Abstract
Reduced-antigen-content diphtheria-tetanus-acellular pertussis (dTpa) vaccine, Boostrix™, is indicated for booster vaccination of children, adolescents and adults. The original prefilled disposable dTpa syringe presentation was recently replaced by another prefilled-syringe presentation with latex-free tip-caps and plunger-stoppers. 671 healthy adolescents aged 10–15 years who had previously received 5 or 6 previous DT(P)/dT(pa) vaccine doses, were randomized (1:1) to receive dTpa booster, injected using the new (dTpa-new) or previous syringe (dTpa-previous) presentations. Immunogenicity was assessed before and 1-month post-booster vaccination; safety/reactogenicity were assessed during 31-days post-vaccination. Non-inferiority of dTpa-new versus dTpa-previous was demonstrated for all antigens (ULs 95% CIs for GMC ratios ranged between 1.03-1.13). 1-month post-booster, immune responses were in similar ranges for all antigens with both syringe presentations. dTpa delivered using either syringe presentation was well-tolerated. These clinical results complement the technical data and support the use of the new syringe presentation to deliver the dTpa vaccine.
Keywords: booster, diphtheria, pertussis, syringe presentation, tetanus
Abbreviations
- ATP
according to protocol
- ANCOVA
analysis of co-variance
- dTpa
reduced-antigen-content diphtheria tetanus and acellular pertussis vaccine
- CI
confidence interval
- El.U
Elisa units
- FHA
filamentous hemagglutinin
- GI
gastrointestinal
- GMC
geometric mean concentration
- IU
international units
- LL
lower limit
- PRN
pertactin
- PT
pertussis toxin
- SAE
serious adverse event
- TVC
total vaccinated cohort
- UL
upper limit
Introduction
Pertussis is a highly infectious disease and remains an important worldwide public health problem, even in countries with sustained high vaccination coverage.1,2 Despite established infant immunization programmes, pertussis continues to circulate, predominantly due to waning immunity beyond childhood and disease transmission from adolescents and adults to vulnerable infants.2,3 The need to maintain antibody levels against pertussis beyond childhood, through booster vaccines is therefore increasingly recognized. Reduced-antigen-content combined diphtheria-tetanus-acellular pertussis (dTpa) vaccines, such as Boostrix™ [dTpa; GlaxoSmithKline (GSK) Vaccines], have been specifically developed to immunize older children from the age of 4 years, adolescents and adults.4,5 Boostrix™, which was first licensed in 1999, is currently available in over 70 countries and has a well-established immunogenicity and tolerability profile in populations ranging from school age to the elderly.6-19 Traditionally, Boostrix™ has been available as a single-dose vial or a prefilled disposable syringe where the tip-cap and plunger stopper contained methylester W1883. However, following the discontinuation of W1883 production by the manufacturer (West), the syringe presentation has recently been replaced using prefilled syringes from a different manufacturer, wherein the tip-cap component contains FM27 (a latex-free non-cytotoxic rubber compound) and the plunger stopper component contains FM457 (an ultra-low extractable bromobutyl compound). Although this presentation change has been approved on the basis of a technical variation, this non-inferiority study was conducted to evaluate the impact on safety and immunogenicity, if any, due to the change in the material used in the rubber plunger of the prefilled syringe as required by a regulatory agency; it compared the immunogenicity and safety of dTpa vaccine injected using the old and new syringe presentations, and thereby support the change with clinical data.
This phase IV, randomized, single-blind, parallel group study (NCT01362322), funded by GlaxoSmithKline Biologicals SA, was conducted across 3 centers in Chile and Mexico between July 2011 and September 2012. The study was approved by Ethics review committees in each country [Av. Francisco I Madero Pte S/N y Dr E Aguirre Pequeno, Col Mitras Centro, Monterrey, Mexico; SubComite de Etica en Investigacion, Hospital General de Ecatepec Las America, Estado de Mexico; Comité de Ética en Investigación, Facultad de Medicina, Pontificia Universidad Católica de Chile; Comité Ético Científico del Servicio de Salud Metropolitano Central, Santiago; Institute of Public Health Chile] and adhered to the Declaration of Helsinki and Good Clinical Practice guidelines. Written, informed consent was obtained from parents/guardians and assent from subjects before enrolment.
Healthy adolescents aged 10–15 years who had received 5 or 6 previous doses of DT(P)/dT(pa) vaccine were randomized (1:1) to receive dTpa booster via the new (dTpa-new) or previous (dTpa-previous) syringe presentations. Due to visual differences in the presentation of the 2 syringes, this study was conducted in a single-blind manner.
Each 0.5 mL dTpa vaccine dose contained ≥2 IU diphtheria toxoid, ≥20 IU tetanus toxoid, 8 µg pertussis toxin (PT), 8 µg filamentous hemagglutinin (FHA) and 2.5 µg pertactin (PRN). The vaccine was supplied in 2 prefilled syringe presentations: dTpa-previous group had syringes with W1833 tip-caps and plunger stoppers (Lot. No: DC37A005B and expiry date: 31 AUG 2013); dTpa-new group had syringes with FM27 tip-caps and FM457 plunger stoppers (Lot. No: DC37A005A and expiry date: 31 AUG 2013).
A single booster dTpa dose was injected intramuscularly into the deltoid region of the non-dominant arm, using a needle ≥2.54 cm length and 22–25 gauge.
Blood samples (5 mL) were collected from all subjects before, and one month post-booster dosing. Antibodies against diphtheria, tetanus and pertussis antigens were measured using standard enzyme-linked immunosorbent assay (ELISA). Seroprotection against diphtheria and tetanus antigens was defined as an antibody concentration ≥0.1 IU/mL. A booster response to diphtheria and tetanus antigens was defined as antibody concentrations ≥4-fold the assay cut-off in initially seronegative subjects or ≥4-fold increase in pre-vaccination antibody concentrations in initially seropositive subjects.
Seropositivity against pertussis antigens was defined as an antibody concentration ≥5 El.U/mL per antigen. A booster response to these antigens was defined as antibody concentrations ≥4-fold the assay cut-off in initially seronegative subjects; a ≥4-fold increase in pre-vaccination antibody concentrations in initially seropositive subjects (pre-vaccination concentrations ≥5 to <20 El.U/mL) or ≥2-fold increase in pre-vaccination antibody concentrations in initially seropositive subjects with pre-vaccination concentrations ≥20 El.U/mL.
Diary cards were used to assess solicited local (injection site pain, redness and swelling) and general (fatigue, headache, fever [axillary temperature ≥37.5°C] and gastrointestinal [GI] symptoms) adverse events for 4 days (day 0–3) after vaccination. The intensity of symptoms was graded on a 3-point scale: Grade 3 redness and swelling: diameter >50 mm; Grade 3 fever: axillary temperature >39.0°C. For all other symptoms “Grade 3” was defined as preventing normal activities. Large injection site reactions (defined as swelling with a diameter >100 mm, noticeable diffuse swelling or noticeable increase of limb circumference) were evaluated by the investigator. All other symptoms, including serious adverse events (SAEs) occurring within 31 days of vaccination were recorded.
The primary objective of the study was to demonstrate that dTpa-new is non-inferior to dTpa-previous, in terms of immune response to all vaccine antigens, one month after booster vaccination. The criteria for evaluation was that the upper limit (UL) of the 95% confidence interval (CI) on the GMC ratios [dTpa-previous over dTpa-new] for anti-diphtheria, anti-tetanus, anti-PT, anti-FHA and anti-PRN antibodies was ≤1.5 (clinical limit for non-inferiority). The 95% CIs for the GMC ratio of the 2 study groups was computed using an analysis of co-variance (ANCOVA) model including the group and number of previous DT doses (5 or 6) as fixed effects and the log-transformed pre-vaccination concentration as co-variable.
With a minimum of 600 evaluable subjects, the study had 94% power (Bonferroni adjustment of β) to achieve the primary objective. Assuming a dropout rate of around 10%, a total of 670 subjects (335 subjects in each group) were to be randomized to ensure a sufficient number of evaluable subjects were available for inclusion in the ATP cohort for analysis of immunogenicity.
Secondary objectives included the evaluation of seroprotection/seropositivity rates, booster response and safety analysis one month after booster vaccination.
The primary analysis of immunogenicity was performed on the according-to-protocol (ATP) cohort, comprising vaccinated subjects who met the eligibility criteria, complied with protocol-defined procedures and for whom immunogenicity data were available. Seroprotection/seropositivity rates and GMCs were calculated with exact 95% CI.
The analysis of safety was performed on the total vaccinated cohort (TVC), which comprised all study participants for whom safety data were available. The safety results are described.
Of 671 subjects enrolled in the current study [376 at Pontificia Universidad, Catolica De Chile, Santiago; 93 at Hospital Universitario, De La UANL, Monterrey; Mexico and 202 at Hospital General De Ecatepec Las Americas, Estado de Mexico, Mexico], 335 received dTpa-new and 336 received dTpa-previous and were included in the TVC. One subject from the dTpa-previous group was eliminated from the ATP cohort for safety after receiving a vaccine forbidden in protocol. Fourteen subjects were eliminated from the ATP cohort in the dTpa-new group due to non-compliance with blood sampling (8) and missing serological data (6); 16 were eliminated from the ATP cohort in the dTpa-previous group due to protocol violation (1), non-compliance with blood sampling (8) and missing serological data (7). The ATP cohort for immunogenicity therefore included 321 and 319 subjects in the dTpa-new and dTpa-previous groups, respectively.
The groups were well-matched demographically. The mean age of the subjects in the ATP cohort for immunogenicity was 11.9 years (standard deviation ±1.61); 50.6% subjects were of Hispanic origin and 53.0% were female.
Before booster vaccination, ≥88.5% subjects in both groups were seroprotected against diphtheria; ≥96.9% subjects in both groups were seroprotected against tetanus (Table 1). At least 54.7% were seropositive against pertussis antibodies before the booster dose in both groups (Table 1).
Table 1.
Seroprotection/seropositivity rates and GMCs one month after booster vaccination (ATP cohort for immunogenicity)
| dTpa-new | dTpa-previous | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibody | Timing | N | n | Seroprotection* (95% CI) | GMC(95% CI) | N | n | Seroprotection* (95% CI) | GMC(95% CI) |
| anti-diphtheria | Pre | 321 | 284 | 88.5 (84.5–91.8) | 0.472 (0.403–0.553) | 319 | 286 | 89.7 (85.8–92.8) | 0.456 (0.392–0.530) |
| Post | 321 | 320 | 99.7 (98.3–100) | 6.784 (6.178–7.450) | 319 | 319 | 100 (98.9–100) | 6.493 (5.915–7.128) | |
| anti-tetanus | Pre | 321 | 311 | 96.9 (94.3–98.5) | 0.956 (0.835–1.095) | 319 | 314 | 98.4 (96.4–99.5) | 0.899 (0.789–1.026) |
| Post | 321 | 321 | 100 (98.9–100) | 18.937 (17.313–20.713) | 319 | 319 | 100 (98.9–100) | 18.515 (16.851–20.342) | |
| dTpa-new | dTpa-previous | ||||||||
| Antibody | Timing | N | n | Seropositivity** (95% CI) | GMC(95% CI) | N | n | Seropositivity** (95% CI) | GMC (95% CI) |
| anti-PT | Pre | 320 | 175 | 54.7 (49.1–60.2) | 7.5 (6.6–8.7) | 319 | 175 | 54.9 (49.2–60.4) | 7.2 (6.3–8.2) |
| Post | 318 | 316 | 99.4 (97.7–99.9) | 140.2 (126.0–156.1) | 318 | 315 | 99.1 (97.3–99.8) | 125.9 (112.7–140.7) | |
| anti-FHA | Pre | 316 | 310 | 98.1 (95.9–99.3) | 48.9 (43.3–55.2) | 315 | 310 | 98.4 (96.3–99.5) | 49.4 (43.6–56.0) |
| Post | 319 | 319 | 100 (98.9–100) | 1080.2 (995.2–1172.5) | 319 | 319 | 100 (98.9–100) | 1013.7 (940.0–1093.2) | |
| anti-PRN | Pre | 321 | 269 | 83.8 (79.3–87.7) | 14.0 (12.3–15.9) | 319 | 272 | 85.3 (80.9–89.0) | 13.4 (11.9–15.0) |
| Post | 321 | 321 | 100 (98.9–100) | 652.4 (572.1–743.9) | 318 | 318 | 100 (98.8–100) | 619.2 (546.0–702.2) | |
dTpa-new = Subjects who received Boostrix™ in new syringe presentation; dTpa-previous = Subjects who received Boostrix™ in previous syringe presentation.
N = Number of subjects with both pre- and post-vaccination results available; 95% CI = 95% confidence interval; D, diphtheria; T, tetanus; PT, pertussis toxin; FHA, filamentous hemagglutinin; PRN, pertactin IU, international unit; El.U, ELISA unit; GMC, geometric mean concentration calculated on all subjects.
Seroprotection=anti-diphtheria and anti-tetanus antibody concentration ≥0.1 IU/mL.
Seropositive=Anti-PT, Anti-FHA and Anti-PRN antibodies ≥5 EU/mL.
One month after booster vaccination, ≥99.7% subjects in both groups were seroprotected against diphtheria antigens; all subjects were seroprotected against tetanus antigens; ≥99.4% were seropositive against the pertussis antigens (Table 1). As the ULs of the 95% CI for the GMC ratios (dTpa-previous/dTpa-new) for all antigens were ≤1.5 (Table 2), non-inferiority of dTpa injected via the new syringe presentation (dTpa-new) against the previously used syringe presentation (dTpa-previous) was demonstrated.
Table 2.
Adjusted GMC ratios between groups (dTpa-previous divided by dTpa-new) one month after booster vaccination (ATP cohort for immunogenicity)
| dTpa-previous | dTpa-new | Adjusted GMC ratio (dTpa-pre Group/ dTpa-new Group) | |||
|---|---|---|---|---|---|
| Antibody | N | Adjusted GMC | N | Adjusted GMC | Value (95% CI) |
| anti-diphtheria | 319 | 6.521 | 321 | 6.765 | 0.96 (0.85–1.09) |
| anti-tetanus | 319 | 18.672 | 321 | 19.171 | 0.97 (0.86–1.10) |
| anti-PT | 318 | 128.340 | 317 | 138.832 | 0.92 (0.82–1.04) |
| anti-FHA | 315 | 1013.167 | 314 | 1096.827 | 0.92 (0.83–1.03) |
| anti-PRN | 318 | 634.592 | 321 | 645.504 | 0.98 (0.85–1.13) |
dTpa-new = Subjects who received Boostrix™ in new syringe presentation; dTpa-previous = Subjects who received Boostrix™ in previous syringe presentation.
N = Number of subjects with both pre- and post-vaccination results available; 95% CI = 95% confidence interval for the adjusted GMC ratio [ANCOVA model: Adjusted for pre-booster concentration and number of previous DT(P)/dT(pa) doses (5 or 6) pooled variance]); LL = lower limit, UL = upper limit.
Adjusted GMC = geometric mean antibody concentration obtained from an ANCOVA model adjusted for pre-booster concentration and number of previous DT(P)/dT(pa) doses (5 or 6).
Irrespective of which different syringe presentation was used for vaccine delivery, robust immune responses were observed and booster response rates for all antigens ranged from 79.0% to 99.7% in the 2 study groups (data not shown).
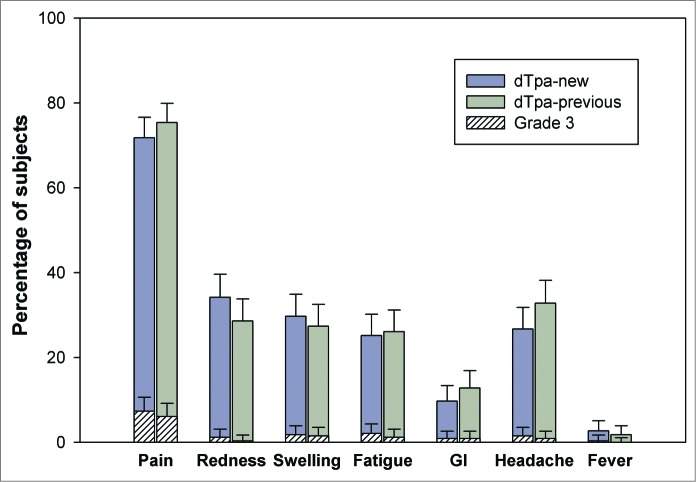
During the 4-day post-vaccination follow-up period, 78.8% and 83.0% subjects reported at least one symptom in the dTpa-new and dTpa-previous groups, respectively. Injection site pain was the most commonly reported solicited local symptom in 71.8% (7.3% Grade 3) and 75.4% (6.1% Grade 3) of the subjects in the dTpa-new and dTpa-previous groups, respectively. Headache, which was reported by 26.7% and 32.8% of the subjects, and fatigue, reported by 25.2% and 26.1% of the subjects, were the most frequently reported solicited general symptoms in the dTpa-new and dTpa-previous groups, respectively (Fig. 1).
Figure 1.
Incidence of solicited local and general symptoms reported during the 4-day post-vaccination follow-up period (Total Vaccinated Cohort).
During the 31-day post-vaccination follow-up period, at least one unsolicited symptom was reported in 13.1% (2.4% Grade 3) and 13.4% (2.7% Grade 3) subjects in the dTpa-new and dTpa-previous groups, respectively.
One subject in the dTpa-new group suffered accidental injury 13 days after vaccination; this SAE was considered to be unrelated to vaccination. No large swelling reactions were observed.
Since launch, over 100 million Boostrix™ doses have been distributed [data on file] and the immunogenicity, reactogenicity and safety of the vaccine has been well established in clinical trials and routine practice across a broad age range.6-20
Owing to a recent technical change in the Boostrix™ syringe presentation, this study was undertaken to compare the immunogenicity and safety of the new and previous syringe presentations, which differed in the nature of the compounds present in the tip-caps and plunger stoppers.
In this study, the dTpa vaccine was immunogenic regardless of which syringe presentation was used to administer the vaccine. The results were consistent with previous reports in adolescents4,6,14,18 and non-inferiority of the new presentation compared to the old presentation was demonstrated. dTpa administered using the new dTpa syringe presentation was also well tolerated and the incidence and nature of adverse events were similar irrespective of the syringe presentation and comparable with previous studies.4,11,12,15 Large swelling reactions, which can be associated with repeated booster doses of dTpa vaccines,6,14 were not observed in either study group.
Due to the resurgence of pertussis in adolescents and adults, the need to maintain antibody levels against pertussis beyond childhood, through booster vaccines is increasingly recognized. Although limited by single-blind design and inconsistent vaccination history [subjects having received either 5 or 6 previous DT(P)/dT(pa) vaccine doses], we demonstrated that a single dTpa booster dose was highly immunogenic and well tolerated in healthy adolescents in Chile and Mexico, irrespective of which syringe presentation was employed.
In conclusion, clinical data from this study support the technical data and the use of the new syringe presentation with FM27 tip-caps and FM457 plunger stoppers to deliver the dTpa vaccine.
Acknowledgments
We thank Adriana Ton, Carolina Frisone, Jonathan Rodriguez Cabo-Mercado, and Martina Kovac (all GlaxoSmithKline) for contributing to the study, Ramandeep Singh (GlaxoSmithKline Vaccines) for providing medical writing services and Julia Donnelly (freelance on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Disclosure of Potential Conflicts of Interest
HHH, SK, GJ, KH, YC and AL are employees of GlaxoSmithKline Vaccines and HHH, GJ, KH and AL declare having GlaxoSmithKline stocks. KA has received grants, personal fees and non-financial support from GlaxoSmithKline and NP-R has received research support from GlaxoSmithKline. MDLO has no conflicts to declare. Boostrix is a trademark of the GlaxoSmithKline group of companies.
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals Vaccines, Belgium. GlaxoSmithKline Vaccines was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with developing and publishing the manuscript.
Trial Number
References
- 1.Gabutti G, Rota MC. Pertussis: a review of disease epidemiology worldwide and in Italy. Int J Environ Res Public Health 2012; 9:4626-38; PMID:23330226; http://dx.doi.org/ 10.3390/ijerph9124626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guiso N, Wirsing von Konig CH, Forsyth K, Tan T, Plotkin SA. The Global Pertussis Initiative: report from a round table meeting to discuss the epidemiology and detection of pertussis, Paris, France, 11–12 January 2010. Vaccine 2011; 29:1115-21; PMID:21168525; http://dx.doi.org/ 10.1016/j.vaccine.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 3.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005; 24:S58-61; PMID:15876927; http://dx.doi.org/ 10.1097/01.inf.0000160914.59160.41 [DOI] [PubMed] [Google Scholar]
- 4.McCormack PL. Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix(R)): a review of its properties and use as a single-dose booster immunization. Drugs 2012; 72:1765-91; PMID:22931522; http://dx.doi.org/ 10.2165/11209630-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5.Scott LJ, McCormack PL. Reduced-antigen, combined diphtheria, tetanus, and acellular pertussis vaccine, adsorbed (boostrix((R))): a guide to its use as a single-dose booster immunization against pertussis. BioDrugs 2013; 27:75-81; PMID:23329401; http://dx.doi.org/ 10.1007/s40259-012-0009-y [DOI] [PubMed] [Google Scholar]
- 6.Mertsola J, Van Der Meeren O, He Q, Linko-Parvinen A, Ramakrishnan G, Mannermaa L, Soila M, Pulkkinen M, Jacquet JM. Decennial administration of a reduced antigen content diphtheria and tetanus toxoids and acellular pertussis vaccine in young adults. Clin Infect Dis 2010; 51:656-62; PMID:20704493; http://dx.doi.org/ 10.1086/655825 [DOI] [PubMed] [Google Scholar]
- 7.Van Damme P, McIntyre P, Grimprel E, Kuriyakose S, Jacquet JM, Hardt K, Messier M, Van Der Meeren O. Immunogenicity of the reduced-antigen-content dTpa vaccine (Boostrix((R))) in adults 55 years of age and over: a sub-analysis of four trials. Vaccine 2011; 29:5932-9; PMID:21718738; http://dx.doi.org/ 10.1016/j.vaccine.2011.06.049 [DOI] [PubMed] [Google Scholar]
- 8.Weston WM, Friedland LR, Wu X, Howe B. Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix((R))): results of two randomized trials. Vaccine 2012; 30:1721-8; PMID:22212127; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.055 [DOI] [PubMed] [Google Scholar]
- 9.Abarca K, Valdivieso F, Potin M, Ibanez I, Vial P. [Immunogenicity and reactogenicity of a reduced antigen content diphtheria, tetanus and acellular pertussis vaccine dTpa) in 10 to 11 years old children and in adults]. Rev Med Chile 2002; 130:502-10; PMID:12143270; http://dx.doi.org/ 10.4067/S0034-98872002000500004 [DOI] [PubMed] [Google Scholar]
- 10.Kosuwon P, Warachit B, Hutagalung Y, Borkird T, Kosalaraksa P, Bock HL, Poovorawan Y. Reactogenicity and immunogenicity of reduced antigen content diphtheria-tetanus-acellular pertussis vaccine (dTpa) administered as a booster to 4-6 year-old children primed with four doses of whole-cell pertussis vaccine. Vaccine 2003; 21:4194-200; PMID:14505898; http://dx.doi.org/ 10.1016/S0264-410X(03)00496-1 [DOI] [PubMed] [Google Scholar]
- 11.Huang LM, Chang LY, Tang H, Bock HL, Lu CY, Huang FY, Lin TY, Lee CY. Immunogenicity and reactogenicity of a reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine in healthy Taiwanese children and adolescents. J Adolesc Health 2005; 37:517; PMID:16310132; http://dx.doi.org/ 10.1016/j.jadohealth.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 12.Pichichero ME, Blatter MM, Kennedy WA, Hedrick J, Descamps D, Friedland LR. Acellular pertussis vaccine booster combined with diphtheria and tetanus toxoids for adolescents. Pediatrics 2006; 117:1084-93; PMID:16585302; http://dx.doi.org/ 10.1542/peds.2005-1759 [DOI] [PubMed] [Google Scholar]
- 13.Knuf M, Zepp F, Meyer C, Grzegowski E, Wolter J, Riffelmann M, Wirsing von König CH. Immunogenicity of a single dose of reduced-antigen acellular pertussis vaccine in a non-vaccinated adolescent population. Vaccine 2006; 24:2043-8; PMID:16356597; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.024 [DOI] [PubMed] [Google Scholar]
- 14.Booy R, Van der Meeren O, Ng SP, Celzo F, Ramakrishnan G, Jacquet JM. A decennial booster dose of reduced antigen content diphtheria, tetanus, acellular pertussis vaccine (Boostrix) is immunogenic and well tolerated in adults. Vaccine 2010; 29:45-50; PMID:20974302; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 15.Blatter M, Friedland LR, Weston WM, Li P, Howe B. Immunogenicity and safety of a tetanus toxoid, reduced diphtheria toxoid and three-component acellular pertussis vaccine in adults 19-64 years of age. Vaccine 2009; 27:765-72; PMID:19041352; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.028 [DOI] [PubMed] [Google Scholar]
- 16.Bose A, Dubey AP, Gandhi D, Pandit A, Raghu MB, Raghupathy P, Rao MI, Verghese VP, Datta SK, Bock HL. Safety and reactogenicity of a low dose diphtheria tetanus acellular pertussis vaccine (Boostrix) in pre-school Indian children. Indian Pediatr 2007; 44:421-4; PMID:17620694 [PubMed] [Google Scholar]
- 17.Zepp F, Habermehl P, Knuf M, Mannhardt-Laakman W, Howe B, Friedland LR. Immunogenicity of reduced antigen content tetanus-diphtheria-acellular pertussis vaccine in adolescents as a sixth consecutive dose of acellular pertussis-containing vaccine. Vaccine 2007; 25:5248-52; PMID:17583395; http://dx.doi.org/ 10.1016/j.vaccine.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 18.Vergara R, Tregnaghi M, Ussher J, Navarro S, Rüttimann R, Potin M, Wolter J, Schuerman L. Reduced-antigen-content-diphtheria-tetanus-acellular-pertussis and inactivated polio vaccine as a booster for adolescents 10 to 14 years of age. Eur J Pediatr 2005; 164:377-82; PMID:15782295; http://dx.doi.org/ 10.1007/s00431-005-1650-y [DOI] [PubMed] [Google Scholar]
- 19.Weston WM, Chandrashekar V, Friedland LR, Howe B. Safety and immunogenicity of a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine when co-administered with influenza vaccine in adults. Human vaccines 2009; 5:858-66; PMID:19838080; http://dx.doi.org/ 10.4161/hv.9961 [DOI] [PubMed] [Google Scholar]
- 20.Sanger R, Behre U, Krause KH, Loch HP, Soemantri P, Herrmann D, Schmitz-Hauss E, Wolter J, Hoet B. Booster vaccination and 1-year follow-up of 4-8-year-old children with a reduced-antigen-content dTpa-IPV vaccine. Eur J Pediatr 2007; 166:1229-36; PMID:17235521; http://dx.doi.org/ 10.1007/s00431-006-0403-x [DOI] [PubMed] [Google Scholar]