Abstract
Hepatitis B and Haemophilus influenzae type b (Hib) infections are major public health problems in developing countries, including India. Hence, combination vaccines containing DTwP, recombinant hepatitis B and Hib conjugate vaccines have been developed. Here, we report a Phase IV study which assessed safety and reactogenicity of a new DTwP-HepB+Hib vaccine. Three doses of DTwP-HepB+Hib vaccine (Pentavac, Serum Institute of India Ltd) or Tritanrix-HB+Hib (GlaxoSmithKline Beecham) were administered to infants at 6, 10 and 14 weeks of age in 2:1 ratio. The subjects were followed till one month after the third dose for safety assessment. Adverse events were captured in structured diaries and physical examinations were performed on each visit. The study was conducted in 1510 infants. Both vaccines caused injection site local and systemic reactions and the incidence was similar in both the groups. The incidence of local solicited reactions was: tenderness 35.9 %–33.6 %; redness 18.1 %–17.2 %; swelling 23.7 %–22.4 %; induration 12.8 % –13.7 %. The percentage of systemic solicited reactions were: diarrhea 2.2 %–2.2 %; drowsiness 3.3 %–3.4 %; fever 14.0 %–11.2 %; irritability 28.1 %–25.4 %; loss of appetite 6.6 %–5.6 %; persistent crying 17.7 %–15.7 %; vomiting 3.5 %–3.0 %. No serious adverse event was caused by the vaccines. The new DTwP-HepB+Hib combination vaccine showed similar safety profile to that of an imported vaccine in Indian infants.
Keywords: DTwP-HepB+Hib vaccine, infants, reactogenicity, safety
Introduction
India started the Expanded Program of immunization (EPI) in 1978 which included 3 doses of DTwP vaccine during infancy.1 With more than 40 million carriers in the country, hepatitis B infection is a major problem in India.2 Haemophilus influenzae type b (Hib) is also a significant problem with an annual estimated 2.4 to 3.0 million cases and 72,000 deaths in under–5 Indian children.3,4 As a result, the National Technical Advisory Group on Immunization (NTAGI) in India recommended inclusion of Hib vaccine in the EPI in year 2009.4
Combination vaccines overcome the constraints of multiple injections. Additionally, they simplify program implementation, increase vaccine acceptance and compliance and also reduce the cost of stocking and administering the vaccine.5 Keeping this in mind, since 1996, DTwP based combination vaccines which included hepatitis B and Hib vaccines have been introduced which have been found immunogenic and safe.6-7
Serum Institute of India Ltd (SIIL), Pune also developed a domestic, low cost DTwP-HepB+Hib vaccine (Pentavac®). The vaccine was licensed in 2007 and was pre-qualified by World Health Organization (WHO) in 2010. After licensure, this study was undertaken to generate additional safety data on the vaccine in comparison with a European manufactured DTwP-HepB+Hib vaccine.
Results
Subject disposition
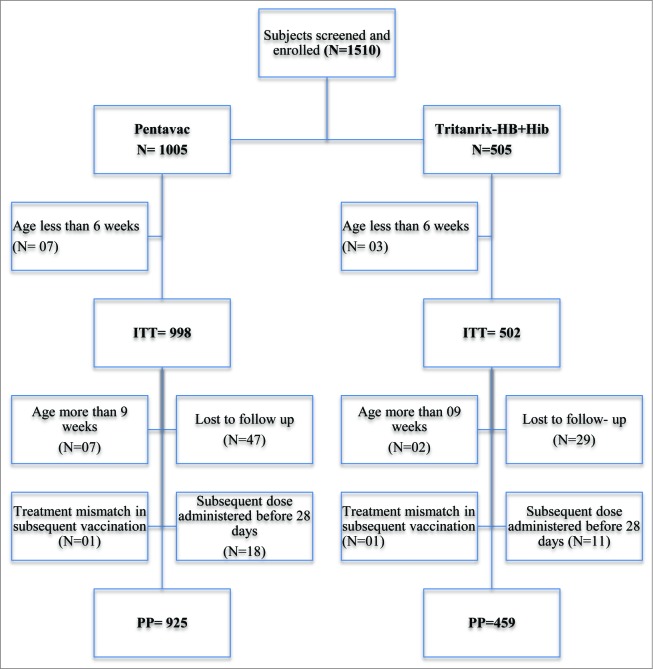
1510 healthy infants aged 6 to 8 weeks were enrolled. 1005 infants received Pentavac and 505 infants received Tritanrix-HB+Hib. Total 10 infants (7 from Pentavac group and 3 from Tritanrix-HB+Hib group) were excluded from ITT analysis because they were younger than 6 weeks. Thus, a total of 1500 infants were included in ITT population (Fig. 1).
Figure 1:
Flowchart for study subject disposition.
Further, 9 infants were more than 9 weeks at the time of enrollment. Two infants, one each from both groups, were treatment mismatch. Subsequent doses of vaccine were administered within 28 d of previous dose in total 29 infants. Additionally 76 subjects were lost to follow-up, out of which 11 were lost after receiving 3 doses as per the schedule. Eventually, 1384 infants were available for PP analysis.
Demographics
Of the 1500 infants included in ITT analysis, 833 were males and 667 were females. (Table 1). Both the groups were well matched at enrollment in terms of gender mix, age, height and weight.
Table 2.
Summary of adverse events-ITT Population
| Pentavac (N=998) | Tritanrix-HB+Hib (N=502) | |||||
|---|---|---|---|---|---|---|
| Category | n | % | 95% CI | n | % | 95% CI |
| Subjects with at least one AE | 742 | 74.3 | (71.6, 77.1) | 370 | 73.7 | (69.9, 77.6) |
| Subjects with at least one Solicited Local AE | 608 | 60.9 | (57.9, 63.9) | 306 | 61.0 | (56.7, 65.2) |
| Subjects with at least one Solicited Systemic AE | 681 | 68.2 | (65.3, 71.1) | 333 | 66.3 | (62.2, 70.5) |
| Subjects with at least one Unsolicited AE | 89 | 8.9 | (7.1, 10.7) | 53 | 10.6 | (7.9, 13.2) |
| n/% = Number / percentage of patients in the given characteristic. | ||||||
Table 3.
Solicited local and systemic reactions: ITT Population
| Pentavac (N=2994) | Tritanrix (N=1506) | |||||
|---|---|---|---|---|---|---|
| AE | E | % | 95 % CI | E | % | 95 % CI |
| Local reactions | ||||||
| Induration | ||||||
| Overall | 384 | 12.8 | 11.68–14.07 | 206 | 13.7 | 12.04–15.51 |
| Severe | 6 | 0.20 | 0.07–0.44 | 2 | 0.13 | 0.02–0.48 |
| Redness | 0.94% (0.62%– 1.35%) | |||||
| Overall | 541 | 18.1 | 16.73–19.49 | 259 | 17.2 | 15.38–19.19 |
| Severe | 7 | 0.23 | 0.09%– 0.48% | 5 | 0.33 | 0.11–0.77 |
| Swelling | ||||||
| Overall | 711 | 23.7 | 22.26–25.30 | 337 | 22.4 | 20.34–24.55 |
| Severe | 28 | 0.94 | 0.62–1.35% | 9 | 0.6 | 0.27–1.13 |
| Tenderness | ||||||
| Overall | 1074 | 35.9 | 34.17–37.61 | 506 | 33.6 | 31.26–36.02 |
| Severe | 70 | 2.34 | 1.83–2.94 | 28 | 1.86 | 1.24–2.68 |
| Systemic reactions | ||||||
| Diarrhea | ||||||
| Overall | 67 | 2.2 | 1.77–2.83 | 33 | 2.2 | 1.56–3.06 |
| Severe | 3 | 0.10 | 0.02–0.29 | 0 | 0 | 0.0–0.24 |
| Drowsiness | ||||||
| Overall | 99 | 3.3 | 2.72–4.01 | 51 | 3.4 | 2.58–4.43 |
| Severe | 1 | 0.03 | 0.0–0.19 | 0 | 0 | 0.0–0.24 |
| Fever | ||||||
| Overall | 858 | 28.7 | 27.07–30.30 | 411 | 27.3 | 25.10–29.60 |
| Severe | 12 | 0.40 | 0.21–0.70 | 5 | 0.33 | 0.11–0.77 |
| Irritability | ||||||
| Overall | 842 | 28.1 | 26.54–29.76 | 382 | 25.4 | 23.23–27.62 |
| Severe | 1 | 0.03 | 0.0–0.19 | 3 | 0.20 | 0.04–0.58 |
| Loss of appetite | ||||||
| Overall | 197 | 6.6 | 5.75–7.52 | 85 | 5.6 | 4.59–6.93 |
| Severe | 2 | 0.07 | 0.0–0.24 | 0 | 0 | 0.0–0.24 |
| Persistent Crying | ||||||
| Overall | 530 | 17.7 | 16.38–19.11 | 236 | 15.7 | 13.92–17.59 |
| Severe | 1 | 0.03 | 0.0–0.19 | 2 | 0.13 | 0.02–0.48 |
| Vomiting | ||||||
| Overall | 105 | 3.5 | 2.91–4.23 | 45 | 3.0 | 2.24–3.97 |
| Severe | 3 | 0.2 | 0.02–0.29 | 1 | 0.2 | 0.0–0.37 |
E – Events
* - Using Binomial proportion test.
Table 4.
Dose-wise solicited reactions: ITT Population
| Pentavac | Tritanrix-HB+Hib | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse event | Severity | Dose 1 (N=998) | Dose 2 (N=998) | Dose 3 (N=998) | Overall (N=2994) | Dose 1 (N=502) | Dose 2 (N=502) | Dose 3 (N=502) | Overall (N=1506 ) |
| Local reactions | |||||||||
| Induration | Any | 154 (15.4%) | 116 (11.6 %) | 114 (11.4 %) | 384 (12.8 %) | 85 (16.9 %) | 67 (13.3 %) | 54 (10.8 %) | 206 (13.7 %) |
| Severe | 4 (0.4 %) | 1 (0.1 %) | 1 (0.1 %) | 6 (0.2 %) | 1 (0.2 %) | 1 (0.2 %) | 0 (0 %) | 2 (0.1 %) | |
| Redness | Any | 235 (23.5%) | 163 (16.3 %) | 143 (14.3 %) | 541 (18.1 %) | 114 (22.7 %) | 79 (15.7 %) | 66 (13.1 %) | 259 (17.2 %) |
| Severe | 5 (0.5 %) | 2 (0.2 %) | 0 (0 %) | 7 (0.2 %) | 2 (0.4 %) | 1 (0.2 %) | 2 (0.4 %) | 5 (0.3 %) | |
| Swelling | Any | 318 (31.9%) | 219 (21.9 %) | 174 (17.4 %) | 711 (23.7 %) | 152 (30.3 %) | 107 (21.3 %) | 78 (15.5 %) | 337 (22.4 %) |
| Severe | 17 (1.7 %) | 6 (0.6 %) | 5 (0.5 %) | 28 (0.9 %) | 5 (1.0 %) | 2 (0.4 %) | 2 (0.4 %) | 9 (0.6 %) | |
| Tenderness | Any | 425 (42.6%) | 345 (34.6 %) | 304 (30.5 %) | 1074 (35.9 %) | 206 (41.0 %) | 167 (33.3 %) | 133 (26.5 %) | 506 (33.6 %) |
| Severe | 44 (4.4 %) | 16 (1.6 %) | 10 (1.0 %) | 70 (2.3 %) | 13 (2.6 %) | 7 (1.4 %) | 8 (1.6 %) | 28 (1.9 %) | |
| Systemic reactions | |||||||||
| Diarrhea | Any | 24 (2.4 %) | 27 (2.7 %) | 16 (1.6 %) | 67 (2.2 %) | 15 (3.0 %) | 10 (2.0 %) | 8 (1.6 %) | 33 (2.2 %) |
| Severe | 2 (0.2 %) | 1 (0.1 %) | 0 (0 %) | 3 (0.1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Drowsiness | Any | 42 (4.2 %) | 30 (3.0 %) | 27 (2.7 %) | 99 (3.3 %) | 22 (4.4 %) | 16 (3.2 %) | 13 (2.6 %) | 51 (3.4 %) |
| Severe | 0 (0 %) | 1 (0.1 %) | 0 (0 %) | 1 (0.03 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Fever | Any | 175 (17.5 %) | 143 (14.3 %) | 101 (10.1 %) | 419 (14.0 %) | 78 (15.5 %) | 50 (10.0 %) | 41 (8.2 %) | 169 (11.2 %) |
| Severe | 3 (0.3 %) | 6 (0.6 %) | 3 (0.3 %) | 12 (0.4 %) | 3 (0.6 %) | 2 (0.4 %) | 0 (0 %) | 5 (0.3 %) | |
| Irritability | Any | 338 (33.9 %) | 253 (25.4 %) | 251 (25.2 %) | 842 (28.1 %) | 158 (31.5 %) | 113 (22.5 %) | 111 (22.1 %) | 382 (25.4 %) |
| Severe | 0 (0 %) | 1 (0.1 %) | 0 (0 %) | 1 (0.03 %) | 2 (0.4 %) | 0 (0 %) | 1 (0.2 %) | 3 (0.2 %) | |
| Loss of appetite | Any | 84 (8.4 %) | 60 (6.0 %) | 53 (5.3 %) | 197 (6.6 %) | 42 (8.4 %) | 25 (5.0 %) | 18 (3.6 %) | 85 (5.6 %) |
| Severe | 0 (0 %) | 1 (0.1 %) | 1 (0.1 %) | 2 (0.1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Persistent crying | Any | 227 (22.7 %) | 162 (16.2 %) | 141 (14.1 %) | 530 (17.7 %) | 104 (20.7 %) | 74 (14.7 %) | 58 (11.6 %) | 236 (15.7 %) |
| Severe | 0 (0 %) | 0 (0 %) | 1 (0.1 %) | 1 (0.03 %) | 1 (0.2 %) | 0 (0 %) | 1 (0.2 %) | 2 (0.1 %) | |
| Vomiting | Any | 37 (3.7 %) | 38 (3.8 %) | 30 (3.0 %) | 105 ( %) | 22 (4.4 %) | 13 (2.6 %) | 10 (2.0 %) | 45 (3.0 %) |
| Severe | 0 (0 %) | 2 (0.2 %) | 1 (0.1 %) | 3 (3.5 %) | 0 (0 %) | 0 (0 %) | 1 (0.2 %) | 1 (0.1 %) | |
Table 5.
Summary of occurrence of unsolicited adverse events—Overall: ITT Population
| Pentavac (N=2994) | TRITANRIX-HB-HIB (N=1506) | |||
|---|---|---|---|---|
| Preferred Term | E | % | E | % |
| Dyspnoea | 0 | 0 | 1 | 0.07 |
| Ear pain | 1 | 0.03 | 0 | 0 |
| Otorrhoea | 2 | 0.06 | 2 | 0.13 |
| Conjunctivitis | 0 | 0 | 1 | 0.07 |
| Lacrimation increased | 2 | 0.06 | 0 | 0 |
| Abdominal pain | 1 | 0.03 | 0 | 0 |
| Aphthous stomatitis | 0 | 0 | 1 | 0.07 |
| Diarrhea | 24 | 0.80 | 14 | 0.93 |
| Dyspepsia | 0 | 0 | 1 | 0.07 |
| Flatulence | 0 | 0 | 1 | 0.07 |
| Mouth ulceration | 2 | 0.06 | 0 | 0 |
| Vomiting | 7 | 0.23 | 3 | 0.20 |
| Induration | 1 | 0.03 | 0 | 0 |
| Influenza like illness | 5 | 0.17 | 3 | 0.20 |
| Irritability | 1 | 0.03 | 1 | 0.07 |
| Nodule | 1 | 0.03 | 0 | 0 |
| Pyrexia | 17 | 0.57 | 8 | 0.53 |
| Swelling | 3 | 0.10 | 0 | 0 |
| Tenderness | 2 | 0.06 | 0 | 0 |
| Bronchopneumonia | 0 | 0 | 1 | 0.07 |
| Pneumonia | 0 | 0 | 1 | 0.07 |
| Respiratory tract infection | 1 | 0.03 | 0 | 0 |
| Decreased appetite | 2 | 0.06 | 0 | 0 |
| Hypocalcaemic seizure | 1 | 0.03 | 0 | 0 |
| Crying | 2 | 0.06 | 1 | 0.07 |
| Hypoglycaemic seizure | 1 | 0.03 | 0 | 0 |
| Infantile colic | 1 | 0.03 | 0 | 0 |
| Cough | 13 | 0.43 | 15 | 1.0 |
| Nasopharyngitis | 48 | 1.60 | 30 | 1.99 |
| Rhinitis | 3 | 0.10 | 1 | 0.07 |
| Rhinorrhoea | 4 | 0.13 | 3 | 0.20 |
| Sneezing | 0 | 0 | 1 | 0.07 |
| Upper respiratory tract infection | 2 | 0.06 | 1 | 0.07 |
| Erythema | 1 | 0.03 | 0 | 0 |
| Rash | 0 | 0 | 1 | 0.07 |
| Rash papular | 0 | 0 | 1 | 0.07 |
| Varicella | 1 | 0.03 | 0 | 0 |
Table 1.
Summary of subject's demographic characteristics at enrollment
| Variable | Statistics | Pentavac N=(998) | Tritanrix-HB+Hib N=(502) |
|---|---|---|---|
| Age (in weeks) | Mean | 7.14 | 7.07 |
| SD | 0.69 | 0.66 | |
| Males | N (%) | 562 ( 56.31 ) | 271 ( 53.98 ) |
| Height (cm) | Mean | 54.10 | 53.94 |
| SD | 2.72 | 2.72 | |
| Weight (kg) | Mean | 4.09 | 4.06 |
| SD | 0.65 | 0.66 |
Safety results
Overall, similar number of subjects experienced at least one adverse event, solicited local and systemic reactions, and unsolicited adverse events in both the groups (Table 2).
Solicited local reactions
Tenderness (35.9 % in Pentavac group and 33.6 % in Tritanrix-HB+Hib group) was the commonest local reaction followed by swelling, redness and induration. Overall incidence of the reactions is compared between 2 groups in the Table 3. No significant difference was found between the groups. Most of these were mild and resolved within 3 d. All reactions resolved without any sequelae. The dose-wise incidence of reactions are presented in Table 4. In general, the reactions reduced in frequency as the doses advanced.
Solicited systemic reactions
Irritability, persistent crying and fever were common reactions in both groups. Other reactions were loss of appetite, drowsiness, vomiting and diarrhea. The incidence was comparable between the groups (Table 3). Most of the reactions were mild and resolved within 3 d without any sequelae. Like local reactions, the incidence reduced in frequency as the doses advanced (Table 4).
Unsolicited adverse events
A total of 241 unsolicited adverse events were reported. The commonest among them were diarrhea, fever and nasopharyingitis. Most of them occurred beyond 7 d after vaccination and resolved within 3 d without any sequelae. Most (75 % in Pentavac group and 71 % in Tritanrix-HB+Hib group) were not caused by the study vaccines (Table 5).
Out of 149 events reported in Pentavac group, 3 events (vomiting, fever and irritability) and 5 events (induration, 2 events of each swelling and tenderness) respectively were assessed as possibly and probably related to study vaccine. Out of 92 events reported in Tritanrix-HB+Hib group, one event of sneezing was possibly related while one event of rash was very likely related to the vaccine (Table 5).
Serious adverse events
A total of 6 SAEs (4 in Pentavac group [0.4%] and 2 in Tritanrix-HB+Hib Group [0.4%]) were reported during the study. These included hospitalizations due to bronchopneumonia and acute febrile illness (Tritanrix-HB+Hib Group); and due to hypocalcemic convulsion, hypoglycemic convulsion, and 2 cases of diarrhea (Pentavac group). The infant with bronchopneumonia from the Tritanrix-HB+Hib Group died. All SAEs were unrelated to study vaccination and all but one recovered uneventfully.
The per protocol (PP) analysis for solicited local and systemic reactions, unsolicited events and SAEs showed a similar pattern as in ITT population.
Discussion
Our study found that the new vaccine had a comparable tolerability profile as the licensed vaccine. In the Pentavac group, 74.3% infants reported at least one AE compared to 73.7 % in Tritanrix-HB+Hib group. The incidence of solicited injection site reactions and systemic reactions was similar in both the groups. Most of these reactions were mild and transient and are expected reactions with whole cell pertussis containing vaccines.
The WHO position paper on pertussis vaccine says that immunization with whole cell pertussis vaccines is frequently (10–50%) associated with minor adverse reactions such as local redness and swelling, fever and agitation.8 Adverse events following immunization against hepatitis B are known to be infrequent and generally mild.9 As regards Hib vaccine, the WHO position paper says that when the Hib vaccine is given at the same time as DTP, the rate of fever or irritability, or both, is no higher than when DTP is given alone.10
A recent Cochrane review comparing combined DTP-HepB-Hib vaccine with separately administered DTP-HepB and Hib vaccines observed that minor adverse events such as pain and redness were more common in children given the combined vaccine; however overall, the direction was in favor of the DTPw-HB-Hib vaccine rather than the DTPa-HB-Hib vaccine when compared to the separate vaccines.11
Our results are also in line with those reported with other DTwP-HepB-Hib vaccine studies. These include a study in Turkey on Tritanrix-HB+Hib,12 a study in India on EasyFive13 and one more study in India14 on Shan5™, EasyFive™ and Tritanrix-HB™ + Hiberix™. Our study showed a reducing frequency of adverse events with the subsequent doses which was also seen in the EasyFive study.13
Though there were 6 SAEs reported in the study, none of them were caused by the vaccine. Among them was also one death due to bronchopneumonia. In the state of Maharashtra, the post-neonatal infant mortality was 5.7 per 1000 in 2005–2006.14 This means that in our study with 3 months follow up, perhaps 3–4 deaths were expected. However, we had only one death, thus indicating a healthy vaccinee effect. This finding is important considering that DTwP-HB-Hib vaccines are now part of EPI in more than 120 countries in the world.15
As per WHO, prolonged crying with whole cell pertussis vaccines occurs in less than 1% of cases.8 In our study, the incidence of crying was much higher. The possible reason for this could be that the parents captured all crying episodes irrespective of their durations and hence, more events were reported. Of course, the incidence of severe crying was less than 1% in both the groups in our study.
Acellular pertussis vaccines are used in developed countries because of their lower reactogenicity as compared to whole cell vaccines. However, the recent pertussis outbreaks in highly vaccinated populations in such countries have demonstrated lower efficacy of the acellular vaccine than whole cell vaccines, thus offsetting their relative safety.16-18 The findings of our study are relevant in this context since it has shown that both the vaccines did not cause any SAE.
Ten medical colleges participated in the study. This multicentric nature is strength of the study. Moreover, the compliance to the study protocol was good. The protocol violations were minimal (3.3 %). Only 5% were lost to follow-up, fewer than usually reported in similar studies. The open label design was a limitation of our study. Since the containers of both vaccines were different, it was not possible to blind the investigators as well as parents. However, the study procedures ensured that this did not affect the collection of data in an unbiased manner. As safety was the objective of this study, immunogenicity of the vaccines could not be assessed.
To conclude, the new DTwP-HepB+Hib vaccine was found safe and tolerable in a large population of Indian infants when administered as primary immunization. Given the public health benefits of the pentavalent combination, the vaccine should be widely used in immunization programs.
Materials and Methods
Study design
This was a Phase IV, open-label, randomized, parallel group, multicentre clinical study conducted in 1510 healthy Indian infants. The study was conducted from December 22, 2009 to January 31, 2011 at the immunization clinics of 10 medical colleges from 10 different cities across the state of Maharashtra. The Maharashtra University of Health Sciences (MUHS), which governs all the medical colleges in Maharashtra, was actively involved in the organization and supervision of the study.
DTwP vaccine is routinely given at 6, 10, and 14 weeks of age along with oral polio vaccine (OPV). The infants who attended immunization clinics for their first dose of DTP vaccine were considered for this study. Such parents were informed about the study and those who were willing participated in the study.
Study subjects
Normal healthy infants of age 6 to 8 weeks whose parents/legal guardians gave written informed consent were included in the study. Infants participating in other clinical trials, infants with immunodeficiency, malignancy, history of allergy, chronic illness, receipt of blood products, bleeding disorder and history of any neurological disorder were excluded from the study. History of acute febrile illness was a temporary exclusion criterion.
Study vaccines
SIIL DTwP-HepB+Hib vaccine (Pentavac, Serum Institute of India Ltd) is composed of Hib vaccine as a freeze-dried powder and DTPw-HB vaccine as a suspension. Freeze dried powder of Hib is reconstituted by using liquid DTPw-HB as a diluent. On reconstitution, each dose of 0.5 ml contains diphtheria toxoid (20 Lf to 30 Lf), tetanus toxoid (5 Lf to 25 Lf), B. Pertussis (4 International Units, IU), recombinant HBsAg (10 mg), purified Hib capsular polysaccharide (10 mg) conjugated with tetanus toxoid (19 to 33 mg), adsorbed on aluminum phosphate, AL+++ 1.25 mg with thiomersal 0.005 % as preservative. The pertussis component is whole cell B. pertussis inactivated by heat. Hepatitis B component is obtained by culturing genetically engineered Hansenula polymorpha yeast cells having the surface antigen gene of the Hepatitis B virus. The Hib polysaccharide is prepared from capsular polysaccharide of H. influenzae type b strain and after activation is coupled to Tetanus Toxoid. Batch numbers 123S008 (Expiry August 2010), 123S9006 (Expiry October 2010) and 123S0006 (Expiry January 2012) were used after release by the Central Drugs Laboratory, Kasauli.
Tritanrix-HB+Hib (GlaxoSmithKline Beecham) is comprised of Tritanrix-HB suspension and Hiberix dried pellet, which are combined just prior to use. Each 0.5 mL dose of reconstituted vaccine contains not less than 30 International Units (IU) of adsorbed diphtheria toxoid, not less than 60 IU of adsorbed tetanus toxoid, not less than 4 IU of whole cell pertussis, 10 μg of recombinant HBsAg protein and 10 μg of purified capsular polysaccharide covalently bound to approximately 20–40 μg tetanus toxoid. The diphtheria and tetanus toxoids are prepared from the toxins of cultures of Corynebacterium diphtheriae and Clostridium tetani by formalin inactivation. The wP component is obtained by heat inactivation of phase I culture of Bordetella pertussis bacteria. The surface antigen of the HBV (HBsAg) is produced by culture of genetically-engineered yeast cells (Saccharomyces cerevisiae) which carry the gene coding for the major surface antigen of the HBV. The Hib polysaccharide is prepared from H. influenzae type b, and is coupled to tetanus toxoid. The vaccine excipients include aluminum hydroxide, aluminum phosphate, 2-phenoxyethanol, polysorbate 20, sodium chloride, thiomersal and water for injection.
Tritanrix-HB batches used were AT15B615BA (expiry August 2011) and AT15B583AH (Expiry November 2010). Hiberix batches used were XHIBB978H1 (Expiry December 2010), XHIBB971A1 (Expiry December 2010), XHIBB817A1 (Expiry May 2010) and XHIBC013A2 (Expiry February 2011).
Study procedures
Eligible subjects were randomized (2:1) to receive 0.5 ml of either SIIL DTwP-HepB+Hib combination vaccine or Tritanrix-HB+Hib. A randomization blocking scheme (2:1) was used. Each Block was of 6 subjects. The statistician generated site-specific randomization lists. The vaccines were administered deep intramuscularly in the right anterolateral thigh at 6, 10, and 14 weeks of age along with OPV. The subjects visited study clinics 4 times during their participation; at 6, 10, 14, and 18 weeks of age. For each visit, a window of + 1 week was allowed.
Parents/legal guardians were trained for documenting solicited adverse reactions, unsolicited adverse events and serious adverse events (SAE) in a structured diary provided to them. They were also provided with measuring scales and thermometers to measure local reactions and temperature, respectively. Prophylactic treatment for potential adverse reactions, including fever, was not allowed.
Solicited adverse reactions were collected within 3 d of administration of each vaccine dose. Solicited reactions occurring beyond 3 d after vaccination, including solicited reactions continuing beyond day 3, were captured in the diary as unsolicited adverse events. The unsolicited adverse events and serious adverse events were collected throughout the participation of subject in the study. At each visit, the subjects were physically examined and parents were asked for adverse events and concomitant medication.
Any missing entries in the diary were filled by the parents/guardians during the visit. Diaries were collected and transcribed into case record forms on the second, third and fourth visits to the study sites. Parents were also asked to report any SAE immediately to the investigator.
All adverse events were graded as mild, moderate and severe based on pre-specified definitions. All solicited local and systemic reactions were assumed to be caused by study vaccines. The causality assessment of all unsolicited adverse events was done on the basis of clinical judgment of the investigators. The causality association was classified as Very likely/Certain, Probable, Possible, Unlikely, Unrelated, and Unclassifiable.
Statistical analyses
The sample size of the study was adequate to detect events with ≥ 1% frequency. At a margin of 30%, the study had more than 80% power to demonstrate non-inferiority of SIIL vaccine as compared to GSK vaccine with respect to severe solicited reactions. Gender distribution was expressed in percentage (%). Age was expressed in mean and SD. The percentage along with 95% confidence interval (CI) of solicited and unsolicited adverse events in both the study groups were calculated. Both intention to treat (ITT) and per protocol (PP) analyses were conducted, however only ITT analysis is presented here as this is more important for safety analysis. All subjects who had received at least one dose of study medication and with minimum age of 6 weeks at the time of first dose of vaccine were included in ITT population. The solicited reactions were compared between groups by binomial proportion test.
Ethical consideration
The study was conducted as per the declaration of Helsinki, Good Clinical Practices guidelines and Indian regulatory and ethical guidelines. The MUHS Ethics Committee, Nasik, Ethics Committee of Serum Institute of India Research Foundation (SIIRF), Pune and Institutional Ethics Committees of all the study sites approved the study. Informed consent forms were used in local languages to obtain consent from the parents. The consent of illiterate parents was attested by an impartial literate witness. The study was independently monitored to assure the quality of data generated throughout all the study sites.
Disclosure of Potential Conflicts of Interest
Prasad S Kulkarni, Prajakt Barde and Somnath Mangrule are employed by Serum Institute of India Ltd., which is the manufacturer of the study vaccine.
Funding
The study was funded by the Serum Institute of India Research Foundation, Pune.
References
- 1. Sokhey J, Kim-Farley RJ, Bhargava I. The expanded programme on immunization: A decade of progress in India. Ann Trop Paediatr 1989 Mar; 9(1):24-9 [DOI] [PubMed] [Google Scholar]
- 2. Dutta S. An overview of molecular epidemiology of hepatitis B virus (HBV) in India. Virol J 2008; 5:156; PMID:19099581; http://dx.doi.org/ 10.1186/1743-422X-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watt JP, Wolfson LJ, O'Brien KL, Henkel E, Deloria-knol M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: Global estimates. Lancet 2009; 374: 903-11; PMID:19748399; http://dx.doi.org/ 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 4. Subcommittee of NTAGI . NTAGI subcommittee recommendations on Haemophilus influenzae type b (Hib) vaccine introduction in India. Indian Pediatr 2009;46:945-54; PMID:19955578. [PubMed] [Google Scholar]
- 5. Combination Vaccines for Childhood Immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). Pediatrics 1999; 103:1064-1077; PMID:10224194; http://dx.doi.org/ 10.1542/peds.103.5.1064. [DOI] [PubMed] [Google Scholar]
- 6. Faingezicht I, Avila-Aguerro ML, Cervantes Y, Fourneau M, Clemens SA. Primary and booster vaccination with DTPw-HB/Hib combination vaccine in Costa Rican children who had received a birth dose of hepatitis B vaccine. Rev Panam Salud Publica 2002; 12(4):247-57; PMID:12431356; http://dx.doi.org/ 10.1590/S1020-498920020010-00005. [DOI] [PubMed] [Google Scholar]
- 7. Hla KH, Thein SA, Aye A, Han HH, Bock HL, David MP, Schuerman L. Reactogenicity and immunogenicity profiles of a novel DTwP-HepB+Hib combination diphtheria-tetanus-whole cell pertussis-hepatitis B and Haemophilus influenzae type B vaccine: A randomized dose ranging trial of the Hib tetanus-conjugate content. Pediatr Infect Dis J 2006; 25(8):706-12; PMID:16874170; http://dx.doi.org/ 10.1097/01.inf.0000223488.80814.df. [DOI] [PubMed] [Google Scholar]
- 8. Pertussis vaccines—WHO position paper. Wkly Epidemiol Rec 2005 Jan 28;80(4):31-9 [PubMed] [Google Scholar]
- 9. Hepatitis B vaccines. Wkly Epidemiol Rec 2009 Oct 1; 84(40):405-19 [PubMed] [Google Scholar]
- 10. Haemophilus influenzae type b (Hib) Vaccination Position Paper—July 2013. Wkly Epidemiol Rec 2013 Sep 27; 88(39):413-26 [PubMed] [Google Scholar]
- 11. Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev 2012 Apr 18; 4:CD005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanra G, Kara A, Demiralp O, Contorni M, Hilbert AK, Spyr C. Safety and immunogenicity of a new fully liquid DTPw-HepB-Hib comination vaccine in infants. Hum Vaccines Jul–Aug 2006; 2(4):155-60; http://dx.doi.org/ 10.4161/hv.2.4.2942. [DOI] [PubMed] [Google Scholar]
- 13. Ali SS, Chandrashekar SR, Singh M, Bansal RK, Sharma DR, Arora D. A multicenter, prospective, open-label, non-comparative study to evaluate the immunogenicity and tolerance of a new, fully liquid DTwP-HepB+Hib combination vaccine (DTwP-HepB-Hib vaccine). Hum Vaccines Jul–Aug 2007;3(4):116-21; http://dx.doi.org/ 10.4161/hv.3.4.4061. [DOI] [PubMed] [Google Scholar]
- 14. Rao R, Dhingra MS, Bavdekar S, et al. A comparison of immunogenicity and safety of indigenously developed liquid (DTwPHB-Hib) DTwP-HepB+Hib combination vaccine (Shan 5) with Easyfive (liq) and TritanrixHB + Hiberix (lyo) in Indian infants administered according to the EPI schedule. Hum Vaccines Jun 2009;5(6):425-9; http://dx.doi.org/ 10.4161/hv.5.6.7816. [DOI] [PubMed] [Google Scholar]
- 15. National Family Health Survey (NFHS-3), 2005–06, India, Volume 1, International Institute for Population Sciences (IIPS) and Macro International, Mumbai, 2007. http://apps.who.int/immunization_monitoring/globalsummary/schedules?sc[r][]=AFRO&sc[r][]=AMRO&sc[r][]=EMRO&sc[r][]=EURO&sc[r][]=SEARO&sc[r][]=WPRO&sc[d]=&sc[v][]=DTWPHIBHEP&sc[v][]=DTWPHIBHEPB&sc[OK]=OK. Accessed on 03 February 2014. [Google Scholar]
- 16. Theofiles AG, Cunningham SA, Chia N, Jeraldo PR, Quest DJ, Mandrekar JN, Patel R. Pertussis outbreak, southeastern Minnesota, 2012. Mayo Clin Proc 2014 Oct; 89(10):1378-88; http://dx.doi.org/ 10.1016/j.mayocp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 2013 Jun; 131(6):e1716-22; http://dx.doi.org/ 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 18. Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 2013 May;56(9):1248-54; http://dx.doi.org/ 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]