Abstract
IL-2 is a pleiotropic cytokine produced by T cell after antigen activation of T cell and it is so called T cell growth factor. A large number of documents suggest that Il-2 plays pivotal roles in the immune response and now Il-2 is an approved drug being used for various kinds of diseases such as cancer and dermatitis.1 The aim of present exploration was to look at effect of IL-2 on structural, phenotypic and functional maturation of murine BMDCs. The structural and phenotypic maturation of BMDCs under influence of IL-2 were evaluated by light microscope and flow cytometry (FCM). The functional maturation of BMDCs was confirmed by cytochemistry assay, FITC-dextran, acid phosphatase (ACP) activity, bio-assay and enzyme linked immunosorbent assay (ELISA).We elucidated that IL-2 up-regulated the expression of key surface markers such as: CD80, CD83, CD86, CD40 and MHC II molecules on BMDCs, down-regulated phagocytosis activity, induced more production of IL-12 and TNF-α secreted by BMDCs. Therefore it can be concluded that IL-2 effectively enhance the maturation of BMDCs. Our results provide direct evidence to support IL-2 would be used as a potent adjuvant in preparation of DC-based vaccines, as well as an immune remedy for cancer situation.
Keywords: Bone marrow derived dendritic cells, Interlukin-2, maturation, surface molecules
Abbreviations
- IL-2
Interlukin-2
- ACP
Acidic phosphatase
- BMDCs
Bone marrow derived dendritic cells
- APCs, LPS
Lipopolysaccharide
- TEM
Transmission electron microscopy
- DAB
3 3′-diaminobenzidine
- MTT
3-(4 5-Dimethylthiazol-2-yl)-2 5-diphenyltetrazolium bromide
- FCM
Fow cytometry
- MACS
Magnetic activated cell sorting
- CTL
Cytotoxic lymphocyte
Introduction
IL-2 is an interleukin, a type of cytokine and a soluble signaling molecule mediating in the immune system. It was first reported simultaneously by Shinpei Kasakura and Louis Lowenstein, plus Julius Gordon and Lloyd MacLean for its mitogenic activity in the culture media of mixed leukocytes.2 IL-2 is also a small protein that regulates white blood cells in body's natural response to infection. As documented, IL-2 mediates a series of immunological effects by binding to IL-2 receptors on the surface of lymphocytes3 and the cellular responses in our body are regulated by the amount of IL-2 produced in response to antigens. It also stimulates the production of IL-2 receptors on the surface of immune cells and acts through autocrine or paracrine way. As result of this the expansion and activation of macrophages, natural killer cells, B lymphocytes etc. would happen.4,5
The unraveled data on IL-2 has considered IL-2 as a major growth and apoptosis factor for activated T lymphocytes. IL-2 also plays necessary role in maintaining self-tolerance and it has been used for over 30 years, and remains one of the most extensively studied cytokines.6 Especially in recent years there were more and more reports on antitumor activity of IL-2.7,8
Dendritic cells (DCs) were first described by Paul Langerhans (Langerhans cells) in late stage of nineteenth century. It wasn't until 1973, however, that the term “dendritic cells” was coined as part of immune cells by Ralph M. Steinman and Zanvil A. Cohn, representing a milestone of immunology. The main function of DCs is to process and present antigen on the surface to be recognized by T cell and therefore initiate T cell response, functioning as antigen-presenting cells (APC).9,10 DCs are not only important for the induction of primary immune responses, but also crucial for the induction of immunological tolerance.11,12
There were many articles describing relation between dendritic cell and IL-2, including transformation of monocyte to DC under influence of IL-2, combined with other cytokines, IL-2 promotes the motility of dendritic cells and their accumulation in lung and skin. IL-2 production by dendritic cells and its immune-regulatory functions, IL-2-induces development of denditric cells from cord blood CD34+ cells.13-17 However, so far there is no systematic study from data bank on the impact of IL-2 on phenotypic and functional maturation of DCs. Therefore we performed the following research to look at phenotypic and functional effect on DC by IL-2.
Results
Confirmation of change of key surface markers on the BMDCc by FCM
Post treatment with 25μg /ml IL-2 for 48h immature BMDCs developed into maturation confirmed by upregulated expression of key surface markers such as: CD86, CD80, CD83 CD40 and MHC-II, which will work as key signal and co-signals to activate T cell. The detailed changes are shown in Figure 1.
Figure 1.
Detailed changes of surface markers on the BMDCs after treatment with IL-2.
T cell activation driven by BMDCs treated with IL-2
Usually immature BMDCs are with a powerful ability to capture and process antigen, but with a lower potential to initiate T-cell response. IL-2 induced maturation of BMDCs with increase in driving T lymphocytes response. The index of induced BMDCs to drive expansion of allogeneic lymphocytes was reflected by a corresponding value of OD492nm (A492) and yielded following numbers as shown in Figure 2.
Figure 2.
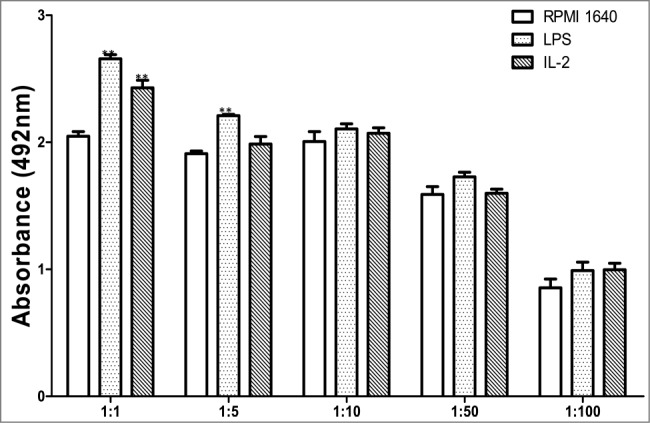
Assay for treated BMDCs to drive T cells.
Immunohistochemical analysis of phagocytosis of BMDCs
Post staining with DAB kit, BMDCs observed under conventional microscope exhibited the process of phagocytosing. The picture revealed a process of phagocytosing labeled horseradish peroxidase by BMDC and exhibited more phagosomes in immature BMDC as compared to less phagosomes in mature BMDC shown in Figure 3. This result was corresponding to ACP activity.
Figure 3.
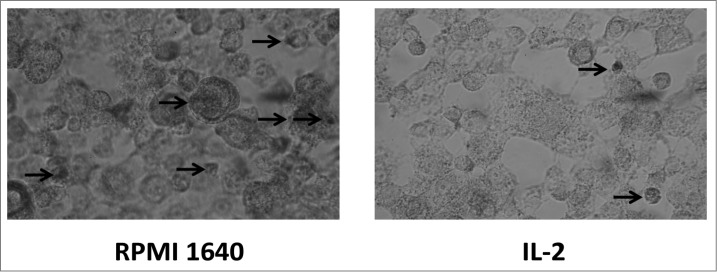
Localization of phagocytosis by BMDC.
Phagocytosis study by FCM
Antigen arrested inside BMDCs also underwent FITC-dextran test. IL-2 weakened the phagocytosis of BMDCs to the labeled dextran. Reversely BMDCs without IL-2 treatment showed strong phagocytosis, as shown Figure 4.
Figure 4.

Phagocytosis study.
Test of ACP activity
With the processing of BMDCs maturation ACP activity changed accordingly. The decrease of ACP activity was an indication of gradual termination of phagocytosis and the number of ACP activity could be reflected by a value of OD520nm (A520) in Figure 5.
Figure 5.

ACP activity measurement.
Assay for production of IL-12 and TNF-α
The immature BMDCs stimulated by 25μg /ml IL-2 for 96h developed into maturation .Besides maturation in morphology as proved by upregulated expression of key surface markers, the BMDCs also underwent functional maturation by secreting higher levels of IL-12 and TNF-α. IL-12 and TNF-α could play important role in regulating a spectrum of innate and adaptive immune responses, as exhibited in Figures 6A,B.
Figure 6.

Secretion of IL-12 and TNF-α by BMDCs.
Discussion
IL-2 is a natural messenger protein of human immune system, called a cytokine which activates parts of immune system and induces substantial increase in number of tumor-reactive T cells in vitro. Experimental data prove that T cells cultured with suitable dose of IL-2 can be more effective in vivo for showing specific tumor cytotoxicity. IL-2 does not kill tumor cells directly like a chemotherapy drug. Instead, IL-2 activates the expansion of T-cells and natural killer cells (NK), both of which can kill cancer cells directly. Now IL-2 has some clinical application in cancer therapy such as: as specific cellular anti-tumor immunity and cytokine induced killer (CIK) cells in cancer therapy.18–20
Early documented research results in data bank have indicated that clinical significance of IL-2 to boost and maintain the balance of both innate and adaptive cellular immunity and even now IL-2 continues to be a key solution for most of melanoma and renal cancer.21-23
In our current research we have proven that IL-2s affects on phenotypes and functions of BMDCs were present. From our results we could see that Il-2 induced the maturation of BMDCs with the following identified fact: (1) 25μg /ml of IL-2 and 48h culture were optimal condition to boost BMDCs; (2) BMDCs under induction of IL-2 showed typical maturation with reduced phagosomes inside BMDCs, which would downregulate phagocytosis of BMDCs, with simultaneously intensified antigen(Fig.3) presentation; (3)IL-2 increased expression of key surface markers of CD80(B7.1), CD40, CD83, CD86(B7.2) and MHC II on the BMDCs(Fig.1), which would mount antigen presentation of BMDCs to initiate specific T cell response(Fig.2) ; (4) IL-2 decreased ACP activity, which is consistent with decreased phagocytosis and increased BMDCS driving T lymphocytes reaction(Figs.3, 4) and (5)IL-2 upregulated secretion of IL-12 and TNF-α, which would be helpful in intensifying the signals between the DC and CD4+T cell, increasing Th1 branch responses and amplifying macrophage function(Fig.6).
Additionally we should pay attention to that higher secretion of IL-12 and TNF-α by BMDCs would not only act on themselves in an autocrine or paracrine manner, resulting in more activated BMDCs, but also will amplify the signal to direct CD4+T cells to be functioning and thus the stability between DCs and CD4+T cells would be firmed. As a positive feedback, this chain signals will enhance more production of cytokines by BMDCs and TNF-β or IFN-γ by CD4+T cells, involved in stimulating macrophage to secret many types of cytokines, coordinating a chain of immune responses in the body.24–27
It is very surprising to us that the results for IL-2 treatment are statistically different from controls only at a 1:1 ratio and the low response obtained with LPS at only 1:1 and 1:5 ratios. These might be caused by our laboratory condition, cell purity or medicines we used.
Really immune system is a diverse network with rather complication, in which all of immune cells are interacting directly or indirectly through mediation of immune molecules, forming a precise feedback. When TNF-α or IL-12 is playing role no matter whether it works alone, or is interacting with other signal molecules, it can exert impact on BMDCs in a direct way or indirect way by activating other cells such as macrophages, neutrophils and NK cells. These activated cells can then secret high levels of cytokines, acting on BMDCs. For example, activated NK or neutrophils can release more IFN-γ to induce BMDCs to maturation and, as consequence of this, BMDCs will secret more IL-12 forming an interacting loop, maintaining the balanced immunity. IL-2 can play a very important role as immune adjuvant to be used in vaccines preparation.28–30
DCs, originally discovered by Steinman (1972), represent marked progress in immunology and in cancer therapy. Due to the most powerful antigen presentation to initiate naïve T cell response they are therefore critical to specific cellular immunity and cellular cytotoxicity.31–34
So far continuous emerging scientific data are supporting DC activation will be one of the best solutions to cancer therapy.
This meaningful exploration with enriched data contribute to the further understanding of immunomodulatory role of IL-2 in optimizing immune system or imkune functions and further understanding of deepening mechanisms of IL-2 action at sub-cellular or molecular level.
Finally this investigation also presents a meaningful mode for IL-2 and highlightens the clinical potential of IL-2 as an key clinical agent used in cancer therapy, an useful adjuvant in vaccine designing.
Conclusion
We believe that it is the first time that we release the data on systematic study demonstrating that IL-2, at defined concentration could enhance both phenotypic and functional maturation of BMDCs. The induced BMDCs could also supply with more IL-12, TNF-α and other key surface markers of CD40, CD80, CD86, CD83 and MHC II to work together to intensify antigen presentation by BMDCs to activate T response.
Materials and Methods
Reagents
rIL-2 was a product of PeproTech USA. The impact of a range of concentrations of IL-2 from 1.56μg/ml to 200 μg/ml on the growth of BMDCs in vitro was tested, and the concentration of 25 μg/ml was found to be optimal concentration. So 25 μg/ml was token for current work. Recombinant murine cytokines of IL-4 and GM-CSF were provided by PeproTech Inc. LPS (from Escherichia coli, serotype 055:B5) was a product of Sigma-Aldrich. The mAbs used in the study include PE-anti-CD83, FITC-conjugated anti-CD40, PE-anti-CD80, PE-anti-CD86 and PE-anti-MHC-II, which were bought either from eBioscience or BD PharMingen. ELISA kits for cytokines assays were bought from R&D System. Other chemicals and reagents, being frequently used in our laboratory were all made in Sigma-Aldrich or BD Pharmingen.
Induction of bone marrow cells to get BMDCs
The preparation of BMDCs from mice referred to a method as described previously.35 The bone marrow cells from the femurs and tibias of female C57BL/6 mice were removed of red cells and counted 107/ml cells were grown in 24-well plates charged with 2 ml of RPMI 1640 containing standard 10% fetal bovine serum, 10ng/ml GM-CSF, 10ng/ml IL-4, 2 mM L-glutamine, 100units/ml penicillin, 100μg/ml streptomycin. After culture for 4h the adherent cells was obtained and 1μg/ml LPS was added to the culture medium on 7d of culture for overnight to get more number to fit purification with CD11c-MACS magnet beads and the process of purification was performed by referring published method and instruction manual included in MACS kit (Miltenyi Biotech.). The CD11c+ BMDCs were sorted out using anti-CD11c-coated magnetic beads and the auto- MACS system and the purity of the purified cells was confirmed by FCM with > 90% purity.36-38
Confirmation of elevated key surface molecules by FCM
The BMDCs co-cultured in presence of 25μg/ml IL-2 for 48h were confirmed of expression of key surface molecules by FCM. The treated BMDCs were reacted together with anti-CD80, anti-CD86, anti-CD83, anti-CD40, and anti-MHC II antibodies at 4℃ for 20 min. After thorough rinsing, the cells were measured with use of FACS Calibur (Becton Dickinson, San Diego, CA) to quantitate change percentage.
T cell activation driven by BMDCs treated with IL-2
The BMDCs post treatment with 25μg /ml IL-2 were collected on 7d. The purified CD8+ T cells(purity>90%, with a similar way to purify DC.) from the splenocytes of C57BL/6 mice were sorted out with magnetic beads and subsequently the CD8+ T cells were grown with10μg/ml ConA for 48h to a number of 107/ml. The CD8+ T cells (5 × 10 5 /well) were incubated in 96-well culture plates with diffrential BMDCs at a ratio of 1:1, 1:5, 1:10, 1:50, 1:100 for 5d. The proliferation of CD8+T cells was determined by testing the OD absorbance at 492nm (A492) using a bichromatic microplate reader. Results from triplicate wells were expressed as the mean ± SE.
ACP activity determination
One × 10 6/ml BMDCs treated with 25μg /ml IL-2 for 48h were used with the phenol-4-AAP (amino anti-pyrine) plus ACP testing kit (Jiancheng Bio-engineering Institute of the South) to test ACP activity inside the BMDCs. OD number of absorbance at 520 nm (A520) represent activity.
Immunohistochemical analysis for phagocytic process by BMDCs
1 × 10 5/ml BMDCs cultured in presence of 25μg /ml IL-2 for 48h were combined with 0.08mg/ml horseradish peroxidase, then fixed with methanol for 4h, followed by with DAB staining and finally the stained sample was observed under microscope.
Phagocytosis study by FCM
The BMDCs incubated with 25μg /ml IL-2 for 48h were collected and100μl FITC-Dextran (40,000 D) 28–30 was added to the BMDCs culture at 4℃ for 2h, subsequently at 37℃ for 1h. Finally the sample was checked with FACS Calibur (Becton Dickinson, San Diego, CA) for localization of phagocytosis process.
Assay of cytokine production of IL-12p70 and TNF-α by ELISA
The BMDCs incubated with 25μg /ml IL-2 for 96h were collected for assay of cytokine production of IL-12p70 and TNF-α by ELISA respectively per instruction manual in the ELISA kit. The absorbance at 450 nm (A450) was obtained using a bichromatic microplate reader (BIO-TEK, USA).
Statistical analysis
All results were processed using statistical program SPSS (Statistical Package for Social Sciences, Version 16.0) for Windows and variables number were presented as mean ± SE. The divergence evaluated by ANOVA indicated statistical significance while P < 0.05.
Acknowledgements
We apologize to the colleagues whose work could not be discussed due to the limited space.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported financially by the Liaoning Science Foundation of China (No. 2009225008–7, 2012225016).
References
- 1. Smith KA, Lachman LB, Oppenheim JJ, Favata MF. The functional relationship of the interleukins. J Exp Med 1980June,151(6):1551-6; http://dx.doi.org/ 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith KA, Gilbride KJ, Favata MF. Lymphocyte activating factor promotes T-cell growth factor production by cloned murine lymphoma cells. Nature 1980 October, 287 (5785):853-5. [DOI] [PubMed] [Google Scholar]
- 3. Robb RJ, Smith KA. Heterogeneity of human T-cell growth factor(s) due to variable glycosylation. Mol Immunol 1981December. 18 (12): 1087-94; http://dx.doi.org/ 10.1016/0161-5890(81)90024-9. [DOI] [PubMed] [Google Scholar]
- 4. Abraham S, Pahwa R, Ye C, Choi JG, Pahwa S, Jaggaiahgari S, et al. . Long-Term Engraftment of Human Natural T Regulatory Cells in NOD/SCID IL2rγc(null) Mice by Expression of Human IL-2. PLoS One 2012;7(12):e51832; PMID:23272176; http://dx.doi.org/ 10.1371/journal.pone.0051832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith KA, Favata MF, Oroszlan S . Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol 1983 October. 131 (4): 1808-15. [PubMed] [Google Scholar]
- 6. Ahn YO, Lee JC, Sung MW, Heo DS. Presence of membrane-bound TGF-beta1 and its regulation by IL-2-activated immune cell-derived IFN-gamma in head and neck squamous cell carcinoma cell lines. J Immunol 2009 May 15; 182(10):6114-20; http://dx.doi.org/ 10.4049/jimmunol.0803725. [DOI] [PubMed] [Google Scholar]
- 7. Wojas-Turek J, Pajtasz-Piasecka E, Rossowska J, Piasecki E, Duś D. Antitumor effect of murine dendritic and tumor cells transduced with IL-2 gene. Folia Histochem Cytobiol. 2012 Oct 8;50(3): 414-9; http://dx.doi.org/ 10.5603/FHC.2012.0056. [DOI] [PubMed] [Google Scholar]
- 8. Lissoni P, Brivio F, Fumagalli L, Messina G, Vigoré L, Parolini D, et al. . Neuroimmunomodulation in medical oncology: application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Res. 2008 Mar-Apr; 28(2B):1377-81. [PubMed] [Google Scholar]
- 9. Liu J, Chen W, Meng J, Lu C, Wang E, Shan F. Induction on differentiation and modulation of bone marrow progenitor of dendritic cell by methionine enkephalin (MENK). Cancer Immunol Immunother 2012 Oct; 61(10):1699-711; http://dx.doi.org/ 10.1007/s00262-012-1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue M, Zhu L, Meng Y, Wang L, Sun H, Wang F, et al. . Detailed modulation of phenotypes and functions of bone marrow dendritic cells (BMDCs) by interferon-gamma (IFN-γ). Int Immunopharmacol 2013 Jul 16;17(2):366-372; http://dx.doi.org/ 10.1016/j.intimp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 11. Meng Y, Wang Q, Zhang Z, Wang E, Plotnikoff NP, Shan F. Synergistic effect of methionine encephalin (MENK) combined with pidotimod(PTD) on the maturation of murine dendritic cells (DCs). Hum Vaccin Immunother 2013 Mar 7;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinman R. and Cohn Z. Identification of a novel cell type in peripheral lymphoid organs of mice. J Exp Med 1973; 137: 1142-62; PMID:4573839; http://dx.doi.org/ 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mnasria K, Lagaraine C, Manaa J, Lebranchu Y, Oueslati R. IL-2/IL-2R pathway in dendritic cell modulates both their cytokine synthesis profiles and their capacity to activate allogeneic CD4+ T lymphocytes. Pathol Biol 2011;59(3): 29-35; PMID:21277703; http://dx.doi.org/ 10.1016/j.patbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14. Kradin RL, Xia W, Pike M, Byers HR, Pinto C. Interleukin-2 promotes the motility of dendritic cells and their accumulation in lung and skin. Pathobiology 1996;64(4):180-6; PMID:9031326; http://dx.doi.org/ 10.1159/000164033. [DOI] [PubMed] [Google Scholar]
- 15. Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immune-regulatory functions. Front Immunol 2012;3:161; PMID:22719740; http://dx.doi.org/ 10.3389/fimmu.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naranjo-Gómez M, Oliva H, Climent N, Ferna´ndez MA, Ruiz-Riol M, Bofill M, et al. . Expression and function of the IL-2 receptor in activated human plasmacytoid dendritic cells. Eur J Immunol 2007;37:1764-1772; http://dx.doi.org/ 10.1002/eji.200636980. [DOI] [PubMed] [Google Scholar]
- 17. Bykovskaja SN, Buffo MJ, Bunker M, Zhang H, Majors A, Herbert M, et al. . Interleukin-2-induces development of denditric cells from cord blood CD34+ cells. J Leukoc Biol 1998;63(5):620-30; PMID:9581807. [DOI] [PubMed] [Google Scholar]
- 18. Cândido EB, Silva LM, Carvalho AT, Lamaita RM, Filho RM, Cota BD, et al. . Immune Response Evaluation Through Determination of Type 1, Type 2, and Type 17 Patterns in Patients With Epithelial Ovarian Cancer. Reprod Sci 2012 Dec 13. [DOI] [PubMed] [Google Scholar]
- 19. Kim D, Suh Y, Lee H, Lee Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int J Mol Med 2012 Nov 29. [DOI] [PubMed] [Google Scholar]
- 20. Gerber SA, Sorensen EW, Sedlacek AL, Lim JY, Skrombolas D, Frelinger JG, et al. . Local expression of IL-2 by B16 melanoma cells results in decreased tumor growth and long-term tumor dormancy. Immunology 2012 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inaba H, Geiger TL. Defective cell cycle induction by IL-2 in naive T-cells antigen stimulated in the presence of refractory T-lymphocytes. Int Immunol 2006 Jul;18(7):1043-54; http://dx.doi.org/ 10.1093/intimm/dxl038. [DOI] [PubMed] [Google Scholar]
- 22. Farrer DG, Hueber S, Laiosa MD, Eckles KG, McCabe MJ., Jr Reduction of myeloid suppressor cell derived nitric oxide provides a mechanistic basis of lead enhancement of alloreactive CD4(+) T cell proliferation. Toxicol Appl Pharmacol 2008 Jun 1;229(2):135-45; http://dx.doi.org/ 10.1016/j.taap.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu MB, Fesler MJ, Armbrecht ES, Fosko SW, Hsueh E, Richart JM. High-Dose Interleukin-2 (HD IL-2) Therapy Should Be Considered for Treatment of Patients with Melanoma Brain Metastases. Chemother Res Pract 2013:726925; http://dx.doi.org/ 10.1155/2013/726925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stark R, Hartung A, Zehn D, Frentsch M, Thiel A. IL-12-mediated STAT4 signaling and TCR signal strength cooperate in the induction of CD40L in human and mouse CD8+ T cells. Eur J Immunol 2013 Apr 4;43(6):1511-1517; http://dx.doi.org/ 10.1002/eji.201243218. [DOI] [PubMed] [Google Scholar]
- 25. Feng X, Feng G, Xing J, Shen B, Li L, Tan W, Xu Y, et al. . TNF-α triggers osteogenic differentiation of human dental pulp stem cells via the NF-κB signaling pathway. Cell Biol Int 2013 Jun 13; http://dx.doi.org/ 10.1002/cbin.10141. [DOI] [PubMed] [Google Scholar]
- 26. Jiang CY, Wang W, Tang JX, Yuan ZR. The adipocytokine resistin stimulates production of proinflammatory cytokines TNF-α and IL-6 in pancreatic acinar cells via NF-κB activation. J Endocrinol Invest 2013 Jun 10. [DOI] [PubMed] [Google Scholar]
- 27. Philips G, Atkins MB. Is interleukin 2 the best initial therapy for many patients with metastatic renal cell carcinoma? Cancer J 2013 May-Jun;19(3):197-8; http://dx.doi.org/ 10.1097/PPO.0b013e318292e6a2. [DOI] [PubMed] [Google Scholar]
- 28. Subramani B, Ratnavelu K, Pullai CR, Krishnan K, Sugadan SD, Deng X, et al. . Autologous immune enhancement therapy: A case report of a stage IV colonic cancer. . Oncol Lett 2013 May;5(5):1611-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiarion-Sileni V, Guida M, Romanini A, Bernengo MG, Ascierto P, Queirolo P, et al. . Diagnostic and therapeutic approaches in italian hospitals: adjuvant and metastatic therapy in melanoma. Dermatology 2013;226 Suppl 1:22-7; PMID:23736267; http://dx.doi.org/ 10.1159/000348870. [DOI] [PubMed] [Google Scholar]
- 30. Yoshizato T, Nannya Y, Imai Y, Ichikawa M, Kurokawa M. Clinical Significance of Serum-Soluble Interleukin-2 Receptor in Patients With Follicular Lymphoma. Clin Lymphoma Myeloma Leuk 2013 Jun 5. pii: S2152-2650(13)00114-6. [DOI] [PubMed] [Google Scholar]
- 31. Morris D, Gonzalez B, Khurasany M, Kassissa C, Luong J, Kasko S, Pandya S, et al. . Characterization of Dendritic Cell and Regulatory T Cell Functions against Mycobacterium tuberculosis Infection. Biomed Res Int 2013;2013:402827; PMID:23762843; http://dx.doi.org/ 10.1155/2013/402827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Uchiyama K, et al. . Fusions between dendritic cells and whole tumor cells as anticancer vaccines. Oncoimmunology 2013 May 1;2(5):e24437; http://dx.doi.org/ 10.4161/onci.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segura E, Amigorena S. Identification of human inflammatory dendritic cells. Oncoimmunology 2013 May 1;2(5):e23851; http://dx.doi.org/ 10.4161/onci.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chung CY, Ysebaert D, Berneman ZN, Cools N. Dendritic cells: cellular mediators for immunological tolerance. Clin Dev Immunol 2013;2013:972865.; PMID:23762100; http://dx.doi.org/ 10.1155/2013/972865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segovia M, Cuturi MC, Hill M. Preparation of mouse bone marrow-derived dendritic cells with immunoregulatory properties. Methods Mol Biol 2011;677:161-8; PMID:20941609; http://dx.doi.org/ 10.1007/978-1-60761-869-0_11. [DOI] [PubMed] [Google Scholar]
- 36. Abbasi H1, Tahmoorespur M, Hosseini SM, Nasiri Z, Bahadorani M, Hajian M, et al. . THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology 2013; 80:923-932; http://dx.doi.org/ 10.1016/j.theriogenology.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 37. Wu J1, Song W, Zhu H, Niu Z, Mu H, Lei A, et al. . Enrichment and characterization of Thy1-positive male germline stem cells (mGSCs) from dairy goat (Capra hircus) testis using magnetic microbeads. Theriogenology 2013; 80:1052-1060; http://dx.doi.org/ 10.1016/j.theriogenology.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 38. Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells.Eur J Immunol 2000;30(5):1453-7; PMID:10820393; http://dx.doi.org/ 10.1002/(SICI)1521-4141(200005)30:5%3c1453::AID-IMMU1453%3e3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]



