Abstract
Despite the benefits of vaccination for health care workers (HCWs), uptake of recommended vaccinations is low, particularly for seasonal influenza and pertussis. In addition, there is variation in uptake within hospitals. While all vaccinations recommended for HCWs are important, vaccination against influenza and pertussis are particularly imperative, given HCWs are at risk of occupationally acquired influenza and pertussis, and may be asymptomatic, acting as a reservoir to vulnerable patients in their care. This study aimed to determine predictors of uptake of these vaccinations and explore the reasons for variation in uptake by HCWs working in different hospital wards. HCWs from wards with high and low influenza vaccine uptake in a tertiary pediatric and obstetric hospital completed a questionnaire to assess knowledge of HCW recommended immunizations. Multiple logistic regression was used to determine predictors of influenza and pertussis vaccination uptake. Of 92 HCWs who responded, 9.8% were able to identify correctly the vaccines recommended for HCWs. Overall 80% of respondents reported they had previously received influenza vaccine and 50.5% had received pertussis vaccine. Independent predictors of pertussis vaccination included length of time employed in health sector (P < 0.001), previously receiving hepatitis B/MMR (measles, mumps, rubella) vaccine (P < 0.001), and a respondent being aware influenza infections could be severe in infants (p = 0.023). Independent predictors of seasonal influenza vaccination included younger age (P < 0.001), English as first language (P < 0.001), considering it important to be vaccinated to protect themselves (P < 0.001), protect patients (p = 0.012) or awareness influenza could be serious in immunocompromised patients (p = 0.030). Independent predictors for receiving both influenza and pertussis vaccinations included younger age (P < 0.001), time in area of work (P = 0.020), previously receiving hepatitis B vaccine (P = 0.006) and awareness influenza could be severe in infants (P < 0.001). A knowledge gap exists around HCW awareness of vaccination recommendations. Assessment of the risk/benefit value for HCWs and their patients, determines uptake of HCW immunization programs and should be considered in promotional HCW vaccination programs.
Keywords: healthcare workers, hospitals, influenza, pertussis, vaccination
Abbreviations
- HCW
health care worker
- MMR
Measles Mumps Rubella
- HUW
high uptake ward
- LUW
low uptake ward
Introduction
The benefits of health care worker (HCW) vaccination are well documented.1-7 Despite this, HCW uptake of recommended vaccines is low2,8-16, particularly for seasonal influenza and pertussis.13,16-19 In Australia, both pertussis and seasonal influenza vaccines are recommended for all HCWs17 however providing free influenza vaccine is a policy decision for each hospital or state and is not uniform across Australia.20
Influenza virus infection causes a wide spectrum of disease, from no or minimal symptoms, to respiratory illness with systemic features, and/or multisystem complications and death from primary viral or secondary bacterial pneumonia.17 Most severe influenza cases and deaths occur among people with chronic medical conditions, in infants and young children, the elderly and pregnant women.2 HCWs are exposed to patients with influenza in the workplace and are at risk of occupationally acquired influenza and transmission of infection to patients and other HCWs.2
Bordetella pertussis is a highly contagious bacterium causing respiratory illness that may result in significant morbidity including pneumonia, convulsions, apnoea, encephalopathy, acute respiratory distress and in death21, with infants aged less than 6 months of age having the highest rate of pertussis related complications.22 HCWs have a higher risk of exposure to pertussis due to occupational contact and may be asymptomatic, acting as a reservoir to vulnerable patients in their care.23-25 With low uptake of pertussis vaccine among HCWs13,18,26 nosocomial outbreaks have been reported.4,27-31
In the workplace environment, it is plausible that vaccination recommendations and practices for some vaccinations may influence the uptake of other vaccines. In addition, if there exists a culture of poor support from management and peers and there is misinformation about official recommendations and vaccination benefits, then it may be difficult for staff to seek out this preventative health measure. The influence that family and friends can have new parents vaccinating their baby it is well documented. Equally, HCWs are likely to be effected by those around them when making decisions related to vaccinations, particularly when their co-workers are also recommended to receive them.
No studies have explored differential uptake within the same occupational category, with little documented about staff attitudes and behavior toward seasonal influenza vaccination within the ward culture.
At one metropolitan pediatric hospital, we identified that despite access to the same seasonal influenza vaccination program, differential uptake between hospital wards exists.
The aims of this study were to: examine reasons for differential uptake of the influenza and pertussis vaccines by HCWs working in different hospital wards and describe and explore motivators and barriers to uptake of the seasonal influenza vaccine by HCWs. A secondary outcome was to explore the relationship between uptake of seasonal influenza vaccination and uptake of pertussis vaccination by HCWs.
Results
Survey population
At the time of the survey the hospital's Workforce Reporting & Analysis Directorate estimated that approximately 196 full or part-time staff worked on the 3 high uptake wards (HUW) and approximately 227 staff worked on the 3 low uptake wards (LUW). We were unable to provide a breakdown of the male/female staff ratios for these wards as we do not have access to demographic data for all ward staff, only those who completed the survey. Ninety-four (94) hard copy questionnaires were returned. We are unaware of how many staff knew about the survey or how many took a survey and didn't return it. No respondents selected to complete the survey online. Data were excluded from 2 respondents; one a nursing student who had not previously worked at the hospital and another had completed only demographic questions. There was an even distribution of respondents by age. The majority of respondents were female (94.6%), worked part-time (63%) or full time (35.9%), from Australia (80.4%) and spoke English as their first language (92.4%) (Table 1). A higher proportion of respondents were from HUWs (59.8%) than LUWs (40.2%) however this difference was not significant (Pearson's Chi-Square 1.840 p = 0.060).
Table 1.
Demographic details of participants
| Variable | Levels | Number of respondents | Percent % |
|---|---|---|---|
| Age Group (years) | 21–30 | 28 | 30.4% |
| 31–40 | 26 | 28.3% | |
| 41–50 | 22 | 23.9% | |
| >50 | 16 | 17.4% | |
| Sex | Male | 5 | 5.4% |
| Female | 87 | 94.6% | |
| Work Status | Full time | 33 | 35.9% |
| Part time | 58 | 63.0% | |
| Casual | 1 | 1.1% | |
| Country of birth | Australia | 74 | 80.4% |
| United Kingdom | 9 | 9.8% | |
| other EU countries | 5 | 5.4% | |
| Asia | 2 | 2.2% | |
| Refused | 1 | 1.1% | |
| Africa | 1 | 1.1% | |
| First language is English | No | 7 | 7.6% |
| Yes | 85 | 92.4% | |
| Highest educational qualification obtained | Certificate/Diploma | 12 | 13.2% |
| High school certificate or less | 3 | 3.3% | |
| Bachelor | 54 | 59.3% | |
| Post Grad certificate/Diploma | 18 | 19.8% | |
| PhD | 1 | 1.1% | |
| Other* | 3 | 3.3% | |
| Category of Health Care Worker | Nurse | 75 | 81.5% |
| Admin Staff | 3 | 3.3% | |
| Doctor | 1 | 1.1% | |
| Allied Health | 2 | 2.2% | |
| Other (not specified) | 5 | 5.4% | |
| Midwife | 6 | 6.5% | |
| Time working in health sector | < 12 months | 5 | 5.4% |
| 1–5 years | 23 | 25.0% | |
| 6–10 years | 15 | 16.3% | |
| 11–15 years | 14 | 15.2% | |
| 16+ years | 35 | 38.0% | |
| Time working in area / ward | < 12 months | 13 | 14.3% |
| 1–5 years | 30 | 33.0% | |
| 6–10 years | 19 | 20.9% | |
| 11–15 years | 8 | 8.8% | |
| 16+ years | 21 | 23.1% | |
| Hours per week in area/ward | <15 hours | 1 | 1.1% |
| 15–30 hours | 33 | 35.9% | |
| 30+hours | 58 | 63.0% | |
| High or Low uptake ward of seasonal influenza vaccine in 2012 | High uptake ward | 55 | 59.8% |
| Low uptake ward | 37 | 40.2% |
*College membership =1, Hospital based nurse/midwife training = 1, Masters =1.
Knowledge and understanding of workplace vaccinations
The majority (95.5%) of HCWs were aware there are vaccines recommended specifically for HCWs, however only 9.8% (n = 9) could correctly identify them (Table 2) and while more respondents from HUWs (10.9%) were able to identify all of them than LUWs (8.1%) this was not significant (Fisher's Exact Test p = 0.736). In terms of vaccination history, only 16.5% of respondents reported having ever received all HCW recommended vaccines (MMR, pertussis, influenza, hepatitis B and varicella) with more respondents from HUWs (20%) receiving them than LUWs (11.1%) (Table 3) however this was not significant (Fisher's Exact Test p = 0.388). There were no significant differences between the proportions of HCWs from HUWs compared to LUWs in terms of identifying vaccines recommended specifically for HCWs or having received any of the recommended vaccines using Fisher's Exact Test.
Table 2.
Knowledge of recommended health care worker vaccines
| Could you please list vaccines you think are recommended for health care workers? | Overall | High uptake wards | Low uptake wards | |||
|---|---|---|---|---|---|---|
| Number | Percent % | Number | Percent % | Number | Percent% | |
| Listed all recommended (MMR, Pertussis, Influenza, Hep B & Varicella) | 9 | 9.8% | 6 | 10.9 | 3 | 8.1 |
| Diptheria | 10 | 10.9% | 8 | 14.5% | 2 | 5.4% |
| Tetanus | 22 | 23.9% | 17 | 30.9% | 5 | 13.5% |
| Pertussis | 51 | 55.4% | 34 | 61.8% | 17 | 45.9% |
| measles-mumps-rubella | 28 | 30.4% | 20 | 36.4% | 8 | 21.6% |
| Measles | 1 | 1.1% | 1 | 1.8% | 0 | 0.0% |
| Mumps | 1 | 1.1% | 1 | 1.8% | 0 | 0.0% |
| Rubella | 2 | 2.2% | 1 | 1.8% | 1 | 2.7% |
| Influenza | 79 | 85.9% | 45 | 81.8% | 34 | 91.9% |
| Polio | 2 | 2.2% | 2 | 3.6% | 0 | 0.0% |
| Hepatitis B | 60 | 65.2% | 35 | 63.6% | 25 | 67.6% |
| Hepatitis A | 19 | 20.7% | 15 | 27.3% | 4 | 10.8% |
| Hepatitis (not specified) | 5 | 5.4% | 4 | 7.3% | 1 | 2.7% |
| Tuberculosis | 13 | 14.1% | 9 | 16.4% | 4 | 10.8% |
| Varicella | 19 | 20.7% | 13 | 23.6% | 6 | 16.2% |
| Othera | 10 | 10.9% | 8 | 14.5% | 2 | 5.4% |
aOther included vaccines and comments: H1N1 n = 1, ‘All childhood vaccines/diseases’ n = 2, ‘Flu A’ n = 1, ‘Swine flu’ n = 1, ‘Hep C’ n = 2, ‘and they should include Hep A’ n = 1, ‘free immunology, TB test’ n = 1.
Table 3.
Health care worker vaccination history
| Which of the following vaccinations have you ever received (either at this workplace or elsewhere)? | Overall | High uptake wards | Low uptake wards | |||
|---|---|---|---|---|---|---|
| Numbera | Percent % | Number | Percent % | Numbera | Percent % | |
| Had received all recommended vaccines (MMR, Pertussis, Influenza, Hepatitis B &Varicella) | 15 | 16.5% | 11 | 20.0% | 4 | 11.1% |
| HepB | 60 | 65.9% | 39 | 70.9% | 21 | 58.3% |
| Pertussis | 46 | 50.5% | 31 | 56.4% | 15 | 41.7% |
| Varicella | 22 | 24.2% | 16 | 29.1% | 6 | 16.7% |
| BCG | 15 | 16.5% | 10 | 18.2% | 5 | 13.9% |
| Influenza | 87 | 95.6% | 53 | 96.4% | 34 | 94.4% |
| MMR | 36 | 39.6% | 24 | 43.6% | 12 | 33.3% |
| HepA | 27 | 29.7% | 14 | 25.5% | 13 | 36.1% |
| Tetanus | 4 | 4.4% | 4 | 7.3% | 0 | 0.0% |
| Typhoid | 5 | 5.5% | 4 | 7.3% | 1 | 2.7% |
| Otherb | 4 | 4.4% | 4 | 4.4% | 0 | 0.0% |
aMissing data n = 1 (one respondent provided no response).
bOther: Diphtheria n = 1, Small pox n = 2, Cholera n = 1, Yellow fever n = 1, whatever was required to commence working’ n = 1.
HCWs reported continuing to work despite having symptoms suggestive of influenza and pertussis such as sore throat (75.8%), rhinorrhoea (69.2%), generalized aches and pains (51.6%), persistent cough (30.8%) and fever (20.9%).
The reasons HCWs receive the influenza vaccine was dominated by self-protection (75.6%), followed by protection of patients (47.4%) and protecting their family (19.2%) (Fig. 1). This was in contrast to reasons HCWs considered vaccines in general to be important in which 47.6% of respondents were motivated by self-protection, followed by protection of patients (25.6%) and preventing spread of infection, (25.6%). Concern about potential side effects, prevented 15.2% of respondents from receiving vaccines, with an allergic reaction being the greatest concern for this group of respondents (50.0%) followed by muscle aches and pains (42.9%) soreness at injection site (35.7%), high fever (35.7%) or getting the disease (35.7%), red arm (14.3%) and fainting (7.1%).
Figure 1.
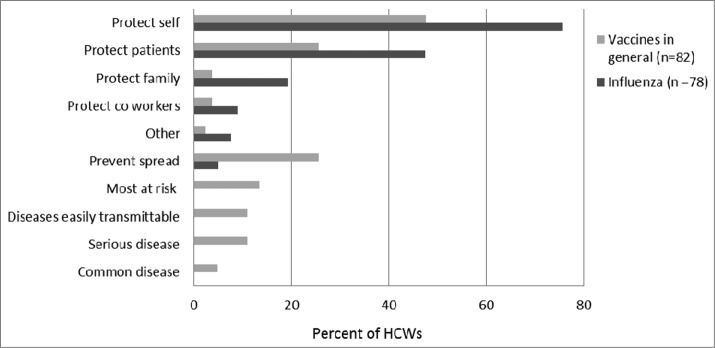
Reasons Health Care Workers receive vaccinations.
Hospital influenza immunization program
All respondents were aware of the hospital's annual influenza vaccination program, with 92.4% reporting they had ever received the seasonal influenza vaccine through the program. A greater proportion of HCWs from HUWs (94.5%) reported that they had ever received the seasonal influenza vaccine through the program than LUWs (89.2%) however this was not significant (Fisher's Exact Test p = 0.433). HCWs were aware of the program through promotional materials (33.8%), ward or department communications (23.1%), mobile influenza vaccine trolley (18.5%) and immunization nurse visits (16.9%). A smaller number found out through other HCWs (12.3%) with 9.2% reporting the program was ‘common knowledge’.
Overall of those who had ever participated in the hospital influenza immunization program (n = 85), 81.0% reported they did so annually. More respondents from HUWs (82.4%) reported receiving the vaccine annually than LUWs (78.8%) however this was not significant (Fisher's Exact Test p = 0.778) A small number (8.6%, n = 7) reported issues accessing the program. Issues included not having time to leave the ward for vaccination once they had missed the ‘flu trolley’ and clinics not being available when they worked i.e. night duty/weekends. The majority of respondents (82.1%, n = 69) reported their supervisor being supportive of them leaving their areas to receive the vaccine, others did not feel supported (3.6%, n = 3) or this was not applicable for them (13.1%, n = 11).
85% of HCWs preferred receiving the influenza vaccine at the workplace, 4.5% preferred their family doctor while 5.6% had no preference or did not want to receive it (4.5%). The greatest influence on their preference was convenience (84.2%).
Uptake of recommended vaccines
Differences between high and low uptake wards
The association between working on a HUW/LUW and pertussis and influenza vaccinations was investigated using logistic regression. ‘Ever having received pertussis vaccine' versus work area showed no significant association (OR 1.8 (CI 0.77, 4.2); p = 0.172). Similarly, there was no significant association for 'received influenza vaccine in the last year' vs. ward area (OR 2.2 (CI 0.77, 6.30); (p = 0.137) or between ‘ever having received pertussis vaccine and received influenza vaccine in the last year’ versus ward area (OR 2.11 (CI 0.87, 5.13); (p = 0.099).
Pertussis
Only 55.4% of respondents knew that the pertussis vaccine was recommended for HCWs, with less (50.5%) stating they had received the vaccine. A high proportion (45.6%) of HCWs had looked after a child with complications from pertussis, with a similar proportion (47.6%) thinking the pertussis vaccine was one of the most important vaccines for HCWs to receive. Only 33.0% thought they were at risk of contracting pertussis.
In terms of disease severity, 96.7% thought that pertussis infection in infants <6 months of age could be severe/very severe, 91.2% thought that pertussis was contagious/very contagious and while 96.7% felt it important/ very important to receive the pertussis vaccine to protect their patients, less (82.4%) thought it was important/very important to receive the pertussis vaccine to protect themselves. Most (88.0%) HCWs strongly agreed that a HCW working in a hospital has an obligation to be vaccinated against pertussis.
Predictors associated with pertussis vaccination
Independent predictors of having ever received a pertussis vaccine included ever having received hepatitis B or MMR vaccine and understanding that influenza could be severe in infants (Table 4a). While a HCWs length of time in the health sector was also significantly associated with pertussis vaccination (global P < 0.001), no pair-wise comparisons were significant (Table 4a).
Table 4a.
Logistic Generalized Estimating Equation for ever had pertussis vaccination versus multiple predictors/confounders, accounting for clustering on ward
| Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|
| Variable | Level | Odds Ratio (95% CI) | Stepdown Bonferroni P value | Adjusted Odds Ratio (95% CI) | Global P value |
| Age | 31–40 | 0.60 (0.44, 0.82) | 0.005 | ||
| 41–50 | 0.54 (0.28, 1.04) | ||||
| >50 | 0.50 (0.25, 1.03) | ||||
| 21–30 | 1.0 | ||||
| Highest Education Level | Bachelor vs Diploma or less | 2.97 (1.87, 4.71) | <0.001 | ||
| Bachelor | 1.65 (1.01, 2.69) | ||||
| Diploma or less | 0.56 (0.26, 1.17) | ||||
| Postgraduate | 1.0 | ||||
| Length of time in health sector | <12 months | 2.25 (0.47, 10.67) | <0.001 | 2.75 (0.59, 12.80) | <0.001 |
| 1–5 years | 1.50 (0.51, 4.45) | 3.35 (0.51, 21.99) | |||
| 6–10 years | 2.25 (1.43, 3.54) | 0.92 (0.41, 2.08) | |||
| 11–15 years | 2.70 (1.11, 6.57) | 1.37 (0.52, 3.62) | |||
| 16+ years | 1.0 | 1.0 | |||
| Length of time in area of work | <12 months | 3.66 (1.28, 10.46) | <0.001 | ||
| 1–5 years | 1.32 (0.55, 3.19) | ||||
| 6–10 years | 1.81 (1.16, 2.81) | ||||
| 11–15 years | 2.71 (0.56, 13.02) | ||||
| 16+ years | 1.0 | ||||
| Received Hepatitis B vaccine | Yes | 11.22 (5.23, 24.07) | <0.001 | 18.16 (3.38, 97.47) | <0.001 |
| No | 1.0 | 1.0 | |||
| Received MMR vaccine | Yes | 12.19 (4.48, 33.18) | 0.001 | 9.35 (4.14, 21.13) | <0.001 |
| No | 1.0 | - | 1.0 | ||
| Received Hepatitis A vaccine | Yes | 5.46 (2.77, 10.77) | <0.001 | ||
| No | 1.0 | - | |||
| How severe do you think influenza infections in infants can be? | For every 1 point increase in severity | 1.36 (1.18, 1.57) | 0.002 | 1.63 (1.07, 2.49) | 0.023 |
Seasonal influenza
Despite 85.9% knowing that the seasonal influenza vaccine was recommended, only 80% of HCWs stated they had received it in the last 12 months (n = 72) with 95.6% ever previously receiving it, either as part of the hospital's seasonal influenza program or elsewhere. The majority (80.7%) thought they were at risk of contracting influenza with 60.7% thinking it was an important vaccine for HCWs to receive. 72.2% of HCWs had looked after a patient with complications from influenza.
Overall 90.0% thought it was important to receive the influenza vaccine to protect patients. In terms of specific patients, 100% thought influenza could be severe/very severe in immunocompromised patients, with less (96.7%) thinking influenza in infants could be severe/very severe and 92.1% thought influenza in pregnant women could be severe/very severe. 79.4 % of HCWs strongly agreed that HCWs working in a hospital have an obligation to be vaccinated against seasonal influenza.
Predictors associated with seasonal influenza vaccination
Independent predictors of seasonal influenza vaccine included younger age, English as a first language, believing it was important to be vaccinated to protect themselves, believing it was important to be vaccinated to protect patients, and being aware influenza could be serious in immunocompromised patients (Table 4b). While ever having received the influenza vaccine at the hospital was significant at the univariate level (p = 0.007) it was not retained in the final model.
Table 4b.
Logistic Generalized Estimating Equation for had influenza vaccination in past year vs. multiple predictors/confounders, accounting for clustering on ward
| Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|
| Variable | Level | Odds Ratio (95% CI) | Stepdown Bonferroni P value | Odds Ratio (95% CI) | Global P value |
| Age group | 31–40 | 0.28 (0.14, 0.55) | <0.001 | 0.12 (0.04, 0.37) | <0.001 |
| 41–50 | 0.43 (0.10, 1.87) | 0.26 (0.04, 1.90) | |||
| >50 | 0.81 (0.21, 3.20) | 0.38 (0.07, 1.99) | |||
| 21–30 | 1.0 | 1.0 | |||
| English as first language | English first language | 6.57 (2.82, 15.33) | 0.002 | 5.29 (1.90, 14.71) | 0.002 |
| English not first language | 1.0 | ||||
| Aware pertussis was a recommended vaccine for health care workers | Yes | 1.75 (1.33, 2.31) | 0.008 | ||
| No | 1.0 | ||||
| How important do you think it is to receive the influenza vaccine annually to protect yourself? | Important | 6.40 (3.79, 10.80) | <.001 | 3.36 (2.04, 5.54) | <0.001 |
| Not important | 1.0 | 1.0 | |||
| How severe do you think influenza infections in immunocompromised patients can be? | For every 1 point increase in severity | 1.89 (1.45, 2.46) | 0.003 | 1.62 (1.05, 2.51) | 0.030 |
| How important do you think it is to receive the influenza vaccine to protect patients? | For every 1 point increase in severity | 1.48 (1.20, 1.82) | 0.026 | 1.29 (1.06, 1.58) | 0.012 |
| Ever received the flu vaccine at this hospital | Yes | 6.57 (2.80, 15.43) | 0.007 | ||
| No | 1.0 | ||||
Predictors of receiving both the pertussis vaccine and seasonal influenza vaccine
A multiple logistic regression model was developed to determine predictors of receiving both pertussis and influenza vaccine, with younger age, receiving the hepatitis B vaccine and thinking influenza could be severe in infants being independent predictors. A HCW's length of time in the health sector was also significantly associated with receiving both the pertussis vaccine and seasonal influenza vaccine (global p = 0.019), no pair-wise comparisons were significant (Table 4c).
Table 4c.
Logistic Generalized Estimating Equation for ever had pertussis and had influenza vaccination in past year versus multiple predictors/confounders, accounting for clustering on ward
| Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|
| Variable | Level | Odds Ratio (95% CI) | Stepdown Bonferroni P value | Odds Ratio (95% CI) | Global P value |
| Age group | 31–40 years | 0.38 (0.22, 0.64) | 0.030 | 0.32 (0.18, 0.58) | <0.001 |
| 41–50 years | 0.46 (0.13, 1.61) | 0.40 (0.03, 4.94) | |||
| >50 years | 0.48 (0.20, 1.15) | 0.47 (0.06, 3.74) | |||
| 21–30 years | 1.0 | 1.0 | |||
| Length of time in area of work | <12 months | 3.20 (0.90, 11.32) | <0.001 | 4.03 (0.37, 43.81) | 0.020 |
| 1–5 years | 1.11 (0.34, 3.59) | 2.15 (0.37, 12.40) | |||
| 6–10 years | 1.45 (0.65, 3.27) | 2.25 (0.21, 24.20) | |||
| 11–15 years | 1.20 (0.28, 5.19) | 1.81 (0.38, 8.52) | |||
| 16+ years | 1.0 | 1.0 | |||
| Received Hepatitis B vaccine | Yes | 6.16 (2.65, 14.32) | 0.003 | 9.70 (1.92, 48.91) | 0.006 |
| No | 1.0 | 1.0 | |||
| How severe do you think influenza infections in infants can be? | For every 1 point increase in severity | 1.83 (1.59, 2.10) | <0.001 | 2.23 (1.44, 3.46) | <0.001 |
Discussion
HCWs had limited awareness of recommendations and only a minority were fully immunized according to HCW recommendations. Our findings are similar to that of a 2002 study that found only 18% of HCWs at a Victorian hospital were fully vaccinated.14 A recent review in Australia found rates of HCW seasonal influenza vaccine coverage to be between 16.3% and 58.7%.19
Our finding that 80.0% of respondents had received the influenza vaccine in the last year, with 95.6% having ever received it is higher than expected compared with the overall hospital uptake, suggesting that respondents were likely to be favorable to vaccination. This may have biased our results, as non-immunizers may have been less inclined to complete the questionnaire.
In terms of having had the influenza vaccination in the past year, the finding that younger HCWs were significantly more likely to have received the influenza vaccine may be explained by more junior staff receiving pre-employment screening as they enter the workforce. This finding differs from a meta-analysis by Riphagen-Dalhuisen32 who found age (>40 years) to be a significant predictor. Higher rates of influenza vaccination usually occur in older individuals as they become more at risk of influenza complications through age and chronic disease onset. The fact that English as a first language was also a significant predictor reveals that language is an important barrier even for HCWs. We were unable to determine whether this barrier is associated with a lack of knowledge of the recommendations or awareness of the hospital seasonal influenza program and/or access to it. Other Australian studies33-35 have reported previous receipt of the influenza vaccine to be associated with vaccination or intention to be vaccinated.
While responses to questions about disease severity indicated awareness, questions about motivation would indicate self-protection to be a strong cue to action. A review36 examining attitudes and predictors for accepting or rejecting vaccination in HCWs found a wide range of misconceptions or lack of knowledge about influenza infection and a lack of convenient access to the vaccine. The majority of reviewed studies reported self-protection to be the most important reason for vaccination. In terms of influenza, as expected, HCW self-protection was a highly significant predictor of vaccination for seasonal influenza, which other studies37-38 have noted. Our finding for support for the obligation to be vaccinated against influenza (79.4%) is higher than that reported by Seale39 who found only 46.8% of respondents in a survey of two tertiary hospitals in Sydney supported a policy of compulsory influenza vaccination for HCWs. This was despite 91.3% of HCWs at these hospitals strongly supporting a policy of compulsory vaccination for other vaccines recommended for HCWs that was introduced in NSW in 2007.39 Believing it was important to be vaccinated to protect patients and that influenza could be serious in immunocompromised patients, were also associated with higher uptake and are similar to findings reported by Riphagen-Dalhuisen 32 who found being willing to protect oneself or protect at-risk patients to be associated with uptake. Our results suggest for influenza vaccine, an understanding of the benefit of patient protection may be a key motivator for HCWs. However, this could also be explained by the high proportion of survey respondents who were vaccinated for influenza relative to the hospital HCW population.
Only 50.5% of respondents had received the pertussis vaccine which is concerning but is higher than in a recent national survey of French HCWs showing coverage to be only 11.4%.26 Our study showed that to receive the pertussis vaccine, acceptance of the receipt of other recommended vaccines for HCWs (hepatitis B and MMR) is important. The underlying reasons for the finding that pertussis immunization is low in a highly vaccinated group requires further investigation. However, it is likely either to be explained by low awareness of adult pertussis vaccination or poor recall of vaccination history. Our positive correlation that receipt of pertussis vaccine was associated with being aware that influenza infections could be severe in infants was unexpected and warrants further investigation.
In terms of following recommendations and receiving both the pertussis and influenza vaccines, the finding that younger HCWs and HCWs who had received the hepatitis B vaccine more likely to have received both vaccines may indicate that processes such as pre-employment screening and acceptance of the other recommended vaccines for HCWs is important. However, this may also be explained by the fact that younger healthcare workers are more likely to have received the hepatitis B vaccine prior to clinical placements while in university.
Overall HCW awareness of recommended vaccines was low. Vaccine prevention measures are not reviewed systematically for individual HCWs and educational provision to HCWs does not appear to be consistent. The use of a structured process such as using HCWs’ required annual registration renewal may be one way to increase awareness of the recommendations and facilitate HCWs to act. The fact that many HCWs reported they came to work exhibiting symptoms suggestive of influenza is concerning. This is similar to findings of Peadon's study that found 85% of HCW who had experienced a cough lasting 2 or more weeks had continued to work.40 Further research to identify the motivators for working against hospital policies in this context is a priority.
The strengths of this study include the chance for HCWs to provide anonymous responses to a detailed questionnaire on topics for which they may have strong opinions or feel they know little about without recourse from peers.
The study results are limited by the number of responses and confinement of the study population to a pediatric and obstetric hospital, although the views and values in relation to vaccination may be similar across hospitals both adult and pediatric. As this was a cross-sectional study, results are limited in time. Also, participation required awareness of the survey on the ward and the majority of respondents were nurses (81.5%) with only 1 doctor responding. In addition, the disproportionately higher number of females reflects the fact that the majority of respondents were nursing staff, which is a predominantly female industry.
Respondents were self-selected; while the incentive used may have helped some to participate it is possible that the majority of respondents may be inherently different from non-respondents. It is also possible that responses were confounded by a HCW's prior experiences and knowledge. We also relied on self-reported vaccination history and HCWs may not have completed the survey independently, but collaborated with colleagues.
Our findings suggest a knowledge gap exists particularly around awareness of vaccination recommendations for HCWs. HCWs assessment of the risk/benefit value for themselves and their patients, appears to be an important factor in determining uptake of HCW vaccination and should be considered in promotional programs. Further studies are required that capture ‘non-vaccinators’ to fully explore the barriers identified and factors related to uptake of the recommended vaccinations for HCWs.
Methods
Study design
This cross-sectional study was performed between July and December 2013 at a tertiary pediatric and obstetric hospital in Adelaide, Australia, with over 5,000 births per year. There are 17 wards at the hospital, 11 pediatric and 6 obstetric wards. The hospital is the leading provider of specialist care for children with acute and chronic conditions in South Australia, as well as the State's largest maternity and obstetric service. In 2011/12, there were 45,000 Pediatric Emergency Department presentations and over 5,000 births. The hospital's 2013 influenza program included a 2 month period of access to an immunization nurse stationed at the hospital cafeteria, a mobile “flu trolley” visiting wards and a clinical practice consultant available to provide vaccinations as required. There were no outbreaks of influenza or pertussis on the wards during the time of the questionnaire.
Survey questionnaire
Questions were designed to assess participants’ immunization history and knowledge associated with recommended HCW immunizations, risks of not being immunized for pertussis and influenza, acceptance of the hospital's seasonal influenza vaccination program and to identify facilitators and barriers to uptake. A scale of 1-10 was used to assess participants’ beliefs about how severe pertussis and influenza could be in infants, with 1 being not severe and 10 being severe. The questionnaire was only available in English.
Participant recruitment
To ensure a broad representation of HCW views on immunization, the 3 highest uptake wards and 3 lowest uptake wards were selected based on an average of the last 3 years uptake in the staff seasonal influenza vaccination program. Three wards had the most consistently high and low uptake. The three highest wards were >73% (73%, 74% and 74%) and 3 lowest wards were below 53 %( 32%, 52% and 53%) in the 2012 staff seasonal influenza vaccination program. Study data were collected through a paper based questionnaire, left on wards for respondents to anonymously complete with the option to complete the questionnaire on-line. To encourage participation, an incentive draw was provided for each ward, with one person from each ward randomly selected to receive a $50 voucher.
Statistical analyses
Logistic regression was used to examine the association between work area (low vs. high influenza vaccine uptake wards based on influenza vaccine uptake in the previous year (2012) and pertussis and influenza vaccinations. Univariate analysis identified potential variables to be included in a multivariate analysis in which logistic regression was used. Multivariable analysis was used to develop 3 models associated with HCW vaccination; (1) ever having received a pertussis vaccine, (2) receiving the seasonal influenza vaccination in the past year and (3) ever having received a pertussis vaccination and receiving an influenza vaccine within the past year to determine predictor variables. A logistic generalized estimating equation was used, taking into account clustering on work area. Stepdown Bonferroni P values were calculated to adjust for multiple comparisons. Variables were included in the initial model and were removed one by one from the model starting with the highest P-value, until only variables with P-values of <0.05 remained. Associations between variables and outcomes are reported as odds ratio's with 95% confidence intervals. Statistical analyses were performed using SPSS version 21 (Armonk, NY: IBM Corp) and SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
The study protocol was reviewed and approved by the Women's and Children's Health Network, Human Research Ethics Committee, Adelaide, South Australia.
Acknowledgments
We thank and acknowledge the statistical support from Susanne Edwards of the Data Management and Analysis Center, University of Adelaide.
Disclosure of Potential Conflicts of Interest
HM is an investigator on vaccine studies sponsored by Industry. Her institution has received grants from GSK, Sanofi Pasteur, Pfizer for investigator led research. HM has not received any personal payments from industry. The other remaining authors report no conflicts of interest. Some of the data contained in this manuscript was presented in a poster at the Public Health Association of Australia 14th National Immunization Conference, 17–19 June 2012 in Melbourne, Australia.
Funding
This study was supported financially by GlaxoSmithKline (GSK). Helen Marshall is a recipient of an NHMRC Career Development Fellowship (1016272).
References
- 1. Burls A, Jordan R, Barton P, Olowokure B, Wake B, Albon E, Hawker J. Vaccinating healthcare workers against influenza to protect the vulnerable–is it a good use of healthcare resources? A systematic review of the evidence and an economic evaluation. Vaccine 2006; 24:4212-21; PMID:16546308; http://dx.doi.org/ 10.1016/j.vaccine.2005.12.043 [DOI] [PubMed] [Google Scholar]
- 2. Shefer A AW, Friedman C, Kuhar DT, Mootrey G, Bialek SR, Cohn A, Fiore A, Grohskopf L, Liang JL, Lorick SA, et al. . Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 2011; 60:1-45. [PubMed] [Google Scholar]
- 3. Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis 2014; 58:50-7; PMID:24046301; http://dx.doi.org/ 10.1093/cid/cit580 [DOI] [PubMed] [Google Scholar]
- 4. Baggett HC, Duchin JS, Shelton W, Zerr DM, Heath J, Ortega-Sanchez IR, Tiwari T. Two nosocomial pertussis outbreaks and their associated costs - King County, Washington, 2004. Infect Control Hosp Epidemiol 2007; 28:537-43; PMID:17464912 [DOI] [PubMed] [Google Scholar]
- 5. Baxi R, Mytton OT, Abid M, Maduma-Butshe A, Iyer S, Ephraim A, Brown KE, O'Moore E. Outbreak report: nosocomial transmission of measles through an unvaccinated healthcare worker–implications for public health. Journal of public health (Oxford, England) 2013; 36(3):375-81. [DOI] [PubMed] [Google Scholar]
- 6. Greer AL, Fisman DN. Keeping vulnerable children safe from pertussis: preventing nosocomial pertussis transmission in the neonatal intensive care unit. Infect Control Hosp Epidemiol 2009; 30:1084-9; PMID:19785517; http://dx.doi.org/ 10.1086/644755 [DOI] [PubMed] [Google Scholar]
- 7. Calugar A, Ortega-Sanchez IR, Tiwari T, Oakes L, Jahre JA, Murphy TV. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin Infect Dis 2006; 42:981-8; PMID:16511764; http://dx.doi.org/ 10.1086/500321 [DOI] [PubMed] [Google Scholar]
- 8. Hollmeyer H, Hayden F, Mounts A, Buchholz U. Review: interventions to increase influenza vaccination among healthcare workers in hospitals. Influenza Other Respir Viruses 2013; 7:604-21; PMID:22984794; http://dx.doi.org/ 10.1111/irv.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baron-Epel O, Bord S, Madjar B, Habib S, Rishpon S. What lies behind the low rates of vaccinations among nurses who treat infants? Vaccine 2012; 30:3151-4; PMID:22446637; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.074 [DOI] [PubMed] [Google Scholar]
- 10. Bull AL, Bennett N, Pitcher HC, Russo PL, Richards MJ. Influenza vaccine coverage among health care workers in Victorian public hospitals. Med J Aust 2007; 186:185-6; PMID:17309419 [DOI] [PubMed] [Google Scholar]
- 11. Betsch C, Wicker S. Personal attitudes and misconceptions, not official recommendations guide occupational physicians' vaccination decisions. Vaccine 2014; 32:4478-84; PMID:24962759; http://dx.doi.org/ 10.1016/j.vaccine.2014.06.046 [DOI] [PubMed] [Google Scholar]
- 12. Botelho-Nevers E, Gautret P, Biellik R, Brouqui P. Nosocomial transmission of measles: an updated review. Vaccine 2012; 30:3996-4001; PMID:22521843; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.023 [DOI] [PubMed] [Google Scholar]
- 13. Leung V, Harper S, Slavin M, Thursky K, Worth L. Are they protected? Immunity to vaccine-preventable diseases in healthcare workers at an Australian hospital. Aust N Z J Public Health 2014; 38:83-6; PMID:24494952; http://dx.doi.org/ 10.1111/1753-6405.12163 [DOI] [PubMed] [Google Scholar]
- 14. Murray SB, Skull SA. Poor health care worker vaccination coverage and knowledge of vaccination recommendations in a tertiary Australia hospital. Aust N Z J Public Health 2002; 26:65-8. [DOI] [PubMed] [Google Scholar]
- 15. Dannetun E, Tegnell A, Torner A, Giesecke J. Coverage of hepatitis B vaccination in Swedish healthcare workers. J Hosp Infect 2006; 63:201-4; PMID:16621139; http://dx.doi.org/ 10.1016/j.jhin.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 16. Lu PJ, Graitcer SB, O'Halloran A, Liang JL. Tetanus, diphtheria and acellular pertussis (Tdap) vaccination among healthcare personnel-United States, 2011. Vaccine 2014; 32:572-8; PMID:24308960; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Australian Technical Advisory Group on Immunization The Australian Immunization Handbook. Canberra: Australian Government Department of Health, 2013. [Google Scholar]
- 18. Faruque MO, Senanayake S, Meyer AD, Dear KB. Emergency department staff and susceptibility to pertussis: a seroprevalence study. Emer Med Aust 2008; 20:45-50; PMID:18062780; http://dx.doi.org/ 10.1111/j.1742-6723.2007.01044.x [DOI] [PubMed] [Google Scholar]
- 19. Seale H, Macintyre CR. Seasonal influenza vaccination in Australian hospital health care workers: a review. Med J Aust 2011; 195:336-8; PMID:21929498 [DOI] [PubMed] [Google Scholar]
- 20. Lim YC, Seale H. Examining the views of key stakeholders regarding the provision of occupational influenza vaccination for healthcare workers in Australia. Vaccine 2014; 32:606-10; PMID:24291538; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.063 [DOI] [PubMed] [Google Scholar]
- 21. O'Brien JA, Caro JJ. Hospitalization for pertussis: profiles and case costs by age. BMC infect Dis 2005; 5:57; PMID:16008838; http://dx.doi.org/ 10.1186/1471-2334-5-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Healy CM, Rench MA, Castagnini LA, Baker CJ. Pertussis immunization in a high-risk postpartum population. Vaccine 2009; 27:5599-602; PMID:19647062; http://dx.doi.org/ 10.1016/j.vaccine.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 23. Sandora TJ, Gidengil CA, Lee GM. Pertussis vaccination for health care workers. Clin Microbiol Rev 2008; 21:426-34; PMID:18625679; http://dx.doi.org/ 10.1128/CMR.00003-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright SW, Decker MD, Edwards KM. Incidence of pertussis infection in healthcare workers. Infect Control Hosp Epidemiol 1999; 20:120-3; PMID:10064216; http://dx.doi.org/ 10.1086/501593 [DOI] [PubMed] [Google Scholar]
- 25. Kuncio DE, Middleton M, Cooney MG, Ramos M, Coffin SE, Feemster KA. Health care worker exposures to pertussis: missed opportunities for prevention. Pediatrics 2014; 133:15-21; PMID:24344101; http://dx.doi.org/ 10.1542/peds.2013-0745 [DOI] [PubMed] [Google Scholar]
- 26. Guthmann JP, Fonteneau L, Ciotti C, Bouvet E, Pellissier G, Levy-Bruhl D, Abiteboul D. Vaccination coverage of health care personnel working in health care facilities in France: results of a national survey, 2009. Vaccine 2012; 30:4648-54. PMID:22579863; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.098 [DOI] [PubMed] [Google Scholar]
- 27. Bryant KA, Humbaugh K, Brothers K, Wright J, Pascual FB, Moran J, Murphy TV. Measures to control an outbreak of pertussis in a neonatal intermediate care nursery after exposure to a healthcare worker. Infect Control Hosp Epidemiol 2006; 27:541-5; PMID:16755471; http://dx.doi.org/ 10.1086/505666 [DOI] [PubMed] [Google Scholar]
- 28. Ward A, Caro J, Bassinet L, Housset B, O'Brien JA, Guiso N. Health and economic consequences of an outbreak of pertussis among healthcare workers in a hospital in France. Infect Control Hosp Epidemiol 2005; 26:288-92; PMID:15796282; http://dx.doi.org/ 10.1086/502541 [DOI] [PubMed] [Google Scholar]
- 29. Alexander EM, Travis S, Booms C, Kaiser A, Fry NK, Harrison TG, Ganpot B, Klein JL. Pertussis outbreak on a neonatal unit: identification of a healthcare worker as the likely source. J Hosp Infect 2008; 69:131-4; PMID:18394752; http://dx.doi.org/ 10.1016/j.jhin.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 30. Boulay BR, Murray CJ, Ptak J, Kirkland KB, Montero J, Talbot EA. An outbreak of pertussis in a hematology-oncology care unit: implications for adult vaccination policy. Infect Control Hosp Epidemiol 2006; 27:92-5; PMID:16418998; http://dx.doi.org/ 10.1086/500420 [DOI] [PubMed] [Google Scholar]
- 31. Baugh V, McCarthy N. Outbreak of Bordetella pertussis among oncology nurse specialists. Occup Med (Lond) 2010; 60:401-5; PMID:20407043; http://dx.doi.org/ 10.1093/occmed/kqq051 [DOI] [PubMed] [Google Scholar]
- 32. Riphagen-Dalhuisen J, Gefenaite G, Hak E. Predictors of seasonal influenza vaccination among healthcare workers in hospitals: a descriptive meta-analysis. Occup Envir Med 2012; 69:230-5; PMID:22172951; http://dx.doi.org/ 10.1136/oemed-2011-100134 [DOI] [PubMed] [Google Scholar]
- 33. Halliday L, Thomson JA, Roberts L, Bowen S, Mead C. Influenza vaccination of staff in aged care facilities in the ACT: how can we improve the uptake of influenza vaccine? Aust N Z J Public Health 2003; 27:70-5; PMID:14705271 [DOI] [PubMed] [Google Scholar]
- 34. Campos W, Jalaludin BB. Predictors of influenza vaccination amongst Australian nurses. Aust J Adv Nurs 2002; 20:19-21; PMID:12537148 [PubMed] [Google Scholar]
- 35. Ballestas T, McEvoy SP, Doyle J. Co-ordinated approach to healthcare worker influenza vaccination in an area health service. J Hosp Infect 2009; 73:203-9; PMID:19783073; http://dx.doi.org/ 10.1016/j.jhin.2009.07.028 [DOI] [PubMed] [Google Scholar]
- 36. Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals–a review of studies on attitudes and predictors. Vaccine 2009; 27:3935-44; PMID:19467744; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.056 [DOI] [PubMed] [Google Scholar]
- 37. Tapiainen T, Bar G, Schaad UB, Heininger U. Influenza vaccination among healthcare workers in a university children's hospital. Infect Control Hosp Eepidemiol 2005; 26:855-8; PMID:16320981; http://dx.doi.org/ 10.1086/502508 [DOI] [PubMed] [Google Scholar]
- 38. Bryant KA, Stover B, Cain L, Levine GL, Siegel J, Jarvis WR. Improving influenza immunization rates among healthcare workers caring for high-risk pediatric patients. Infect Control Hosp Epidemiol 2004; 25:912-7; PMID:15566023; http://dx.doi.org/ 10.1086/502319 [DOI] [PubMed] [Google Scholar]
- 39. Seale H, Leask J, Macintyre CR. Awareness, attitudes and behavior of hospital healthcare workers towards a mandatory vaccination directive: two years on. Vaccine 2011; 29:3734-7; PMID:21458607; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.050 [DOI] [PubMed] [Google Scholar]
- 40. Peadon E, Cooper C. Whooping cough: are health-care workers putting children at risk? J Paediatr Child Health 2007; 43:398-402; PMID:17489832; http://dx.doi.org/ 10.1111/j.1440-1754.2007.01087.x [DOI] [PubMed] [Google Scholar]


