Abstract
Epstein–Barr virus (EBV) has been recently shown to be present in human breast cancer worldwide, which could play an important role in the initiation and progression of this cancer. In this regard, we aimed to explore the prevalence of EBV in 108 breast cancer tissues from Syrian women using polymerase chain reaction (PCR) and tissue microarray (TMA) analysis. We found that EBV is present in 56 (51.85%) of breast cancers samples. Additionally, we report that the expression of LMP1 gene of EBV is associated with a cancer invasive phenotype in the majority of the cancer samples. These data imply that EBV is present in breast cancer worldwide including Syria and its presence is associated with more aggressive cancer phenotype. Thus, future investigations are needed to elucidate the exact role of EBV in breast carcinogenesis and metastasis.
Keywords: breast cancer, Epstein–Barr virus, invasive cancer, Syrian women
Introduction
Epstein-Barr virus (EBV) is a ubiquitous gamma-herpesvirus that infects more than 90% of the population worldwide.1 Besides infectious mononucleosis EBV is well known to be linked to several human cancers, including Burkitt's lymphoma, Hodgkin's disease, nasopharyngeal carcinomas (NPC), gastric cancer and T-cell/natural killer cell lymphoma.1,2
EBV-infect cells express a small subset of genes, including 6 nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -LP), 3 latent membrane proteins (LMP-1, -2A, and -2B), 2 small noncoding RNAs (EBER-1 and 2), and BamHI-A rightward transcripts (BART).3 The differential expression pattern of these latent genes defines the distinct latency programs associated with the types of cancers.2,4 For instance, type II latency is characterized by a more restricted latent gene expression pattern (EBNA-1, LMP-1, -2), and is associated with Hodgkin's lymphoma and NPC as well as other carcinomas including gastric and probably breast.4,5 Thus, LMP1 is considered the major EBV-encoded oncogenic protein, as it induces a multitude of effects promoting cell growth, protecting cells from apoptosis, enhancing cell motility, promoting angiogenesis and it is frequently expressed in EBV-associated human NPC.6,7 On the other hand, EBNA2 is considered the central transcriptional regulator of viral and cellular genes that are involved in B cell immortalization.8 LMP2A plays a key role in regulating viral latency and it functionally mimicks signals induced by the B cell receptor, altering normal B cell development; LMP2A can also immortalize epithelial cells and influence their adhesion and motility.6,9 Alternatively, EBV is the first, and one of the few viruses known to encode micro-RNAs (EBV-miRNAs).9 Accumulating evidence suggests that miRNAs play important roles in carcinogenesis, modulating apoptosis and host innate defense mechanisms.9,10 All this implicates EBV in the initiation and progression of human malignancies including several types of lymphoma and carcinomas.
Several recent studies reported that approximately 30–50% of human breast cancers, worldwide, are positive for EBV;11-15 controversially a few studies revealed that EBV could not be detected in human breast cancers.16,17 Nevertheless, earlier studies pointed out that the presence of EBV in human breast cancers is associated with more aggressive features and poor prognosis.15,18 Thus, these studies suggest that EBV could play an important role in the progression of breast cancer.
This study aims to investigate the presence of EBV and its association with tumor aggressiveness in breast cancer in Syrian women.
Results
In order to determine the incidence and the role of EBV infections in human breast carcinogenesis in Middle Eastern women, we investigated the presence of this virus in a cohort of 108 breast cancer samples from Syrian women by PCR and Immunohistochemistry (IHC) analysis using specific primers for LMP1 and/or EBNA1 genes of EBV (Table 1) and a monoclonal antibody for LMP1 (please refer to Materials and methods section and below). Our study revealed that 56 (51.85%) of the 108 samples are EBV positive; in contrast, 52 (48.15%) cancer tissues were negative for EBV (Table 2A).
Table 1.
The specific primer sets for LMP1 and EBNA1 genes of EBV used for PCR amplification
| Genes | Primers |
|---|---|
| LMP1 | 5'- TTGGAGATTCTCTGGCGACT -3' |
| 5'- AGTCATCGTGGTGGTGTTCA -3' | |
| EBNA1–297 | 5'- AAGGAGGGTGGTTTGGAAAG -3' |
| 5'- AGACAATGGACTCCCTTAGC -3' | |
| EBNA1–207 | 5'- ATCGTGGTCAAGGAGGTTCC -3' |
| 5'- ACTCAATGGTGTAAGACGAC -3' | |
| GAPDH | 5'- GAAGGC-CATGCCAGTGAGCT -3' |
| 5'- CCGGGAAACTGTGGCGTGAT -3' |
Table 2A.
EBV detection in breast carcinomas by PCR. The incidence of this virus was found in 58 samples out of 108 examined using specific primers for LMP1 and EBNA1 genes of EBV
| Tested cases | Positive | Percentage | |
|---|---|---|---|
| Breast cancer tissues | 108 | 56 | 51.85 |
| Table 2B. Presence of EBV and its association with invasive breast cancer phenotype. The presence of EBV is more frequent in invasive breast cancer in comparison to In Situ breast cancer tissues (P = 0.0013); the presence of EBV was assessed by PCR for LMP1 and EBNA1 genes and confirmed by IHC analysis of LMP1 expression using Tissue Microarray (TMA) methodology, as described in the Materials and Methods section. | |||
| Breast cancer | No of cases | EBV+(%) | |
| Invasive | 84 | 51/84 (60.7) | |
| In situ | 24 | 5/24 (20.8) | |
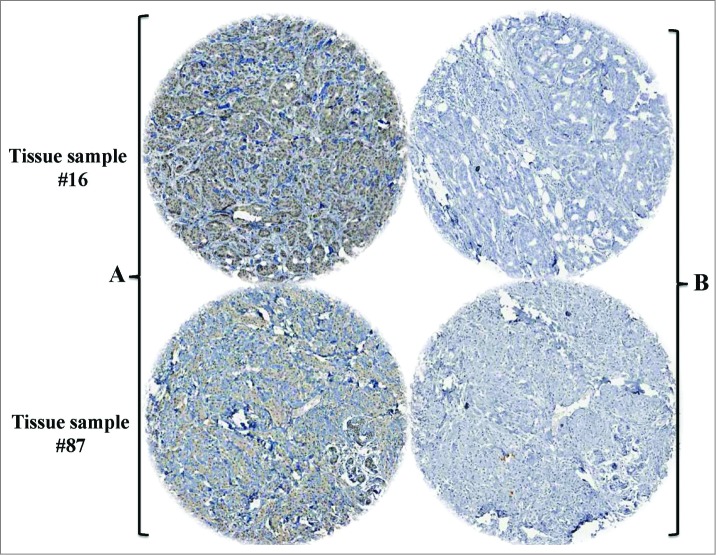
On the other hand and to assess the association between the presence of EBV and tumor aggressiveness in breast cancer in Syrian women, we examined the expression of the LMP1 onco-protein of EBV and its association with invasive cancer phenotype in our breast tissue samples by H&E and IHC using tissue microarray methodology. We found that the LMP1 expression, a cytoplasmic dot staining pattern, is correlated with invasive breast cancer phenotype in 60.7% of breast cancer samples as opposed to 20.8% of in-situ cancer tissues (Table 2B and Fig. 1); while we presume that these in situ breast carcinomas, which are EBV-positive, will ultimately progress into invasive carcinomas under the effect of EBV, since they are already intermediate to high nuclear grade. On the other hand, we noted that more than 90% of the cancer cells in our samples were positive for LMP1 in comparison with the adjacent normal mammary epithelial cells, which were revealed negative for LMP1 (Fig. 1). The IHC data were confirmed by the presence of both LMP1 and EBNA1 in 54 of our samples using PCR analysis with all the necessary controls including Mec1 and MCF7 cell lines as well as normal mammary epithelial cells (Fig. 2). Accordingly, we were able to prove that EBV is present in the majority of invasive breast carcinomas in comparison with in situ cancers in Syrian women (Table 2B and Figs. 1 and 2). Thus, the presence of EBV in breast cancer could play an important role in its progression.
Figure 1.
Representative IHC revealing LMP1 expression of EBV in 2 invasive breast cancer tissue samples (A): with LMP1 antibody; (B): without antibody, control). Magnification is 100X. This analysis was performed using TMA methodology with all the necessary controls; and presence of EBV was confirmed by PCR using specific primers for LMP1 and EBNA1 genes in 54 cases of our samples including these samples.
Figure 2.
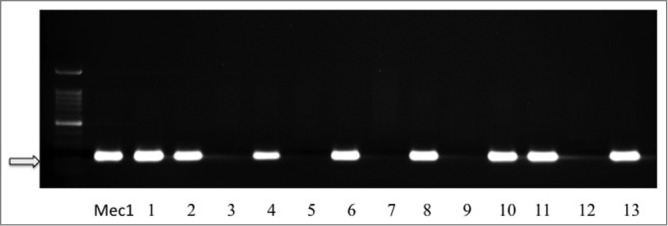
Representative PCR reactions for LMP1 of EBV in 13 different breast cancer tissue samples. LMP1 and EBNA1 are present in 8 of 13 samples (line 1: Mec1 cell line was used as a positive control; MCF7 and normal mammary epithelial cells were used as negative controls, data not shown).
Discussion
This is, to the best of our knowledge, the first study on the presence of EBV and its relation with tumor aggressiveness in breast cancer in Syrian women. In fact, recently there has been an increasing interest in investigating the possible role of viral infection in breast cancer specially EBV and human papillomaviruses (HPVs) based on their roles in various types of cancers such as lymphoma and cervical, respectively. Accordingly, we have recently reported that 61% of human breast cancers in the Syrian population are positive for high-risk HPVs.19 In addition, our study revealed that the presence of high-risk HPVs is correlated with an invasive and metastatic breast cancer form. Regarding the presence of EBV in breast cancers worldwide, several recent investigations reported that approximately 30–50% of these cancers are positive for EBV;11-15 however, a small number of studies were unable to detect EBV in breast cancer.16,17 In parallel, it is important to emphasize that there are only few studies about the presence of EBV in the Middle East which can be outlined as follows: Kalkan et al.,20 revealed that 58% of human breast cancer and normal mammary tissues are positive for EBV in the Turkish population. Zekri et al.21 found that, 45% and 28% of breast cancer in Egyptian and Iraqi women, respectively, are positive for EBV. In parallel, Hachana et al.22 demonstrated that 27% of breast cancer tissues in Tunisia are positive for EBV. However, a recent study from Iran showed that EBV is not detectable in breast cancer and normal mammary tissues in Iranian women.17 Therefore, investigations from different countries of the Middle East area, except one study from one country, showed that the presence of EBV in breast cancer and mammary tissues vary from 27 to 58%, which is approximately similar to its presence in breast cancer worldwide. In this study, we report that EBV is present in approximately 52% of breast cancer in Syrian women. Therefore, our data point-out that the prevalence of EBV in breast cancer tissues in Syria is similar to the incidence of this virus in women worldwide including the Middle East. On the other hand and based on our previous HPVs investigations in human breast cancer and the present study, as well as our recent paper about HPV and EBV cooperation in human oral cancer,19,23 we believe that high-risk HPVs could cooperate with EBV in human breast carcinogenesis and metastasis; this presumption is presently under-investigation. In this context, it is important to mention that ∼20% of our human breast cancer samples revealed positive for both HPV and EBV together.
Regarding the association between EBV and tumor aggressiveness, recent studies have reported that the presence of EBV is correlated with human invasive carcinomas including breast and nasopharyngeal.11,18,24 Moreover, earlier investigations demonstrated that LMP1 and EBNA1 onco-proteins of EBV enhance cancer progression and metastasis of nasopharyngeal malignancy though the initiation of the epithelial-mesenchymal transition (EMT) phenomenon,24-26 which is a crucial event during cancer metastasis progression.27 Therefore and based on this study and others, we believe that the presence of EBV in breast cancer could enhance cancer progression through the initiation of EMT event via EGF-receptor and/or Akt signaling pathways, as previously described in gastric and nasopharyngeal carcinomas;9,24 in addition, we assume that Wnt/β-catenin signaling can be involved in this progression since it has been demonstrated that LMP1 of EBV regulate this pathway in nasopharyngeal carcinomas.28 These signaling pathways can deregulate the expression patterns of several key genes of cell adhesion and invasion such as E-cadherin, Fascin as well as others and consequently enhance breast cancer progression (this hypothesis is presently under investigation in our laboratory).
In conclusion, we demonstrate that EBV is present in human breast cancer in Syrian women. In addition, EBV infection was found in approximately 52% in breast cancer, which is similar to its presence in breast cancer in Middle Eastern women, based on Kalkan et al.20, Zekri et al.21 and Hachana et al.22 studies. In parallel, we report that the presence of EBV in breast cancer is associated with more aggressive phenotype. Our findings provide clear evidence on the presence of EBV in human breast cancer and its relation to the progression of this cancer. However, we believe that further studies are required to elucidate the role and pathogenesis of EBV in human breast cancer, especially since EBV vaccine is presently under clinical trial investigations. Therefore, it is of great interest to gain a better understanding of the association between EBV infection and breast cancer initiation and progression.
Materials and Methods
EBV detection and PCR analysis
A total of 108 blocks from breast cancer patients with a median age of 52 (range 26–66) years were used in this study. Formalin fixed (buffered neutral aqueous 10% solution), paraffin embedded tumor materials were obtained from the department of Pathology/Faculty of Medicine, University of Aleppo. The use of these specimens and data in research was approved by the Ethics Committee of the Faculty of Medicine of Aleppo University.19 Five μg of purified DNA (from each sample) was analyzed for EBV by PCR using specific primers for LMP1 (Table 1) and EBNA1 genes as described by Glenn et al.11 Briefly, LMP1 gene was amplified for 35 cycles of 95°C for 30s, 60°C for 30s, and 72°C for 30s. In parallel, EBNA1 was amplified in 2 rounds; the first one produced a 297 bp fragment using EBNA1–297 primers. The product of the first round was used as a template for the second amplification using EBNA1–207 primers, generating a 209 bp fragment. The amplification condition of the EBNA1 was 35 cycles of 95°C for 30s, 55°C for 30s, and 72°C for 30s. DNA from the chronic B cell leukemia, Mec1, and MCF7 cell lines as well as specific primers for the GAPDH gene were used as controls for the presence of EBV genes and PCR analysis, respectively, (Table 1). On the other hand, DNAs from human normal mammary epithelial cells were used as negative controls for our PCR analysis.
Tissue microarray
The tissue microarray (TMA) construction was performed as described previously by our group.19 Briefly, tissue cylinders with a diameter of 0.6 mm were punched from representative tumor areas of a ‘donor’ tissue block using a semiautomatic robotic precision instrument. Two sections of the TMA blocks were transferred to an adhesive coated slide system (Instrumedics Inc.., Hackensack, New Jersey). Slides of the finished blocks were used for H&E and immunohistochemisty analysis.
Immunohistochemistry (IHC) analysis
Immunohistochemical procedures examining the expression of LMP1 was carried out using standard procedures as previously described.19 Primary LMP1 antibody was obtained from Dako. Briefly, TMA sections were deparaffinised, rehydrated and endogenous peroxidase activity within the rehydrated tissue was blocked with a solution of 3% hydrogen peroxide in methanol for 10 min at room temperature. The TMA slides were cooled and equilibrated in OptimaxTM wash buffer then incubated overnight (15 h) at 4°C with the LMP1 primary antibody (mouse monoclonal; clone 1–4; Dako, Canada). Sections were then washed (4x1 min), and the appropriate secondary HRP-conjugated antibody was applied for 1 h at room temperature (Calbiochem, Canada). The slides were counterstained with haematoxylin and mounted. Finally, sections were analyzed by Leica DMLB microscopy equipped with a Leica DFC-480 Camera (Leica Microsystems, Germany). Immuno-satainig analysis on human normal mammary epithelial cells were used as control for LMP1 expression using the same antibodies.
Statistical analysis
Statistical analysis was performed using SPSS (version 22) and R. The data were assessed as nonparametric data. We used χ2 test with Yates correction to assess the significance of the relationship between tumors aggressiveness and EBV presence.
Acknowledgments
We are thankful to Ms A. Kassab and Mr. B. Al Moustafa for their reading of the manuscript. We also thank Dr. N. Benlimame for her technical assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by the Canadian Institutes for Health Research and the Fonds de la Recherche en Santé du Québec (FRSQ- Réseau du Cancer).
References
- 1. Murata T, Tsurumi T. Switching of EBV cycles between latent and lytic states. Rev Med Virol 2014; 24:142-53; PMID:24339346; http://dx.doi.org/ 10.1002/rmv.1780 [DOI] [PubMed] [Google Scholar]
- 2. Münz C, Moormann A. Immune escape by Epstein-Barr virus associated malignancies. Semin Cancer Biol 2008; 18:381-7; PMID:18996483; http://dx.doi.org/ 10.1016/j.semcancer.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol 2003; 45:1-36; PMID:12482570; http://dx.doi.org/ 10.1016/S1040-8428(02)00078-1 [DOI] [PubMed] [Google Scholar]
- 4. Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res 2004; 10:803-21; PMID:14871955; http://dx.doi.org/ 10.1158/1078-0432.CCR-0670-3 [DOI] [PubMed] [Google Scholar]
- 5. Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J Cancer Res Clin Oncol 2009; 135:329-37; PMID:19009309; http://dx.doi.org/ 10.1007/s00432-008-0511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 2012; 22:144-53; PMID:22249143; http://dx.doi.org/ 10.1016/j.semcancer.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 7. Morris MA, Dawson CW, Young LS. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol 2009; 5:811-25; PMID:19663731; http://dx.doi.org/ 10.2217/fon.09.53 [DOI] [PubMed] [Google Scholar]
- 8. Schlee M, Krug T, Gires O, Zeidler R, Hammerschmidt W, Mailhammer R, Laux G, Sauer G, Lovric J, Bornkamm GW. Identification of Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA2) target proteins by proteome analysis: activation of EBNA2 in conditionally immortalized B cells reflects early events after infection of primary B cells by EBV. J Virol 2004; 78:3941-52; PMID:15047810; http://dx.doi.org/ 10.1128/JVI.78.8.3941-3952.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raab-Traub N. Novel mechanisms of EBV-induced oncogenesis. Curr Opin Virol 2012; 2:453-8; PMID:22858118; http://dx.doi.org/ 10.1016/j.coviro.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He ML, Luo MX, Lin MC, Kung HF. MicroRNAs: potential diagnostic markers and therapeutic targets for EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta 2012; 1825:1-10; PMID:21958739; http://dx.doi.org/ 10.1016/j.bbcan.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 11. Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One 2012; 7:e48788; PMID:23183846; http://dx.doi.org/ 10.1371/journal.pone.0048788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joshi D, Quadri M, Gangane N, Joshi R, Gangane N. Association of Epstein Barr virus infection (EBV) with breast cancer in rural Indian women. PLoS One 2009; 4:e8180; PMID:19997605; http://dx.doi.org/ 10.1371/journal.pone.0008180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorenzetti MA, De Matteo E, Gass H, Martinez Vazquez P, Lara J, Gonzalez P, Preciado MV, Chabay PA. Characterization of Epstein Barr virus latency pattern in Argentine breast carcinoma. PLoS One 2010; 5:e13603; PMID:21042577; http://dx.doi.org/ 10.1371/journal.pone.0013603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xue SA, Lampert IA, Haldane JS, Bridger JE, Griffin BE. Epstein-Barr virus gene expression in human breast cancer: protagonist or passenger? Br J Cancer 2003; 89:113-9; PMID:12838311; http://dx.doi.org/ 10.1038/sj.bjc.6601027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fawzy S, Sallam M, Awad NM. Detection of Epstein-Barr virus in breast carcinoma in Egyptian women. Clin Biochem 2008; 41:486-92; PMID:18258188; http://dx.doi.org/ 10.1016/j.clinbiochem.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 16. Deshpande CG, Badve S, Kidwai N, Longnecker R. Lack of expression of the Epstein-Barr Virus (EBV) gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer cells. Lab Invest 2002; 82:1193-9; PMID:12218080; http://dx.doi.org/ 10.1097/01.LAB.0000029150.90532.24 [DOI] [PubMed] [Google Scholar]
- 17. Kadivar M, Monabati A, Joulaee A, Hosseini N. Epstein-Barr virus and breast cancer: lack of evidence for an association in Iranian women. Pathol Oncol Res 2011; 17:489-92; PMID:21207256; http://dx.doi.org/ 10.1007/s12253-010-9325-z [DOI] [PubMed] [Google Scholar]
- 18. Mazouni C, Fina F, Romain S, Ouafik L, Bonnier P, Brandone JM, Martin PM. Epstein-Barr virus as a marker of biological aggressiveness in breast cancer. Br J Cancer 2011; 104:332-7; PMID:21179039; http://dx.doi.org/ 10.1038/sj.bjc.6606048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer 2008; 99:404-7; PMID:18648363; http://dx.doi.org/ 10.1038/sj.bjc.6604503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalkan A, Ozdarendeli A, Bulut Y, Yekeler H, Cobanoglu B, Doymaz MZ. Investigation of Epstein-Barr virus DNA in formalin-fixed and paraffin- embedded breast cancer tissues. Med Princ Pract 2005; 14:268-71; PMID:15961939; http://dx.doi.org/ 10.1159/000085748 [DOI] [PubMed] [Google Scholar]
- 21. Zekri AR, Bahnassy AA, Mohamed WS, El-Kassem FA, El-Khalidi SJ, Hafez MM, Hassan ZK. Epstein-Barr virus and breast cancer: epidemiological and molecular study on Egyptian and Iraqi women. J Egypt Natl Canc Inst 2012; 24:123-31; PMID:22929918; http://dx.doi.org/ 10.1016/j.jnci.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 22. Hachana M, Amara K, Ziadi S, Romdhane E, Gacem RB, Trimeche M. Investigation of Epstein-Barr virus in breast carcinomas in Tunisia. Pathol Res Pract 2011; 207:695-700; PMID:22024152; http://dx.doi.org/ 10.1016/j.prp.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 23. Al Moustafa AE, Chen D, Ghabreau L, Akil N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses 2009; 73:184-6; PMID:19361933; http://dx.doi.org/ 10.1016/j.mehy.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 24. Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, Xiong D, Guan S, Guo BH, Mai HQ, Chen QY, Zhang X, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog 2010; 6:e1000940; PMID:20532215; http://dx.doi.org/ 10.1371/journal.ppat.1000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer 2011; 104:1160-7; PMID:21386845; http://dx.doi.org/ 10.1038/bjc.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X, et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer 2014; 120:363-72; PMID:24190575; http://dx.doi.org/ 10.1002/cncr.28418 [DOI] [PubMed] [Google Scholar]
- 27. Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci (Schol Ed) 2012; 4:671-84; PMID:22202084; http://dx.doi.org/ 10.2741/S292 [DOI] [PubMed] [Google Scholar]
- 28. QingLing Z, LiNa Y, Li L, Shuang W, YuFang Y, Yi D, Divakaran J, Xin L, YanQing D. LMP1 antagonizes WNT/β-catenin signalling through inhibition of WTX and promotes nasopharyngeal dysplasia but not tumourigenesis in LMP1(B95-8) transgenic mice. J Pathol 2011; 223:574-83; PMID:21394719; http://dx.doi.org/ 10.1002/path.2820 [DOI] [PubMed] [Google Scholar]