Abstract
Interstitial lung disease (ILD) is a prognostic factor for poor outcome in polymyositis (PM)/dermatomyositis (DM). The appropriate management of ILD is very important to improve the prognosis of patients with PM/DM. ILD activity and severity depend on the disease subtype. Therefore, clinicians should determine therapeutic strategies according to the disease subtype in each patient with PM/DM. Anti–melanoma differentiation-associated gene 5 antibody and hyperferritinemia predict the development and severity of rapidly progressive (RP) ILD, particularly in East Asian patients. Combination therapy with corticosteroids, intravenous cyclophosphamide pulse, and calcineurin inhibitors should be administered in RP-ILD. In contrast, patients with anti–aminoacyl-tRNA synthetase (ARS) show better responses to corticosteroids alone. However, ILDs with anti-ARS often display disease recurrence or become refractory to corticosteroid monotherapy. Recent studies have demonstrated that the administration of tacrolimus or rituximab in addition to corticosteroids may be considered in ILD patients with anti-ARS. Large-scale, multicenter randomized clinical trials should be conducted in the future to confirm that the aforementioned agents exhibit efficacy in ILD patients with PM/DM. The pathophysiology of ILD with PM/DM should also be elucidated in greater detail to develop effective therapeutic strategies for patients with ILD in PM/DM.
Keywords: interstitial lung disease, idiopathic inflammatory myopathies, dermatomyositis, polymyositis, treatment
Introduction
Polymyositis (PM)/dermatomyositis (DM) are idiopathic inflammatory myopathies (IIM) that are occasionally complicated with extramuscular lesions, such as interstitial lung disease (ILD), cardiomyopathy, and malignancy. These complications are poor prognostic factors in PM/DM patients. ILD is found in approximately 50% of patients with PM/DM.1 ILD associated with DM is more refractory to treatment and more strongly associated with a poorer prognosis than ILD associated with PM.2 Recent research has revealed that myositis-specific autoantibodies (MSAs) are closely linked to clinical phenotypes in PM/DM. ILD activity and severity are dependent on the subtype of PM/DM. Therefore, physicians should evaluate MSAs and determine the therapeutic strategies for ILD according to the subtype of PM/DM in each patient.
Classification of ILD with PM/DM
ILD is classified by clinical course or pathohistological findings. The clinical course of ILD is divided into two subtypes: acute/subacute interstitial pneumonia (A/SIP), also called rapidly progressive ILD (RP-ILD), and chronic IP (CIP). The pathohistological classification includes usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), diffuse alveolar damage (DAD), desquamative interstitial pneumonia, and lymphoid interstitial pneumonia.3 NSIP is a typical form of ILD in PM/DM patients. UIP, OP, and DAD are also complicated in patients with PM/DM.4,5 DAD is typically characterized by RP-ILD, and pulmonary function deteriorates daily or weekly in RP-ILD with PM/DM patients. NSIP is usually recognized as CIP. ILD develops gradually (monthly) in CIP but develops acutely/subacutely in some cases. Some patients with NSIP or UIP with PM/DM do not exhibit respiratory symptoms, although ILD is demonstrated by radiological imaging, such as X-ray radiography and/or high-resolution computed tomography (HRCT).
Clinical Characteristics of ILD with PM/DM
The measurement of MSAs is useful for predicting clinical course, clinical characteristics, response to treatment, and prognosis in PM/DM patients. The MSAs that are strongly associated with ILD include the anti–melanoma differentiation-associated gene 5 (MDA5) and anti–aminoacyl-tRNA synthetase (ARS) antibodies (Table 1).6,7
Table 1.
Differences in clinical characteristics between anti-MDA5 and anti-ARS antibodies in PM/DM.
| ANTI-MDA5 | ANTI-ARS | |
|---|---|---|
| Type of PM/DM | CADM | PM/DM/CADM |
| Clinical course of ILD | RP* | Chronic progressive |
| Response to corticosteroids in ILD | Poor* | Favorable |
| Recurrence of ILD | Rare* | Frequent |
Note:
These findings are frequently found in East Asia. ILD in Caucasians is generally not RP. Clinical manifestations may depend on race.
The presence of anti-MDA5 is associated with clinically amyopathic DM (CADM),8,9 especially cutaneous ulcers. CADM involves the typical skin lesions that are revealed in DM with amyopathy or hypomyopathy. Therefore, patients with CADM do not typically present with muscle symptoms, such as myalgia and muscle weakness.10 The risk of ILD development is increased in patients with anti-MDA5 in East Asian and Western countries.8,9,11–13 However, the clinical course and severity of ILD are different between Asians and Westerners. ILD in Japan and China is generally RP and frequently causes fatal outcomes in patients with anti-MDA5. RP-ILD is not frequently found in the United States and Europe. The clinical characteristics of ILD may depend on race in patients with anti-MDA5. Approximately half of patients with anti-MDA5 present with RP-ILD in East Asia.8,14,15 RP-ILD is generally complicated with CADM, although it is not found in all patients with CADM. Anti-MDA5 is also not found in all patients with CADM. Therefore, anti-MDA5 is a useful predictor for the complication of RP-ILD in patients with CADM, especially in East Asia. Ferritin is the other useful predictive marker for RP-ILD, and serum ferritin levels predict the development and severity of RP-ILD in PM/DM patients. Hyperferritinemia is frequently found in RP-ILD with anti-MDA5, and serum ferritin levels correlate with the disease activity of ILD with anti-MDA5. Gono et al demonstrated that serum ferritin levels were higher in PM/DM patients with A/SIP than in patients with CIP.16
Anti-ARS is associated with clinical manifestations, including arthritis, mechanic’s hand, Raynaud’s phenomenon, myositis, and ILD, which are known as antisynthetase syndromes. This type of ILD generally manifests as NSIP or UIP and typically exhibits chronic progressive pulmonary dysfunction, which may acutely/subacutely deteriorate. ILD with anti-ARS exhibits a better response to corticosteroid therapy and better prognosis than ILD without anti-ARS in PM/DM.17 However, corticosteroid therapy alone often causes a recurrence of myositis and/or ILD in patients with anti-ARS.18 Therefore, combination therapy of corticosteroids and immunosuppressive agents should be considered.
Anti-ARS includes anti–Jo-1, anti-EJ, anti-OJ, anti–PL-7, anti–PL-12, anti-KS, anti-Zo, and anti-Ha. Hamaguchi et al demonstrated the similarities and differences of clinical manifestations in Japanese patients with individual anti-ARSs.19 DM-specific rashes, such as heliotrope and Gottron’s sign, have been frequently observed in patients with anti–Jo-1, anti-EJ, anti–PL-7, and anti–PL−12. ILD alone has been frequently found in patients with anti-OJ and anti-KS, and myositis is not complicated with ILD in some patients with anti-ARS.
Prognosis
The overall 5-year survival rate is 77%–95% in PM/DM,20–22 and cardiac dysfunction, malignancy, respiratory failure, and infection are the major causes of fatal outcome. Previous studies reported that cardiac involvement and respiratory muscle involvement are significant prognostic factors for death in patients with PM/DM, except in patients with cancer.22 Yamasaki et al retrospectively investigated 197 patients with PM, DM, and CADM.1 Survival in the entire group at 1, 5, and 10 years was 85%, 75%, and 67%, respectively, and the mortality in CADM and DM was 61% and 77% at 5 years, respectively. DM patients exhibited significantly lower survival compared to PM (91% at 5 years), and ILD was the major cause of death in CADM (71%) and DM (60%) patients. Most of these patients died within the first few months.
The overall 5-year mortality rate of ILD with PM/DM patients is 50%.23 Dankó et al reported survival curves of patients with PM/DM patients with and without ILD.22 The cumulative survival rate was significantly worse in DM with ILD patients than DM without ILD patients, although there was no difference in survival rate between PM with and without ILD patients. One Japanese study revealed that acute/subacute ILD, forced vital capacity (FVC), age, neutrophils in bronchoalveolar lavage fluid, and CADM were significantly associated with poor outcome.24 The overall 6-month survival rate of ILD with anti-MDA5 is 50%–60% in Japan.14,15 Serum ferritin levels also predict clinical outcome in ILD with PM/DM. The survival rate in patients with serum ferritin levels higher than 1500 ng/mL on admission is lower than patients with levels less than 1500 ng/mL. The 10-year survival rate in another study was significantly higher in ILD patients with anti-ARS than in ILD patients without anti-ARS (91.6% vs. 58.7%). Therefore, prognosis is dependent upon clinical phenotype, serum ferritin levels, and MSAs.17
Efficacy of Each Drug in ILD with PM/DM
Corticosteroids and immunosuppressive agents are considered a first-line therapy in ILD with PM/DM patients. Some specialists recommend the use of immunosuppressive agents with corticosteroids for myositis as the first-line therapy because these drugs exhibit corticosteroid-sparing effects.25,26 Immunosuppressive agents should be considered in PM/DM patients, particularly when ILD severely or progressively develops, to reduce the side effects of corticosteroids and improve the response to treatment. Combination therapy with corticosteroids and immunosuppressive agents is commonly used, although there have been no large randomized clinical trials (RCTs) in ILD with PM/DM patients.27,28 However, it is difficult to conduct RCTs because ILD with PM/DM is a relatively rare disease and is occasionally progressive and fatal. Herein, we review each agent that was previously reported as effective for the treatment of ILD with PM/DM. Table 2 summarizes the characteristics of each agent. We initially describe conventional agents for the treatment of ILD with PM/DM. Subsequently, we review the efficacy of novel agents from recent studies that provided evidence for the treatment of ILD with PM/DM, especially tacrolimus (TAC) and rituximab (RTX).
Table 2.
Characteristics of each drug therapy for ILDs with PM/DM.
| DRUG THERAPY | CHARACTERISTICS |
|---|---|
| Corticosteroids | First-line therapy for ILD with PM/DM. |
| Corticosteroids monotherapy is generally not effective for RP-ILD with PM/DM. | |
|
| |
| MTX | Inhibitor of folic acid metabolism. |
| Useful for corticosteroid-sparing agents. | |
| AZA | A prodrug of 6-mercaptopurine, inhibition of purine synthesis. |
| Useful for corticosteroid-sparing agents. | |
| CY | Alkylating agent, nitrogen mustard derivative. IVCY is administered in RP-ILD or refractory ILD. |
|
| |
| MMF | Anti-metabolite that blocks de novo purine synthesis and the production of B and T cells. |
| Efficacy for corticosteroid-resistant ILD with PM/DM has been shown. | |
|
| |
| IVIG | The mechanism of drug action is variable. |
| Efficacy for refractory myositis has been demonstrated. | |
| Efficacy was also found in several PM/DM cases with ILD. | |
|
| |
| CSA | CNI, one of the T-cell-targeting therapies. |
| Cornerstone for the PM/DM-ILD treatment. | |
| Usually administered in antisynthetase syndrome or RP-ILD. | |
|
| |
| TAC | CNI. TAC has a 100-fold greater potency than CSA in inhibiting T-cell activation. |
| Efficacy for CSA-refractory ILD in PM/DM has been demonstrated. | |
|
| |
| RTX | Chimeric monoclonal anti-CD20 antibody, B-cell targeting agent. |
| Efficacy for refractory ILD or antisynthetase syndrome has been shown. | |
Abbreviation: CD, cluster of differentiation.
Conventional Agents
Corticosteroids
Frazier and Miller first reported the use of oral corticosteroids in ILD with PM/DM in 1974.29 Oral high-dose corticosteroids (>1 mg/kg/day of prednisone) or pulse therapy of methylprednisolone (1000 mg intravenously for 3 days) are still used as a first-line therapy for ILD with PM/DM. Approximately half of patients respond well to initial corticosteroid therapy.2,30–33 However, there is a difference in the response to corticosteroid monotherapy between ILD with PM patients and ILD with DM patients. Fujisawa et al investigated the differential responses of 28 ILD with PM/DM patients (16 PM and 12 DM) to the therapy.2 Corticosteroid monotherapy achieved favorable responses in six (37.5%) ILD with PM patients but only one (8.3%) ILD with DM patient. The overall 2.5-year survival rate of ILD with DM patients was 58%, and the 5-year survival of ILD with PM patients was 81%. RP-ILD is generally not responsive to corticosteroid monotherapy.8,9,14,34–36 RP-ILD is frequently complicated with CADM. Nawata et al reported that ILD with PM/DM patients with normal creatine kinase (CK) exhibited significantly more resistance to corticosteroid therapy and poorer prognosis than patients with high CK (1-year survival, 31% vs. 89%).32 Patients with normal CK levels may present with the clinical manifestation of CADM. The efficacy of corticosteroids alone as an initial therapy for ILD is limited. Clinicians should also consider the corticosteroid-sparing effects of the immunosuppressive agents described below, although corticosteroids remain the standard therapy for ILD with PM/DM.
Methotrexate/azathioprine
Methotrexate (MTX) is an inhibitor of folic acid metabolism and suppresses T-cell activation and adhesion molecule expression.37 MTX is widely used in the treatment of arthritis and myositis as an adjunctive agent after corticosteroid failure38,39 or as a corticosteroid-sparing agent in PM/DM patients. There is no evidence of pulmonary-specific efficacy, although MTX is accepted in the treatment of ILD with PM/DM.40,41 Azathioprine (AZA) is a prodrug of 6-mercaptopurine that inhibits purine synthesis. AZA prevents lymphocyte proliferation, which suppresses the production of antibodies and cytokines. AZA has been widely used in ILD with PM/DM patients as a corticosteroid-sparing agent in maintenance therapy after IVCY.23,40,42,43
Cyclophosphamide
Cyclophosphamide (CY) is used in RP or refractory ILD. CY is generally administered orally or intravenously and is commonly used in combination with corticosteroids. IVCY is a recent standard treatment for lupus and PM/DM because there are fewer adverse effects associated with monthly IVCY than the daily oral administration of CY. The efficacy of CY for the treatment of ILD with PM/DM was demonstrated in several case studies.43–47 Yamasaki et al demonstrated the efficacy of IVCY in refractory ILD with PM/DM in a small open-label trial.46 IVCY (300–800 mg/m2, at least six times every 4 weeks) was administered in combination with corticosteroids (0.5–1 mg/kg/day) in 17 cases of refractory ILD with PM/DM. Eight of the 17 patients exhibited improvement in vital capacity, and 9 of 17 patients showed improved findings on HRCT. Mok et al reported improvement when CY was administered orally followed by AZA as a maintenance therapy in RP-ILD with DM patients.48 CY has also been used with other immunosuppressive agents in refractory ILD.45,49 Kameda et al demonstrated the efficacy of combination therapy that included prednisolone (PSL), IVCY (10–30 mg/kg, every 3–4 weeks), and cyclosporin A (CSA, 2–4 mg/kg/day).49 In this study, 10 DM patients with acute/subacute ILD were initially given combination therapy with IVCY, PSL, and CSA, and this group exhibited significantly lower mortality (50% vs. 75%) over 3 months compared to treatment with corticosteroids alone. However, five patients died of respiratory failure within 3 months. Biweekly IVCY administration with corticosteroids and calcineurin inhibitors (CNIs) was recently recommended in Japanese patients with RP-ILD who harbor hyperferritinemia and/or anti-MDA5.50 Nakashima et al demonstrated that the 6-month survival rate was increased to 75% in patients with an early intensive combination therapy with corticosteroids, IVCY, and CNI than patients treated with conventional therapy. Therefore, early intensive combination therapy may improve prognosis in RP-ILD with PM/DM patients.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an antimetabolite that blocks de novo purine synthesis and targets the production of activated B and T lymphocytes and fibroblasts. Several case series revealed the potential efficacy of MMF in the stabilization of progressive ILD and the reduction of corticosteroid dose in ILD with connective tissue disease (CTD) patients, including PM/DM.42,51–58 An open trial of MMF was conducted in 28 patients with ILD in CTD, including five patients with PM/DM. These patients received MMF (30 mg/kg/day), although pulmonary function tests (FVC and diffusion lung capacity for carbon monoxide [DLco]) showed no significant improvement at 18-month follow-up.52 In another case series, three patients with ILD in PM/DM who received corticosteroids and MMF showed improvement in ILD.53
A retrospective study was conducted to identify differences in the efficacy of AZA, CY, and MMF for corticosteroid-resistant ILD with PM/DM.42 Thirteen, 24, and 9 patients were treated with AZA, CY, and MMF, respectively. There were no differences in baseline pulmonary function in each subset. Pulmonary function improved and the severity of dyspnea decreased in each subset at the 6-month assessment. The corticosteroid dose was also reduced. This result suggests that the addition of immunosuppressive agents is useful for refractory cases and corticosteroid-sparing effects in ILD with PM/DM patients.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) is widely used for the treatment of numerous autoimmune diseases. IVIG is variously involved in the suppression or neutralization of autoantibodies and cytokines and complements the blockade of several cell surface molecules and specific immune cell surface receptors.59 The efficacy of IVIG in the treatment of refractory myositis has been demonstrated,60,61 although its efficacy for ILD with PM/DM is not certain. One case series treated five patients with severe and refractory ILD in PM/DM with IVIG as a salvage therapy in combination with conventional treatments, including corticosteroids, IVCY, and CNIs.62 Another case report demonstrated improvements in pulmonary function tests and chest CTs following the use of IVIG alone without other immunosuppressive therapies, including corticosteroids, in a patient with ILD in PM/DM.63
Plasmapheresis/hemoperfusion
Plasma exchange is used to remove circulating autoantibodies, cytokines, and immune complexes. There have been two case reports of the efficacy of plasmapheresis in patients with antisynthetase syndrome who were refractory to corticosteroids and other immunosuppressive therapies.64,65 Lee et al reported a case series of DAD associated with PM/DM66 in which plasmapheresis was performed, although all three patients died within 5 months after the development of ILD.
Recent reports suggest that hemoperfusion is promising in certain cases with ILD associated with PM/DM.67–70 Direct hemoperfusion using a polymyxin B–immobilized fiber column (PMX-DHP) exhibits efficacy for Gram-negative–induced sepsis. PMX-DHP reduces endotoxin levels and inflammatory chemical mediators, such as cytokines.71 Ichiyasu et al reported three cases of RP-ILD with CADM, in which the PaO2/FiO2 ratio and CT findings improved, and all patients survived after treatment with PMX-DHP.67 The mechanism of the efficacy of PMX-DHP in RP-ILD is not sufficiently known, although PMX-DHP likely inhibits monocyte activation.72
Novel Agents
TAC and RTX have been the focus of novel treatments of ILD with PM/DM in the last several years. TAC is a CNI, and RTX is a biologic agent. The efficacies of other CNIs and biologic agents have been reported in ILD with PM/DM patients. These CNIs and biologic agents are described below.
Calcineurin inhibitors
CNIs are T-cell–targeting agents that may become the cornerstone for the treatment of ILD with PM/DM based on clinical findings. CNIs inhibit interleukin (IL)-2 production and T-cell proliferation. CNIs were recently indicated as an appropriate choice for the early phase of ILD with PM/DM. Zou et al. reported that CADM-acute ILD patients treated with CNIs exhibited a significantly better outcome than patients who received treatment without CNIs.73 CNIs may improve the outcome of CADM patients with acute ILD. CSA and TAC are described below.
Cyclosporin A
Several retrospective and open-label studies analyzed the efficacy of CSA in ILD with PM/DM patients.2,31,32,74,75 Takada et al reported a retrospective multicenter study of 38 cases with acute ILD with PM/DM. Patients who received a combination therapy of corticosteroids and CSA as a first-line therapy had a better survival rate than patients who received corticosteroids alone. Nine cases with ILD in PM and five cases with chronic ILD in DM exhibited good CSA efficacy and a good prognosis, whereas 17 cases with RP-ILD in DM showed poor prognosis, and 7 of these 17 patients died.75 A further analysis of 32 cases with RP-ILD in DM demonstrated that 9 of 13 (69%) patients who started CSA within 2 weeks of initial corticosteroid treatment survived, whereas all 17 cases who received only corticosteroids for more than 2 weeks as the initial therapy died within 9 months from the initiation of therapy. Another study revealed that the early use of cyclosporine was beneficial for survival outcome in DM-associated ILD. The mortality rate was significantly lower in the initial treatment group compared to the delayed-treatment group (0.02 person-years vs. 0.18 person-years; P = 0.0092, log-rank test).76 These results suggest that combination therapy with CSA and corticosteroids during the early phase of ILD is superior to corticosteroid monotherapy in the treatment of ILD with PM/DM.
The monitoring of serum CSA concentrations is important for achieving maximum efficacy and reducing toxicity. There is marked interpatient variability in CSA absorption. Nagai et al. suggested that preprandial once-daily administration of CSA is beneficial, rather than twice daily, because C0 was significantly lower and adverse effects may be reduced using a once-daily administration of CSA.77 The 2-hour postdose level (C2) was correlated with the therapeutic effect.77,78 Recent studies indicated that the C2 level should reach 1000 ng/mL to achieve a maximal immunosuppressive effect.79
Tacrolimus
TAC has a 100-fold greater potency than CSA for the inhibition of T-cell activation. The medication concentration in blood is also more stable, and dose adjustments of medication are easier in TAC than CSA. Therefore, TAC is more often used than CSA in recent treatments of CTD, including ILD with PM/DM, especially in Japan. TAC was previously used in refractory ILD with PM/DM as an alternative to CSA. Several case series and retrospective studies demonstrated the efficacy and tolerability of TAC in ILD in PM/DM patients, including patients who were refractory to CSA.75,80–85 Kurita et al reported the efficacy of TAC for the treatment of ILD with PM/DM. Forty-nine patients were treated with the addition of TAC to conventional therapy (25 cases) or conventional therapy alone (24 cases, PSL, IVCY, and/or CSA). The group treated with TAC exhibited significantly longer survival than the other group, although the concomitant use of IVCY was more frequent in the group treated with TAC than the other group. This study encourages the use of TAC in progressive or refractory ILD in which conventional treatments, such as corticosteroids and other immunosuppressive agents, have no efficacy.
TAC also appears more effective in ILD with anti-ARS patients.81–83,86 Wilkes et al retrospectively assessed TAC efficacy in 13 patients with ILD harboring anti-ARS.82 The authors suggested that TAC is a well-tolerated and effective therapy for the management of ILD with anti-ARS. Labirua-Iturburu et al demonstrated the efficacy of CNIs (TAC or CSA) for ILD management in 15 patients with anti-ARS.86 A greater than 10% increase in FVC was observed in 13 patients treated with CNIs. Taken together, these reports demonstrate that CNIs are effective in refractory cases and as a first-line therapy in ILD with PM/DM patients.
Biologic agents
Biologic agents, such as anti-tumor necrosis factor (anti-TNF), anti–IL-6 receptor, and anti-CD20, have exhibited sufficient efficacies in improvements of disease status in rheumatoid arthritis. These agents were also used in PM/DM patients. The anti-CD20 antagonist RTX improved clinical outcome in PM/DM patients. Herein, we review recent studies of the efficacy of RTX or other biologics in PM/DM patients.
Rituximab
RTX is a biologic agent consisting of a chimeric monoclonal anti-CD20 antibody. This molecule targets B cells and results in B-cell depletion.87 Several case reports and case series reported RTX efficacy in patients with refractory myositis or ILD in PM/DM.88–94 Sem et al demonstrated the short-term efficacy of RTX in 11 patients with antisynthetase syndrome, including severe and progressive ILD, in a retrospective case series.88 RTX stabilized or improved the disease activity of ILD in 7 of 11 patients during the first 6 months. Krystufková et al demonstrated that serum levels of B-cell–activating factor (BAFF) were significantly higher in patients with PM/DM, especially those patients with anti–Jo-1, DM, or ILD.95 BAFF is necessary for B-cell maturation and function. These findings indicate that BAFF may also be a potential therapeutic target in patients with ILD in PM/DM. Aggarwal et al investigated predictors of clinical improvement in PM/DM patients treated with RTX.96 Patients with anti–Mi-2 or anti-ARS exhibited greater improvement than patients with other MSAs, such as anti-signal recognition particle (anti-SRP), anti–TIF-1γ, and anti-MJ. Andersson et al reported the efficacy of ILD with anti-ARS in 24 ILD patients with anti-ARS.97 Sixteen patients were treated with glucocorticoid steroids and/or immunosuppressive agents prior to RTX administration. Acute onset/exacerbation of ILD was revealed in 50% of these 24 patients. Pulmonary function, the extent of ILD, and myositis improved after RTX treatment in most patients with anti-ARS. These findings indicate that RTX was more effective in patients with antisynthetase syndrome with acute-onset ILD. RTX administration should be considered in patients with anti-ARS.
Anti-TNF agents
RCTs of anti-TNF agents were conducted to confirm improvements in myositis,98 although the efficacy of anti-TNF agents is controversial. There have been no large RCTs of anti-TNF agents to investigate the efficacy of these agents in ILD with PM/DM. Therefore, only small case series are described below.
Infliximab (IFX) is a chimeric monoclonal antibody against soluble and membrane-bound TNF-α with a murine Fv region. There is only one relevant case report and a literature review that described a retrospective study of 14 patients with DM and acute ILD (10 CADM, 4 DM). These 14 patients received conventional immunosuppressive therapies and IFX (5 mg/kg).99 IFX was administered at 5 mg/kg intravenously once weekly at weeks 0, 2, and 6, and every 8 weeks thereafter in available patients. Ten cases (71.4%) exhibited favorable responses in chest CT findings. These 10 patients were treated with IFX at an early stage of the disease. The other four patients were treated with IFX after respiratory failure was progressive. These four patients died of respiratory failure.
Adalimumab is a fully human monoclonal antibody that successfully improved ILD with DM in a case report and exhibited marked improvements in DLco and radiographic findings.100
PM/DM may be induced or exacerbated in chronic inflammatory diseases, such as RA, during anti-TNF therapy.101 One literature review revealed that 20 cases, including 17 RA patients, developed new PM/DM during anti-TNF therapies,98 and myositis and ILD with anti-ARS was complicated in 6 cases. Thus, physicians should carefully consider the use of anti-TNF in PM/DM.
Symptomatic treatment
Symptomatic treatment should be considered in patients with irreversible lesions in the lung, such as severe pulmonary fibrosis, and patients with poor response to immunosuppressive therapy. Home oxygen therapy (HOT) is a valuable option for patients suffering from hypoxia associated with ILD, and HOT can improve functional performance in daily life. Pulmonary rehabilitation may also improve respiratory muscle strength and functional daily performance levels in patients with ILD.102
Therapeutic Strategy for ILD with PM/DM
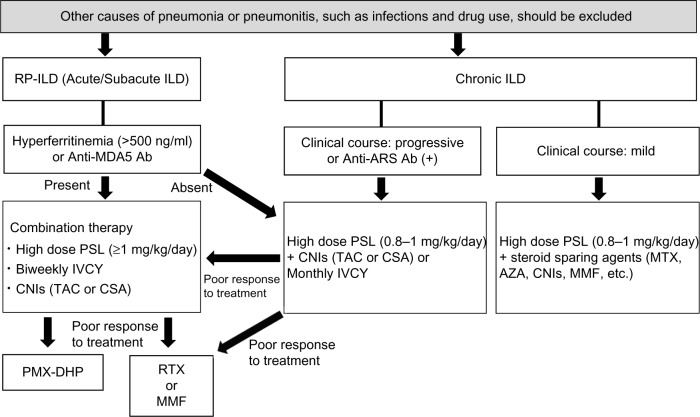
Clinicians should determine when and how patients with ILD should be treated in PM/DM. However, there are no large controlled trials to confirm the efficacy of treatments in ILD with PM/DM patients. Figure 1 provides a flowchart that illustrates the process for determining the optimal therapeutic strategy based on our experiences and the findings described above. The clinical course of ILD shows a less rapid progression in Caucasians than in East Asians. For example, ILD with anti-MDA5 more frequently shows rapid progression in Asian patients than in Westerners. Clinical manifestations and treatment responses for each MSA may depend on race, although MSAs are useful predictors of clinical manifestations and prognoses. Thus, therapeutic strategies should be considered individually in each race.
Figure 1.
Therapeutic strategy for ILDs associated with PM/DM.
Abbreviation: Ab, antibodies.
Corticosteroids and immunosuppressive agents should be co-administered as soon as possible in RP-ILD with PM/DM patients. However, other causes of pneumonia or pneumonitis, such as infections and drug use, should be excluded before treatment initiation. The development of RP-ILD should be considered particularly in Asian patients with anti-MDA5 and/or hyperferritinemia, which is defined as serum ferritin levels greater than 500 ng/mL. Moreover, RP-ILD patients with hyperferritinemia and/or anti-MDA5 have a poorer prognosis than other patients with PM/DM in Japan. Therefore, a combination therapy of corticosteroids, IVCY, and CNIs, such as CSA and TAC, should be immediately administered in RP-ILD patients with hyperferritinemia and/or anti-MDA5. However, ILD is not generally RP in Caucasian patients with anti-MDA5.
CNIs or IVCY should be added to corticosteroids in patients with chronic progressive ILD if pulmonary function is gradually deteriorating and/or the lesions of ILD are spreading on CT findings. CNIs should be administered with PSL in ILD with anti-ARS patients to prevent the progression and recurrence of ILD. The administration of steroid-sparing agents (eg, MTX, AZA, CNIs, and MMF) should be also considered in patients with chronic mild ILD. RTX or MMF administration may be considered when RP-ILD or chronic ILD with anti-ARS is refractory to the therapies described above.
Perspectives for Future Therapies for ILD with PM/DM
The agents described above are not sufficient to improve the clinical outcome of ILD in PM/DM. For example, approximately 20%–40% of RP-ILD with anti-MDA5 patients die within 6 months after diagnosis, even if a combination therapy of corticosteroids, IVCY, and CNIs is immediately administered.14
Therefore, there is a need to elucidate the pathophysiology of ILD with PM/DM in greater detail to improve the outcome of ILD. Recent studies demonstrated novel findings in the pathophysiology of PM/DM. Th17, CD28null T cells, BAFF, IL-1, IL-6, type 1 interferon, high-mobility group box 1, and leukotriene B4 are potential molecular targets for the treatment of patients with PM/DM.103 Anti-BAFF, belimumab, and anti–IL-6 receptor tocilizumab are commonly administered in lupus and RA patients, respectively. These agents may be relatively easy to use as a novel therapy in PM/DM in the future.
Recent differences in cytokine profiles were demonstrated between RP-ILD (ILD with anti-MDA5) and the chronic form ILD (ILD with anti-ARS) in PM/DM.104 Serum IL-8 levels were significantly higher in ILD with anti-MDA5 patients than ILD with anti-ARS patients, although IL-6, TNF-α, and IP-10 levels were high in both subsets. Therefore, IL-6–targeting therapy, such as tocilizumab, may be efficacious in ILD with anti-MDA5 and ILD with anti-ARS patients. We also found that IL-6 and IL-8 were significant contributors to hyperferritinemia in PM/DM-ILD.105 Inflammatory alveolar macrophages synthesize ferritin and become activated in lung, liver, spleen, and bone marrow in RP-ILD with anti-MDA5 patients.106 These findings suggest that activated alveolar macrophages and inflammatory cytokines are more selectively and strongly regulated to improve clinical outcome in RP-ILD with PM/DM.
Conclusion
ILD is associated with poor prognosis in PM/DM. The appropriate management of ILD is very important for improving the prognosis of patients with PM/DM. ILD activity and severity depend on the disease subtype. Therefore, clinicians should determine therapeutic strategies according to the subtype in each patient. Anti-MDA5 antibody and hyperferritinemia predict the development and severity of RP-ILD, particularly in East Asians. Combination therapy with corticosteroids, IVCY, and CNIs should be started immediately in RP-ILD patients. In contrast, ILD patients with anti-ARS exhibit a better response to corticosteroid monotherapy in the short term, although these patients often experience a recurrence and progression of ILD. Therefore, CNIs or RTX may be added to corticosteroids if pulmonary function gradually deteriorates.
Large-scale, multicenter RCTs should be conducted to confirm that the agents described above exhibit efficacy for ILD with PM/DM. We should also elucidate the pathophysiology of ILD with PM/DM in greater detail. Investigations of the pathophysiology of these conditions may lead to the development of novel agents to improve the prognosis in patients with ILD with PM/DM.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: HK, TG, YK, HY. Analyzed the data: HK. Wrote the first draft of the manuscript: HK. Contributed to the writing of the manuscript: TG. Agree with manuscript results and conclusions: HK, TG, YK, HY. Jointly developed the structure and arguments for the paper: HK, TG. Made critical revisions and approved final version: HK, TG, YK, HY. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Yamasaki Y, Yamada H, Ohkubo M, et al. Long-term survival and associated risk factors in patients with adult-onset idiopathic inflammatory myopathies and amyopathic dermatomyositis: experience in a single institute in Japan. J Rheumatol. 2011;38(8):1636–43. doi: 10.3899/jrheum.101002. [DOI] [PubMed] [Google Scholar]
- 2.Fujisawa T, Suda T, Nakamura Y, et al. Differences in clinical features and prognosis of interstitial lung diseases between polymyositis and dermatomyositis. J Rheumatol. 2005;32(1):58–64. [PubMed] [Google Scholar]
- 3.Society AT, Society ER, American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Yousem SA, Schneider F, Bi D, Oddis CV, Gibson K, Aggarwal R. The pulmonary histopathologic manifestations of the anti-PL7/antithreonyl transfer RNA synthetase syndrome. Hum Pathol. 2014;45(6):1199–204. doi: 10.1016/j.humpath.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Schneider F, Yousem SA, Bi D, Gibson KF, Oddis CV, Aggarwal R. Pulmonary pathologic manifestations of anti-glycyl-tRNA synthetase (anti-EJ)-related inflammatory myopathy. J Clin Pathol. 2014;67(8):678–83. doi: 10.1136/jclinpath-2014-202367. [DOI] [PubMed] [Google Scholar]
- 6.Mimori T, Nakashima R, Hosono Y. Interstitial lung disease in myositis: clinical subsets, biomarkers, and treatment. Curr Rheumatol Rep. 2012;14(3):264–74. doi: 10.1007/s11926-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 7.Labirua A, Lundberg IE. Interstitial lung disease and idiopathic inflammatory myopathies: progress and pitfalls. Curr Opin Rheumatol. 2010;22(6):633–8. doi: 10.1097/BOR.0b013e32833f1970. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52(5):1571–6. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 9.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerami P, Schope J, McDonald L, Walling H, Sontheimer R. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54(4):597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Narang NS, Casciola-Rosen L, Li S, Chung L, Fiorentino DF. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res (Hoboken) 2015;67(5):667–72. doi: 10.1002/acr.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JC, Casciola-Rosen L, Samedy LA, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 2013;65(8):1307–15. doi: 10.1002/acr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceribelli A, Fredi M, Taraborelli M, et al. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol. 2014;32(6):891–7. [PubMed] [Google Scholar]
- 14.Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 2010;49(3):433–40. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 15.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49(9):1713–9. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 16.Gono T, Kawaguchi Y, Hara M, et al. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49(7):1354–60. doi: 10.1093/rheumatology/keq073. [DOI] [PubMed] [Google Scholar]
- 17.Hozumi H, Enomoto N, Kono M, et al. Prognostic significance of anti-aminoacyl-tRNA synthetase antibodies in polymyositis/dermatomyositis-associated interstitial lung disease: a retrospective case control study. PLoS One. 2015;10(3):e0120313. doi: 10.1371/journal.pone.0120313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011;37(1):100–9. doi: 10.1590/s1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaguchi Y, Fujimoto M, Matsushita T, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8(4):e60442. doi: 10.1371/journal.pone.0060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie I, Hachulla E, Hatron PY, et al. Polymyositis and dermatomyositis: short term and long-term outcome, and predictive factors of prognosis. J Rheumatol. 2001;28(10):2230–7. [PubMed] [Google Scholar]
- 21.Sultan SM, Ioannou Y, Moss K, Isenberg DA. Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology (Oxford) 2002;41(1):22–6. doi: 10.1093/rheumatology/41.1.22. [DOI] [PubMed] [Google Scholar]
- 22.Dankó K, Ponyi A, Constantin T, Borgulya G, Szegedi G. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 2004;83(1):35–42. doi: 10.1097/01.md.0000109755.65914.5e. [DOI] [PubMed] [Google Scholar]
- 23.Cottin V, Thivolet-Béjui F, Reynaud-Gaubert M, et al. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. Eur Respir J. 2003;22(2):245–50. doi: 10.1183/09031936.03.00026703. [DOI] [PubMed] [Google Scholar]
- 24.Fujisawa T, Hozumi H, Kono M, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS One. 2014;9(6):e98824. doi: 10.1371/journal.pone.0098824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimojima Y, Ishii W, Matsuda M, Tazawa K, Ikeda S. Coadministration of tacrolimus with corticosteroid accelerates recovery in refractory patients with polymyositis/dermatomyositis: a retrospective study. BMC Musculoskelet Disord. 2012;13:228. doi: 10.1186/1471-2474-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama Y, Furuta S, Ikeda K, Hirose K, Nakajima H. Corticosteroid-sparing effect of tacrolimus in the initial treatment of dermatomyositis and polymyositis. Mod Rheumatol. 2015 Apr;30:1–5. doi: 10.3109/14397595.2015.1029239. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Gordon P, Gooptu B. Tacrolimus in idiopathic inflammatory myopathy- associated interstitial lung disease: defining roles and responders. Rheumatology (Oxford) 2015;54(1):3–4. doi: 10.1093/rheumatology/keu335. [DOI] [PubMed] [Google Scholar]
- 28.Hallowell RW, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: recent advances. Curr Opin Rheumatol. 2014;26(6):684–9. doi: 10.1097/BOR.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 29.Frazier AR, Miller RD. Interstitial pneumonitis in association with polymyositis and dermatomyositis. Chest. 1974;65(4):403–7. doi: 10.1378/chest.65.4.403. [DOI] [PubMed] [Google Scholar]
- 30.Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):233–41. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 31.Koreeda Y, Higashimoto I, Yamamoto M, et al. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010;49(5):361–9. doi: 10.2169/internalmedicine.49.2889. [DOI] [PubMed] [Google Scholar]
- 32.Nawata Y, Kurasawa K, Takabayashi K, et al. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/polymyositis: prediction and treatment with cyclosporine. J Rheumatol. 1999;26(7):1527–33. [PubMed] [Google Scholar]
- 33.Songcharoen S, Raju SF, Pennebaker JB. Interstitial lung disease in polymyositis and dermatomyositis. J Rheumatol. 1980;7(3):353–60. [PubMed] [Google Scholar]
- 34.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 2010;49(9):1726–33. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]
- 35.Kang EH, Nakashima R, Mimori T, et al. Myositis autoantibodies in Korean patients with inflammatory myositis: anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord. 2010;11:223. doi: 10.1186/1471-2474-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaguchi Y, Kuwana M, Hoshino K, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol. 2011;147(4):391–8. doi: 10.1001/archdermatol.2011.52. [DOI] [PubMed] [Google Scholar]
- 37.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114(2):154–63. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Malaviya AN, Many A, Schwartz RS. Treatment of dermatomyositis with methotrexate. Lancet. 1968;2(7566):485–8. doi: 10.1016/s0140-6736(68)90649-1. [DOI] [PubMed] [Google Scholar]
- 39.Sokoloff MC, Goldberg LS, Pearson CM. Treatment of corticosteroid-resistant polymyositis with methotrexate. Lancet. 1971;1(7688):14–16. doi: 10.1016/s0140-6736(71)80005-3. [DOI] [PubMed] [Google Scholar]
- 40.Douglas WW, Tazelaar HD, Hartman TE, et al. Polymyositis-dermatomyositis- associated interstitial lung disease. Am J Respir Crit Care Med. 2001;164(7):1182–5. doi: 10.1164/ajrccm.164.7.2103110. [DOI] [PubMed] [Google Scholar]
- 41.Uribe L, Ronderos DM, Díaz MC, Gutierrez JM, Mallarino C, Fernandez-Avila DG. Antisynthetase antibody syndrome: case report and review of the literature. Clin Rheumatol. 2013;32(5):715–9. doi: 10.1007/s10067-013-2207-5. [DOI] [PubMed] [Google Scholar]
- 42.Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107(6):890–6. doi: 10.1016/j.rmed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida T, Koga H, Saitoh F, et al. Pulse intravenous cyclophosphamide treatment for steroid-resistant interstitial pneumonitis associated with polymyositis. Intern Med. 1999;38(9):733–8. doi: 10.2169/internalmedicine.38.733. [DOI] [PubMed] [Google Scholar]
- 44.Shinohara T, Hidaka T, Matsuki Y, et al. Rapidly progressive interstitial lung disease associated with dermatomyositis responding to intravenous cyclophosphamide pulse therapy. Intern Med. 1997;36(7):519–23. doi: 10.2169/internalmedicine.36.519. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka F, Origuchi T, Migita K, et al. Successful combined therapy of cyclophosphamide and cyclosporine for acute exacerbated interstitial pneumonia associated with dermatomyositis. Intern Med. 2000;39(5):428–30. doi: 10.2169/internalmedicine.39.428. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007;46(1):124–30. doi: 10.1093/rheumatology/kel112. [DOI] [PubMed] [Google Scholar]
- 47.Schnabel A, Reuter M, Biederer J, Richter C, Gross WL. Interstitial lung disease in polymyositis and dermatomyositis: clinical course and response to treatment. Semin Arthritis Rheum. 2003;32(5):273–84. doi: 10.1053/sarh.2002.50012. [DOI] [PubMed] [Google Scholar]
- 48.Mok CC, To CH, Szeto ML. Successful treatment of dermatomyositis-related rapidly progressive interstitial pneumonitis with sequential oral cyclophosphamide and azathioprine. Scand J Rheumatol. 2003;32(3):181–3. doi: 10.1080/03009740310002542. [DOI] [PubMed] [Google Scholar]
- 49.Kameda H, Nagasawa H, Ogawa H, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32(9):1719–26. [PubMed] [Google Scholar]
- 50.Nakashima R, Mimori T. Anti-MDA5 (melanoma differentiation-associated gene 5) antibody and dermatomyositis with rapidly progressive interstitial pneumonia. Nihon Rinsho Meneki Gakkai Kaishi. 2013;36(2):71–6. doi: 10.2177/jsci.36.71. [DOI] [PubMed] [Google Scholar]
- 51.Saketkoo LA, Espinoza LR. Experience of mycophenolate mofetil in 10 patients with autoimmune-related interstitial lung disease demonstrates promising effects. Am J Med Sci. 2009;337(5):329–35. doi: 10.1097/MAJ.0b013e31818d094b. [DOI] [PubMed] [Google Scholar]
- 52.Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130(1):30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 53.Morganroth PA, Kreider ME, Werth VP. Mycophenolate mofetil for interstitial lung disease in dermatomyositis. Arthritis Care Res (Hoboken) 2010;62(10):1496–501. doi: 10.1002/acr.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum. 2011;63(11):3439–47. doi: 10.1002/art.30513. [DOI] [PubMed] [Google Scholar]
- 55.Hervier B, Masseau A, Mussini JM, Audrain M, Hamidou MA. Long-term efficacy of mycophenolate mofetil in a case of refractory antisynthetase syndrome. Joint Bone Spine. 2009;76(5):575–6. doi: 10.1016/j.jbspin.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Sundaragiri PR, Vallabhajosyula S, Kanaan JP. Interstitial lung disease in antisynthetase syndrome without clinical myositis. BMJ Case Rep. 2014 Apr 3;:pii: bcr2014204296. doi: 10.1136/bcr-2014-204296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cozzani E, Cinotti E, Felletti R, Pelucco D, Rebora A, Parodi A. Amyopathic dermatomyositis with lung involvement responsive to mycophenolate mofetil. Immunopharmacol Immunotoxicol. 2013;35(6):687–92. doi: 10.3109/08923973.2013.833624. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchiya H, Tsuno H, Inoue M, et al. Mycophenolate mofetil therapy for rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis. Mod Rheumatol. 2014;24(4):694–6. doi: 10.3109/14397595.2013.874762. [DOI] [PubMed] [Google Scholar]
- 59.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367(21):2015–25. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 60.Cherin P, Pelletier S, Teixeira A, et al. Results and long-term follow-up of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum. 2002;46(2):467–74. doi: 10.1002/art.10053. [DOI] [PubMed] [Google Scholar]
- 61.Donofrio PD, Berger A, Brannagan TH, 3rd, et al. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009;40(5):890–900. doi: 10.1002/mus.21433. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki Y, Hayakawa H, Miwa S, et al. Intravenous immunoglobulin therapy for refractory interstitial lung disease associated with polymyositis/dermatomyositis. Lung. 2009;187(3):201–6. doi: 10.1007/s00408-009-9146-6. [DOI] [PubMed] [Google Scholar]
- 63.Bakewell CJ, Raghu G. Polymyositis associated with severe interstitial lung disease: remission after three doses of IV immunoglobulin. Chest. 2011;139(2):441–3. doi: 10.1378/chest.10-0360. [DOI] [PubMed] [Google Scholar]
- 64.Bozkirli DE, Kozanoglu I, Bozkirli E, Yucel E. Antisynthetase syndrome with refractory lung involvement and myositis successfully treated with double filtration plasmapheresis. J Clin Apher. 2013;28(6):422–5. doi: 10.1002/jca.21285. [DOI] [PubMed] [Google Scholar]
- 65.Omotoso BA, Ogden MI, Balogun RA. Therapeutic plasma exchange in antisynthetase syndrome with severe interstitial lung disease. J Clin Apher. 2015 Feb 25; doi: 10.1002/jca.21387. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Lee CS, Chen TL, Tzen CY, et al. Idiopathic inflammatory myopathy with diffuse alveolar damage. Clin Rheumatol. 2002;21(5):391–6. doi: 10.1007/s100670200104. [DOI] [PubMed] [Google Scholar]
- 67.Ichiyasu H, Horio Y, Tsumura S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol. 2014;24(2):361–5. doi: 10.3109/14397595.2013.852847. [DOI] [PubMed] [Google Scholar]
- 68.Teruya A, Kawamura K, Ichikado K, Sato S, Yasuda Y, Yoshioka M. Successful polymyxin B hemoperfusion treatment associated with serial reduction of serum anti-CADM-140/MDA5 antibody levels in rapidly progressive interstitial lung disease with amyopathic dermatomyositis. Chest. 2013;144(6):1934–6. doi: 10.1378/chest.13-0186. [DOI] [PubMed] [Google Scholar]
- 69.Ichiyasu H, Horio Y, Tsumura S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol. 2012 Oct 16; doi: 10.3109/14397595.2013.852847. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70.Kakugawa T, Mukae H, Saito M, et al. Rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis successfully treated with polymyxin B-immobilized fiber column hemoperfusion. Intern Med. 2008;47(8):785–90. doi: 10.2169/internalmedicine.47.0639. [DOI] [PubMed] [Google Scholar]
- 71.Antonelli M, Fumagalli R, Cruz DN, Brienza N, Giunta F, Group ES. PMX endotoxin removal in the clinical practice: results from the EUPHAS trial. Contrib Nephrol. 2010;167:83–90. doi: 10.1159/000315922. [DOI] [PubMed] [Google Scholar]
- 72.Hara S, Ishimoto H, Sakamoto N, et al. Direct hemoperfusion using immobilized polymyxin B in patients with rapidly progressive interstitial pneumonias: a retrospective study. Respiration. 2011;81(2):107–17. doi: 10.1159/000321958. [DOI] [PubMed] [Google Scholar]
- 73.Zou J, Guo Q, Chi J, Wu H, Bao C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin Rheumatol. 2015;34(4):707–14. doi: 10.1007/s10067-015-2866-5. [DOI] [PubMed] [Google Scholar]
- 74.Maeda K, Kimura R, Komuta K, Igarashi T. Cyclosporine treatment for polymyositis/dermatomyositis: is it possible to rescue the deteriorating cases with interstitial pneumonitis? Scand J Rheumatol. 1997;26(1):24–9. doi: 10.3109/03009749709065660. [DOI] [PubMed] [Google Scholar]
- 75.Takada K, Nagasaka K, Miyasaka N. Polymyositis/dermatomyositis and interstitial lung disease: a new therapeutic approach with T-cell-specific immunosuppressants. Autoimmunity. 2005;38(5):383–92. doi: 10.1080/08916930500124023. [DOI] [PubMed] [Google Scholar]
- 76.DJ G, EH K, JK P, et al. Survival benefit of early use of cyclosporine in dermatomyositis-associated interstitial lung disease. Ann Rheum Dis. 2014;73(suppl 2):97. [Google Scholar]
- 77.Nagai K, Takeuchi T, Kotani T, et al. Therapeutic drug monitoring of cyclosporine microemulsion in interstitial pneumonia with dermatomyositis. Mod Rheumatol. 2011;21(1):32–6. doi: 10.1007/s10165-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 78.Kotani T, Makino S, Takeuchi T, et al. Early intervention with corticosteroids and cyclosporin A and 2-hour postdose blood concentration monitoring improves the prognosis of acute/subacute interstitial pneumonia in dermatomyositis. J Rheumatol. 2008;35(2):254–9. [PubMed] [Google Scholar]
- 79.Levy G, Thervet E, Lake J, Uchida K, Group CoNCERiTC Patient management by Neoral C(2) monitoring: an international consensus statement. Transplantation. 2002;73(9 suppl):S12–8. doi: 10.1097/00007890-200205151-00003. [DOI] [PubMed] [Google Scholar]
- 80.Ochi S, Nanki T, Takada K, et al. Favorable outcomes with tacrolimus in two patients with refractory interstitial lung disease associated with polymyositis/dermatomyositis. Clin Exp Rheumatol. 2005;23(5):707–10. [PubMed] [Google Scholar]
- 81.Oddis CV, Sciurba FC, Elmagd KA, Starzl TE. Tacrolimus in refractory polymyositis with interstitial lung disease. Lancet. 1999;353(9166):1762–3. doi: 10.1016/S0140-6736(99)01927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum. 2005;52(8):2439–46. doi: 10.1002/art.21240. [DOI] [PubMed] [Google Scholar]
- 83.Bongartz T, Ryu JH, Matteson EL. Is tacrolimus effective for treating antisynthetase-associated interstitial lung disease? Nat Clin Pract Rheumatol. 2005;1(2):80–1. doi: 10.1038/ncprheum0067. [DOI] [PubMed] [Google Scholar]
- 84.Kurita T, Yasuda S, Oba K, et al. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology (Oxford) 2015;54(1):39–44. doi: 10.1093/rheumatology/keu166. [DOI] [PubMed] [Google Scholar]
- 85.Ando M, Miyazaki E, Yamasue M, et al. Successful treatment with tacrolimus of progressive interstitial pneumonia associated with amyopathic dermatomyositis refractory to cyclosporine. Clin Rheumatol. 2010;29(4):443–5. doi: 10.1007/s10067-009-1358-x. [DOI] [PubMed] [Google Scholar]
- 86.Labirua-Iturburu A, Selva-O’Callaghan A, Martínez-Gómez X, Trallero-Araguás E, Labrador-Horrillo M, Vilardell-Tarrés M. Calcineurin inhibitors in a cohort of patients with antisynthetase-associated interstitial lung disease. Clin Exp Rheumatol. 2013;31(3):436–9. [PubMed] [Google Scholar]
- 87.Dalakas MC. Immunotherapy of myositis: issues, concerns and future prospects. Nat Rev Rheumatol. 2010;6(3):129–37. doi: 10.1038/nrrheum.2010.2. [DOI] [PubMed] [Google Scholar]
- 88.Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the antisynthetase syndrome: a retrospective case series. Rheumatology (Oxford) 2009;48(8):968–71. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- 89.Vandenbroucke E, Grutters JC, Altenburg J, Boersma WG, ter Borg EJ, van den Bosch JM. Rituximab in life threatening antisynthetase syndrome. Rheumatol Int. 2009;29(12):1499–502. doi: 10.1007/s00296-009-0859-x. [DOI] [PubMed] [Google Scholar]
- 90.Ball EM, Savage EM, Pendleton A. Refractory anti-synthetase syndrome treated with rituximab. Rheumatology (Oxford) 2010;49(5):1013. doi: 10.1093/rheumatology/kep438. [DOI] [PubMed] [Google Scholar]
- 91.Brulhart L, Waldburger JM, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis. 2006;65(7):974–5. doi: 10.1136/ard.2005.045898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambotte O, Kotb R, Maigne G, Blanc FX, Goujard C, Delfraissy JF. Efficacy of rituximab in refractory polymyositis. J Rheumatol. 2005;32(7):1369–70. [PubMed] [Google Scholar]
- 93.Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52(2):601–7. doi: 10.1002/art.20849. [DOI] [PubMed] [Google Scholar]
- 94.Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. 2012;106(4):581–7. doi: 10.1016/j.rmed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Krystufková O, Vallerskog T, Helmers SB, et al. Increased serum levels of B cell activating factor (BAFF) in subsets of patients with idiopathic inflammatory myopathies. Ann Rheum Dis. 2009;68(6):836–43. doi: 10.1136/ard.2008.091405. [DOI] [PubMed] [Google Scholar]
- 96.Aggarwal R, Bandos A, Reed AM, et al. RIM Study Group Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66(3):740–9. doi: 10.1002/art.38270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersson H, Sem M, Lund MB, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford) 2015 Mar 3;:pii: kev004. doi: 10.1093/rheumatology/kev004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 98.Brunasso AM, Aberer W, Massone C. New onset of dermatomyositis/polymyositis during anti-TNF-α therapies: a systematic literature review. ScientificWorld-Journal. 2014;2014:179180. doi: 10.1155/2014/179180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen D, Wang XB, Zhou Y, Zhu XC. Efficacy of infliximab in the treatment for dermatomyositis with acute interstitial pneumonia: a study of fourteen cases and literature review. Rheumatol Int. 2013;33(10):2455–8. doi: 10.1007/s00296-012-2653-4. [DOI] [PubMed] [Google Scholar]
- 100.Park JK, Yoo HG, Ahn DS, Jeon HS, Yoo WH. Successful treatment for conventional treatment-resistant dermatomyositis-associated interstitial lung disease with adalimumab. Rheumatol Int. 2012;32(11):3587–90. doi: 10.1007/s00296-011-2220-4. [DOI] [PubMed] [Google Scholar]
- 101.Liu SW, Velez NF, Lam C, et al. Dermatomyositis induced by anti-tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol. 2013;149(10):1204–8. doi: 10.1001/jamadermatol.2013.5220. [DOI] [PubMed] [Google Scholar]
- 102.Criner GJ. Ambulatory home oxygen: what is the evidence for benefit, and who does it help? Respir Care. 2013;58(1):48–64. doi: 10.4187/respcare.01918. [DOI] [PubMed] [Google Scholar]
- 103.Venalis P, Lundberg IE. Immune mechanisms in polymyositis and dermatomyositis and potential targets for therapy. Rheumatology (Oxford) 2014;53(3):397–405. doi: 10.1093/rheumatology/ket279. [DOI] [PubMed] [Google Scholar]
- 104.Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford) 2014;53(12):2196–203. doi: 10.1093/rheumatology/keu258. [DOI] [PubMed] [Google Scholar]
- 105.Kawasumi H, Gono T, Kawaguchi Y, et al. IL-6, IL-8, and IL-10 are associated with hyperferritinemia in rapidly progressive interstitial lung disease with poly-myositis/dermatomyositis. Biomed Res Int. 2014;2014:815245. doi: 10.1155/2014/815245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gono T, Miyake K, Kawaguchi Y, Kaneko H, Shinozaki M, Yamanaka H. Hyper-ferritinaemia and macrophage activation in a patient with interstitial lung disease with clinically amyopathic DM. Rheumatology (Oxford) 2012;51(7):1336–8. doi: 10.1093/rheumatology/kes012. [DOI] [PubMed] [Google Scholar]