Abstract
This observer-blind study (clinicaltrials.gov NCT01462357) compared the immunogenicity and safety of 2 doses of the HPV-16/18 AS04-adjuvanted vaccine (HPV-16/18(2D)) vs. 2 or 3 doses of the HPV-6/11/16/18 vaccine (HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D)) in healthy girls aged 9–14 y. Girls were randomized (1:1:1) to receive HPV-16/18(2D) at months (M) 0,6 (N = 359), HPV-6/11/16/18(2D) at M0,6 (N = 358) or HPV-6/11/16/18(3D) at M0,2,6 (N = 358). The primary objective was non-inferiority/superiority of HPV-16/18 antibodies by ELISA for HPV-16/18(2D) vs. HPV-6/11/16/18(2D) at M7 in the according-to-protocol immunogenicity cohort (ATP-I) and total vaccinated cohort, respectively. Secondary objectives included non-inferiority/superiority of HPV-16/18(2D) vs. HPV-6/11/16/18(3D) at M7, non-inferiority/superiority at M12, HPV-16/18 neutralizing antibodies, frequencies of T-cells/B-cells, reactogenicity and safety. Antibody responses at M7 for HPV-16/18(2D) were superior to those for HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) (lower limit of 95% confidence interval for geometric mean titer ratio (GMR) was >1): HPV-16/18(2D)/HPV-6/11/16/18(2D) GMRs were 1.69 [1.49–1.91] for anti-HPV-16 and 4.52 [3.97–5.13] for anti-HPV-18; HPV-16/18(2D)/HPV-6/11/16/18(3D) GMRs were 1.72 [1.54–1.93] for anti-HPV-16 and 3.22 [2.82–3.68] for anti-HPV-18; p = 0.0001 for all comparisons. Non-inferiority/superiority was also demonstrated at M12. Among initially seronegative girls in the ATP-I, neutralizing antibody titers were at least 1.8-fold higher for HPV-16/18(2D) vs. HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) at M7 and M12. Frequencies of HPV-16/18-specific T-cells and B-cells were in similar ranges between groups. Reactogenicity and safety were in line with the known profile of each vaccine. In conclusion, superior HPV-16/18 antibody responses were elicited by 2 doses of the HPV-16/18 AS04-adjuvanted vaccine compared with 2 or 3 doses of the HPV-6/11/16/18 vaccine in girls (9–14 years).
Keywords: administration schedule, female adolescents, immunogenicity, human papillomavirus (HPV) vaccines, safety
Abbreviations
- 2D
2-dose
- 3D
3-dose
- AAHS
aluminum hydroxyphosphate sulfate
- ANOVA
analysis of variance
- AS04
Adjuvant System containing 50 µg 3-O-desacyl-4 ′-monophosphoryl lipid A (MPL) adsorbed on aluminum salt (500 µg Al3+)
- ATP-I
according-to-protocol immunogenicity cohort
- CI
confidence interval
- CMI
cell-mediated immunity
- ED50
effective dose producing 50% response
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immunosorbent spot assay
- EU
ELISA unit
- GMR
geometric mean titer ratio
- GMT
geometric mean antibody titer
- HPV
human papillomavirus
- HPV-16/18(2D)
2-dose schedule of the HPV-16/18 vaccine
- HPV-6/11/16/18(2D)
2-dose schedule of the HPV-6/11/16/18 vaccine
- HPV-6/11/16/18(3D)
3-dose schedule of the HPV-6/11/16/18 vaccine
- IFNγ
interferon
- M
month(s)
- IgG
immunoglobulin G
- PBMC
peripheral blood mononuclear cells
- PBNA
pseudovirion-based neutralisation assay
- pIMD
potential immune-mediated disease
- SAE
serious adverse event
- TVC
total vaccinated cohort
- VLP
virus-like particle
- y
year(s)
Introduction
Two human papillomavirus (HPV) vaccines, the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, GSK group of companies) and the HPV-6/11/16/18 vaccine (Gardasil®, Merck & Co., Inc.), are currently approved for the prevention of premalignant genital lesions and cervical cancer. Both vaccines contain L1 virus-like particles (VLPs) from the 2 most prevalent oncogenic HPV types, HPV-16 and HPV-18.1 These 2 HPV types are responsible for more than 70% of cervical cancers worldwide.2-4 The main differences in the composition of the vaccines are the inclusion of HPV-6 and -11 L1 VLPs in the HPV-6/11/16/18 vaccine and AS04 adjuvantation of the HPV-16/18 vaccine. In addition, they are produced using different methods.1
The observation that standard 3-dose (3D) schedules of these vaccines elicited antibody titers in girls that were approximately 2-fold higher than those elicited in young women,5,6 the age group in which efficacy has been demonstrated in clinical studies,7-13 prompted the evaluation of alternative dosing schedules for younger subjects. Two doses (2D) of the HPV-16/18 vaccine administered at months (M) 0,6 or 0,12 to girls aged 9–14 y were shown to elicit non-inferior HPV-16 and −18 antibody responses 1M and 6M after the last vaccine dose compared with the standard 3D schedule in young women aged 15–25 years,14,15 and antibody titers were sustained at high levels for up to 4 y after first vaccination.16 Similarly, a 2D schedule of the HPV-6/11/16/18 vaccine administered at M0,6 to girls aged 9–13 y elicited non-inferior antibody responses to the 4 HPV vaccine types 1M after the last vaccine dose compared with the standard 3D schedule in young women aged 16–26 years, with persistently high antibody titers observed for up to 3 y.17 Consequently, 2D schedules of both HPV vaccines have now been approved for girls in a number of countries in Europe, Latin America, Africa and Asia. For the HPV-16/18 vaccine there is some flexibility around administration of the second dose, from 5 to 13 months after the first dose;18 for the HPV-6/11/16/18 vaccine the second dose is recommended 6 months after the first dose.19
Comparison of data for 2D schedules of the 2 HPV vaccines across studies is not appropriate given the differences in study design and serological assays utilized. Thus, the current study was designed to evaluate the immunogenicity and safety of 2D schedules of the HPV-16/18 vaccine (HPV-16/18(2D) study group) and the HPV-6/11/16/18 vaccine (HPV-6/11/16/18(2D) study group) in girls aged 9–14 y in a head-to-head comparison. We also compared the 2D schedule of the HPV-16/18 vaccine with the 3D schedule of the HPV-6/11/16/18 vaccine (HPV-6/11/16/18(3D) study group).
Results
Study population
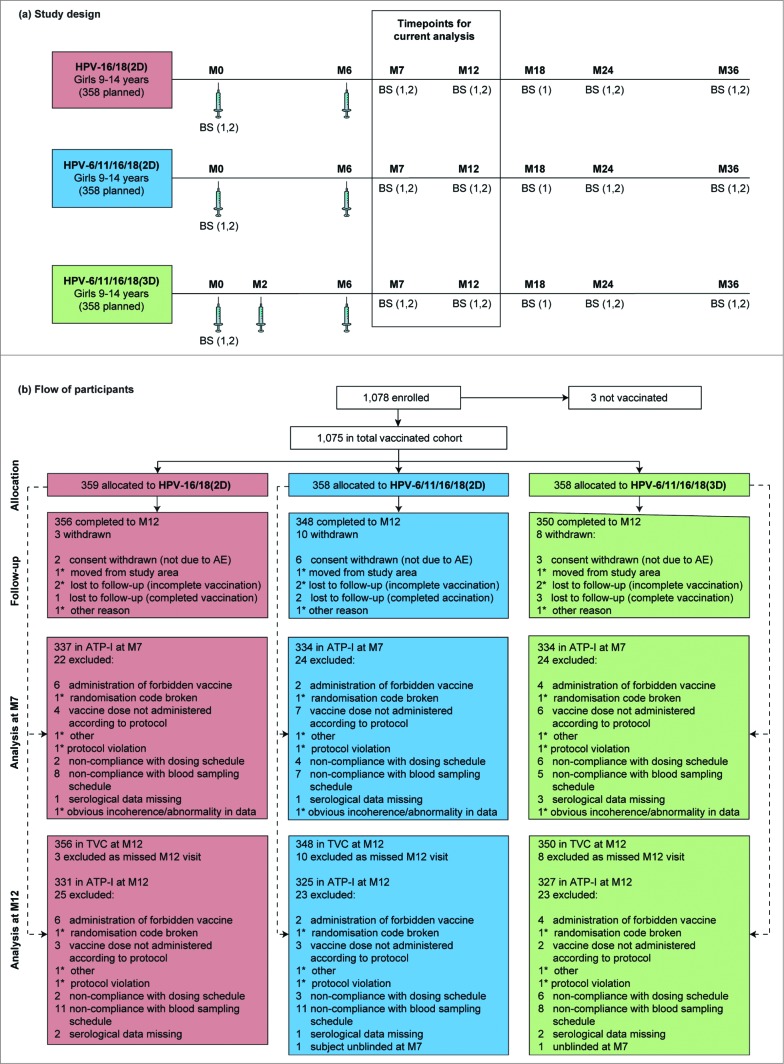
A total of 1,075 girls received at least one vaccine dose (359 for HPV-16/18(2D), 358 for HPV-6/11/16/18(2D), 358 for HPV-6/11/16/18(3D)) and 1,054 (98%) completed the study to M12 (Fig. 1). 1,005 (93%) and 983 (91%) girls were included in the according-to-protocol cohort for immunogenicity (ATP-I) at M7 and M12, respectively. The reasons for exclusion from the ATP-I were balanced between groups. Compliance with the planned vaccination schedule was high (≥98 % in each group). The three groups were well matched with regard to demographic characteristics (Table 1). For all cohorts, the majority of girls in each group were seronegative for anti-HPV-16 (>96%) and anti-HPV-18 (>99%) at baseline (Table 1).
Figure 1.
For figure legend, see page 1692. Figure 1 (See previous page). Study design (A) and flow of participants through the trial to month 12 (B). Syringe symbols represent vaccine administration. AE, adverse event; ATP-I, according to protocol immunogenicity cohort; BS (1), blood sample for assessment of antibodies (by enzyme-linked immunosorbent assay in all subjects and by pseudovirion-based neutralisation assay in a subset of subjects); BS (2), blood sample for assessment of cell-mediated immunity in a subcohort of subjects; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine at months, 0, 2 and 6; M, month; TVC, total vaccinated cohort. *number present in one group only, but replicated to avoid unblinding.
Table 1.
Demographic characteristics and baseline serostatus in the TVC and ATP-I (at one (M7) and 6 months (M12) after the last vaccine dose)
| HPV-16/18(2D) | HPV-6/11/16/18(2D) | HPV-6/11/16/18(3D) | |
|---|---|---|---|
| Demographic characteristics | |||
| TVC | N = 359 | N = 358 | N = 358 |
| Age (years) at time of first vaccine dose, mean (SD) | 11.5 (1.64) | 11.5 (1.56) | 11.6 (1.64) |
| Geographic ancestry, n (%) | |||
| Asian Heritage | 261 (72.7) | 257 (71.8) | 264 (73.7) |
| White Heritage | 94 (26.2) | 93 (26.0) | 90 (25.1) |
| African Heritage / African American | 4 (1.1) | 6 (1.7) | 4 (1.1) |
| Other | 0 (0.0) | 2 (0.6) | 0 (0.0) |
| Baseline serology | |||
| TVC | N=359 | N=358 | N=358 |
| HPV-16 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 352 (98.1) | 349 (97.5) | 345 (96.4) |
| Seropositive | 7 (1.9) | 9 (2.5) | 13 (3.6) |
| HPV-18 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 356 (99.2) | 355 (99.2) | 357 (99.7) |
| Seropositive | 3 (0.8) | 3 (0.8) | 1 (0.3) |
| ATP-I at M7 | N=337 | N=334 | N=334 |
| HPV-16 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 330 (97.9) | 327 (97.9) | 322 (96.4) |
| Seropositive | 7 (2.1) | 7 (2.1) | 12 (3.6) |
| HPV-18 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 334 (99.1) | 331 (99.1) | 333 (99.7) |
| Seropositive | 3 (0.9) | 3 (0.9) | 1 (0.3) |
| ATP-I at M12 | N=331 | N=325 | N=327 |
| HPV-16 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 325 (98.2) | 318 (97.8) | 315 (96.3) |
| Seropositive | 6 (1.8) | 7 (2.2) | 12 (3.7) |
| HPV-18 baseline serostatus by ELISA, n (%) | |||
| Seronegative | 328 (99.1) | 322 (99.1) | 326 (99.7) |
| Seropositive | 3 (0.9) | 3 (0.9) | 1 (0.3) |
ATP-I, according-to-protocol immunogenicity cohort; ELISA, enzyme-linked immunosorbent assay; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6; M, month; N, total number of subjects; n (%), number (percentage) of subjects in a given category; SD, standard deviation; TVC, total vaccinated cohort. Seronegative status defined as an antibody titer lower than the assay cut-off (19 ELISA units [EU]/mL for anti-HPV-16 and 18 EU/mL for anti-HPV-18).
Immunological non-inferiority and superiority
Non-inferior immunogenicity was demonstrated for HPV-16/18(2D) compared with HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) at M7, since the upper limits of the 95% confidence intervals (CIs) for the differences in seroconversion rates (HPV-6/11/16/18(2D) or –(3D) minus HPV-16/18(2D)) for both anti-HPV-16 and -HPV-18 by enzyme-linked immunosorbent assay (ELISA) were below 5% and the upper limits of the 95% CIs for the geometric mean titers (GMT) ratios (HPV-6/11/16/18(2D) or –(3D) divided by HPV-16/18(2D)) for both antibodies were below 2 in the ATP-I (Table 2). Additionally, HPV-16/18(2D) was demonstrated to be superior to HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) at M7, since the lower limits of the 95% CIs for the anti-HPV-16 and -18 GMT ratios (HPV-16/18(2D) divided by HPV-6/11/16/18(2D) or –(3D)) were above 1 in the total vaccinated cohort (TVC) regardless of baseline serostatus (p = 0.0001 for each antigen for both comparisons). Non-inferiority/superiority was also successfully demonstrated at M12.
Table 2. Non-inferiority (a) and superiority (b) testing for HPV-16 and −18 antibody responses by ELISA one and 6 months after the last vaccine dose
| (a) Non-inferiority testing: initially seronegative girls in the ATP-I | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | Antibody | Group | N | Serocon-version%(95% CI) | GMT EU/mL (95% CI) | Seroconversion difference % (95% CI)1 | GMT ratio (95% CI)2 | ||
| HPV-6/11/16/18(2D) - HPV-16/18(2D) | HPV-6/11/16/18(3D) - HPV-16/18(2D) | HPV-6/11/16/18(2D)/HPV-16/18(2D) | HPV-6/11/16/18(3D) /HPV-16/18(2D) | ||||||
| M7 | HPV-16 | HPV-16/18(2D) | 330 | 100 (98.9, 100) | 8244.1 (7678.3, 8851.7) | 0.00 (−1.16, 1.15) | 0.00 (−1.18, 1.15) | 0.61 (0.54, 0.69) | 0.58 (0.52, 0.65) |
| HPV-6/11/16/18(2D) | 327 | 100 (98.9, 100) | 5056.0 (4596.5, 5561.5) | ||||||
| HPV-6/11/16/18(3D) | 322 | 100 (98.9, 100) | 4807.4 (4420.8, 5227.7) | ||||||
| HPV-18 | HPV-16/18(2D) | 334 | 100 (98.9, 100) | 5277.4 (4858.6, 5732.4) | 0.00 (−1.15, 1.14) | 0.00 (−1.14, 1.14) | 0.23 (0.20, 0.26) | 0.31 (0.27, 0.36) | |
| HPV-6/11/16/18(2D) | 331 | 100 (98.9, 100) | 1207.2 (1092.9, 1333.4) | ||||||
| HPV-6/11/16/18(3D) | 333 | 100 (98.9, 100) | 1653.5 (1484.4, 1841.8) | ||||||
| M12 | HPV-16 | HPV-16/18(2D) | 325 | 99.7 (98.3, 100) | 2217.5 (2022.9, 2430.7) | 0.31 (−0.89, 1.72) | 0.31 (−0.90, 1.72) | 0.58 (0.50, 0.67) | 0.72 (0.63, 0.82) |
| HPV-6/11/16/18(2D) | 318 | 100 (98.8, 100) | 1285.3 (1151.4, 1434.8) | ||||||
| HPV-6/11/16/18(3D) | 315 | 100 (98.8, 100) | 1591.3 (1448.8, 1747.7) | ||||||
| HPV-18 | HPV-16/18(2D) | 328 | 99.7 (98.3, 100) | 1312.6 (1187.5, 1451.0) | −0.01 (−1.46, 1.42) | 0.30 (−0.86, 1.71) | 0.20 (0.17, 0.23) | 0.36 (0.31, 0.43) | |
| HPV-6/11/16/18(2D) | 322 | 99.7 (98.3, 100) | 263.9 (234.2, 297.3) | ||||||
| HPV-6/11/16/18(3D) | 326 | 100 (98.9, 100) | 477.1 (421.9, 539.6) | ||||||
| (b) Superiority testing: TVC regardless of baseline serostatus | |||||||
|---|---|---|---|---|---|---|---|
| Time | Antibody | Group | N | Seroconversion % (95% CI) | GMT EU/mL (95% CI) | GMT ratio (95% CI)3 | |
| HPV-16/18(2D) / HPV-6/11/16/18(2D) | HPV-16/18(2D) / HPV-6/11/16/18(3D) | ||||||
| M7 | HPV-16 | HPV-16/18(2D) | 357 | 100 (99.0, 100) | 8256.4 (7650.3, 8910.6) | 1.69 (1.49, 1.91)** | 1.72 (1.54, 1.93)** |
| HPV-6/11/16/18(2D) | 353 | 100 (99.0, 100) | 4886.1 (4435.4, 5382.6) | ||||
| HPV-6/11/16/18(3D) | 351 | 100 (99.0, 100) | 4789.2 (4409.6, 5201.4) | ||||
| HPV-18 | HPV-16/18(2D) | 357 | 100 (99.0, 100) | 5267.8 (4857.1, 5713.2) | 4.52 (3.97, 5.13)** | 3.22 (2.82, 3.68)** | |
| HPV-6/11/16/18(2D) | 353 | 100 (99.0, 100) | 1166.3 (1056.0, 1288.2) | ||||
| HPV-6/11/16/18(3D) | 351 | 100 (99.0, 100) | 1635.8 (1470.0, 1820.4) | ||||
| M12 | HPV-16 | HPV-16/18(2D) | 355 | 99.7 (98.4, 100) | 2217.5 (2024.4, 2429.0) | 1.76 (1.53, 2.03)** | 1.41 (1.24, 1.61)** |
| HPV-6/11/16/18(2D) | 347 | 99.7 (98.4, 100) | 1260.0 (1131.1, 1403.6) | ||||
| HPV-6/11/16/18(3D) | 348 | 100 (98.9, 100) | 1567.4 (1429.7, 1718.5) | ||||
| HPV-18 | HPV-16/18(2D) | 355 | 99.7 (98.4, 100) | 1296.1 (1178.1, 1426.0) | 4.96 (4.27, 5.75)** | 2.76 (2.37, 3.22)** | |
| HPV-6/11/16/18(2D) | 347 | 99.7 (98.4, 100) | 261.3 (233.0, 293.1) | ||||
| HPV-6/11/16/18(3D) | 348 | 99.7 (98.4, 100) | 469.2 (415.7, 529.5) | ||||
Non-inferiority with respect to seroconversion was confirmed if the upper limit of the 95% CI for difference in seroconversion rates (HPV-6/11/16/18 minus HPV-16/18) was less than the predefined limit of 5%.
Non-inferiority with respect to GMTs was confirmed if the upper limit of the 95% CI for the GMT ratio (HPV-6/11/16/18 divided by HPV-16/18) was below the predefined limit of 2.
Superiority with respect to GMTs was confirmed if the lower limit of the 95% CI for the GMT ratio (HPV-16/18 divided by HPV-6/11/16/18) was above the predefined limit of 1.
p = 0.0001. ATP-I, according-to-protocol immunogenicity cohort; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6; M, month; N, number of subjects with available results; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA units per milliliter; GMT, geometric mean antibody titer; TVC, total vaccinated cohort. Seronegative status defined as an antibody titer lower than the assay cut-off at baseline (19 EU/mL for anti-HPV-16 and 18 EU/mL for anti-HPV-18).
In the TVC at M7, GMT ratios [95% CI] for HPV-16/18(2D)/HPV-6/11/16/18(2D) were 1.69 [1.49, 1.91] for anti-HPV-16 and 4.52 [3.97, 5.13] for anti-HPV-18. GMT ratios [95% CI] for HPV-16/18(2D)/HPV-6/11/16/18(3D) were 1.72 [1.54, 1.93] for anti-HPV-16 and 3.22 [2.82, 3.68] for anti-HPV-18. Similar GMT ratios were observed in the TVC at M12: 1.76 [1.53, 2.03] for anti-HPV-16 and 4.96 [4.27, 5.75] for anti-HPV-18 for HPV-16/18(2D)/HPV-6/11/16/18(2D) and 1.41 [1.24, 1.61] and 2.76 [2.37, 3.22] for HPV-16/18(2D)/HPV-6/11/16/18(3D) (Table 2).
Antibody responses
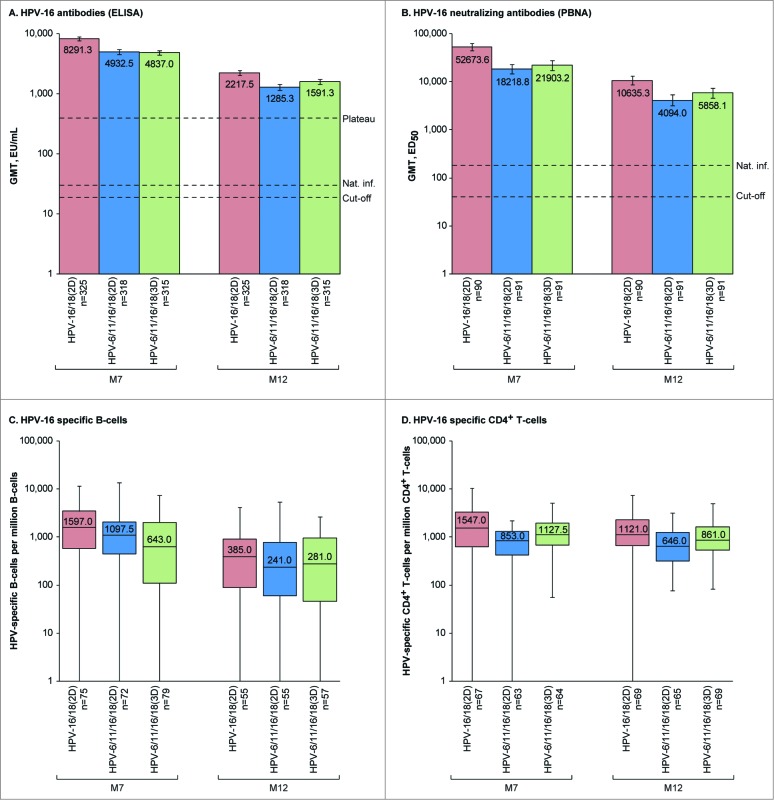
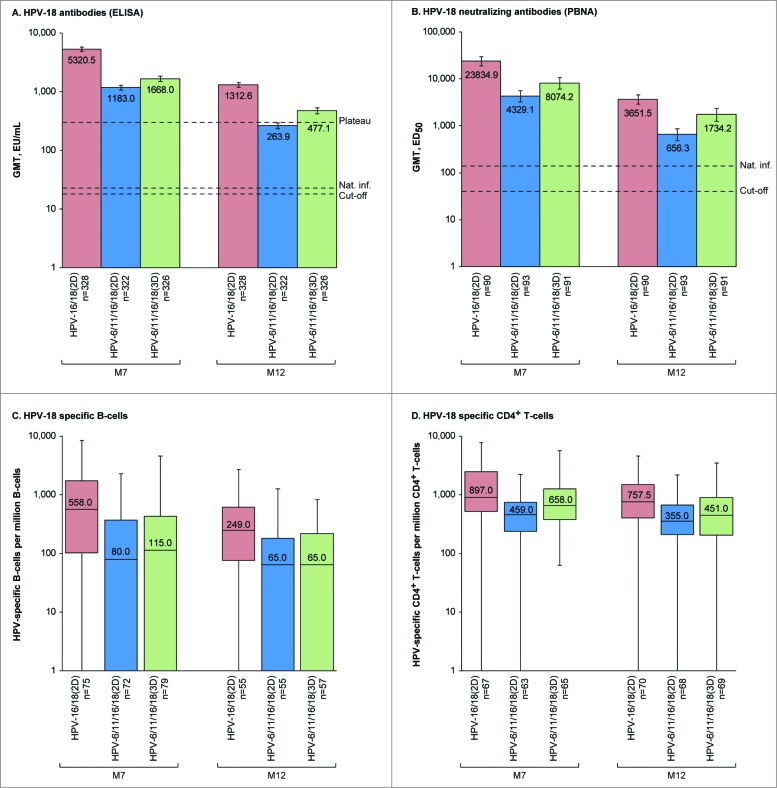
In each group, all initially seronegative subjects in the ATP-I had seroconverted for HPV-16 and −18 antibodies by ELISA and pseudovirion-based neutralization assay (PBNA) at M7. More than 99% of subjects in each group remained seropositive by ELISA and PBNA at M12. There was a decline in HPV-16 and −18 antibodies from M7 to M12 (Fig. 2, Fig. 3, Tables S1 and S2) in all groups, but GMTs (by ELISA and PBNA) at M12 remained well above those previously observed in women who had cleared a natural infection and mounted an immune response (HPV-16 and −18 GMTs by ELISA were at least 43.1- and 11.7-fold higher, respectively, and HPV-16 and −18 GMTs by PBNA were at least 22.7- and 4.8-fold higher, respectively for HPV-6/11/16/18(2D) where titers were the lowest of all groups).1,20 In the HPV-16/18(2D) and HPV-6/11/16/18(3D) groups, anti-HPV-16 and −18 GMTs by ELISA at M12 were also above those observed during the plateau phase of a long-term efficacy study in women aged 15–25 y associated with sustained efficacy,8 but anti-HPV-18 GMTs in the HPV-6/11/16/18(2D) group fell below this benchmark at M12.
Figure 2.
HPV-16-specific humoral and cellular immune responses for initially seronegative subjects in the ATP-I at one (M7) and 6 months (M12) after the last vaccine dose. For panels (A and B), bars show geometric mean antibody titers (GMTs) and associated 95% confidence intervals; the numbers within each bar are the GMTs for each group. For panels (C and D), boxplots show median, lower quartile, upper quartile, minimum and maximum frequency of cells; the numbers within each bar are the median values for each group. ATP-I, Month 12 according-to-protocol immunogenicity cohort; ED50, effective dose producing 50% response; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA units per milliliter; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine at months, 0, 2 and 6; M, month; n, number of subjects with available results; Nat. inf., historical GMTs for women who had cleared a natural infection (29.8 EU/mL for ELISA; 180.1 ED50 for PBNA);1,20 PBNA, pseudovirion-based neutralisation assay; Plateau, historical GMT (ELISA) at months 45–50 for women aged 15–25 y participating in a long-term efficacy trial (397.8 EU/mL).8 The cut-off values for the ELISA and PBNA assays were 19 EU/mL and 40 ED50, respectively.
Figure 3.
HPV-18-specific humoral and cellular immune responses for initially seronegative subjects in the ATP-I at one (M7) and 6 months (M12) after the last vaccine dose. For panels (A and B), bars show geometric mean antibody titers (GMTs) and associated 95% confidence intervals; the numbers within each bar are the GMTs for each group. For panels (C and D), boxplots show median, lower quartile, upper quartile, minimum and maximum frequency of cells; the numbers within each bar are the median values for each group. ATP-I, Month 12 according-to-protocol immunogenicity cohort; ED50, effective dose producing 50% response; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA units per milliliter; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6; M, month; n, number of subjects with available results; Nat. inf., historical GMTs for women who had cleared a natural infection (22.6 EU/mL for ELISA; 137.3 ED50 for PBNA);1,20 PBNA, pseudovirion-based neutralisation assay; Plateau, historical GMT (ELISA) at months 45–50 for women aged 15–25 y participating in a long-term efficacy trial (297.3 EU/mL).8 The cut-off values for the ELISA and PBNA assays were 18 EU/mL and 40 ED50, respectively.
Among initially seronegative girls in the M12-ATP-I, HPV-16 neutralizing antibody titers were at least 2.4-fold higher at M7 and at least 1.8-fold higher at M12 for HPV-16/18(2D) vs. HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D), and HPV-18 neutralizing antibody titers were at least 3.0-fold higher at M7 and at least 2.1-fold higher at M12 for HPV-16/18(2D) vs. HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D). Similar results were observed in the TVC regardless of the baseline serostatus (Tables S1 and S2).
Cell-mediated immunity (CMI)
In descriptive analyses, frequencies of HPV-16 and −18-specific memory B-cell and CD4+ T-cells for initially seronegative girls in the ATP-I were within similar ranges in all groups at M7 and M12, but median frequencies were numerically highest for girls who received 2D of the HPV-16/18 vaccine (Figs. 2 and 3). Similar results were observed in the TVC, regardless of baseline serostatus (Table S3 and Table S4). No substantial HPV-16 and −18 specific CD8+ T-cell response was detected at M7 or M12 in any group.
Reactogenicity and safety
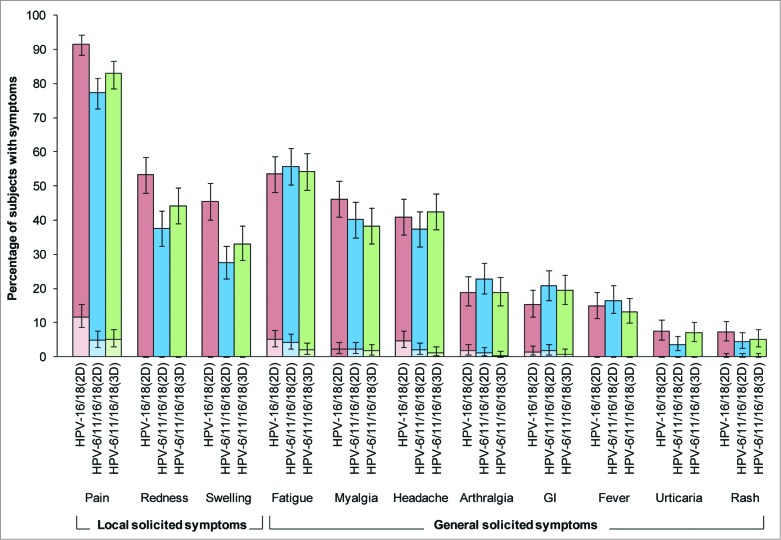
There was a higher incidence of solicited local symptoms in the HPV-16/18(2D) group than the HPV-6/11/16/18(2D) or HPV-6/11/16/18(3D) groups, but the incidence of solicited general symptoms was generally similar across groups (Fig. 4 and Table S5). Solicited local symptoms were reported by 93%, 81% and 86% of girls in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) groups, after 75%, 62% and 67% of doses, respectively. Pain at the injection site was the most frequently reported solicited local symptom, being reported by 92%, 77% and 83% of girls in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D), respectively; grade 3 pain was reported by 12%, 5% and 5% of girls, respectively. Solicited general symptoms were reported by 74%, 75% and 74% of girls in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) groups, after 52%, 50% and 53% of doses, respectively. Fatigue, myalgia and headache were the most frequently reported solicited general symptoms in each group. Each individual grade 3 solicited general symptom was reported by no more than 5% of girls. Solicited local and general symptoms were generally transient, with only a small percentage of girls (<2 %) having a symptom ongoing beyond the 7-day observation period. At least one unsolicited symptom was reported by 25%, 27% and 28% of girls in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) groups, respectively, with at least one grade 3 unsolicited symptom reported by 5%, 2% and 6% of girls, respectively. There was no increase in the frequency of AEs with increasing number of doses (data not shown).
Figure 4.
Incidence of local and general solicited symptoms during the 7-day post-vaccination period overall per subject for the total vaccinated cohort. For each group, the darker and lighter shaded bars, respectively, represent the percentage of subjects with any symptom or any grade 3 symptom with exact 95% confidence intervals. GI, gastrointestinal; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6 (n = 359); HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6 (n = 357); HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6 (n = 356); girls in the 2D groups received placebo at dose 2. Fever was defined as oral or axillary temperature ≥37.5°C; grade 3 fever was defined as oral or axillary temperature >39.0°C. Grade 3 redness/swelling were defined as an area at the local injection site with diameter >50 mm. For all other symptoms, a grade 3 event was defined as one which prevented normal everyday activities.
Up to M12, at least one medically significant adverse event was reported by 14%, 16% and 13% of girls in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) groups, respectively. No deaths were reported. Sixteen girls had serious adverse events (SAEs) (13 [3.6%], 2 [0.6%] and 1 [0.3%] in HPV-16/18(2D), HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) groups, respectively) which all resolved. None of the SAEs was considered by the investigators to have a causal relationship to vaccination. No discernible pattern in the nature and time to onset of the SAEs was observed.
Six girls had potential immune-mediated diseases (pIMDs) (3 [0.8%] in the HPV-16/18(2D) group and 3 [0.8%] in the HPV-6/11/16/18(2D) group). The reported pIMDs were reactive arthritis, juvenile idiopathic arthritis, erythema nodosum, alopecia areata, ulcerative colitis and celiac disease; 2 of these events were categorized as serious (erythema nodosum and juvenile idiopathic arthritis) and one non-serious event was considered by the investigator to have a possible causal relationship to vaccination (reactive arthritis). No subject was withdrawn from the study due to an adverse event and no pregnancy was reported.
Discussion
The recent licensure of 2D schedules of the HPV-16/18 AS04-adjuvanted vaccine and the HPV-6/11/16/18 vaccine for preteens/adolescents is important for global public health, as this could facilitate the introduction of vaccination programs in lower income countries, where cost and infrastructure are significant barriers to program implementation. This could also help to improve the relatively low vaccine coverage and series completion rates observed in some higher income countries.21-24 The current head-to-head trial was undertaken to compare the immunogenicity and safety of a 2D schedule of the HPV-16/18 AS04-adjuvanted vaccine vs. 2D and 3D schedules of the HPV-6/11/16/18 vaccine in girls aged 9–14 y. We found that anti-HPV-16 and −18 GMTs, measured by ELISA, were significantly higher one and 6 months after the last vaccine dose following administration of 2D of the HPV-16/18 vaccine than 2D or 3D of the HPV-6/11/16/18 vaccine. Statistical testing was not performed for secondary immunogenicity endpoints but in descriptive comparisons, neutralizing anti-HPV-16 and −18 GMTs were at least 1.8-fold higher following administration of the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine.
Our finding of higher antibody responses in girls vaccinated with 2D of the HPV-16/18 AS04-adjuvanted vaccine is in accord with previous head-to-head comparisons between the HPV-16/18 and HPV-6/11/16/18 vaccines administered according to a 3D schedule.1,25,26 Following administration of a 3D schedule, higher anti-HPV-16 and −18 GMTs (by ELISA and PBNA) were observed for the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine up to 60 months after first vaccination in healthy women aged 18–45 y,1,25 and higher neutralizing antibody titers against vaccine (HPV-16 and −18) and non-vaccine (HPV-31 and −45) types were observed for the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine up to 12 months after first vaccination in healthy girls aged 12–15 y.26 Additionally, following administration of a 3D schedule, significantly higher HPV-18 antibody titers were observed for the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine up to 12 months after first vaccination in HIV-positive adult men and women, although no significant difference in HPV-16 antibody titers was found.27 Furthermore, the HPV-16/18 vaccine induced significantly higher HPV-16 and −18 neutralizing antibody titers than the HPV-6/11/16/18 vaccine in girls vaccinated within organized vaccination programmes.28
An immunological correlate of protection has not yet been defined for HPV infection and associated cervical lesions, although previous studies have shown that higher titers of naturally-acquired HPV-16 antibodies (by ELISA), and to a lesser extent HPV-18 antibodies, are associated with a lower risk of newly detected infection and cervical abnormalities due to the same HPV type.29,30 In our study, all 3 vaccine regimens induced HPV-16 and −18 antibody titers that were many fold higher (at least 11.7-fold by ELISA and at least 4.8-fold by PBNA) than the levels of naturally acquired antibodies previously observed in women who had cleared a natural infection.1,20 Furthermore, in girls who received 2D of the HPV-16/18 vaccine or 3D of the HPV-6/11/16/18 vaccine, both anti-HPV-16 and −18 GMTs at M12 were above the plateau level of antibodies observed in women aged 15–25 y participating in an efficacy trial, in whom sustained protection against HPV-16/18-associated infection and cervical lesions was shown.8 However, in girls who received 2D of the HPV-6/11/16/18 vaccine, anti-HPV-18 GMTs at M12 fell below this benchmark plateau level. The clinical relevance of this observation is not known, although a high magnitude of vaccine-induced antibody titers could influence persistence of immunity.
The role of CMI in the control of HPV infection is not well established, although induction of antigen-specific memory B-cells, a process in which CD4+ T-cells play an essential role, are thought to be important for long-term vaccine-induced protection.31-33 Our trial was not powered to make statistical comparisons for CMI endpoints and descriptive analyses showed large overlaps in the range of CMI responses between groups. However, the median frequencies of HPV-16 and −18-specific memory B-cells and CD4+ T-cells at M7 and M12 were numerically highest for girls who received 2D of the HPV-16/18 vaccine. Further investigations may help to better understand the correlation between vaccine-induced CMI and humoral responses.
Both vaccines had a clinically acceptable safety profile in girls aged 9–14 years, in line with the safety profile observed in previous clinical trials and post-licensure experience.34-36 Compliance with the vaccination schedule was equally high in all groups, and no subject withdrew due to an adverse event. There was a tendency for a higher incidence of local injection site reactions for girls administered 2D of the HPV-16/18 vaccine than those administered 2D or 3D of the HPV-6/11/16/18 vaccine. In previous studies, a larger proportion of vaccinees reported pain or swelling with AS04-adjuvanted vaccines regardless of the antigen, compared with placebo or aluminum salt-only-adjuvanted formulations, possibly reflecting involvement of CMI induced by AS04 adjuvantation.37 A higher incidence of local injection site reactions was also observed for the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine in the head-to-head comparison of these 2 vaccines administered according to the standard 3D schedule in women aged 18–45 y.1 The solicited local symptoms were generally transient and resolved without sequelae. Incidences of general solicited symptoms, unsolicited symptoms and medically significant conditions were comparable between groups. A larger number of girls in the HPV-16/18(2D) group than the HPV-6/11/16/18 groups experienced an SAE. An analysis of type and time to onset of SAEs did not reveal any association or cluster in the nature of the events reported in the study. Except for one non-serious pIMD (reactive arthritis), all SAEs and remaining pIMDs were considered as not related to vaccination by investigators.
The differences in immunogenicity and reactogenicity between the 2 HPV vaccines may be due to the different production methods and adjuvants used in each vaccine. VLPs for the HPV-16/18 vaccine are produced in Trichoplusia ni Rix4446 cell substrate using a baculovirus expression vector system and formulated with AS04, which contains aluminum hydroxide plus an additional immunostimulant, the toll-like receptor 4 agonist monophosphoryl lipid A.38 AS04 has been shown to enhance humoral and cell-mediated immune responses39 by triggering a local and transient cytokine response, leading to increased activation of antigen-presenting cells and an improved presentation of antigen to CD4+ T-cells.38 However, the incidence of transient local solicited symptoms, such as pain, is also increased in vaccines formulated with AS04 compared with aluminum salt alone.37 VLPs for the HPV-6/11/16/18 vaccine are produced in the yeast Saccharomyces cerevisiae and formulated with amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant, which has an increased capacity to bind to L1 VLPs compared with aluminum salts.40 Mice immunized with HPV-16 L1 VLPs adsorbed onto AAHS had significantly higher antibody titers than mice immunized with VLPs adsorbed to aluminum hydroxide and the AAHS-formulated vaccine induced a substantial L1-specific interferon (IFNγ) secreting T-cell response.40 However, there has not been any direct comparison between AS04 and AAHS adjuvants using identical HPV antigens.
A strength of the present study is that assessments were performed according to the same schedule and using the same methodology in all groups, allowing a valid head-to-head comparison of immunogenicity and reactogenicity for the 2 vaccines. The study was also conducted in the age group of young girls that is targeted by most immunization programs. We endeavored to minimize factors which might have introduced bias against either vaccine. The study was conducted in an observer-blind manner to enable the vaccines to be administered according to their recommended schedules. The 2D regimens of each vaccine were administered at months 0 and 6 and the 3D regimen of the HPV-6/11/16/18 vaccine was administered at months 0, 2 and 6, with a placebo administered at month 2 for girls in the 2D groups to maintain blinding. The administration of aluminum hydroxide alone at month 2 would not be expected to affect HPV-specific immune responses. It is possible that in vitro stimulation of memory B-cells with the L1 VLP constructs from the HPV-16/18 vaccine in the B-cell enzyme-linked immunosorbent spot (ELISPOT) assay might have introduced bias against the HPV-6/11/16/18 vaccine. However, results were not expected to be significantly affected as the HPV-16 and −18 L1 patented sequences for the 2 vaccines are 99.6% and 99.4% identical, respectively, by protein level comparison, with the main differences between the constructs being 33 and 35 amino acid C-terminal truncations of the L1 sequences used in the HPV-16/18 vaccine. Although the ELISA also used the VLPs present in the HPV-16/18 vaccine as the coating antigen for the assay, there did not appear to be a notable bias in favor of the HPV-16/18 vaccine since the magnitude of differences in GMT ratios between groups were similar when neutralizing antibody titers were measured by PBNA (which used pseudovirions with structures that were as close as possible to those of natural HPV-16 and −18 virus particles41). Good correlation has been previously demonstrated between results from the ELISA measuring total anti-HPV-16/18 IgG independently of their neutralising activity and PBNA detecting neutralizing antibodies.41 Good correlation has also been shown between ELISA, PBNA and the competitive Luminex immunoassay (measuring only a subset of neutralizing antibodies),42 which is primarily used to evaluate the immunogenicity of the HPV-6/11/16/18 vaccine in clinical trials, providing further reassurance that the likelihood of assay bias is minimal. The assay used to evaluate antigen-specific CD4+ T-cell responses was also unlikely to favor either vaccine, since the HPV-16 and −18 peptide pools used for in vitro stimulation covered the entire L1 VLP sequences of each vaccine.
In summary, the current study showed that a 2D schedule of the HPV-16/18 AS04-adjuvanted vaccine elicited superior antibody responses in girls to those elicited by 2D and 3D of the HPV-6/11/16/18 vaccine 1 and 6 months after the last vaccine dose. Follow-up over the planned 3-y observation period will show if such differences are sustained over the longer term. Population-based studies are necessary to determine the clinical relevance, if any, of the immunological differences observed following administration of 2 doses of the HPV-16/18 AS04-adjuvanted vaccine vs. the HPV-6/11/16/18 vaccine to girls.
Methodology
Study design and ethics
This is an ongoing observer-blind, randomized, age-stratified study with 3 parallel groups (Fig. 1) conducted at 21 sites in France, Hong Kong, Singapore and Sweden. Subject enrolment started in November 2011. The last visit of the vaccination phase was in July 2013. This article presents data from baseline to M12 (ie, 6M after the last vaccine dose) and the study will continue until M36. The trial is registered with ClinicalTrials.gov (NCT01462357) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. A summary of the protocol is available at www.gsk-clinicalstudyregister.com (GSK Study Identifier 115411). The research protocol and other study materials were approved by the independent ethics committee or equivalent body for each participating center.
The primary objective was to evaluate if immunogenicity to HPV types 16 and 18 (by ELISA) of the 2D schedule of the HPV-16/18 vaccine was non-inferior/superior, to the 2D schedule of the HPV-6/11/16/18 vaccine 1M after the last vaccine dose. A secondary objective was to evaluate if the 2D schedule of the HPV-16/18 vaccine was non-inferior/superior to the 3D schedule of the HPV-6/11/16/18 vaccine 1M after the last dose. Other secondary immunogenicity objectives included evaluation of non-inferiority/superiority at other time points, HPV-16 and −18 neutralizing antibodies (by PBNA), HPV-specific CMI, frequencies of HPV-16 and −18-specific memory B-cells and T-cells. Reactogenicity and safety were also evaluated.
Study participants
Healthy girls aged 9–14 y were eligible to participate. Girls of childbearing potential could be enrolled if they were abstinent or practiced adequate contraception for 30 d prior to vaccination, had a negative pregnancy test on the day of each vaccination, and agreed to continue contraception up to 2 months after completion of the vaccination series. Informed written assent and consent were obtained from subjects and their parents/legally acceptable representatives, respectively.
Vaccines, randomization and masking
Girls were stratified by age (approximately 50% aged 9–11 y and 50% aged 12–14 years) and randomized (1:1:1 ratio in each age stratum) to receive 0.5 mL doses of the HPV-16/18 L1 VLP AS04-adjuvanted vaccine at M0,6 (HPV-16/18(2D) group), the HPV-6/11/16/18 L1 VLP vaccine at M0,6 (HPV-6/11/16/18(2D) group), or the HPV-6/11/16/18 L1 VLP vaccine at M0,2,6 (HPV-6/11/16/18(3D) group) into deltoid muscle of the non-dominant arm. Compositions of the 2 vaccines have been described previously.1 AS04 is a GSK proprietary Adjuvant System containing 50 µg of 3-O-desacyl-4 ′-monophosphoryl lipid A (MPL) adsorbed on aluminum hydroxide salt [500 µg Al(OH)3].
The study was conducted in an observer-blind manner (ie, vaccines were prepared and administered by qualified medical personnel not otherwise involved in the conduct of this study). Personnel involved in subject evaluation and subjects themselves were blinded to group assignments. Girls in the 2D groups received placebo [Al(OH)3] at M2 to maintain the observer-blinding. The randomization code was generated using MATEX, a program developed for use in SAS (Cary, NC, USA), by GSK Vaccines, Belgium. Treatment allocation at the investigator site was performed using a centralized internet-based randomization system.
A subset of 100 subjects in each group (300 in total) were randomly selected for measurement of neutralizing HPV-16 and −18 antibody titers by PBNA. Additionally, the first 50 subjects in each age stratum in each group at preselected sites in France and Sweden (300 subjects in total) underwent cell-mediated immunity testing for measurement of subpopulations of circulating HPV-specific B- and T-lymphocytes.
Immunogenicity assessments
Blood sampling was scheduled at M0 (pre-vaccination), 7, 12, 18, 24 and 36 for assessment of HPV-16 and −18 antibodies (by ELISA in all subjects and by PBNA in a subset). An additional blood sample was scheduled at M0, 7, 12, 24 and 36 for girls assigned to the CMI subcohort.
HPV-16 and −18 antibodies were determined by ELISA, using the purified type-specific recombinant VLPs present in the HPV-16/18 vaccine as coating antigen.7,41 Seronegativity was defined as a titer lower than the assay cut-off (19 ELISA units [EU]/mL for anti-HPV-16 and 18 EU/mL for anti-HPV-18). Neutralizing HPV-16 and −18 antibodies were determined by PBNA.1,43 Pseudovirions were produced in a manner that was independent of vaccine constructs, as described previously.41 The structures of the pseudovirions were as close as possible to those of the natural HPV-16 and −18 viral particles. Seronegativity was defined as a titer lower than the assay cut-off (40 ED50 [effective dose producing 50% response] for each antigen).
The frequencies of HPV-specific memory B-lymphocytes were measured by ELISPOT assay that contained L1 VLP antigens present in the HPV-16/18 vaccine (truncated at the C-terminus).1,39 A conventional immunoenzymatic procedure was applied to detect antibody/antigen spots enumerating total and specific antibody-secreting cells.44
The frequencies of HPV-16 and −18-specific CD4+ and CD8+ T-lymphocytes were evaluated using a pool of HPV peptides that stimulated peripheral blood mononuclear cells (PBMCs) to produce cytokines in vitro.45,46 The HPV peptide pool used for in vitro stimulation included the HPV-16 and −18 L1 VLP sequences used in the HPV-16/18 vaccine and the portions truncated from the HPV-16/18 vaccine but present in the HPV-6/11/16/18 vaccine. Overall, this peptide pool comprised 55 and 53 peptides (including 20-mer peptides overlapping by 10 amino acids) covering the L1 VLP sequences for HPV-16 and HPV-18, respectively. Following intracellular cytokine staining, flow cytometry was used to quantify the number of lymphocytes producing at least 2 of 4 different immune markers [CD40L, interleukin (IL) 2, tumor necrosis factor (TNF)α and IFNγ] in response to the HPV peptide pool. The effector cytokines IL2, TNFα and IFNγ were selected as they have been shown to be the most relevant to define populations of antigen-specific CD4+ and CD8+ T-cells.47 CD40L, a member of the TNF superfamily of molecule which is expressed on activated T-cells, has also been shown to be a reliable functional marker for the detection of antigen-specific T-cells.48,49
Reactogenicity and safety
Solicited local and general symptoms were recorded on diary cards for 7 d after each vaccination. Unsolicited symptoms were recorded for 30 d after each vaccination. Grade 3 symptoms were defined as redness or swelling >50 mm in diameter, fever >39°C and, for other symptoms, as preventing normal activity. SAEs, medically significant adverse events, pIMDs and pregnancy were reported throughout the study.
Statistical methods
The hierarchy of testing for immunological non-inferiority and superiority comparisons was pre-specified in the protocol. The most conservative dataset was chosen for each analysis, as guided by principles from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.50 Thus, for superiority comparisons the full analysis set (TVC of all subjects with at least one documented vaccine dose) was used as the primary analysis because it tends to avoid over-optimistic estimates resulting from a per protocol analysis. In contrast, the more stringent per protocol analysis set (ATP-I) was used as the primary analysis for non-inferiority comparisons. The ATP-I included subjects who received their planned doses of study vaccine, met all eligibility criteria, were compliant with all study procedures and requirements and for whom immunogenicity data were available. The M7 ATP-I was used for the assessment of non-inferiority 1M after the last vaccine dose. The M12 ATP-I was used for the assessment of non-inferiority 6M after the last vaccine dose and for descriptive summaries of immunogenicity.
The primary objective was to evaluate sequentially if HPV-16 and −18 antibody responses (by ELISA) of HPV-16/18(2D) were non-inferior, then superior, to HPV-6/11/16/18(2D) in initially seronegative girls in the ATP-I, 1M after the last dose (at M7). Non-inferiority with respect to seroconversion was shown if, for both anti-HPV-16 and −18, the upper limits of the 95% CIs for the differences (HPV-6/11/16/18(2D) minus HPV-16/18(2D)) were below 5%. Non-inferiority with respect to the GMTs was shown if, for both anti-HPV-16 and −18, the upper limits of the 95% CIs for the GMT ratios (HPV-6/11/16/18(2D) divided by HPV-16/18(2D)) were below 2. If non-inferiority was proven and the lower limits of the 2-sided 95% CIs for the ratio of GMTs of a given antigen were above 1 in the ATP-I, superiority was assessed sequentially in the TVC. First, superiority for HPV-18 was shown if the lower limit of the 95% CI for the ratio of GMTs (HPV-16/18(2D) divided by HPV-6/11/16/18(2D)) was above 1 for anti-HPV-18 with the associated p-value. If superiority for HPV-18 was proven, superiority for HPV-16 was assessed using the same criteria. If the primary non-inferiority objective was proven, the non-inferiority/superiority of HPV-16/18(2D) compared to HPV-6/11/16/18(3D) at M7 was evaluated sequentially as a secondary objective based on the criteria defined above. Similarly, non-inferiority/superiority of HPV-16/18(2D) to HPV-6/11/16/18(2D) and HPV-6/11/16/18(3D) was assessed at M12.
For the non-inferiority analysis, 285 evaluable subjects per group in the ATP-I would allow the detection of a 5% difference between HPV-16/18(2D) and HPV-6/11/16/18(2D) in terms of anti-HPV-16 and −18 seroconversion rates 1M after the last dose with 95% power and the detection of a 2-fold difference between HPV-16/18(2D) and HPV-6/11/16/18(2D) in terms of anti-HPV-16 and −18 GMTs with at least 99% power. A sample size of 322 subjects per group in the TVC would allow the demonstration of superiority in terms of anti-HPV-16 and −18 GMTs 1M after the last dose with at least 99% power. Assuming that 20% of subjects would withdraw or not be evaluable for immunogenicity 1M after the last dose (at M7), the target sample size for enrolment was 1,074 subjects (358 per group).
Seroconversion and seropositivity rates (with exact 95% CIs) and GMTs (with 95% CIs) for HPV-16 and −18 antibodies were calculated by baseline serostatus. GMTs were computed by taking the anti-log of the mean of the log titer transformations; antibody titers below the cut-off of the assay were given an arbitrary value of half the cut-off in this calculation. The 2-sided standardized asymptotic 95% CI of the difference between the percentages of seroconverted subjects was calculated. The 2-sided 95% CIs of GMT ratios between groups (HPV-6/11/16/18(2D) or –(3D)/HPV-16/18(2D) for non-inferiority comparisons; HPV-16/18(2D)/HPV-6/11/16/18(2D) or –(3D) for superiority comparisons) were computed using an analysis of variance (ANOVA) model on the log10 transformation of the titers. The ANOVA model included the vaccine group as fixed effect. Frequencies of HPV-16 and −18-specific memory B- and T-lymphocytes at each time point were summarized for each group using descriptive statistics. Immunogenicity analyses focused on subjects who were seronegative at baseline; supplementary analyses were done by baseline serostatus. Supplementary analyses were also performed on the TVC. Confirmatory testing was not performed for secondary immunogenicity endpoints. Descriptive comparisons were made between anti-HPV-16 and −18 GMTs measured in our trial and historical data (measured using similar assays at the same laboratory), ie, GMTs for women aged 15–25 y (by ELISA) or 18–45 y (by PBNA) who had cleared a natural infection and mounted an immune response1,20 and GMTs (by ELISA) from the plateau phase (months 45–50) of a long-term efficacy study in women aged 15–25 y.8
Safety data were summarized descriptively for the TVC. Statistical testing was not planned or conducted for safety and reactogenicity endpoints and inferences are based on descriptive comparisons. Group assignment was not disclosed if there was only one event of a particular type, to avoid unblinding this ongoing study. Statistical analyses were performed using SAS 9.2 and PROC StatXact 8.1.
Disclosure of Potential Conflicts of Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare the following potential conflicts of interest. Ting Fan Leung, Fong Seng Lim, Bee Wah Lee, Ngiap Chuan Tan or their respective Institutions received grant for the conduct of the clinical trial from the GSK group of companies. Ting Fan Leung received honorarium as speaker from PT Wyeth Nutrition Indonesia, consultant fee from MSD Ltd and sponsorship for attending conferences from the GSK group of companies and Nestle outside of the submitted work. Fong Seng Lim received support for travel to meetings from the GSK group of companies outside of the submitted work. Franck Thollot received personal fees during the conduct of the study from the GSK group of companies. Lars Rombo received honorarium for lectures from the GSK group of companies and from Sanofi-Pasteur MSD. Bee Wah Lee received honorarium for lectures from the GSK group of companies. Roman Rouzier received consulting fees from the GSK group of companies. Damien Friel, Benoit De Muynck, Stéphanie De Simoni, Pemmaraju Suryakiran, Nicolas Folschweiller, Florence Thomas and Frank Struyf are employees of the GSK group of companies. Marjan Hezareh is a Chiltern International consultant for the GSK group of companies. Florence Thomas holds stock options from the GSK group of companies. Frank Struyf holds restricted shares and stock options from the GSK group of companies. Anthony Liu and Helen May Lin Oh have no conflict of interest to declare.
Acknowledgments
The authors thank the study participants and their families, as well as the study investigators and their staff members who are not named as authors but who substantially contributed to the study, in particular Jean-Pierre Arsène (Pediatric Office, Le Havre, France), Jean-Paul Boyes (Medical Office, Seysses, France), Poh Chong Chan (National University Hospital, Singapore), Yu-Lung Lau (The University of Hong Kong), Christian Petit (Medical Office, Draguignan, France), Kumudhini Rajasegaran (KK Women's and Children's Hospital, Singapore) and Denis Taminau (Medical Office, Le Bourg Rosiers d'Egletons, France). In addition, the authors would like to thank the following contributors to the study and/or publication: from the Chinese University of Hong Kong: Edmund Anthony Severn Nelson, Paul Kay-sheung Chan, Albert Martin Li, Eva Lai-wah Fung, Assunta Chi-hang Ho, Ho-chung Yau, Kate Ching-ching Chan, Grace Pui-king Chiang, Terence Ping-yuen Ma, Alice Ka-wah Yiu, Yuen-mei Chan, Wanny Tze-wan Leung, Elsa Sau-ping Chan, Clara Yuen-fan Yu, Lucia Siu-shan Ko, Nancy Shuk-man Li, Suk-Tak Lee and Po-yin Cheng; from the University of Hong Kong: Tsz-leung Lee, Marco Hok-kung Ho, Brian Hon-yin Chung, Chun-yin Chong, Pamela Pui-wah Lee, Herman Hoi-ham Tsang, Winnie Wai-sim Lau, Sau-man Chan, Stella Yuk-suim Lau, Amanda Sio-peng Mok, Wai-fong Wong; from the National Healthcare Group Polyclinics, Singapore: Simon Lee.
The authors are indebted to the global and regional clinical teams of the GSK group of companies for their overall contribution to the design and conduct of the study, the running of the laboratory analyses, the coordination of the data management and statistical analyses, and the writing of the clinical protocol and the clinical study report. The authors are in particular grateful to: Grégory Catteau (Project Biostatistician), Brigitte Colau (Global Vaccine Clinical Laboratories Representative), Anaëlle Delhage (Global Study Manager), Dominique Descamps (Director Global Vaccine Development), Monique Dodet-Boutsona (Project Manager, Global Vaccine Clinical Laboratories), Nele Martens (Scientific Writer), Amoolya Modi (Study Delivery Lead), Philippe Moris (Clinical Immunology Representative), Bhakthi Pereira (Scientific Writer), Sylviane Poncelet (Expert Scientist), Alina Stoyanova (Clinical Data Coordinator), Sheetal Verlekar (Study Delivery Manager) and Christine Van Hoof (Scientific Writer).
The authors would like to thank Julie Taylor (Peak Biomedical Ltd., UK, on behalf of GSK Vaccines) for publication writing assistance, Jean-Michel Heine and Jérôme Leemans (Keyrus Biopharma, Belgium, on behalf of GSK Vaccines, Wavre, Belgium) and Bruno Baudoux (Business & Decision Life Science, Belgium, on behalf of GSK Vaccines, Wavre, Belgium) for the coordination of the manuscript development and editorial assistance.
Funding
GlaxoSmithKline Biologicals SA funded this study and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Authors' Contribution
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Trademark Information
Cervarix is a registered trade mark of the GSK group of companies. Gardasil is a registered trade mark of Merck & Co., Inc.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al.. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705-19; PMID:19684472; http://dx.doi.org/ 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- 2.de Sanjosé S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al.. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048-56; PMID:20952254; http://dx.doi.org/ 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 3.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927-35; PMID:20473886; http://dx.doi.org/ 10.1002/ijc.25396 [DOI] [PubMed] [Google Scholar]
- 4.Bruni L, Barrionuevo-Rosas L, Serrano B, Brotons M, Cosano R, Muñoz J, Bosch FX, de Sanjosé S. ICO information centre on HPV and cancer (HPV Information Centre). Human papillomavirus and related diseases in World. Summary Report 2014-08-22. Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf. Accessed 7April2015.
- 5.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, Dubin G. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564-71; PMID:17531764; http://dx.doi.org/ 10.1016/j.jadohealth.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 6.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al.. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006; 118:2135-45; PMID:17079588; http://dx.doi.org/ 10.1542/peds.2006-0461 [DOI] [PubMed] [Google Scholar]
- 7.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al.. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757-65; PMID:15541448; http://dx.doi.org/ 10.1016/S0140-6736(04)17398-4 [DOI] [PubMed] [Google Scholar]
- 8.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247-55; PMID:16631880; http://dx.doi.org/ 10.1016/S0140-6736(06)68439-0 [DOI] [PubMed] [Google Scholar]
- 9.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al.. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301-14; PMID:19586656; http://dx.doi.org/ 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 10.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al.. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89-99; PMID:22075171; http://dx.doi.org/ 10.1016/S1470-2045(11)70286-8 [DOI] [PubMed] [Google Scholar]
- 11.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, et al.. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100-10; PMID:22075170; http://dx.doi.org/ 10.1016/S1470-2045(11)70287-X [DOI] [PubMed] [Google Scholar]
- 12.FUTURE II Study Group . Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 2007; 196:1438-46; PMID:18008221; http://dx.doi.org/ 10.1086/522864 [DOI] [PubMed] [Google Scholar]
- 13.Ault KA, FUTURE II Study Group . Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369:1861-8; PMID:17544766; http://dx.doi.org/ 10.1016/S0140-6736(07)60852-6 [DOI] [PubMed] [Google Scholar]
- 14.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al.. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared to the licensed 3-dose schedule: Results from a randomized study. Hum Vaccin 2011; 7:1374-86; PMID:22048171; http://dx.doi.org/ 10.4161/hv.7.12.18322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthanakit T, Huang L, Tang R, Schwarz T, Esposito S, Frenette L, McNeil S, Rheault P, Horn M, Klar M, et al.. Non-inferiority of HPV-16/18 AS04-adjuvanted vaccine administered as 2-dose schedules in girls (9-14 years) versus 3 doses in women (15-25 years): a randomised trial. Abstract presented at ESPID 2014. May 6-10, 2014 Dublin, Ireland Available at: http://cmoffice.kenes.com/cddemo/data/HtmlApp/main.html#. Accessed 7April2015 [Google Scholar]
- 16.Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al.. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: Results from a randomized study. Hum Vaccin Immunother 2014; 10:1155-65; PMID:24576907; http://dx.doi.org/ 10.4161/hv.28022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al.. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793-802; PMID:23632723; http://dx.doi.org/ 10.1001/jama.2013.1625 [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency European public assessment report. Cervarix: human papillomavirus vaccine [types 16, 18] (recombinant, adjuvanted, adsorbed). EMEA/H/C/000721 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000721/WC500024634.pdf. Ac-cessed 7April2015. [Google Scholar]
- 19.European Medicines Agency European public assessment report. Gardasil: human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, adsorbed). EMEA/H/C/000703 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000703/WC500021146.pdf. Accessed 7April2015. [Google Scholar]
- 20.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsagué X, et al.. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 21.Harper DM, Verdenius I, Ratnaraj F, Arey AM, Rosemergey B, Malnar GJ, Wall J. Quantifying clinical HPV4 dose inefficiencies in a safety net population. PLoS One 2013; 8:e77961; PMID:24223131; http://dx.doi.org/ 10.1371/journal.pone.0077961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, Brotherton JM. Human papillomavirus vaccine introduction-the first five years. Vaccine 2012; 30 Suppl 5:F139-48; PMID:23199957; http://dx.doi.org/ 10.1016/j.vaccine.2012.05.039 [DOI] [PubMed] [Google Scholar]
- 23.Hopkins TG, Wood N. Female human papillomavirus (HPV) vaccination: global uptake and the impact of attitudes. Vaccine 2013; 31:1673-9; PMID:23375978; http://dx.doi.org/ 10.1016/j.vaccine.2013.01.028 [DOI] [PubMed] [Google Scholar]
- 24.Harper DM, Verdenius I, Harris GD, Barnett AL, Rosemergey BE, Arey AM, Wall J, Malnar GJ. The influence of free quadrivalent human papillomavirus vaccine (HPV4) on the timely completion of the three dose series. Prev Med 2014; 61:20-5; PMID:24440159; http://dx.doi.org/ 10.1016/j.ypmed.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 25.Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David MP, Lin L, Struyf F, et al.. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: End-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother 2014;10:3435-45; PMID:25483701; http://dx.doi.org/ 10.4161/hv.36121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S. A randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® human papillomavirus vaccines in 12-15 year old girls. PLoS One 2013; 8:e61825; PMID:23650505; http://dx.doi.org/ 10.1371/journal.pone.0061825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, Østergaard L, Søgaard OS. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis 2014; 209:1165-73; PMID:24273179; http://dx.doi.org/ 10.1093/infdis/jit657 [DOI] [PubMed] [Google Scholar]
- 28.Barzon L, Squarzon L, Masiero S, Pacenti M, Marcati G, Mantelli B, Gabrielli L, Lazzarotto T, Caputo A, Palu G. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine 2014; 32:5357-62; PMID:25045814; http://dx.doi.org/ 10.1016/j.vaccine.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Castellsagué X, Naud P, Chow SN, Wheeler CM, Germar MJ, Lehtinen M, Paavonen J, Jaisamrarn U, Garland SM, Salmeron J, et al.. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis 2014; 210:517-34; PMID:24610876; http://dx.doi.org/ 10.1093/infdis/jiu139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn LJ, Sherman ME, Xhenseval V, et al.. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and −18 infections. J Natl Cancer Inst 2010; 102:1653-62; PMID:20944077; http://dx.doi.org/ 10.1093/jnci/djq384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002; 298:2199-202; PMID:12481138; http://dx.doi.org/ 10.1126/science.1076071 [DOI] [PubMed] [Google Scholar]
- 32.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003; 171:4969-73; PMID:14607890; http://dx.doi.org/ 10.4049/jimmunol.171.10.4969 [DOI] [PubMed] [Google Scholar]
- 33.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine 2003; 21 Suppl 2:S35-7; PMID:12763680; http://dx.doi.org/ 10.1016/S0264-410X(03)00198-1 [DOI] [PubMed] [Google Scholar]
- 34.Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, Struyf F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014; 23:466-79; PMID:24644063; http://dx.doi.org/ 10.1002/pds.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angelo MG, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf 2014; 23:456-65; PMID:24644078; http://dx.doi.org/ 10.1002/pds.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block SL, Brown DR, Chatterjee A, Gold MA, Sings HL, Meibohm A, Dana A, Haupt RM, Barr E, Tamms GM, et al.. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95-101; PMID:19952863; http://dx.doi.org/ 10.1097/INF.0b013e3181b77906 [DOI] [PubMed] [Google Scholar]
- 37.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723-39; PMID:17931153; http://dx.doi.org/ 10.1586/14760584.6.5.723 [DOI] [PubMed] [Google Scholar]
- 38.Garçon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011; 25:217-26; PMID:21815697; http://dx.doi.org/ 10.2165/11591760-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 39.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al.. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24:5937-49; PMID:16828940; http://dx.doi.org/ 10.1016/j.vaccine.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 40.Caulfield MJ, Shi L, Wang S, Wang B, Tobery TW, Mach H, Ahl PL, Cannon JL, Cook JC, Heinrichs JH, et al.. Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Hum Vaccin 2007; 3:139-45; PMID:17581283; http://dx.doi.org/ 10.4161/hv.3.4.4309 [DOI] [PubMed] [Google Scholar]
- 41.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425-34; PMID:18948732; http://dx.doi.org/ 10.4161/hv.4.6.6912 [DOI] [PubMed] [Google Scholar]
- 42.Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, Gonzalez P, Schiller J, Lowy D, Poncelet S, et al.. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front Oncol 2014; 3:328; PMID:24455487; http://dx.doi.org/ 10.3389/fonc.2013.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger KS, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 2004; 321:205-16; PMID:15051381; http://dx.doi.org/ 10.1016/j.virol.2003.12.027 [DOI] [PubMed] [Google Scholar]
- 44.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111-22; PMID:15087226; http://dx.doi.org/ 10.1016/j.jim.2003.12.015 [DOI] [PubMed] [Google Scholar]
- 45.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al.. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: Follow-up from Months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7:1343-58; PMID:22048173; http://dx.doi.org/ 10.4161/hv.7.12.18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443-54; PMID:21174144; http://dx.doi.org/ 10.1007/s10875-010-9490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, Chiu YL, McElrath MJ, De Rosa SC. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 2007; 323:39-54; PMID:17451739; http://dx.doi.org/ 10.1016/j.jim.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc 2006; 1:1-6; PMID:17406204; http://dx.doi.org/ 10.1038/nprot.2006.1 [DOI] [PubMed] [Google Scholar]
- 49.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 2005; 11:1118-24; PMID:16186818; http://dx.doi.org/ 10.1038/nm1292 [DOI] [PubMed] [Google Scholar]
- 50.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline. Statistical Principles for Clinical Trials (E9) 5 February 1998 Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed 7April2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.