Abstract
High-dose inactivated, influenza vaccine was licensed by the FDA in December 2009 for adults aged 65 y and older. The ACIP did not issue or state a preference for a specific vaccine in the elderly population. The extent of its on-label and off-label use is unknown. Using the MarketScan Commercial Claims and Encounters and the Medicare Supplemental database, we identified individuals who received the high-dose influenza vaccine or the standard, seasonal trivalent influenza vaccine between January 1, 2010 and December 31, 2012. For people aged ≥65 y, we used multivariable regression to assess the association between patient and provider level variables and high-dose influenza vaccine versus standard influenza vaccine. We characterized all off-label high-dose vaccine administered to people younger than 65 y of age, and investigated whether sicker patients were targeted for off-label use by examining the association between various comorbid conditions and receipt of the high-dose vaccine among adults aged 18–64. Among patients aged ≥65 y who received an influenza vaccine, 18.4% received the high-dose vaccine. Uptake was minimal in 2010, but 25% and 32% of influenza shots were the high-dose formulation in 2011 and 2012, respectively. Almost 27,000 seniors received a second high-dose vaccine with a median of 368 d (IQR: 350–387 days) between doses. Older age, family practice physicians, and having PPO insurance were positively associated with receiving high-dose vaccine. There were 36,624 off-label high-dose vaccines administered. Half of the patients receiving off-label doses were aged 50–64. Adults aged 18–64 y receiving high-dose vaccine were more likely to have chronic comorbidities than people receiving standard influenza vaccine; however, there was not one specific illness that seemed to be targeted by physicians. In the first 3 y since licensure, use of the high-dose vaccine among seniors has been limited. The safety of this vaccine should be monitored closely among 2 groups of people - seniors receiving repeat doses and people <65.
Keywords: adult vaccination, high-dose influenza vaccine, influenza vaccine, influenza prevention
Abbreviations
- ACIP
Advisory Committee on Immunization Practices
- CPT
Current Procedural Terminology
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
Interquartile range
- HMO
Health Maintenance Organization
- PPO
Preferred provider organization
Introduction
Each year, it is estimated that on average 36,000 people die from influenza in the United States, with a majority occurring in the elderly population.1 It is becoming increasingly clear that elderly people have a decreased immune response to standard influenza vaccines as compared to healthy adults, most likely due to immune senescence.2,3 In December 2009, a new high-dose influenza vaccine was licensed for elderly people aged 65 y and older.4 This vaccine contains the same 3 strains as standard seasonal influenza vaccines, but has 4 times as much antigen (60 μg per strain compared to 15 μg per strain for the standard vaccine). Although small efficacy studies have been conducted and show increased immune response after administration of the high-dose vaccine,5–9 no population-based effectiveness studies have been performed. Results from a post-licensure randomized controlled efficacy trial including ∼32,000 patients suggested that the high-dose vaccine was 24.2 % (95% CI: 9.7%, 36.5%) more effective in preventing influenza than the standard vaccine.10 However, these results have not been formally published, and the recommendation for the 2013–2014 influenza season by the Advisory Committee on Immunization Practices (ACIP) remained the same as previous years – that there was no preference between the high-dose and standard, seasonal vaccines for the elderly aged ≥65 y.11 Therefore, the decision is left to the physician/provider as to which vaccine to administer. As the market for different types and formulations of influenza vaccines is increasing each year, it is important to understand administration patterns for both on-label use among people aged ≥65 y and off-label use among people aged <65 y. Understanding the uptake of this vaccine will also encourage further monitoring of safety and effectiveness in the populations that are using it.
We use the first 3 y of post-licensure data from a large segment of commercially insured adults and from elderly patients with employee sponsored Medicare supplemental insurance to study the uptake of high-dose influenza vaccine. We investigate individual and provider level predictors of on-label use and characterize comorbidity patterns among patients who receive off-label vaccine.
Results
Among the 1,208,173 patients ≥65 y who received an influenza vaccine between 2010 and 2012, 221,853 (18.4%) received the high-dose vaccine. Uptake was minimal in 2010 (5.6% of all influenza shots administered between August and December), but 25% and 32% of influenza shots were the high-dose formulation in 2011 and 2012, respectively (Fig. 1). Fifteen percent of seniors meeting inclusion criteria and who received an initial high-dose vaccine had a second high dose vaccine (N = 26,857) with a median of 368 d (IQR: 350–387 days) between doses, indicating that most of these repeat vaccinees received one high dose vaccine per influenza season. Patients 75 y or older were more likely to receive the high-dose vaccine, while males, patients with HIV and patients with liver disease were less likely to receive the vaccine. There was some indication that the high-dose vaccine was being used more frequently in patients with past comorbidities (i.e. transplant patients, stroke, pneumonia); however, these effects were very small (Table 1). All other comorbidities were not strongly associated with high-dose vaccine. These relationships were similar when using a 6-month window and an 8-month window to assess comorbidities (Appendix 2). Provider level characteristics that were associated with high-dose vaccine included provider type and insurance type (Table 1). Family practice physicians, pharmacists and nurses were more likely to administer the vaccine compared to internal medicine physicians, and patients with health maintenance organization (HMO) insurance were less likely to receive high-dose vaccine compared with preferred provider organization (PPO) insurance. Finally, there may be some indication of regional variation, with the North Central and the South regions being more likely to administer the high-dose vaccine.
Figure 1.
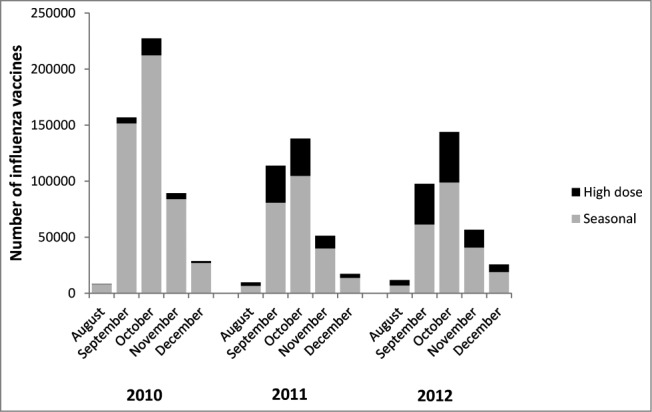
Doses of influenza vaccine administered to people aged ≥65 y by month and vaccine type, 2010–2012.
Table 1.
Predictors of high-dose influenza vaccine use for eligible seniors 65 y and over, 2010–2012
| High dose vaccine, N | % | Crude OR (95% CI) | Multivariable adjusted* OR (95% CI) | |
|---|---|---|---|---|
| Total vaccinated | 212,030 | 18.6 | — | — |
| Age | ||||
| 65–74 | 108,285 | 51.0 | Reference | Reference |
| 75+ | 103,745 | 48.9 | 1.19 (1.18, 1.21) | 1.27 (1.26, 1.29) |
| Male sex | 93,851 | 44.3 | 0.94 (0.93, 0.95) | 0.94 (0.93, 0.95) |
| Comorbidities | ||||
| Hypertension | 130,062 | 61.3 | 1.04 (1.03, 1.05) | 1.09 (1.08, 1.10) |
| Infection | 81,375 | 38.4 | 1.04 (1.03, 1.05) | 1.08 (1.07, 1.10) |
| IHD | 77,029 | 36.3 | 1.03 (1.02, 1.04) | 1.07 (1.06, 1.09) |
| Diabetes | 59,109 | 27.9 | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| COPD | 30,796 | 14.5 | 0.98 (0.97, 0.99) | 1.02 (1.00, 1.03) |
| Cancer | 28,515 | 13.5 | 0.97 (0.96, 0.99) | 1.05 (1.03, 1.07) |
| Autoimmune | 12,194 | 5.8 | 1.01 (0.99, 1.03) | 1.06 (1.04, 1.08) |
| Stroke | 11,428 | 5.4 | 1.06 (1.04, 1.08) | 1.09 (1.06, 1.11) |
| Pneumonia | 5,665 | 2.7 | 1.05 (1.02, 1.08) | 1.02 (0.99, 1.06) |
| Transplant | 3,104 | 1.5 | 1.09 (1.05, 1.14) | 1.08 (1.03, 1.13) |
| Liver disease | 2,169 | 1.0 | 0.85 (0.81, 0.89) | 0.94 (0.89, 0.99) |
| MI | 1,839 | 0.9 | 1.00 (0.95, 1.05) | 0.97 (0.91, 1.02) |
| Sepsis | 1,451 | 0.7 | 0.90 (0.86, 0.96) | 0.87 (0.83, 0.95) |
| HIV | 80 | 0.04 | 0.36 (0.29, 0.45) | 0.49 (0.38, 0.63) |
| Hospitalization | 18,895 | 8.9 | 1.05 (1.04, 1.07) | 1.02 (1.00, 1.04) |
| Pneumococcal vaccine | 18,395 | 8.7 | 1.11 (1.09, 1.12) | 1.08 (1.06, 1.10) |
| Provider type | ||||
| Internal medicine | 58,260 | 30.3 | Reference | Reference |
| Family practice | 93,386 | 48.5 | 1.81 (1.79, 1.83) | 1.64 (1.62, 1.66) |
| Specialist | 14,241 | 7.4 | 0.78 (0.76, 0.79) | 0.81 (0.80, 0.83) |
| Pharmacist | 3,321 | 1.7 | 1.65 (1.58, 1.72) | 1.72 (1.65, 1.79) |
| Nurse | 2,123 | 1.1 | 1.52 (1.45, 1.59) | 1.19 (1.13, 1.25) |
| Hospital | 8,059 | 4.2 | 1.00 (0.98, 1.03) | 0.84 (0.81, 0.86) |
| Other | 13,223 | 6.9 | 0.74 (0.72, 0.75) | 0.69 (0.68, 0.71) |
| Medicare Plan type | ||||
| PPO | 97,506 | 47.3 | Reference | Reference |
| Comprehensive | 65,858 | 32.0 | 1.38 (1.36, 1.40) | 1.59 (1.57, 1.61) |
| HMO | 33,747 | 16.4 | 0.52 (0.52, 0.53) | 0.62 (0.61, 0.63) |
| Other | 8,938 | 4.3 | 0.80 (0.78, 0.81) | 1.04 (1.01, 1.07) |
| Region | ||||
| Northeast | 39,902 | 18.8 | Reference | Reference |
| North central | 75,171 | 35.5 | 2.29 (2.26, 2.32) | 1.87 (1.84, 1.90) |
| South | 59,853 | 28.2 | 1.91 (1.88, 1.94) | 1.84 (1.81, 1.87) |
| West | 33,867 | 16.0 | 1.03 (1.01, 1.05) | 1.09 (1.08, 1.11) |
| Unknown | 3,237 | 1.5 | 1.78 (1.71, 1.85) | 1.40 (1.35, 1.47) |
| Rurality | ||||
| Metropolitan | 22,905 | 10.8 | Reference | — |
| Urban, nonmetro | 2,899 | 1.4 | 0.90 (0.86, 0.93) | — |
| Rural | 340 | 0.2 | 1.09 (0.98, 1.22) | — |
| Missing | 185,886 | 87.7 |
IHD = Ischemic heart disease; COPD = Chronic obstructive pulmonary disease; MI = Myocardial infarction; HIV = Human immunodeficiency virus.
*Adjusted for month of vaccine receipt and all other variables in the table except for rurality
**Rurality was assessed in 2010 only. All other variables were measured in all years.
There were 36,624 doses of off-label high-dose vaccine administered to patients less than 65 y of age. Off-label administration increased 49% from 2010 to 2011, but was slightly less in 2012 (Fig. 2). Among patients with 4 months of enrollment prior to vaccination (N = 34,497), the majority of off-label doses were given to adults aged 51–64 (Table 2). However; 7.3% of off-label doses were given to children. There were no clear comorbidity patterns among off-label vaccinees – only 26% had some immune-compromising or chronic condition. Other comorbidities that were the most common among off-label recipients were hypertension and diabetes (Table 2). When compared to adults aged 18–64 who received the standard seasonal vaccine, high-dose recipients were more likely to have an immune-compromising condition (23% more likely), heart disease (30%), chronic lung disease (25%), diabetes (28%) and hypertension (17%) (Table 3). Family practice physicians, pharmacists and nurses were much less likely to administer the high-dose vaccine compared to internal medicine physicians (Table 3).
Figure 2.
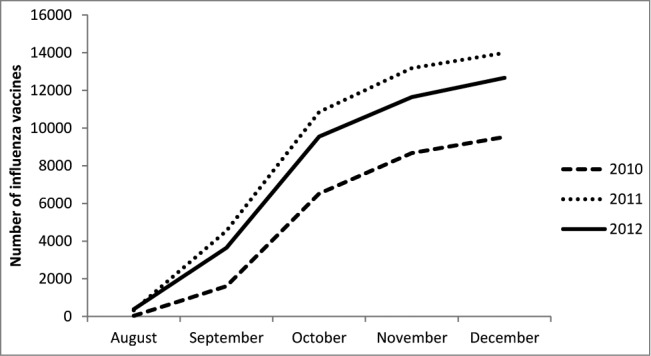
Cumulative number of off-label doses of high dose influenza vaccine administered by month, 2010–2012.
Table 2.
Characteristics of patients meeting enrollment eligibility and receiving off-label high dose vaccine, 2010–2012
| N | % | |
|---|---|---|
| Total | 34,497 | |
| Age | ||
| 0–18 | 2,508 | 7.3 |
| 19–35 | 5,373 | 15.6 |
| 36–50 | 8,870 | 25.7 |
| 51–64 | 17,746 | 51.4 |
| Male sex | 15,493 | 44.9 |
| Physician type | ||
| Family practice doctor | 9,697 | 28.1 |
| Internal medicine doctor | 8,229 | 23.9 |
| Other | 5,832 | 16.9 |
| Specialist | 5,212 | 15.1 |
| Hospital doctor | 493 | 1.4 |
| Nurse | 222 | 0.6 |
| Pharmacist | 154 | 0.5 |
| Unknown | 4,658 | 13.5 |
| Region | ||
| Northeast | 5,930 | 17.2 |
| North central | 11,472 | 33.3 |
| South | 12,339 | 35.8 |
| West | 4,219 | 12.2 |
| Unknown | 537 | 1.6 |
| Immune related comorbidities | ||
| Immunesuppression (HIV, cancer, autoimmune, transplant) | 3,713 | 10.8 |
| Heart disease* | 3,576 | 10.4 |
| Chronic liver disease | 472 | 1.4 |
| Chronic lung disease | 3,372 | 9.8 |
| Any | 9,044 | 26.2** |
| Other comorbidities | ||
| Diabetes | 5,701 | 16.5 |
| Hypertension | 9,749 | 28.3 |
*IHD or MI
**27.3% of adults <50 had any immune related
Table 3.
Predictors of off-label high-dose influenza vaccine use among adults aged 18–64 y, 2010–2012
| % High-dose influenza vaccine | % Standard influenza vaccine | Multivariable adjusted OR (95% CI) | |
|---|---|---|---|
| Total (N) | 31,989 | 8,608,982 | |
| Age | |||
| 18–35 | 16.8 | 19.0 | 0.83 (0.80, 0.87) |
| 36–50 | 27.7 | 30.7 | 0.83 (0.81, 0.86) |
| 51–64 | 55.5 | 50.3 | Reference |
| Male sex | 44.5 | 41.2 | 1.08 (1.05, 1.10) |
| Physician type | |||
| Family practice doctor | 27.6 | 36.1 | 0.62 (0.61, 0.64) |
| Internal medicine doctor | 25.3 | 20.0 | Reference |
| Other | 14.8 | 14.1 | 0.92 (0.89, 0.96) |
| Specialist | 15.7 | 13.7 | 1.01 (0.98, 1.05) |
| Hospital doctor | 1.3 | 1. | 0.55 (0.50, 0.60) |
| Nurse | 0.6 | 3.2 | 0.17 (0.14, 0.19) |
| Pharmacist | 0.5 | 4.8 | 0.08 (0.07, 0.10) |
| Region | |||
| Northeast | 17.6 | 22.0 | Reference |
| North central | 34.2 | 24.9 | 1.45 (1.40, 1.50) |
| South | 34.6 | 34.5 | 1.40 (1.35, 1.44) |
| West | 12.0 | 16.6 | 0.98 (0.94, 1.03) |
| Unknown | 1.6 | 2.0 | 0.98 (0.90, 1.08) |
| Immune related comorbidities | |||
| Immunesuppression (HIV, cancer, autoimmune, transplant) | 13.4 | 10.5 | 1.23 (1.18, 1.27) |
| Heart disease* | 13.1 | 9.1 | 1.30 (1.25, 1.35) |
| Chronic liver disease | 1.8 | 1.4 | 1.08 (0.99, 1.18) |
| Chronic lung disease | 11.4 | 8.9 | 1.25 (1.20, 1.29) |
| Other comorbidities | |||
| Diabetes | 19.0 | 14.1 | 1.28 (1.24, 1.32) |
| Hypertension | 34.2 | 28.2 | 1.17 (1.14, 1.21) |
*IHD or MI
Discussion
In the first 3 influenza seasons since licensure, the uptake of high-dose influenza vaccine among the elderly has been limited. Among this population of patients with Medicare Supplemental insurance, we show that only 18.4% of influenza shots were high-dose vaccine. Interestingly, almost 27,000 people received multiple high-dose vaccines. While small studies have reported a potential for an increased rate of minor adverse events after one shot (i.e., redness and pain at the injection site, gastrointestinal upset),12–14 there has been no safety assessment for multiple doses.
There may be several reasons why uptake of this vaccine has been slow. Physicians may be hesitant to suggest this new vaccine to their patients, especially since the ACIP has not issued a preference for the high dose vaccine. Additionally, access to the vaccine may be another barrier. Stocking another type of influenza vaccine may increase cost and logistics for physician offices, particularly for physicians who don't have a history of stocking adult vaccines. Our study suggested that family practice physicians were more likely to administer the high dose vaccine than internal medicine physicians, which may be explained by a higher proportion of family physicians stocking all adult vaccines.15 Cost and inadequate reimbursement have also been documented as significant barriers for physicians in administering other adult vaccines.16,17 In an environment with increasing options of influenza vaccine type (administration route, dose, strain) further research is needed to determine the rationale for which vaccine is administered by providers.
Older age was one of the stronger predictors of on-label high dose vaccine use. Providers may be administering the high dose vaccine to older patients in response to immune senescence. Physicians may also be more willing to try a new vaccine in patients where the risk of severe disease far outweighs the potential for minor adverse effects, which could be the scenario in the very old.
There was a surprising amount of off-label use of the high dose vaccine. While off-label use of medications is common particularly for oncology treatment,18 antipsychotic medications19 and the use of biologics to treat rheumatoid arthritis,20 use of vaccines off-label is rare. We found only one recent study that reported the use of Tdap among elderly people.21 It is possible that some of the off-label use we report is due to coding errors, particularly in children. The code for the high-dose vaccine is only 1 digit different from the live virus vaccine, commonly given to children. However, it is less likely coding errors can explain the adult usage, as the codes for all other adult influenza vaccine formulations have 2 digits that are different from the high-dose code.
We did not see a clear pattern of specific comorbidities among adults aged 18–64 y given high-dose vaccine. Only 26% of all off-label users had a recent diagnosis of an immune-related condition (27% of off-label adults less than 50 y). However; when compared to adults aged 18–64 who received the standard influenza vaccine, high-dose recipients did appear to be sicker. Adult patients receiving high-dose vaccine were more likely to have an immune-compromising condition, heart disease, chronic lung disease, diabetes and hypertension. However; the majority of the population receiving off-label high-dose vaccine did not have a severe, immune-related condition. Therefore, additional studies should investigate further why health care personnel are administering high dose vaccine to people younger than 65 y.
There were several limitations to this study. First, we may have underestimated the use of all types of influenza vaccine in both the elderly and the general population if people paid for the vaccine in other ways than through their insurance (i.e., paid out-of-pocket or received the vaccine through their employer). For the analysis done in the elderly population, if people were more likely to pay out-of-pocket for the standard vaccine because it is cheaper, then the absolute estimates would be biased upward and actual use of the high-dose vaccine could be lower. However, Medicare covers both vaccines under Part B and therefore, missing vaccine may be less likely in the elderly population. For the younger population, we felt that it was likely that claims for the standard, seasonal vaccine could be missing if people received the vaccine through an employer or paid out-of-pocket. One study suggested that workplaces were the third most common place that adults received the influenza vaccine,22 therefore it is likely that some claims for standard vaccine were missing. If this missingness was different for those who had chronic disease vs. those who did not, then we may have overestimated the association between the comorbidity and high-dose vaccine use. Additionally, it is possible that we missed off-label use of the high-dose vaccine. It is unclear whether insurance companies would pay for these shots for people younger than 65, and therefore, it is possible that physicians did not submit claims for off-label use. Therefore, we suggest interpreting the comparison between high-dose and seasonal vaccine in the younger population with caution. Second, we used a 4 month window to search for comorbidities that could be associated with both on and off-label use. This would only identify people with recent claims for these conditions and could miss people with well managed chronic illness. When we increased the window to 6 and 8 months prior to administration of the vaccine, we did identify more comorbidities; however the association with receiving high-dose vaccine remained similar. Third, we used ICD-9-CM codes to identify comorbidities. This method can be less sensitive in identifying conditions, and certain conditions contain many codes making them less specific. We did provide all codes that were used to allow others to replicate these comorbidity groupings. Finally, we assessed use of on-label vaccine among a population of seniors with Medicare supplemental insurance. This population could be different than the general Medicare population, which could limit generalizability. However, in 2012, 1 in 3 Medicare beneficiaries had supplemental insurance through an employer23 – therefore our study would directly apply to a large portion of the elderly population.
We document limited uptake of the high dose influenza vaccine among seniors and substantial use of this vaccine off-label in people younger than 65 y. Further research on the effectiveness and safety of the high-dose vaccine in both on-label and off-label populations is necessary. In particular, studies assessing the safety of this vaccine in seniors receiving multiple does and among people younger than 65 are warranted.
Materials and Methods
We analyzed data from the MarketScan Medicare Supplemental and Commercial Claims and Encounters databases (Copyright © Thomson Truven Healthcare, Inc.). Both databases capture patient-level data on inpatient, outpatient, and prescription drug claims, including vaccinations. The Medicare Supplemental database includes retirees with Medicare insurance paid for by employers, and captures claims for the Medicare-covered and the employer-paid portions of payment, as well as out-of-pocket expenses. Approximately one-third of all Medicare beneficiaries have employer-sponsored supplemental insurance.23,24 The Commercial Claims and Encounters database includes claims from approximately 150 large employers and health plans that insure employees, early retirees and dependents. These databases have been used in previous vaccine studies among infants,25 adolescents,26 and to characterize trends in drug usage among adults.27,28
To understand patterns of use of the high-dose vaccine in the elderly, we constructed a cohort of people ≥65 y, who received an influenza vaccine between January 1, 2010 and December 31, 2012. We stratify by calendar year, and focus on vaccinations administered in August-December of each influenza season (i.e., vaccinations given in August through December of 2010 were included for the year 2010). We chose to structure the analysis into calendar years rather than influenza seasons, because the vast majority of vaccinations (∼98% in the 2010 and 2011 influenza seasons) were administered in the fall of each influenza season. This allowed us to maximize the data that were available and include vaccinations that were administered in the fall of 2012, without requiring data from the spring of 2013. Vaccinations were identified using the following Current Procedural Terminology (CPT) codes: high-dose vaccine code = 90662, and standard trivalent vaccine codes = 90655, 90656, 90657, 90658, and 90660. Medical records were not reviewed to identify additional vaccine use. General patterns of vaccine use were described in this population that was not subject to further enrollment criteria. We evaluated doses administered by influenza season and report frequencies of patients who received high-dose vaccine in multiple seasons.
To further investigate associations between high-dose and seasonal vaccine use in the elderly population, we subsequently limited the above cohorts to patients with continuous enrollment from May 1 through August 31 of each year, where we assessed patient and provider characteristics. We conducted a sensitivity analysis to require a longer time to assess predictors and started eligibility on March 1 (6 month window) and January 1 (8 month window). We used multivariable logistic regression to assess the association between patient and provider variables and high-dose influenza vaccine receipt versus standard influenza vaccine. All predictors were identified a priori and included age, sex, comorbidities, hospitalization, pneumococcal vaccination (CPT codes 90670, 90732), type of provider who administered the vaccine (general practitioner, specialist, pharmacist, other), health plan type, region of residence, and rurality of residence. Comorbidities were assessed using inpatient and outpatient diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Rurality was measured by linking the US Department of Agriculture (USDA), Economic Research Service 2003 rural-urban continuum codes to the claims database via 5-digit Federal Information Processing Standard (FIPS) codes. These codes classify counties into metropolitan or non-metropolitan categories, based on population size and degree of urbanization. This variable was only available in 2010; therefore we did not include it in multivariable models. Data on race and socioeconomic status were unavailable.
To understand patterns of off-label use of the vaccine, we constructed a cohort of people younger than 65 y, who received an influenza vaccine during the same time period. Vaccinations were identified using the same CPT codes as above and the same enrollment criteria were applied to assess associations with comorbid conditions. We categorized certain comorbidities including: immune-compromising conditions (HIV, cancer, transplant recipients) and general comorbidities (diabetes, hypertension) and report the most frequent conditions. We used multivariable logistic regression to assess the association between specific comorbidities and high-dose influenza vaccine receipt vs. standard influenza vaccine among adults aged 18–64 in this younger population.
Appendix 1 shows the ICD-9-CM codes used to define comorbidities for both on and off-label analyses. Analyses were conducted with SAS, version 9.3 (SAS Institute, Cary, NC). This study was approved by the institutional review board at the University of North Carolina.
Acknowledgment
We thank Virginia Pate for providing assistance with statistical programming and data management.
Appendix 1. Diagnosis codes used to identify comorbidities.
| Comorbidity | ICD-9-CM diagnosis codes | ICD-9-CM Procedure codes/HCPCS codes |
|---|---|---|
| Pneumonia | 486 | |
| Transplant | 238.77, 279.5, 996.8, V42, V5844, E8784 | 001.8, 009.1, 009.2, 009.3, 375.1, 419.4, 469.7, 335, 336, 410, 505, 528, 556 |
| HIV | 042, 795.71, V08 | |
| Autoimmune disease | 279.4, 564.1, 696.0, 696.1, 695.4, 701.0, 710, 714, 720, 725 | |
| Cancer | 140–172, 174, 175, 179–201, 173.3, 173.9, 202.0, 202.1, 202.3, 203.01, 203.8, 238.6, 273.3, 202.5, 202.6, 202.7, 202.8, 202.9, 232.9, 233.0, 233.1, 338.3, 789.51, 795.82, 799.4, V672 | 992.5 |
| Infection | 001–139, 320–326, 460–466, 480–490, 680–686, 254.1, 373.0, 373.1, 373.2, 382.0, 382.1, 382.2, 382.3, 382.4, 383.0, 422.0, 474.0, 491.1, 513.0, 518.6, 522.5, 522.7, 527.3, 528.3, 569.5, 573.1, 573.2, 573.3, 575.0, 575.1, 599.0, 607.1, 607.2, 608.0, 608.4, 611.0, 616.1, 616.3, 616.4, 616.8, 706.0, 730.0, 730.1, 730.2, 730.3, 730.8, 730.9, 790.7, 790.8, 998.5, 999.3, 372.0, 372.1, 372.2, 372.3, 421.0, 421.1, 572.0, 572.1, 595.0, 595.1, 595.2, 595.3, 595.4, 996.6, 254.1, 373.0, 373.1, 373.2, 382.0, 382.1, 382.2, 382.3, 382.4, 383.0, 422.0, 474.0, 491.1, 513.0, 518.6, 522.5, 522.7, 527.3, 528.3, 569.5, 573.1, 573.2, 573.3, 575.0, 575.1, 599.0, 607.1, 607.2, 608.0, 608.4, 611.0, 616.1, 616.3, 616.4, 616.8, 706.0, 730.0, 730.1, 730.2, 730.3, 730.8, 730.9, 790.7, 790.8, 998.5, 999.3, 372.0, 372.1, 372.2, 372.3, 421.0, 421.1, 572.0, 572.1, 595.0, 595.1, 595.2, 595.3, 595.4, 996.6', 331.81, 386.33, 386.35, 388.60 422.91, 422.92, 422.93 478.21, 478.22, 478.24 478.29, 519.01, 997.62 | J3370, J0690, J0713, J0692, J0696, J1580, J3260, J0278, J1840, J1956 |
| Sepsis | 038, 995.90, 995.91, 995.92 | |
| Diabetes mellitus | 250 | |
| Stroke | 434.01, 434.11, 434.91, 435–438, V1254 | |
| Myocardial infarction | 410 | |
| Chronic obstructive pulmonary disease | 490–496, 505, 506.4 | |
| Liver disease | 070.32, 070.33, 070.54, 456.0, 456.1, 456.20, 456.21, 571, 572.3, 572.8, V427 | |
| Hypertension | 401, 402.0, 402.90, 402.10, 403, 404.0, 404.10, 404.12, 404.90, 404.92, 405, | |
| Ischemic heart disease | 093.2, 402.11, 402.91, 404.11, 404.12, 404.91, 404.93, 411–414, 420–429, 441–445, 447.1, 557.1, 557.9, 746.3, 746.4, 746.5, 746.6, 785.0, V422, V433, V434,V450, V533, | 006.6, 360.6, 360.7, 929.82, 929.85, 929.80, 335.10–335.14, 335.16–335.19 |
Appendix 2. Predictors of high dose influenza vaccine among seniors 65 and older with extended baseline windows, 2010–2012
| 6-month window | 8-month window | |||
|---|---|---|---|---|
| High dose vaccinees with selected condition, % | Multivariable adjusted* OR (95% CI) | High dose vaccinees with selected condition, % | Multivariable adjusted* OR (95% CI) | |
| Total vaccinated | 18.8 | - | 18.9 | - |
| Comorbidities | ||||
| Hypertension | 67.0 | 1.08 (1.07, 1.09) | 70.9 | 1.07 (1.06, 1.09) |
| Infection | 46.7 | 1.10 (1.08, 1.11) | 53.7 | 1.10 (1.08, 1.11) |
| IHD | 41.6 | 1.07 (1.06, 1.08) | 45.3 | 1.06 (1.05, 1.08) |
| Diabetes | 29.4 | 1.00 (0.99, 1.01) | 30.5 | 1.00 (0.99, 1.01) |
| COPD | 16.9 | 1.01 (0.99, 1.02) | 19.1 | 0.99 (0.98, 1.01) |
| Cancer | 16.2 | 1.04 (1.02, 1.05) | 18.2 | 1.02 (1.01, 1.04) |
| Autoimmune | 7.0 | 1.06 (1.04, 1.09) | 7.9 | 1.05 (1.03, 1.08) |
| Stroke | 6.9 | 1.08 (1.06, 1.10) | 8.4 | 1.07 (1.05, 1.09) |
| Pneumonia | 3.8 | 1.03 (1.00, 1.06) | 5.0 | 1.04 (1.01, 1.07) |
| Transplant | 2.0 | 1.08 (1.04, 1.12) | 2.5 | 1.08 (1.04, 1.12) |
| Liver disease | 1.3 | 0.93 (0.89, 0.98) | 1.6 | 0.94 (0.91, 0.98) |
| MI | 1.2 | 0.97 (0.93, 1.02) | 1.5 | 0.96 (0.92, 1.00) |
| Sepsis | 1.0 | 0.90 (0.85, 0.95) | 1.2 | 0.90 (0.85, 0.94) |
| HIV | 0.04 | 0.49 (0.38, 0.62) | 0.04 | 0.50 (0.39, 0.64) |
| Hospitalization | 12.9 | 1.04 (1.02, 1.06) | 16.6 | 1.05 (1.03, 1.07) |
| Pneumococcal vaccine | 9.3 | 1.09 (1.07, 1.11) | 10.3 | 1.10 (1.08, 1.12) |
IHD = Ischemic heart disease; COPD = Chronic obstructive pulmonary disease; MI = Myocardial infarction; HIV = Human immunodeficiency virus.
*Adjusted for month of vaccine receipt and all other predictor variables in Table 1 except for rurality.
Disclosure of Potential Conflicts of Interest
MAB receives investigator-initiated research funding from the National Institutes of Health (R01 AG042845, R21 HD080214, R01 AG023178) and through contracts with the Agency for Healthcare Research and Quality's DEcIDE program and the Patient Centered Outcomes Research Institute. He has served as a scientific advisor for Amgen, Merck, GSK (honoraria/payment received by the institution). He received consulting fees from RxAnte and World Health Information Consultants. LM has no conflicts of interests.
Authors’ Contributions
LM designed the study, analyzed and interpreted the data, drafted and revised the manuscript. MAB acquired and interpreted the data and revised the manuscript. The final manuscript has been approved by both authors.
References
- 1. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289(2):179–86; PMID:12517228; http://dx.doi.org/ 10.1001/jama.289.2.179 [DOI] [PubMed] [Google Scholar]
- 2. McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30(12):2060-7; PMID:22289511; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine 2007; 25(16):3066-9; PMID:17275144; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.025 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (fluzone high-dose) and guidance for use — United States, 2010. MMWR 2010; 59(16):485-6 [PubMed] [Google Scholar]
- 5. Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine 2011; 29(16):2865-73; PMID:21352939; http://dx.doi.org/ 10.1016/j.vaccine.2011.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson R, Poland GA, Sullivan SJ. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vac 2010; 9:1127+; PMID:20923264; http://dx.doi.org/ 10.1586/erv.10.117 [DOI] [PubMed] [Google Scholar]
- 7. Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200(2):172-80; PMID:19508159; http://dx.doi.org/ 10.1086/599790 [DOI] [PubMed] [Google Scholar]
- 8. Cate TR, Rayford Y, Niño D, Winokur P, Brady R, Belshe R, Chen W, Atmar RL, Couch RB. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 2010; 28(9):2076-9; PMID:20044052; http://dx.doi.org/ 10.1016/j.vaccine.2009.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine 2013; 31(6):861-6; PMID:23261045; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 10. Greenberg DP. Fluzone high-dose vaccine FIM12 efficacy trial summary results: ACIP meeting, October 24, 2013; Atlanta, GA: Centers for Disease Control and Prevention; 2013. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2013/04-Fluzone-Greenberg.pdf. Accessed February 13, 2014. [Google Scholar]
- 11. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines - recommendations of the advisory committee on immunization practices (ACIP), 2013-2014. MMWR 2013; 62(7):1-43 [PubMed] [Google Scholar]
- 12. Keitel Wa, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, Couch RB. SAfety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166(10):1121-7; PMID:16717175; http://dx.doi.org/ 10.1001/archinte.166.10.1121 [DOI] [PubMed] [Google Scholar]
- 13. Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, Sigurdardottir B, Hoeper A, Graham IL, Edelman R, et al. . Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25(44):7656-63; PMID:17913310; http://dx.doi.org/ 10.1016/j.vaccine.2007.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moro PL, Arana J, Cano M, Menschik D, Yue X, Lewis P, Haber P, Martin D, Broder K. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the vaccine adverse event reporting system, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54(11):1608-14; PMID:22441652; http://dx.doi.org/ 10.1093/cid/cis256 [DOI] [PubMed] [Google Scholar]
- 15. Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine 2011; 29(9):1850-4; PMID:21216314; http://dx.doi.org/ 10.1016/j.vaccine.2010.12.097 [DOI] [PubMed] [Google Scholar]
- 16. Szilagyi PG, Shone LP, Barth R, Kouides RW, Long C, Humiston SG, Jennings J, Bennett NM. Physician practices and attitudes regarding adult immunizations. Prev Med 2005; 40(2):152-61; PMID:15533524; http://dx.doi.org/ 10.1016/j.ypmed.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 17. Hurley LP, Lindley MC, Harpaz R, Stokley S, Daley MF, Crane LA, Dong F, Beaty BL, Tan L, Babbel C, et al. . Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152(9):555-60; PMID:20439573; http://dx.doi.org/ 10.7326/0003-4819-152-9-201005040-00005 [DOI] [PubMed] [Google Scholar]
- 18. Levêque D. Off-label use of anticancer drugs. Lancet Oncol 2008; 9(11):1102-7; http://dx.doi.org/ 10.1016/S1470-2045(08)70280-8 [DOI] [PubMed] [Google Scholar]
- 19. Maher A, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 2011; 306(12):1359-69; PMID:21954480; http://dx.doi.org/ 10.1001/jama.2011.1360 [DOI] [PubMed] [Google Scholar]
- 20. Furst DE, Fleischman R, Kalden J, Kavanaugh A, Sieper J, Mease P, Smolen J, Breedveld F. Documentation of off-label use of biologics in Rheumatoid Arthritis. Ann Rheum Dis 2013; 72(suppl 2):ii35-ii51; PMID:23532442 [DOI] [PubMed] [Google Scholar]
- 21. Tseng HF, Sy LS, Qian L, Marcy SM, Jackson LA, Glanz J, Nordin J, Baxter R, Naleway A, Donahue J, et al. . Safety of a tetanus-diphtheria-acellular pertussis vaccine when used off-label in an elderly population. Clin Infect Dis 2013; 56(3):315-21; PMID:23196953; http://dx.doi.org/ 10.1093/cid/cis871 [DOI] [PubMed] [Google Scholar]
- 22. Lu P-j, O’Halloran A, Ding H, Williams WW, Bridges CB, Kennedy ED. National and state-specific estimates of place of influenza vaccination among adult populations – United States, 2011–12 influenza season. Vaccine 2014; 32(26):3198-204; PMID:24731815; http://dx.doi.org/ 10.1016/j.vaccine.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaiser Family Foundation Medicare at a glance. Menlo Park, CA: Kaiser Family Foundation; 2012. Available at: http://kff.org/medicare/fact-sheet/medicare-at-a-glance-fact-sheet/. Accessed July 26, 2013. [Google Scholar]
- 24. Umans B, Nonnemaker KL The medicare beneficiary popoluation. Washington, DC: AARP Public Policy Institue; 2009. Available at: http://assets.aarp.org/rgcenter/health/fs149_medicare.pdf. Accessed August 1, 2014. [Google Scholar]
- 25. Panozzo CA, Becker-Dreps S, Pate V, Jonsson Funk M, Stürmer T, Weber DJ, Brookhart MA. Patterns of rotavirus vaccine uptake and use in privately-insured US infants, 2006–2010. PLoS One 2013; 8(9):e73825; PMID:24066076; http://dx.doi.org/ 10.1371/journal.pone.0073825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsai Y, Zhou F, Wortley P, Shefer A, Stokley S. Trends and characteristics of preventive care visits among commercially insured adolescents, 2003-2010. J Pediat 2014; 164(3):625-30; PMID:24286572; http://dx.doi.org/ 10.1016/j.jpeds.2013.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mintzer S, Maio V, Foley K. Use of antiepileptic drugs and lipid-lowering agents in the United States. Epilepsy Behav 2014; 34(0):105-8; PMID:24735835; http://dx.doi.org/ 10.1016/j.yebeh.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mark TL, Joish VN, Hay JW, Sheehan DV, Johnston SS, Cao Z. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiat 2011; 19(3):211-21; PMID:21425504; http://dx.doi.org/ 10.1097/JGP.0b013e3181f1803d [DOI] [PubMed] [Google Scholar]


