Abstract
Various components of mulberry leaves, such as iminosugars, flavonoids and polysaccharides, have been reported to exert anti-diabetic activity. The purpose of our present study was to examine the modulating effect of mulberry leaf polysaccharides (MLPs) on murine bone-marrow-derived dendritic cells (BMDCs). The ultrastructure, phenotype and functional maturation of BMDCs were studied using transmission electron microscopy (TEM), flow cytometry (FCM), and tested for phagocytosis, acid phosphatase (ACP) activity using an enzyme linked immunosorbent assay (ELISA). Our results demonstrated that MLPs could markedly induce BMDC maturation by up-regulating the expression of membrane phenotypic markers, such as CD80, CD86, CD83,CD40, and MHC II, down-regulating phagocytosis and ACP activity, and by enhancing the production of interleukin 12 (IL-12) and tumor necrosis factor α (TNF-α) secreted by BMDCs. We therefore concluded that MLPs can positively modulate BMDCs.
Keywords: bone-marrow-derived dendritic cells, immunoregulation, mulberry leaf polysaccharides, maturation
Abbreviations
- MLP
mulberry leaf polysaccharides
- BMDCs
bone-marrow-derived dendritic cells
- ACP
acidic phosphatase
- LPS
lipopolysaccharide
- TEM
transmission electron microscopy
- DAB
3, 3′-diaminobenzidine
- MTS
methyl tolyl sulfide
- FCM
flow cytometry
- MACS
magnetic activated cell sorting
Introduction
Mulberry leaves, in addition to their use in the silkworm and poultry industries, have historically been used as a traditional Chinese medicinal herb to treat such conditions as diabetes and hyperglycemia.1,4 With advances in the field of biochemistry, our understanding about the composition of mulberry leaves has substantially increased.2
MLPs are the major components of mulberry leaves2 and are used as a treatment for hyperglycemia, inflammation, cancer, and as an antioxidant.3 One Chinese medical journal even reported that MLPs could increase the transformation rate of splenic lymphocytes in mice, including T cells, B cells and natural-killer (NK) cells.4 In this study, the purified MLPs were composed of complex carbohydrates that include D-rhamnose, arabinose, xylose, glucose, lactose, and mannose. Their molecular weight was 65KD and molecular ratios of D-rhamnose, arabinose, xylose, glucose, lactose, and mannose were 14:9:7:14:9:10:14.3,4 Some MLPs are used to treat diabetes, cancer, hyperglycemia, hypertension, and to protect the liver.5As documented, MLPs positively induced and activated both macrophages and neutrophils.5-12
Dendritic cells (DCs) were first reported by Paul Langerhans in the late 19thcentury.16 However, it was not until 1973 that Ralph M. Steinman and Zanvil A. Cohn coined the term “dendritic cell,” introducing a new epoch in immunology.16 The primary function of DCs is to present antigens to T cells and, therefore, to activate their expansion and function. DCs not only induce primary immune responses, but are also crucial for inducing immune tolerance. Today, DC-based vaccines are increasingly important in cancer immunotherapy.13-16
To our knowledge, there are no published data concerning DC regulation by MLPs, and little information exists detailing the changes that MLPs induce upon DCs. Therefore, due to the increasing value of MLPs in medicine and healthcare, we conducted the following investigations to examine the role of MLPs in DC regulation.
Results
Using a series of experimental techniques, we analyzed BMDCs both before and post treatment with MLPs. During the process of maturation, immature BMDCs underwent phenotypic and functional changes in phagosome number, alterations in key membrane phenotypic markers, acidic phosphatase (ACP) activity, phagocytosis, and cytokine secretion. Our findings are presented as follows.
Observation of MLP induced BMDC changes in ultrastructure by TEM
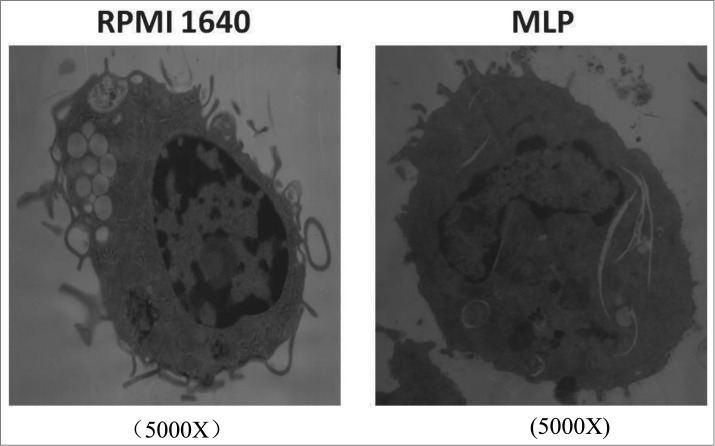
Commonly, immature BMDCs are more active in antigen phagocytosis and contain more phagosomes. As BMDCs mature, phagocytic activity and phagosome numbers are markedly reduced leading in association with an increased expression of membrane co-stimulatory markers. Figure 1 shows an electron micrograph documenting a reduction in phagosomes following co-incubation with MLP, as compared to control BMDCs.
Figure 1.
Structural changes of bone marrow-derived cells (BMDCs) treated with mulberry leaf polysaccharides(MLPs) under transmission electron microscopy (TEM). BMDCs, post treatment with MLPs for 48h, were centrifuged, dispensed in 0.5 mL 0.05M pH 7.2 PBS, fixed in 2.5% glutaraldehyde by 1% osmium teroxide overnight, dehydrated in ethanol and embedded in epon. Prepared sections placed on a Reichert-Jung Ultracut E were stained with uranyl acetate and lead citrate. The sample was analyzed under TEM for BMDC intracellular structure changes.
ACP activity changes induced by MLP
Post treatment with 50 µg/ml MLP for 48 h, BMDCs matured, and ACP activity decreased in association with a decrease in phagocytosis activity as shown in Figure 2.
Figure 2.
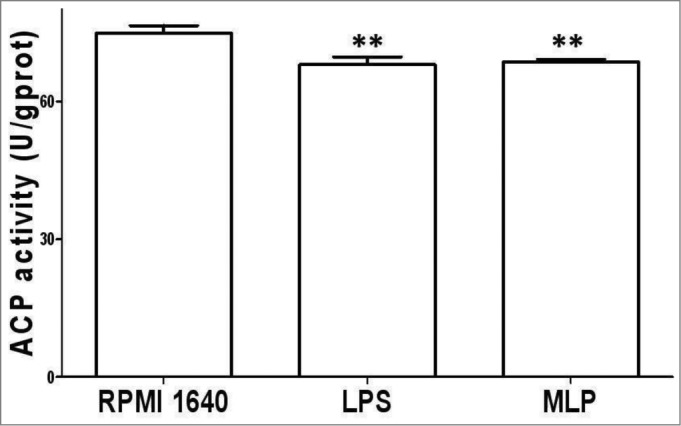
Acidic phosphatase (ACP) activity change. 106/ml bone marrow-derived cells (BMDCs) treated with 50 µg/mL MLPs for 48 h were reacted using an ACP testing kit, plus reagent phenol-4-AAP (amino anti-pyrine) to test for ACP activity within the BMDCs by measuring absorbance numbers at 520 nm(A520). ACP inside BMDCs reduced significantly, which corresponded to the termination of phagocytosis.
Phagocytosis of dextran particles by MLP activation of BMDCs
We further analyzed the phagocytic activity of BMDCs using a labeled FITC-dextran particle phagocytosis test. Here we found that MLP-induced mature BMDCs had downregulate phagocytosis activity as compared to immature BMDCs without MLP treatment as shown in Figure 3. This was also consistent with the results found with the analysis of ACP activity levels.
Figure 3.
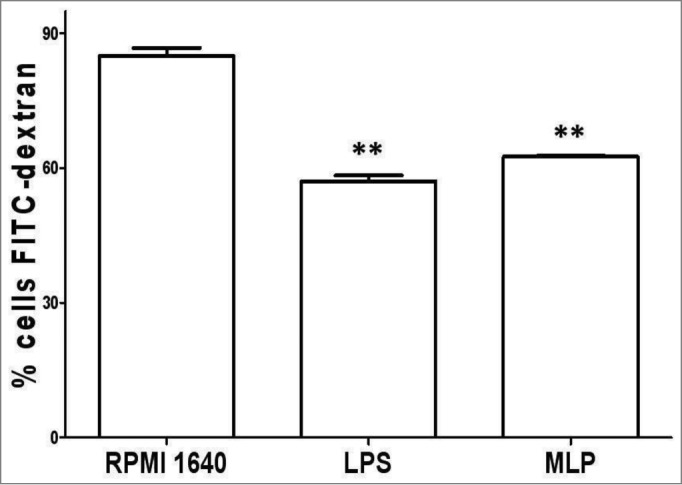
Phagocytosis down-regulation. 100 μl FITC-dextran particles (40,000 D) 28–30 were added to the bone marrow-derived cell (BMDC) culture and then incubated with 50 µg/mL mulberry leaf polysaccharide (MLP) for 2 h at 4°, followed by 1 h at 37°. Finally, treated cells were analyzed using FACSCalibur→ (Becton Dickinson, San Diego, CA) to determine phagocytosis levels. The figure shows the difference of BMDCs to phagocyte-labeled antigen particles both before and after treatment with MLP.
Confirmation of elevated expression of co-stimulatory molecules
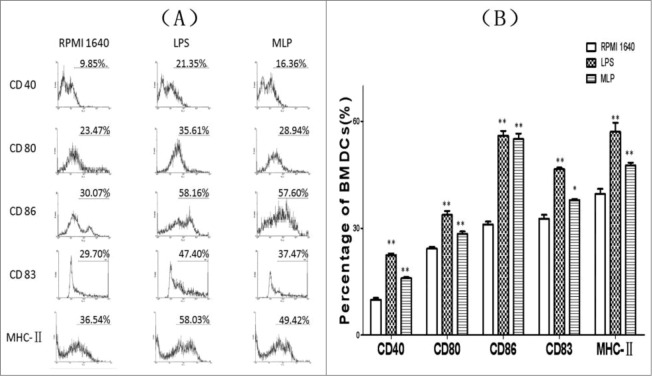
BMDCs treated with 50µg/mL MLPs for 48 h matured and exhibited upregulated expression of CD80, CD86, CD83, CD40 and MHCII as shown in (Fig. 4).
Figure 4.
Changes of expression of surface markers on the bone marrow-derived cells (BMDCs). Anti-CD80, anti-CD86, anti-CD83, anti-CD40, and anti-MHC II antibodies were added to the culture of mulberry leaf polysaccharide (MLP)-treated BMDCs at 4°C for 20 min. After thoroughly rinsing, the cells were examined using a FACSCalibur® (Becton Dickinson, San Diego, CA) to quantitate the percentage of changed surface markers.
Cytokine assay of IL-12 and TNF-α
In addition to the BMDC structural and phenotypic changes induced by MLPs, these functionally mature BMDCs were found to also secrete increased levels of IL-12 and TNFα. Figure 5 details the changes in cytokine production before and after treatment with MLP.
Figure 5.
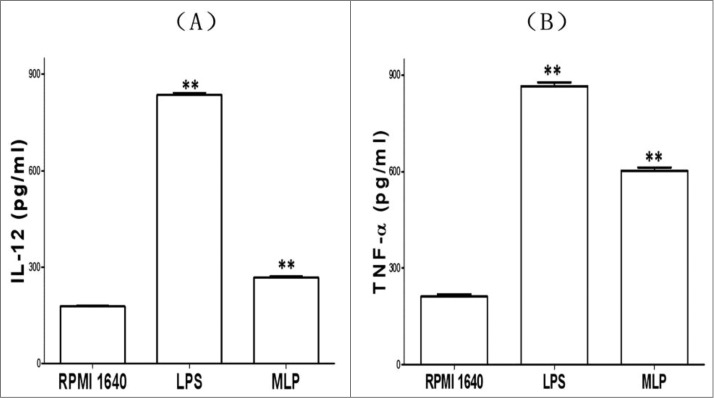
Changes of cytokines production. The bone marrow-derived cell (BMDC) supernatant incubated with 50 µg/mL mulberry leaf polysaccharide (MLP) for 48 h was assayed by ELISA for interleukin 12 (IL-12)p70 and tumor necrosis factor α (TNF-α)production, respectively. The absorbance number at 450 nm (A450) was then measured using a bichromatic microplate reader according to the instructions.
Discussion
Commonly, immature DCs process and present antigens at secondary lymphoid organs, to naïve T cells (T-cell activation). During this process, DCs mature and develop both phenotypic and functionally distinct abilities, including reduced phagocytosis ability, as identified by TEM and FCM of FITC labeled dextran beads. In addition, the activity of key digesting enzymes in DCs will decrease, signaling the gradual termination of phagocytosis.17 Simultaneously, as confirmed by FCM analysis, the membrane of DCs increase their expression of co-stimulatory molecules that help to activate T cells resulting in specific cellular immunity. Furthermore, matured DCs secret increased levels of cytokines, which then serve to coordinate immune, host defense responses.
Mulberry leaves are primarily used as food for silkworms or cattle and are sometimes eaten as a vegetable by humans. As an immunological agent from mulberry leaves, MLPs stimulate immunity and have anti-cancer properties.5,18-21 Early research data both in vitro and in vivo have proven that MLPs can increase both humoral and cellular immunity as evidenced by increased levels of antibody production and through the prevention of death in mice by bovine Pasteurella multocida.
The effect of MLPs on the immune system was evaluated using various experimental models, such as a carbon-clearance test, cyclophosphamide-induced neutropenia, a neutrophil-adhesion test. These data indicated that MLPs increased the phagocytic index in the carbon-clearance assay, a significant protection against cyclophosphamide-induced neutropenia, and increased neutrophil adhesion.5,21,22
There were articles published previously on the induction of DC by many polysaccharides from plant resources and proved that these polysaccharides played immune enhancing role through DC driving. The results from these studies indicated that the polysaccharides could modulate the number of phagosomes and phagocytosis of DC, ACP activity of DC, expression of key surface co-signals molecules on DC and cytokines production by DC.22-24 Our current results confirmed MLPs' role as an immune enhancer by inducing BMDC maturation in that BMDCs, induced by MLPs, showed typical maturation both phenotypically and functionally. These impact on BMDCs by MLP will help vaccines with intensification of special immunity initiation and generation of cellular responses.
Immune systems include a complex, diverse network of leukocytes cells that form a feedback loop regulated by secreted cytokines. Individually, TNF-α or IL-12 alone or in combination with other cytokines, can affect the functions of BMDCs either directly or indirectly by activating other immune cells, such as NK cells, macrophages, or neutrophils, which then produce other cytokines, thereby completing the network loop. For example, NK cells can secrete IFN-γ, which can then induce BMDC maturation, and as a result of this activation, additional IL-12 and TNF-αare secreted. This secretion of IFN-γ, may also entice B cells to produce opsonizing antibodies, which aids in phagocytosis through the macrophage- and antibody-dependent, cell-mediated cytotoxicity of NK cells. Thus, MLPs have immune enhancing properties in immune-handicapped individuals or in patients with immune systems damaged by chemotherapy or radiotherapy.
Our current studies have increased our understanding of the immunoregulatory role of MLPs and how they work on cellular or molecular levels by modulating BMDCs. Our efforts also suggest clinical potential for MLPs as an immune modulator and their potential benefit in immune dysfunctional patients and in vaccine development.25,27-29 There are numerous reports of immune modification by polysaccharides, some of which are derived from plant resource, although to date none of these have received regulatory approval. Two advantages of MLPs, as compared to other polysaccharide immune adjuvants, include their lower molecular weight (65KD) and their lower dose levels with bioactivity including DC stimulation. However, it should be clarified that higher dose levels of MLPs can induce differing immunoregulatory results. While these results are encouraging, additional work is still necessary in order to develop MLPs for clinical use.
Conclusion
We have reported herein that MLPs, administered at given concentrations, can induce BMDC maturation. These MLP-induced, matured BMDCs is with deceased phagosomes and phagocytosis, contrarily with elevated expression of key surface markers ofCD83, CD80, CD86, CD40and MHC II molecules. Moreover, such mature BMDCs also generate additional IL-12, TNF-α, which serve to coordinate the resultant chain of immune responses.
Materials and Methods
Key chemicals
The MLPs were provided by the China Liaoning Institute of Microbiology (purity>98.5%). A range of concentrations of MLPs from 20 μg/mL to200 µg/mL was tested in vitro on the expansion of BMDCs, and the optimal concentration was found to be50 μg/mL. Therefore, 50 μg/mL was used in the current investigation. LPS (from Escherichia coli, serotype 055:B5, Sigma-Aldrich, St. Louis, MO) was used as a positive control throughout. Recombinant murine cytokines, IL-4 and GM-CSF, were provided by PeproTech, Inc., Rocky Hill, NJ. The mAbs used in these experiments included PE-anti-CD80, PE-anti-CD83, PE-anti-CD86, PE-anti-MHC-II and FITC-conjugated anti-CD40 (eBioscience, San Diego, CA, or BD PharMingen, San Jose, CA). ELISA kits for cytokines assays were products of R&D Systems, Minneapolis, MN. Other frequently used chemicals and reagents were acquired from BD PharMingen, eBioscience, or Sigma-Aldrich.
BMDCs induction from mice BM cells
Female C57BL/6 mice, 4–6 weeks old, were obtained from a pathogen-free animal facility and housed in our animal facility under conditions of 12 h photoperiod at 22 ± 1°. The mice received both food and water for 10d before the experiments. All mice were treated humanely according to the Guide for the Care and Use of Laboratory Animals of China Medical University, and all surgical procedures were approved by the Committee of Experimental Animals, China Medical University.
BMDC induction from mice BM cells was performed according to the previously described method.26 BM cells from the femurs and tibias of female C57BL/6 mice were obtained and the red blood cells removed. The counted107/mL cells were then transferred into a 24-well plate filled with 2 mL of enriched RPMI 1640 medium, containing 10% fetal bovine serum, 10ng/mL granulocyte macrophage-colony stimulating factor (GM-CSF), 10ng/mL IL-4, 2 mM L-glutamine, 100units/mL of penicillin and100μg/mL of streptomycin. After inoculation or 4h, non-adherent cells were removed and the adherent cells cultured for 7d. Following culture, non-adherent cells were harvested and incubated with anti-mouse CD11c+mAb in MACS buffer (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min. The cells were then isolated using MACS magnet beads using a MACS column in a magnetic field. The column was removed from the magnet, and the positive cells were flushed. Flow cytometric (FCM) analysis revealed that more than 93% of the purified cells expressed CD11c.All CD11c+ cells were counted and seeded for subsequent study.
Ultrastructural characterization of BMDCs Treated with MLPs
After treatment with MLPs for 48 h, BMDCs were centrifuged, resuspended in 0.5 mL 0.05M pH 7.2 PBS, fixed in 2.5% glutaraldehyde by 1% osmium teroxide overnight, dehydrated in ethanol and embedded in epon resin. Prepared sections were placed on a Reichert-Jung Ultracut E and stained with uranyl acetate and lead citrate. Finally, the sample was checked for BMDC intracellular structure changes under TEM (JEOL JEM-1200EX, JEOL, Ltd., Tokyo, Japan).
ACP activity change
106/mL BMDCs treated with 50 µg/mL MLPs for 48 h were studied using an ACP test kit (Jiancheng Bioengineering Institute of the South, Nanjing, China) plusphenol-4-AAP (amino anti-pyrine) by measuring absorbance at 520 nm(A520).27 All procedures were performed according to the instruction manual.
Phagocytosis study by FCM
100 μl FITC-dextran particles (40,000 D)28-30 were added to the BMDC culture and incubated with 50µg/ml MLPs for 48 h, let it stand for 2 h at 4°C and subsequently for 1h at 37°C. Treated cells were then analyzed using a FACS Calibur to determine the phagocytosis.
Upregulated expression of cell surface markers determined by FCM
The BMDCs treated with MLPs for 48 h were analyzed by FCM for the expression of cell surface markers. Anti-CD80, anti-CD86, anti-CD83, anti-CD40, and anti-MHC II antibodies were added to the culture of MLP-treated BMDCs at 4°C for 20 min. After rinsing thoroughly, the cells were examined using a FACS Calibur® flow cytometer (Becton Dickinson, San Diego, CA) to quantify the percentage of phenotypic marker expression.
Cytokine production of IL-12and TNF-α
The supernatant of BMDCs cultured and incubated with 50 µg/mL MLPs for 48 h was then assayed by ELISA for IL-12 and TNF-α production, respectively (R&D Systems, Minneapolis, MN). The absorbance number at 450 nm (A450) was measured using a bichromatic microplate reader (BIO-TEK, Winooski, VT) according to the instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
The data were expressed as mean and standard error of mean (SEM), and the analysis for multiple groups was completed using ANOVA testing. All analyses of the statistical data were performed using Statistical Package for Social Sciences version 16 software (SPSS, SPSS Inc.., Chicago, IL) and all statistical graphs were produced using Prism version 6.01(GraphPad Software, Inc.., La Jolla, CA). Differences were considered statistically significant when the p-value was less than 0.05(*p<0.05; **p<0.01).
Funding
This work received funding from China Liaoning Science Foundation (2009225008–7, 2012225016).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhou Z, Zhou B, Ren L, Meng Q. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on finishing steer growth performance, blood biochemical parameters, and carcass characteristics. PLoS One 2014 Jan 10; 9(1):e85406; http://dx.doi.org/ 10.1371/journal.pone.0085406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao XY, Zhang DW, Chen RD, Yin YZ, Zou JH, Xie D, Yang L, Wang CM, Dai JG. Chemical constituents from cell cultures of Morusalba. Zhongguo Zhong Yao Za Zhi 2012 Dec; 37(24):3738-42. Chinese; PMID:23627170 [PubMed] [Google Scholar]
- 3.Fang F, Luo ML, Su N, Wu XR. Effect of mulberry leaves extracts on glucose uptake of insulin-resistant HepG2 cells and the mechanism. Yao Xue Bao 2012 Nov; 47(11):1452-6; PMID:23387076 [PubMed] [Google Scholar]
- 4.Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One 2012; 7(11):e50619; PMID:23185641; http://dx.doi.org/ 10.1371/journal.pone.0050619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharani SE, Asad M, Dhamanigi SS, Chandrakala GK. Immunomodulatory activity of methanolic extract of Morusalba Linn. (Mulberry) leaves. Pak J Pharm Sci 2010 Jan; 23(1):63-8 [PubMed] [Google Scholar]
- 6.Park S, Kim YS, Lee HA, Lim Y, Kim Y. Mulberry leaf extract inhibits invasive potential and down-regulates hypoxia-inducible factor-1α (HIF-1α) in SK-N-BE2C neuroblastoma cells. Biosci Biotechnol Biochem 2013; 77(4):722-8 [DOI] [PubMed] [Google Scholar]
- 7.Chao WW, Kuo YH, Li WC, Lin BF. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morusalba ethyl acetate fractions. J Ethnopharmacol 2009 Feb 25; 122(1):68-75; http://dx.doi.org/ 10.1016/j.jep.2008.11.029 [DOI] [PubMed] [Google Scholar]
- 8.Deepa M, Sureshkumar T, Satheeshkumar PK, Priya S. Purified mulberry leaf lectin (MLL) induces apoptosis and cell cycle arrest in human breast cancer and colon cancer cells. Chem Biol Interact 2012 Oct 25; 200(1):38-44; http://dx.doi.org/ 10.1016/j.cbi.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 9.Park S, Kim J, Kim Y. Mulberry leaf extract inhibits cancer cell stemness in neuroblastoma. Nutr Cancer 2012 Aug;64(6):889-98; http://dx.doi.org/ 10.1080/01635581.2012.707280 [DOI] [PubMed] [Google Scholar]
- 10.Kim GN, Kwon YI, Jang HD. Mulberry leaf extract reduces postprandial hyperglycemia with few side effects by inhibiting α-glycosidase in normal rats. J Med Food 2011 Jul-Aug;14(7-8):712-7; http://dx.doi.org/ 10.1089/jmf.2010.1368 [DOI] [PubMed] [Google Scholar]
- 11.Lee YJ, Choi DH, Kim EJ, Kim HY, Kwon TO, Kang DG, Lee HS. Hypotensive, hypolipidemic, and vascular protective effects of Morusalba L. in rats fed an atherogenic diet. Am J Chin Med 2011; 39(1):39-52; PMID:21213397; http://dx.doi.org/ 10.1142/S0192415X11008634 [DOI] [PubMed] [Google Scholar]
- 12.Hunyadi A, Liktor-Busa E, Márki A, Martins A, Jedlinszki N, Hsieh TJ, Báthori M, Hohmann J, Zupkó I. Metabolic effects of mulberry leaves: exploring potential benefits in type 2 diabetes and hyperuricemia. Evid Based Complement Alternat Med 2013; 2013:948627; PMID:24381639; http://dx.doi.org/ 10.1155/2013/948627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mody N, Sharma R, Agrawal U, Vyas SP. Dendritic-cell based vaccine research against cancer. Expert Rev Clin Immunol 2015 Feb; 11(2):213-32. [DOI] [PubMed] [Google Scholar]
- 14.Weber JS. Current perspectives on immunotherapy. Semin Oncol 2014 Oct;41 Suppl 5:S14-29; http://dx.doi.org/ 10.1053/j.seminoncol.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Bedoui S, Greyer M. The role of dendritic cells in immunity against primary herpes simplex virus infections. Front Microbiol 2014 Oct 21;5:533; http://dx.doi.org/ 10.3389/fmicb.2014.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman R, Cohn Z. Identification of a novel cell type in peripheral lymphoid organs of mice. J Exp Med 1973; 137: 1142-62; PMID:4573839; http://dx.doi.org/ 10.1084/jem.137.5.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia L, Gao X, Wang Y, Yao N, Zhang X. Structural, phenotypic and functional maturation of bone marrow dendritic cells (BMDCs) induced by Chitosan (CTS). Biologicals 2014 Nov;42(6):334-8; http://dx.doi.org/ 10.1016/j.biologicals.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Lee WJ, Choi SW. Quantitative changes of polyphenolic compounds in mulberry (Morusalba L.) leaves in relation to varieties, harvest period, and heat processing. Prev Nutr Food Sci 2012 Dec; 17(4):280-5; http://dx.doi.org/ 10.3746/pnf.2012.17.4.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyahara C, Miyazawa M, Satoh S, Sakai A, Mizusaki S. Inhibitory effects of mulberry leaf extract on postprandial hyperglycemia in normal rats. J NutrSci Vitaminol (Tokyo) 2004 Jun; 50(3):161-4 [DOI] [PubMed] [Google Scholar]
- 20.Deepa M, Priya S. Purification and characterization of a novel anti-proliferative lectin from Morusalba L. leaves. Protein Pept Lett 2012 Aug; 19(8):839-45; http://dx.doi.org/ 10.2174/092986612801619516 [DOI] [PubMed] [Google Scholar]
- 21.Zhang EH, Wang RF, Guo SZ, Liu B. An update on antitumor activity of naturally occurring chalcones. Evid Based Complement Alternat Med 2013;2013:815621; PMID:23690855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng J, Cao Y, Meng Y, Luo H, Gao X, Shan F. Maturation of mouse bone marrow dendritic cells (BMDCs) induced by Laminaria japonica polysaccharides (LJP). Int J Biol Macromol 2014 Aug;69:388-92; http://dx.doi.org/ 10.1016/j.ijbiomac.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 23.Meng J, Meng Y, Liang Z, Du L, Zhang Z, Hu X, Shan F. Phenotypic and functional analysis of the modification of murine bone marrow dendritic cells (BMDCs) induced by neutral Ginseng polysaccharides (NGP). Hum Vaccin Immunother 2013 Feb;9(2):233-41; http://dx.doi.org/ 10.4161/hv.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Meng Y, Guo Y, He X, Liu Q, Wang X, Shan F. Rehmannia glutinosa polysaccharide induces maturation of murine bone marrow derived Dendritic cells (BMDCs).Int J Biol Macromol 2013 Mar;54:136-43; http://dx.doi.org/ 10.1016/j.ijbiomac.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 25.Coosemans A, Vergote I, van Gool SW. Dendritic cell-based immunotherapy in ovarian cancer. Oncoimmunology 2013 Dec 1;2(12):e27059; http://dx.doi.org/ 10.4161/onci.27059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salman B, Zhou D, Jaffee EM, Edil BH, Zheng L. Vaccine therapy for pancreatic cancer. Oncoimmunology 2013 Dec 1;2(12):e26662 Epub 2013 Oct 22. Review; http://dx.doi.org/ 10.4161/onci.26662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spel L, Boelens JJ, Nierkens S, Boes M. Antitumor immune responses mediated by dendritic cells: How signals derived from dying cancer cells drive antigen cross-presentation. Oncoimmunology 2013 Nov 1;2(11):e26403 Epub 2013 Oct 21. Review; http://dx.doi.org/ 10.4161/onci.26403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segovia M, Cuturi MC, Hill M. Preparation of mouse bone marrow-derived dendritic cells with immunoregulatory properties. Methods Mol Biol 2011; 677:161-8; PMID:20941609; http://dx.doi.org/ 10.1007/978-1-60761-869-0_11 [DOI] [PubMed] [Google Scholar]
- 29.Belfield A, Goldberg DM. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme 1971; 12(5):561-73; PMID:5169852 [DOI] [PubMed] [Google Scholar]