M, Shringarpure R, Burger R, Hideshima T, Chauhan D, Mitsiades N, et al.. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3'-kinase/AKT signaling. Cancer Res 2003; 63:5850–8; PMID:14522909 [PubMed] [Google Scholar]
- 171.Valentinis B, Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol Pathol 2001; 54:133–7; PMID:11376123; http://dx.doi.org/ 10.1136/mp.54.3.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vartanian T, Goodearl A, Lefebvre S, Park SK, Fischbach G. Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in schwann cells. Biochem Biophys Res Commun 2000; 271:414–7; PMID:10799311; http://dx.doi.org/ 10.1006/bbrc.2000.2624 [DOI] [PubMed] [Google Scholar]
- 173.Yang XH, Flores LM, Li Q, Zhou P, Xu F, Krop IE, Hemler ME. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res 2010; 70:2256–63; PMID:20197472; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bryant P, Zheng Q, Pumiglia K. Focal adhesion kinase controls cellular levels of p27/Kip1 and p21/Cip1 through Skp2-dependent and -independent mechanisms. Mol Cell Biol 2006; 26:4201–13; PMID:16705171; http://dx.doi.org/ 10.1128/MCB.01612-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, et al.. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 2008; 68:8022–30; PMID:18829560; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1385 [DOI] [PubMed] [Google Scholar]
- 176.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One 2008; 3:e3065; PMID:18725974; http://dx.doi.org/ 10.1371/journal.pone.0003065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, Shapiro CL, Chen CY, Dunn SE, Pollak M. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxib-derived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol 2006; 70:1534–41; PMID:16887935; http://dx.doi.org/ 10.1124/mol.106.023911 [DOI] [PubMed] [Google Scholar]
- 178.Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia 2008; 13:485–98; PMID:19034632; http://dx.doi.org/ 10.1007/s10911-008-9107-3 [DOI] [PubMed] [Google Scholar]
- 179.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, et al.. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A 2011; 108:5021–6; PMID:21385943; http://dx.doi.org/ 10.1073/pnas.1016140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kamath AV, Lu D, Gupta P, Jin D, Xiang H, Wong A, Leddy C, Crocker L, Schaefer G, Sliwkowski MX, et al.. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol 2012; 69(4):1063-69; PMID:22203367; http://dx.doi.org/10:1007/s00280-011-1806-6 [DOI] [PubMed] [Google Scholar]
- 181.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al.. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472–86; PMID:22014573; http://dx.doi.org/ 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 182.Yen L, Benlimame N, Nie ZR, Xiao D, Wang T, Al Moustafa AE, Esumi H, Milanini J, Hynes NE, Pages G, et al.. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell 2002; 13:4029–44; PMID:12429844; http://dx.doi.org/ 10.1091/mbc.E02-02-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rajkumar T, Stamp GW, Hughes CM, Gullick WJ. c-erbB3 protein expression in ovarian cancer. Clin Mol Pathol 1996; 49:M199–202; PMID:16696074; http://dx.doi.org/ 10.1136/mp.49.4.M199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lollini PL, De Giovanni C, Nanni P. Preclinical HER-2 Vaccines: From Rodent to Human HER-2. Front Oncol 2013; 3:151; PMID:23772419; http://dx.doi.org/ 10.3389/fonc.2013.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nomura T, Tamaoki N, Takakura A, Suemizu H. Basic concept of development and practical application of animal models for human diseases. Curr Top Microbiol Immunol 2008; 324:1–24; PMID:18481450 [DOI] [PubMed] [Google Scholar]
- 186.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al.. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002; 100:3175–82; PMID:12384415; http://dx.doi.org/ 10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 187.De Giovanni C, Nicoletti G, Landuzzi L, Romani F, Croci S, Palladini A, Murgo A, Antognoli A, Ianzano ML, Stivani V, et al.. Human responses against HER-2-positive cancer cells in human immune system-engrafted mice. Br J Cancer 2012; 107:1302–9; PMID:22929887; http://dx.doi.org/ 10.1038/bjc.2012.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004; 304:104–7; PMID:15064419; http://dx.doi.org/ 10.1126/science.1093933 [DOI] [PubMed] [Google Scholar]