Abstract
The aim of this Phase IIIb, open-label, randomized study was to demonstrate the non-inferiority of immune responses and to assess the safety of a purified chick-embryo cell rabies vaccine (PCECV) in healthy Chinese children (6 to 17 years) and older adults (≥51 years) following 2 alternative intramuscular (IM) simulated post-exposure prophylaxis (PEP) regimens: 4-dose Zagreb or 5-dose Essen regimen. Serum samples were collected prior to vaccination on Days 1 and 15 and on day 43 to assess immune response by rabies virus neutralizing antibody (RVNA) concentrations. Solicited adverse events (AEs) were recorded for up to 7 days following each vaccine dose, and unsolicited AEs throughout the entire study period. PCECV vaccination induced a strong immune response at Day 15, and the non-inferiority in immune response of the Zagreb vs. the Essen regimen was demonstrated in children and older adults. At Day 15,100% of children (N = 224), and 99% of subjects ≥51 years of age (N = 376) developed adequate RVNA concentrations (≥0.5 IU/mL); at Day 43 all subjects achieved RVNA concentrations ≥0.5 IU/mL, for both PEP regimens. The well-known tolerability and safety profile of the PCECV was again observed in this study following either Zagreb or Essen regimens. Rabies PEP vaccination with PCECV following a Zagreb regimen induced immune responses non-inferior to those of the Essen regimen, and had a similar safety and tolerability profile to the Essen regimen in Chinese children, adolescents, and adults over 51 years. ClinicalTrials.gov identifier: NCT01680016.
Keywords: Essen regimen, immunogenicity, intramuscular post-exposure prophylaxis; purified chick-embryo cell rabies vaccine, rabies, rabies virus neutralizing antibody, Zagreb regimen
Abbreviations
- AE
adverse event
- CI
confidence interval
- GMC
geometric mean concentration
- IM
intramuscular
- NIFDC
National Institutes for Food and Drug Control
- PCECV
purified chick-embryo cell rabies vaccine
- PEP
post-exposure prophylaxis
- PPS
per-protocol set
- RFFIT
Rapid Fluorescent Focus Inhibition Test
- RVNA
rabies virus neutralizing antibody
Introduction
Rabies is a zoonotic disease caused by a lyssavirus infection, which is endemic in more than 150 countries and territories worldwide, and is conservatively estimated to cause 60,000 deaths every year.1 More human deaths due to rabies are reported annually (about 30,000, half of the global estimate) in Asia than in other continents, and one of the most important rabies enzootic areas is found in China, where in 2012 rabies was the second leading cause of death due to infectious diseases.1-3 Three major epidemics were reported between 1950 and 2007, the last one in 2000 after a rapid increase in the pet dog population in urban areas.4 Although declining in recent years, more than 1,400 human deaths were reported in China in the year 2012.3-7
Following an incubation period of approximately 1–3 months after virus inoculation, the virus travels to the central nervous system, and causes an acute progressive encephalomyelitis followed by coma and death within 1–2 weeks in almost 100% of cases.8 Although after the onset of clinical symptoms there is no known cure for rabies, timely prophylaxis by vaccination can avert the development of the disease even after exposure to the virus. In the event of suspected or confirmed contact with a rabid animal, the WHO recommends immediate post-exposure prophylaxis (PEP) based on thorough local wound cleaning, timely active vaccination with cell culture or embryonated egg-based rabies vaccines, and simultaneous passive immunization with rabies immune globulin, depending on the category of exposure.1
The WHO recommendation for PEP vaccination via IM injection in healthy, fully immune competent subjects following exposure to rabies is 2 different regimens: 5 doses of the vaccine given at 5 separate visits (Essen regimen; 1-1-1-1-1), namely on Days 0, 3, 7,14, and 28; or a 4-dose regimen (Zagreb regimen; 2-1-1) consisting of 2 doses given on Day 0 (1 dose in the right arm, and 1 dose in the left arm), and 1 dose given on each of Days 7 and 21. The Zagreb regimen, relative to Essen regimen, has been shown to induce earlier protective titers, to reduce healthcare costs, and to have a potential favorable impact on vaccination compliance, as it involves a reduced number of visits and vaccine doses.9-11
Clinical trials conducted with purified chick-embryo cell vaccines (PCECV) have consistently reported that protective virus-neutralizing antibodies (RVNA) are usually induced by Day 14 following first vaccine dose, and the immunogenicity/efficacy and safety profiles of the vaccine have been well established in children and adults in previously simulated PEP studies involving healthy subjects or post exposure studies in subjects exposed to suspected or confirmed rabid animals.12-19 Moreover, a study assessing the anamnestic response following a single booster dose administered 2 years after a primary 3-dose immunization with PCECV indicated that it was potentially able to elicit long-lasting immune responses even after 14 years.20
In China, where 12–15 million doses of rabies vaccine are estimated to be administered annually,7,21,22 the traditional 5-dose Essen regimen recommended by the WHO has been widely adopted since years,23 while the Zagreb regimen has been only recently approved for PCECV preparations. A previous clinical trial conducted in healthy adult Chinese subjects aged 18–50 years indicated that immunization following the Zagreb regimen with PCECV was non-inferior to that following the Essen regimen, and had an acceptable and similar if not more favorable safety profile.23
Although all age groups are susceptible to rabies, there is a need to specifically study children and elderly populations. Children are at higher risk of rabies exposure than adults for several reasons, including the increased likelihood of receiving extensive bites to the face and head, which is associated with a higher possibility of contracting rabies, their curiosity and attraction toward animals, and their lack of awareness of the potential dangers.1,24 Indeed, the highest incidence of rabies across all developing countries is observed in children aged <15 years, with 60% of cases occurring between 0 and 12 years of age.24,25 It is also well documented that older subjects above 65–70 years of age have a decrease in the quality and quantity of immune responses because of immunosenescence that leads to decreased efficacy of vaccines.26-28
In the present study of simulated PEP, the primary objective was to determine the non-inferiority of immune response induced by PCECV (Rabipur®, Chiron Behring Vaccines Pvt. Ltd., Ankleshwar, India) following the Zagreb regimen compared to the 5-dose Essen regimen by measuring RVNA geometric mean concentrations (GMC) at 14 days after the first vaccine dose in 2 age cohorts: healthy children and adolescents (6 to 17 years) and older adults (≥51 years).
Materials and Methods
Study design and objectives
This was a Phase IIIb, open label, age-stratified, randomized study conducted between September 2012 and January 2013 at the Center for Disease Control and Prevention of Mengshan, Guangxi province, China (ClinicalTrials.gov identifier: NCT01680016). The protocol was approved by the appropriate Independent Ethics Review Committee, and was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines and local regulations. All participant subjects or the subject's parents/legal guardian, as applicable, provided written informed consent before enrollment.
As per Novartis convention and the Clinical Data Interchange Standards Consortium (CDISC), the day of first vaccination in this study was study Day 1. WHO and ACIP consider the first day of vaccination (treatment) as Day 0.1,29 Ensuring vaccination days are shifted accordingly, study Days 1, 4, 8, 15 and 29 in this clinical trial were equivalent to vaccination Days 0, 3, 7, 14 and 28 of WHO and ACIP recommendations.
The primary objectives of the study were to establish non-inferiority of immune response after simulated PEP with PCECV (Rabipur®, Chiron Behring Vaccines Pvt. Ltd., Ankleshwar India) following the 2-1-1 Zagreb regimen to 1-1-1-1-1 Essen regimen in healthy children 6–17 years of age and in older adults ≥51 years of age by means of the GMC of RVNA at Day 15. Moreover, the study evaluated the percentage of subjects with RVNA concentrations ≥0.5 IU/mL at Days 15 and 43 following the first vaccine dose (Day 1). The total study participation for the subjects was 43 days. Secondary objectives included the assessment of antibody response by means of GMC at Day 43, the percentage of subjects with RVNA levels ≥0.5 IU/mL (defined as adequate to confer protection from rabies virus infection)30 at Day 15 and Day 43, and also the safety and tolerability of the vaccine according to each regimen, in both age cohorts.
Subjects
The study planned to enroll a total of 640 healthy Chinese volunteers: 240 children aged 6 to 17 years, further divided into 2 age subsets of children: ≥6 to ≤11 years and ≥12 to ≤17 years of age, and 400 older adults aged ≥51, further divided into 2 age subsets of adults: ≥51 to ≤60 years and ≥61 years of age. Subjects within each age subset were randomized in a 1:1 ratio to receive 4 vaccine doses following the Zagreb regimen or 5 doses following the Essen regimen.
Both age cohorts had not been studied previously with PCECV in the Chinese population. The sample sizes estimation and the noninferiority margin used for this clinical trial were derived from the study design and the data obtained in a previous study with PCECV in adult Chinese subjects23. In the previous study, a standard deviation of 2 in log2 scale was observed in the Essen group. Same standard deviation (2 in log2 scale) was used in estimating the sample size for the children cohort and a higher value (2.59 in log2 scale) was assumed for the older adult cohort. With these standard deviations and a 2-sided Type I error of 5%, 105 evaluable subjects in the children cohort, and 176 in the older adults cohort per regimen were needed to assess each of the primary non-inferiority objectives at a 95% power using a 0.5 fold non-inferiority criterion in ratio of GMCs between Zagreb and Essen regimens. The planned enrollment accounted for an approximately 10–15% drop-out rate.
Enrolled subjects were of both genders, and in good health at study entry as judged by the clinical investigator through medical history and physical examination. Main exclusion criteria were allergy to any of the vaccine components; having previously received any rabies vaccine or rabies immune globulin; and receiving or planning to receive antimalarial medications 14 days prior to first vaccination through to study termination; any progressive or severe neurologic disorder, seizure disorder or Guillian-Barré syndrome; known or suspected impairment of the immune system; known bleeding diathesis or any condition that might be associated with a prolonged bleeding time. An additional specific exclusion criterion for subjects 6 to 17 years was to ever have had a malignancy; for adults aged ≥51 years, to have had a malignancy (excluding nonmelanotic skin cancer) or lymphoproliferative disorder within the past 5 years.
PEP Vaccination regimens
After a dose reconstitution, 1 mL of the rabies PCECV (Rabipur®, Chiron Behring Vaccines Pvt. Ltd. manufactured in Ankleshwar, India; Lot number 1980) containing inactivated rabies virus (Flury Low-Egg Passage [LEP] strain), with a potency ≥2.5 IU/mL, was administered intramuscularly to the deltoid muscle based on the regimen assigned after randomization: 2 doses on Day 1, and one dose on each of Days 8 and 22 for subjects in the Zagreb regimen, and one dose on each of Days 1, 4, 8, 15, and 29 for subjects in the Essen regimen.
Immunogenicity assessment
Blood samples (approximately 5 mL) for immunogenicity testing were obtained from subjects prior to vaccination (Day 1 and Day 15), and at Day 43 (−2/+3 days). Blood draw at day 7 for immunogenicity testing was not included in the study design because it was considered not adding additional information; results from a previous clinical registration trial in Chinese adults23 showed in fact no more than 10% of subject with RVNA concentrations ≥0.5 IU/mL after only 2 vaccine doses (day 7). RVNA concentration levels were determined by means of a Rapid Fluorescent Focus Inhibition Test (RFFIT),31 with rabies virus strain CVS-11 as the challenge virus for the assay, carried out at the National Institutes for Food and Drug Control (NIFDC) laboratory in China.
Safety assessment
The occurrence of immediate adverse reactions was monitored for 30 minutes after each vaccination at the site; the frequency and severity of all solicited adverse events (AEs) were recorded for up to 7 days following each vaccination; unsolicited AEs were recorded throughout the study up to Day 43. Solicited local AEs were erythema, induration, and pain at the site of injection; solicited systemic AEs included loss of appetite, nausea, headache, myalgia, fatigue, arthralgia; other indicators of vaccine reactogenicity were fever (defined as an axillary temperature ≥38ºC) and use of analgesics/antipyretics. The relationship of the study treatment to an AE was to be determined by the investigator, who also determined the severity and the seriousness of unsolicited AEs.
Statistical analysis
Descriptive demographic statistics at enrollment were summarized by PEP regimen. For the immunogenicity objectives, the per-protocol set (PPS) was used as the primary analysis set, and defined to include subjects who correctly received the vaccine according to the regimen that they were randomized to, who provided an evaluable serum sample at Day 15 or Day 43 and who had no major protocol deviations.
GMCs of RVNA and associated 2-sided 95% CIs were calculated by exponentiating the least square means and the lower and upper limits of the 95% CIs of the log transformed titers for each PEP regimen. The ratio of GMCs for each age cohort at day 15 between the Zagreb and Essen PEP regimens was computed by a 2-way Analysis of Variance (ANOVA) adjusting for factors of regimen and age subset.
Non-inferiority of the Zagreb regimen to the Essen regimen was demonstrated if the lower limit of the 2-sided 95% CI for the ratio of GMCs between regimens was >0.5. Moreover, for each age cohort the percentage of subjects with RVNA concentrations ≥0.5 IU/mL and the associated 2-sided 95% Clopper-Pearson CIs was computed by PEP regimen at all applicable visits. Safety was analyzed for all subjects exposed to PCECV who provided post-vaccination safety data, and was summarized by regimen, providing the frequency and proportion of subjects reporting an event. All statistical analyses were conducted at the Biostatistics and Clinical Data Management (BCDM) group of Novartis Vaccines using SAS software version 9.2.
Results
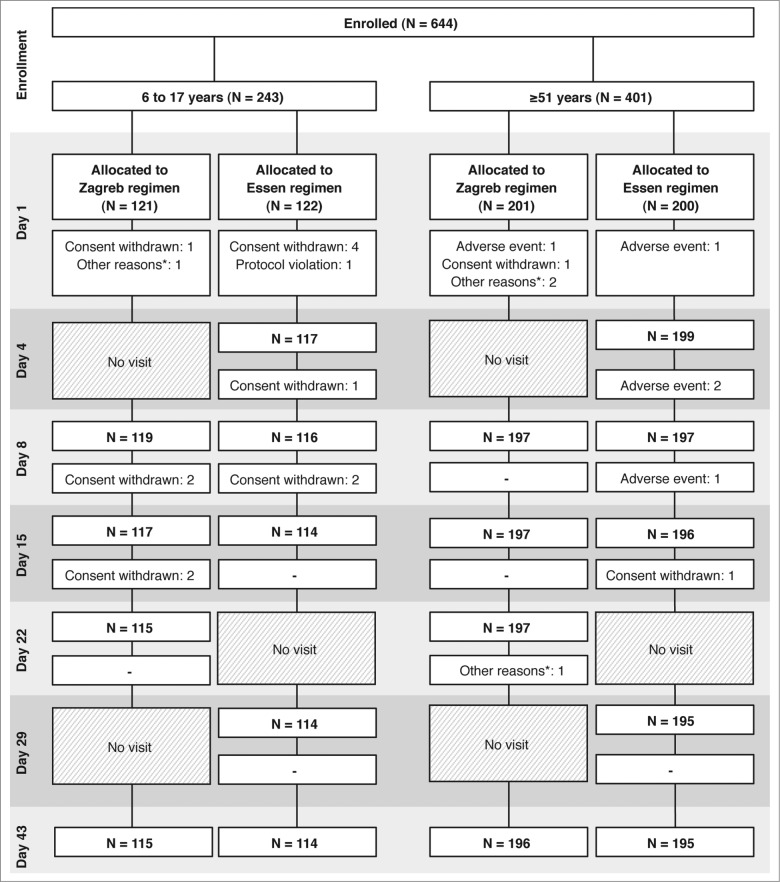
A total of 243 children aged 6 to 17 years were enrolled; 121 of them were assigned to the Zagreb regimen, and 122 to the Essen regimen; 115 (95%) and 114 (93%) of subjects completed the study on Day 43, respectively (Fig. 1). A total of 401 subjects ≥51 years were enrolled; 201 were assigned to the Zagreb regimen, and 200 to the Essen regimen; 196 (98%) and 195 (98%) subjects completed the study on Day 43, respectively (Fig. 1).
Figure 1.
Flow diagram of the trial. *Other reasons included that the subject went out (2 subjects at Day 1, both in the Zagreb regimen, 1 in the 6 to 17 years cohort, the other in the ≥51 years cohort); screening failure (1 subject at Day 1, in the Zagreb regimen, and in the ≥51 years cohort); and withdrawal of consent for continuing study participation (1 subject at Day 22 in the Zagreb regimen, and in the ≥51 years cohort).
Except for sex, the demographics and other baseline characteristics of subjects in both age cohorts were balanced across the 2 vaccine regimens (Table 1). The overall mean age in the 6 to 17 cohort was 10.9 ± 3 years; in the ≥51 age cohort, the overall mean age was 62.0 ± 6.6 years. In both age cohorts, overall the majority of subjects was female (51% in the 6 to 17 years, and 60% in the ≥51 cohort), and in the Zagreb regimen the proportion of enrolled females was lower than in the Essen regimen for both age cohorts (40% vs. 62% in the 6 to 17 years cohort, and 59% vs. 62% in the ≥51 cohort).
Table 1.
Summary of demographic characteristics of subjects enrolled in the study, by age cohort
| Children 6 to 17 years (N = 243) | Adults ≥51 years (N = 401) | |||
|---|---|---|---|---|
| Characteristic | Zagreb regimen (N = 121) | Essen regimen (N = 122) | Zagreb regimen (N = 201) | Essen regimen (N = 200) |
| Age, mean (SD), years | 11.0 (3.0) | 10.8 (2.9) | 62.1 (6.5) | 61.9 (6.8) |
| Gender, n (%) | ||||
| Male | 72 (60) | 46 (38) | 83 (41) | 77 (39) |
| Female | 49 (40) | 76 (62) | 118 (59) | 123 (62) |
| Weight, mean (SD), kg | 33.59 (11.57) | 32.85 (10.95) | 52.93 (9.36) | 52.57 (8.53) |
| Height, mean (SD), cm | 139.1 (16.1) | 137.9 (16) | 153.5 (7.3) | 153.2 (8.1) |
Immunogenicity
At Day 15 there was an increase in GMCs from baseline following both PEP regimens in children aged 6 to 17 years (12 and 14 IU/mL in Zagreb and Essen regimen, respectively) (Fig. 2). The ratio of GMCs between the Zagreb and Essen regimens (GMR) was 0.84 (95% CI: 0.69 −1.02), therefore meeting the non-inferiority criterion of the Zagreb to the Essen regimen (lower limit of the 95% CI GMR >0.5). At Day 43, GMCs were similar to those observed at Day 15 in the 4-dose Zagreb regimen (13.0 IU/mL), while, as expected, there was a further increase following a 5-dose Essen regimen (24 IU/mL).
Figure 2.
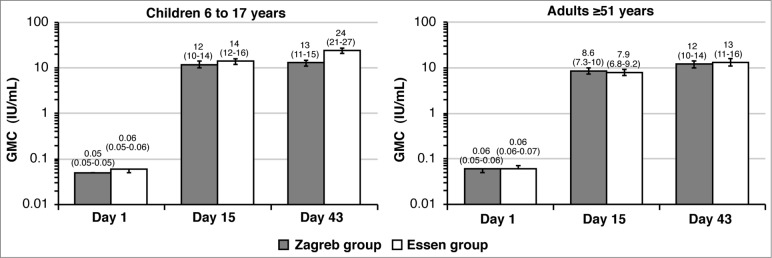
Rabies virus neutralizing antibody concentrations (GMC) in the Zagreb and Essen regimens (PP set) on Days 1, 15, and 43, by age cohort. Error bars and values in parenthesis represent 95% CI.
In older adults ≥51 years there was also a robust increase in GMCs from baseline, which at Day 15 reached a mean of 8.57 IU/mL for the Zagreb regimen, and 7.89 IU/mL in the Essen regimen (Fig. 2). The GMR Zagreb/Essen was 1.1 (95% CI: 0.87 −1.35), also demonstrating non-inferiority of the Zagreb to the Essen regimen in this age cohort. At Day 43, GMCs had increased from Day 15, with a similar trend between groups: 12 IU/mL and 13 IU/mL for the Zagreb and Essen regimen, respectively.
All children achieved RVNA concentrations ≥0.5 IU/mL at Day 15 and at Day 43 (Fig. 3) following both PEP regimens. In older adults, the percentage of subjects with adequate antibody concentrations was 99% at Day 15, and 100% at Day 43, irrespectively of the PEP regimen (Fig. 3).
Figure 3.
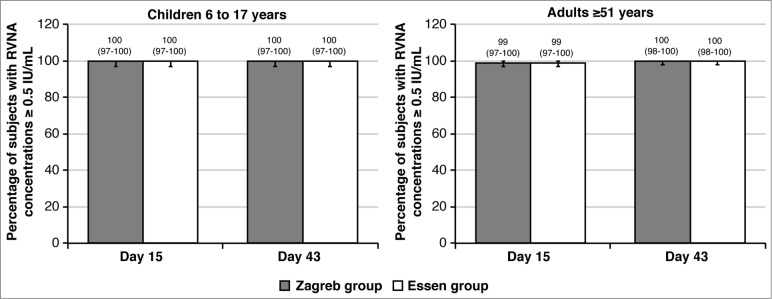
Percentage of subjects with RVNA concentrations ≥0.5 IU/mL in the Zagreb and Essen regimens (PP set) on Days 15, and 43, by age cohort. Error bars and values in parenthesis represent 95% CI.
The analysis by age subset (children [6 to 11 years], adolescents [12 to 17 years], adults aged 51 to 60 years and ≥61 years) showed comparable results for all immunogenicity outcome variables as the overall cohorts of children or older adults.
Safety
Overall, 52% and 51% of the children aged 6 to 17 years reported solicited AEs after any vaccine dose following the Zagreb and Essen regimens, respectively. In older adults aged ≥51 years, the overall percentage of subjects reporting solicited AEs after any vaccination was 19% in the Zagreb regimen and 24% in the Essen regimen. After any vaccination, pain at the injection site was the most frequently reported solicited local AE, in both age cohorts (Table 2). Injection site pain was in fact observed in 38% and 40% of subjects 6 to 17 years in the Zagreb and Essen regimen, respectively, and in 9% and 11% of older adults, respectively. The most commonly observed systemic AE was fatigue in both age cohorts, reported in 15% and 13% of subjects of 6 to 17 years in the Zagreb and Essen regimens, respectively, and in 5% of the Zagreb and 4% of the Essen regimen in the older adults. Most of the solicited local and systemic AEs were mild to moderate in intensity.
Table 2.
Percentage of subjects with any and severe solicited local and systemic AEs, and other indicators of reactogenicity, from 6 hours to 7 days following any vaccination
| Children 6 to 17 years | Adults ≥51 years | |||
|---|---|---|---|---|
| Zagreb regimen (N = 119) | Essen regimen (N = 118) | Zagreb regimen (N = 197) | Essen regimen (N = 200) | |
| Local AEs, n (%) | ||||
| Erythema | ||||
| Any | 2 (2) | 1 (1) | 1 (1) | 3 (2) |
| Severe (>100 mm) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Induration | ||||
| Any | 2 (2) | 0 (0) | 1 (1) | 1 (1) |
| Severe (>100 mm) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Injection site pain | ||||
| Any | 45 (38) | 47 (40) | 18 (9) | 22 (11) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Systemic AEs, n (%) | ||||
| Loss of appetite | ||||
| Any | 14 (12) | 10 (8) | 0 (0) | 2 (1) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | ||||
| Any | 7 (6) | 10 (8) | 2 (1) | 2 (1) |
| Severe | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Headache | ||||
| Any | 11 (9) | 10 (8) | 7 (4) | 8 (4) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myalgia | ||||
| Any | 11 (9) | 13 (11) | 0 (0) | 2 (1) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | ||||
| Any | 18 (15) | 15 (13) | 9 (5) | 7 (4) |
| Severe | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Arthralgia | ||||
| Any | 3 (3) | 1 (1) | 3 (2) | 3 (2) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fever (≥38°C) | ||||
| Yes | 8 (7) | 3 (3) | 5 (3) | 5 (3) |
| No | 111 (93) | 115 (97) | 192 (97) | 195 (98) |
| Other | ||||
| Use of analgesics/antipyretics | N = 119 | N = 118 | N = 197 | N = 200 |
| Yes | 11 (9) | 8 (7) | 13 (7) | 15 (8) |
| No | 108 (91) | 110 (93) | 184 (93) | 185 (93) |
| Body temperature ≥ 37.1°C | N = 30 | N = 25 | N = 13 | N = 19 |
| Low (37.1°C–37.5°C) | 17 (57) | 18 (72) | 6 (46) | 11 (58) |
| Medium (37.6°C–39°C) | 13 (43) | 7 (28) | 6 (46) | 8 (42) |
| (High >39ºC) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
The incidence of unsolicited AEs in children was 21% in the Zagreb regimen and 24% in the Essen regimen, and only 7% and 5% of the cases, respectively, were reported as at least possibly related to the vaccination. In older adults, 18% in the Zagreb regimen and 20% in the Essen regimen reported unsolicited AEs; 3% and 8% of them, respectively, were considered at least possibly related to the study vaccine. In both age cohorts, and regardless of the PEP regimen followed, the most commonly reported unsolicited AE (≥2% subjects) was upper respiratory tract infection. No SAEs were reported in the children's cohort, and none of the subjects withdrew prematurely due to an AE. In adults ≥51 years, 1 subject experienced a SAE, which was judged as not vaccine-related (a case of moderate acute pancreatitis in a 72 years old female with onset 40 day after first vaccine dose; the subject had significant medical history of gall stones; the subject was discharged from the hospital after 15 days, with complete resolution of the AE), and 5 subjects were prematurely withdrawn due to AEs: 1 in the Zagreb regimen (due to myocardial ischemia), and 4 in the Essen regimen (1 subject had mild tachycardia, 1 subject had mild headache, pain and pyrexia; 1 subject experienced moderate back pain, and 1 subject had moderate fatigue and headache persisting more than 7 days after vaccination). No deaths were reported during the study.
Discussion
IM rabies vaccination, after exposure to the virus (PEP) and when administered in a timely manner, is the only effective treatment and life-saving intervention to prevent the disease. The 5-dose PEP Essen regimen gives reliable post-exposure immunization, and has been widely used in developed and developing countries for several decades. In 1992, the WHO started to recommend the abbreviated 2-1-1 Zagreb vaccine regimen in order to reduce costs and offer a more simple and economical vaccination course with acceptable safety, immunogenicity and efficacy profiles.32 Since 2010, the Zagreb regimen is recommended over the 5-dose Essen regimen by the Advisory Committee of Immunization Practices, as well as a reduced 4-dose Essen regimen (each dose on days 0, 3, 7, and 14) for healthy immunocompetent adults.10
In this study, PEP with PCECV via IM route elicited a strong immune response at Day 15 when administered under either the Zagreb or Essen regimen, and immune responses following Zagreb regimen were found to be non-inferior to those induced by 5-dose Essen regimen in all age cohorts.
All subjects at the end of the study, in both PEP regimens and in both age cohorts achieved RVNA concentrations ≥0.5 IU/mL. These results are in agreement with previous results obtained with healthy adult subjects (18 to 50 years) vaccinated under the Zagreb PEP regimen with PCECV or other licensed vaccines.9,11,19,23 As expected, RVNA concentrations resulted higher in the children than in older adults, especially after the fifth dose of vaccine received following Essen regimen.
The results suggested a lower overall rate of AEs after the first vaccination among subjects ≥51 years compared to the children and adolescents. However, there were no significant differences in the frequency and nature of reported AEs between either post-exposure regimens in any of the age cohorts. The incidence of AEs resulting in premature withdrawal from the study was low and was observed at a similar frequency for both regimens.
In China, 90% of human rabies cases occur in rural areas,5,33 and besides difficult or delayed access to public health services, the lack of proper or complete PEP is one of the major causes of treatment failure.2,6 Indeed, recent epidemiological data indicate that among subjects who sought medical advice and received PEP, only 77% to 78% were actually compliant with the full vaccination course for contact categories requiring vaccination, and the study noted that compliance dropped significantly after the third dose.34 Therefore, it is foreseeable that beyond strict adherence to standard WHO and ACIP recommendations, compliance with the full vaccine course might be eased by the adoption of the abbreviated Zagreb regimen.
In summary, the results of the present study confirm that post-exposure rabies vaccination with PCECV is well tolerated and immunogenic with an acceptable safety profile in healthy Chinese children and older populations, and that the immune response induced under the abbreviated 4-dose Zagreb IM regimen is non-inferior to the response obtained following the 5-dose Essen regimen in subjects 6-years of age and older.23
Acknowledgments
The authors wish to thank Dr. Hari Sai Priya Baddela (Novartis Healthcare Private Limited, Hyderabad, India) and Dr. Mònica Gratacòs (CHC-Europe) for editorial assistance in the preparation of this manuscript.
Disclosure of Potential Conflicts of Interest
Dr. M. Pellegrini is a Novartis Vaccines and Diagnostics employee; F. Xie is a statistical consultant to Novartis Vaccines and Diagnostics. All other authors declare no conflicts of interest.
Funding
The study was funded by Novartis Vaccines.
Author Contributions
All authors contributed to the content, drafting, critical revision and approval of this manuscript.
References
- 1. WHO Expert Consultation on Rabies . Second report. World Health Organ Tech Rep Ser 2013; 982:1-139, back cover; PMID:24069724 [PubMed] [Google Scholar]
- 2. Yin W, Dong J, Tu C, Edwards J, Guo F, Zhou H, Yu H, Vong S. Challenges and needs for China to eliminate rabies. Infect Dis Poverty 2013; 2:23; PMID:24088366; http://dx.doi.org/ 10.1186/2049-9957-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Health and Family Planning Commission Chinese notificable disease surveillance monthly report. http://www.moh.gov.cn/zhuzhan/yqxx/201304/b540269c8e5141e6bb2d00ca539bb9f7.shtml [Google Scholar]
- 4. Zhang L, Wilson DP. Trends in notifiable infectious diseases in China: implications for surveillance and population health policy. PLoS ONE 2012; 7:e31076; PMID:22359565; http://dx.doi.org/ 10.1371/journal.pone.0031076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin CP, Zhou H, Wu H, Shen XX, Wang LH, Yin WW, Wang SM, Tang Q. Epidemiological analysis of rabies in 2010, China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011; 25:434-6; PMID:22734228 [PubMed] [Google Scholar]
- 6. Si H, Guo ZM, Hao YT, Liu YG, Zhang DM, Rao SQ, Lu JH. Rabies trend in China (1990–2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis 2008; 8:113; PMID:18717989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu R, Tang Q, Tang J, Fooks AR. Rabies in China: an update. Vector Borne Zoonotic Dis 2009; 9:1-12; PMID:18803503; http://dx.doi.org/ 10.1089/vbz.2008.0046 [DOI] [PubMed] [Google Scholar]
- 8. Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. J Neurovirol 2005; 11:93-100; PMID:15804967 [DOI] [PubMed] [Google Scholar]
- 9. Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. . Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009; 27:7141-8; PMID:19925944; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.029 [DOI] [PubMed] [Google Scholar]
- 10. Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, et al. . Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2010; 59:1-9. [PubMed] [Google Scholar]
- 11. Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7:220-4; PMID:21311216 [DOI] [PubMed] [Google Scholar]
- 12. Scheiermann N, Baer J, Hilfenhaus J, Marcus I, Zoulek G. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC)-rabies vaccine in man. Zentralbl Bakteriol Mikrobiol Hyg A 1987; 265:439-50; PMID:2445127 [DOI] [PubMed] [Google Scholar]
- 13. Quiambao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerczyk C. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine 2005; 23:1709-14; PMID:15705476; http://dx.doi.org/ 10.1016/j.vaccine.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 14. Dobardzic A, Izurieta H, Woo EJ, Iskander J, Shadomy S, Rupprecht C, Ball R, Braun MM. Safety review of the purified chick embryo cell rabies vaccine: Data from the Vaccine Adverse Event Reporting System (VAERS), 1997-2005. Vaccine 2007; 25:4244-51; PMID:17382435 [DOI] [PubMed] [Google Scholar]
- 15. Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S, Wasi C. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ 2000; 78:693-8; PMID:10859864 [PMC free article] [PubMed] [Google Scholar]
- 16. Sampath G, Parikh S, Sangram P, Briggs DJ. Rabies post-exposure prophylaxis in malnourished children exposed to suspect rabid animals. Vaccine 2005; 23:1102-5; PMID:15629352 [DOI] [PubMed] [Google Scholar]
- 17. Ambrozaitis A, Laiskonis A, Balciuniene L, Banzhoff A, Malerczyk C. Rabies post-exposure prophylaxis vaccination with purified chick embryo cell vaccine (PCECV) and purified Vero cell rabies vaccine (PVRV) in a four-site intradermal schedule (4-0-2-0-1-1): an immunogenic, cost-effective and practical regimen. Vaccine 2006; 24:4116-21; PMID:16545510 [DOI] [PubMed] [Google Scholar]
- 18. Madhusudana SN, Anand NP, Shamsundar R. Economical multi-site intradermal regimen with purified chick embryo cell vaccine (Rabipur) prevents rabies in people bitten by confirmed rabid animals. Int J Infect Dis 2002; 6:210-4; PMID:12718837; http://dx.doi.org/ 10.1016/S1201-9712(02)90113-X [DOI] [PubMed] [Google Scholar]
- 19. Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother 2014; 10:1645-9; PMID:24632727; http://dx.doi.org/ 10.4161/hv.28420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malerczyk C, Briggs DJ, Dreesen DW, Banzhoff A. Duration of immunity: an anamnestic response 14 years after rabies vaccination with purified chick embryo cell rabies vaccine. J Travel Med 2007; 14:63-4; PMID:17241256 [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization , WHO expert consultation on rabies. First report, WHO Tech Rep Ser 931, Geneva, WHO. 2005. [PubMed] [Google Scholar]
- 22. Ministry of Health, People's Republic of China. Situation of rabies prevention and control in China http://www.moh.gov.cn, 2009. [Google Scholar]
- 23. Ma J, Wang H, Li J, Xie Y, Liu Z, Zhao Y, Malerczyck C. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb Regimen (2-1-1) sompared with the Essen Regimen in Chinese adults. Human Vaccines and Immunotherapeutics 2014; 10:2805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eng TR, Fishbein DB, Talamante HE, Hall DB, Chavez GF, Dobbins JG, Muro FJ, Bustos JL, de los Angeles Ricardy M, Munguia A, et al. Urban epizootic of rabies in Mexico: epidemiology and impact of animal bite injuries. Bull World Health Organ 1993; 71:615-24; PMID:8261565 [PMC free article] [PubMed] [Google Scholar]
- 25. Coleman PG, Fevre EM, Cleaveland S. Estimating the public health impact of rabies. Emerg Infect Dis 2004; 10:140-2; PMID:15078611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leder K, Weller PF, Wilson ME. Travel vaccines and elderly persons: review of vaccines available in the United States. Clin Infect Dis 2001; 33:1553-66; PMID:11588700 [DOI] [PubMed] [Google Scholar]
- 27. Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078-84; PMID:18444828; http://dx.doi.org/ 10.1086/529197 [DOI] [PubMed] [Google Scholar]
- 28. Dorrington MG, Bowdish DM. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol 2013; 4:171; PMID:23825474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al. . Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1-28; PMID:18496505 [PubMed] [Google Scholar]
- 30. WHO Publication . Rabies vaccines: WHO position paper–recommendations. Vaccine 2010; 28:7140-2; PMID:20831913 [DOI] [PubMed] [Google Scholar]
- 31. Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand 1979; 7:213-20; PMID:387798 [DOI] [PubMed] [Google Scholar]
- 32. WHO expert committee on rabies Guide for post-exposure treatment. 8th report. WHO tecnical report 824. Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
- 33. Song M, Tang Q, Wang DM, Mo ZJ, Guo SH, Li H, Tao XY, Rupprecht CE, Feng ZJ, Liang GD. Epidemiological investigations of human rabies in China. BMC Infect Dis 2009; 9:210; PMID:20025742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang CL, Zhang XW, Yu YX. Study on the compliance and economic cost of rabies vaccination. Zhongguo Yi Miao He Mian Yi 2010; 16:254-7; PMID:20726270 [PubMed] [Google Scholar]