Abstract
Rickettsia rickettsii is the etiological agent of Rocky Mountain spotted fever (RMSF). YbgF and TolC are outer membrane-associated proteins of R. rickettsii that play important roles in its interaction with host cells. We investigated the immunogenicity of YbgF and TolC for protection against RMSF. We immunized C3H/HeN mice with recombinant R. rickettsii YbgF (rYbgF) or TolC (rTolC). Rickettsial burden and impairment in the lungs, spleens, and livers of rYbgF-immunized mice were significantly lower than in rTolC-immunized mice. The ratio of IgG2a to IgG1 in rYbgF-immunized mice continued to increase over the course of our experiments, while that in rTolC-immunized mice was reduced. The proliferation and cytokine secretion of CD4+ and CD8+ T cells isolated from R. rickettsii-infected mice were analyzed following antigen stimulation. The results indicated that proliferation and interferon (IFN)-γ secretion of CD4+ or CD8+ T cells in R. rickettsii-infected mice were significantly greater than in uninfected mice after stimulation with rYbgF. YbgF is a novel protective antigen of R. rickettsii. Protection conferred by YbgF is dependent upon IFN-γ-producing CD4+ and CD8+ T cells and IgG2a, which act in synergy to control R. rickettsii infection.
Keywords: cell-mediated immune response, Rickettsia rickettsii, Rocky Mountain spotted fever, YbgF, protective antigen
Introduction
Rickettsia rickettsii is the etiological agent of Rocky Mountain spotted fever (RMSF), a life-threatening tick-transmitted infection.1 Infection with R. rickettsii damages blood vessels throughout the body, causing increased vascular permeability, diminished serum oncotic pressure, and reduced perfusion of various organs.2 The case fatality rate of RMSF in untreated patients is as high as 60%, with a range of 5–10% in treated patients. Fatality rates tend to rise if treatment with antibiotics (tetracycline or chloramphenicol) is delayed.3 There have been concerted efforts to develop an effective vaccine against RMSF.
Three different vaccines containing nonviable R. rickettsii have been prepared from R. rickettsii-infected tick material,4 egg-grown R. rickettsii,5 and chicken embryonic cell-grown R. rickettsii.6 These vaccines have been tested in human volunteers but have only been proven to be minimally effective in preventing RMSF.7,8 Furthermore, preparation of these rickettsia vaccines involves large-scale culturing of R. rickettsii cells, which is difficult and hazardous because R. rickettsii is highly infectious. A good alternative would therefore be subunit vaccines against RMSF comprising R. rickettsii protective antigens that can be efficiently and safely produced using modern techniques.
In recent years, many surface proteins have been recognized in the spotted fever group (SFG) rickettsiae. The Sca0 (OmpA) and Sca1 proteins are involved in the attachment of rickettsiae to host cells,9,10 while Sca5 (OmpB) is associated with the rickettsial invasion of host cells.4 Sca2 serves as a formin mimic that is associated with actin-based motility of rickettsiae in host cells.4 Sca4 can activate vinculin and interacts with the actin cytoskeleton of host cells.11 Both OmpA and OmpB have the ability to elicit efficient protection against R. rickettsii infection.12,13 Other Sca proteins have not been demonstrated to be protective antigens of R. rickettsii.
We previously showed that YbgF, a highly abundant surface-exposed protein (SEP) of Rickettsia heilongjiangensis, could confer protection against R. heilongjiangensis infection in a murine model.14 TolC, a bacterial membrane-associated protein, is recognized as a major SEP through the proteomic analysis of biotinylated cell surface proteins of R. rickettsii. In the current study, we used recombinant YbgF (rYbgF) and TolC (rTolC) to immunize C3H/HeN mice and determine their efficacies in protecting against R. rickettsii infection.
Results
Immunoblotting
Purified rYbgF (44 kDa) and rTolC (63 kDa) were recognized by immunoblotting of sera collected from R. rickettsii-infected mice at 28 days post-infection (dpi; Fig. 1).
Figure 1.
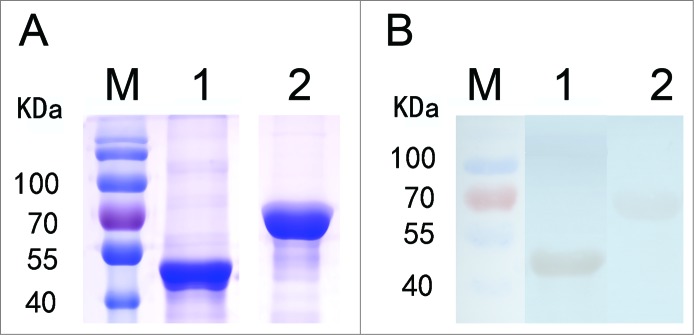
Immunoblotting analysis of rYbgF and rTolC. Recombinant YbgF and TolC purified from E. coli cell lysates were separated by 12% SDS-PAGE and stained with G-250 Coomassie brilliant blue (A). Immunoblotting analysis of rYbgF and rTolC. Lane M, protein molecular mass markers; lane 1, rYbgF; lane 2, rTolC (B).
Microscopy detection of YbgF and TolC
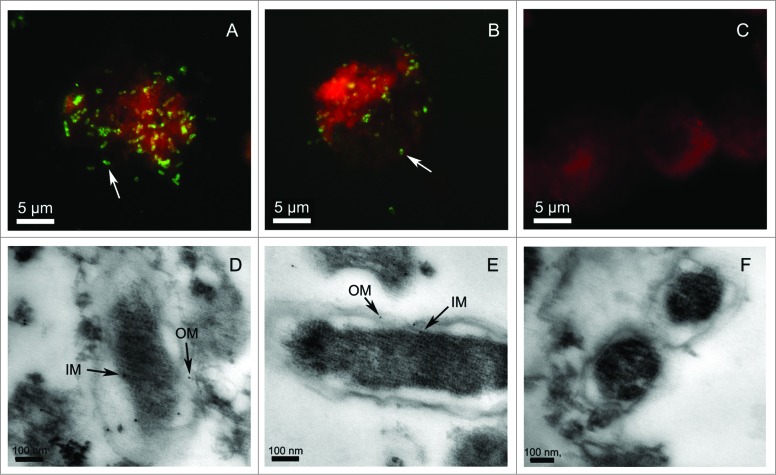
The presence of R. rickettsii YbgF or TolC was determined by indirect immunofluorescence assays (IFA) and transmission electron microscopy (TEM). Distinct fluorescent spots were observed in rickettsial cells stained with sera collected from mice immunized with rYbgF (Fig. 2A) or rTolC (Fig. 2B). Fluorescence was not observed in rickettsial cells stained with sera from mock-immunized mice (Fig. 2C). From our TEM results, the outer membrane (OM) and inner membrane (IM) of R. rickettsii cells were covered with colloidal gold particles when sera from mice immunized with rYbgF (Fig. 2D) or rTolC (Fig. 2E) were used.
Figure 2.
Microscopy analysis of YbgF and TolC in R. rickettsii cells. Rickettsia rickettsii were cultured in Vero cells and incubated with antibodies against rYbgF (A) or rTolC (B), or with naïve (C) serum. Secondary antibodies conjugated to fluorescein isothiocyanate (FITC) were then applied. The white arrows indicate YbgF or TolC in R. rickettsii cells. TEM analysis of R. rickettsii in Vero cells involved staining with antibodies against rYbgF (D), rTolC (E), or with naïve (F) serum. A goat anti-mouse IgG labeled with colloidal gold particles was then added to samples. The black arrows indicate the locations of YbgF or TolC in the inner membrane (IM) and outer membrane (OM) of R. rickettsii cells.
Immune protection against R. rickettsii challenge
Using quantitative polymerase chain reaction (qPCR) assays, the loads of rickettsia in the spleens (Fig. 3A), livers (Fig. 3B), or lungs (Fig. 3C) of mice immunized with rYbgF or whole cell antigen (WCA) were significantly lower than those in mock-immunized mice. The rickettsial loads in the spleens or lungs of mice immunized with rTolC were not significantly different from those in PBS-immunized mice.
Figure 3.
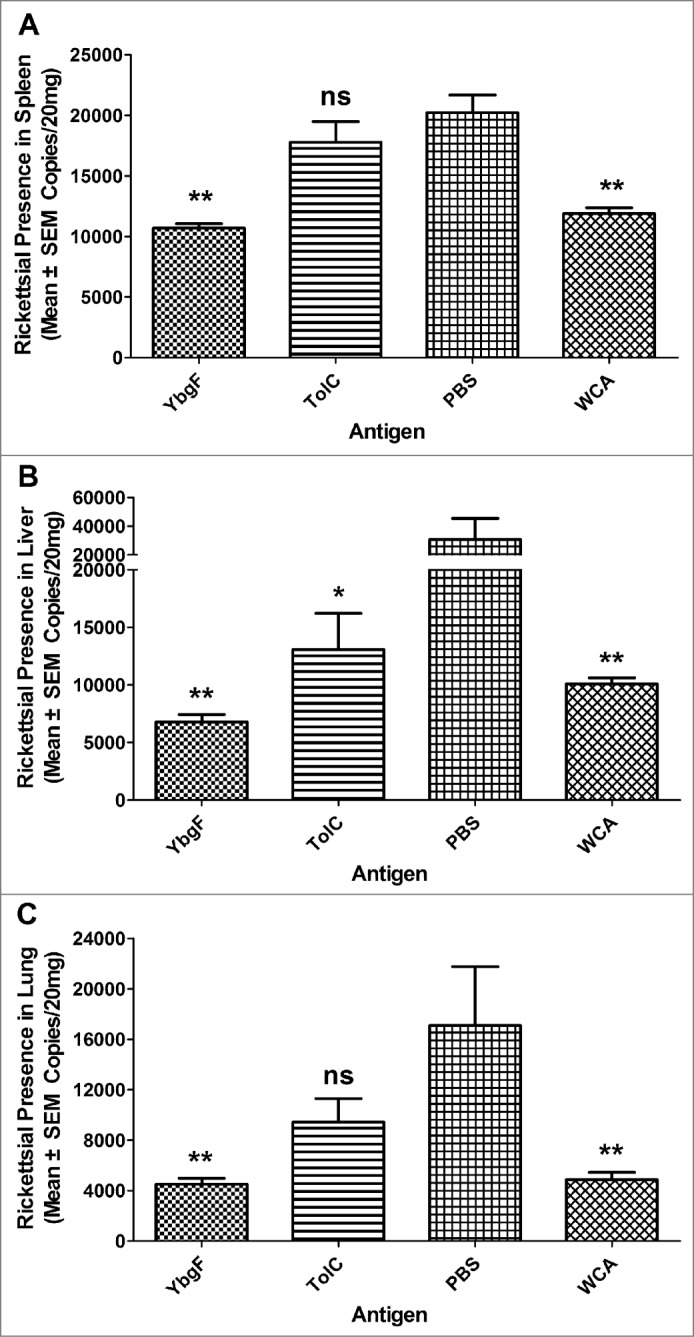
Immune protection against R. rickettsii infection. C3H/HeN mice were immunized 3 times with rYbgF, rTolC, WCA, or PBS. At day 14 after the last immunization, mice were challenged with R. rickettsii. At day 6 after challenge, mice were sacrificed and their spleens (A), livers (B), and lungs (C) were collected for use in qPCR assays. Rickettsial DNA copies among groups are expressed as mean copies + standard deviations. The statistically significant differences between PBS immunized mice and other groups were analyzed using the T test or Wilcoxon Two-Sample Test based on their normality and equality of variances and are indicated as follows: *, P < 0.05; ***, P < 0.001; ns, no significance. *P < 0.05; **P < 0.01; ns, no significance, respectively.
Serious pathological damages were observed in rTolC- and PBS-immunized mice (Fig. 4). In liver tissues, we observed hydropic degeneration, nuclear pyknosis, inflammatory infiltrates consisting of mononuclear cells and polymorphonuclear leukocytes that were focused on the portal area of the liver. In spleen tissues, large numbers of macrophages were observed. For lung lesions, interstitial pneumonia was characterized by numerous inflammatory infiltrates of mononuclear cells, polymorphonuclear leukocytes, alveolar interstitial thickening, and alveolar hemorrhage. The lesions in the organs of mice immunized with rYbgF or WCA were not as severe as those in other mice (Fig. 4).
Figure 4.
Pathological lesion in the organs of immunized mice after challenge. Infiltration of inflammatory cells is shown with thick arrows in the livers and lungs, macrophages in spleen tissues are shown with thin arrows, severe interstitial thickened alveolar walls are shown with short lines, and alveolar hemorrhage in lungs is shown with circles (400× magnification, bar = 200 μm).
Humoral immune responses
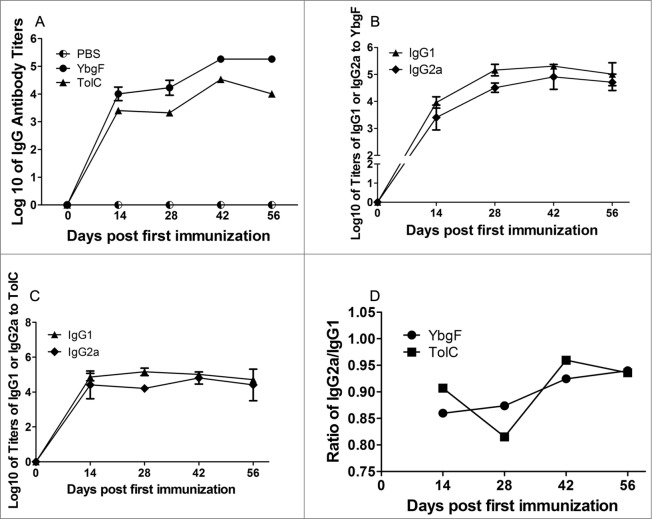
The mean log10 of IgG titers for rYbgF-immunized sera were 4.01, 4.23, 5.26, and 5.26, while those for rTolC-immunized sera were 3.40, 3.32, 4.53, and 4.01 on 14, 28, 42, and 56 dpi (Fig. 5). The titer ratio of IgG2a to IgG1 in rYbgF-immunized mice continued to increase over the course of our experiments, while that in rTolC-immunized mice was decreased on 42 dpi (Fig. 5).
Figure 5.
Humoral immune responses induced by different antigens. Log10 of titer kinetics for IgGs against rYbgF and rTolC (A). Titer kinetics of IgG1 and IgG2a against rYbgF (B). Titer kinetics of IgG1 and IgG2a against rTolC (C). IgG2a:IgG1 ratios for rYbgF and rTolC (D). Antibody titers were determined by ELISA and presented as log10, with the standard deviation indicated by an error bar.
Serum neutralization of R. rickettsii
The quantities of rickettsia for groups treated with sera from mice immunized with rYbgF (0.45 rickettsia/cell) or rTolC (0.43 rickettsia/cell) were lower than those treated with sera from PBS-immunized mice (0.61 rickettsia/cell; P < 0.05; Fig. 6). The amount of rickettsia in the group treated with sera from mice immunized with R. rickettsii WCA was higher than that in the group treated with sera from PBS-immunized mice.
Figure 6.
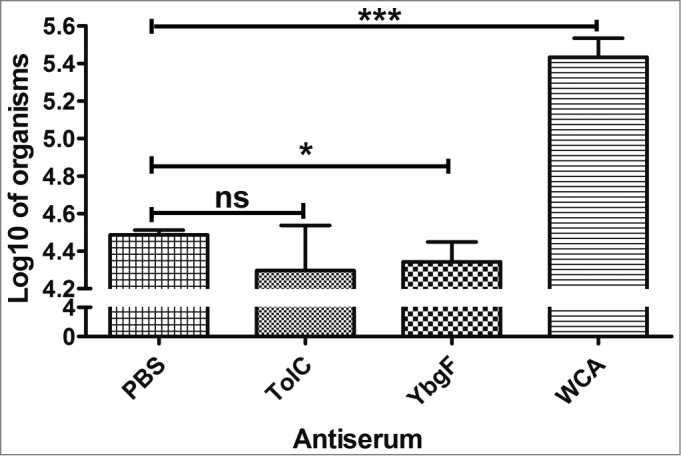
Neutralization of R. rickettsii by sera from mice immunized with various antigens. Purified R. rickettsii were incubated with sera from mice immunized with rYbgF, rTolC, WCA, or PBS for 60 min. Rickettsiae treated with these sera were then inoculated into EA.hy 926 cells. The total amount of R. rickettsii in host cells was determined by qPCR. Values are presented as the mean with standard deviations (n = 3). The statistically significant differences between anti-PBS immune serum and other immune serum groups were analyzed using the T test or Wilcoxon Two-Sample Test based on their normality and equality of variances and are indicated as follows: *, P < 0.05; ***, P < 0.001; ns, no significance.
Proliferation and cytokine production of T cells
CD4+ or CD8+ T cells from R. rickettsii-infected or uninfected mice were stimulated in vitro by rYbgF, rTolC, WCA, or ConA. Their proliferation and levels of cytokine secretion were analyzed (Table 1). After stimulation with rYbgF, the proliferation of CD4+ and CD8+ T cells from infected mice was significantly higher than that of uninfected cognate T cells (P < 0.01). The secretion of interferon (IFN)-γ and tumor necrosis factor (TNF)-α by CD4+ T cells from infected mice stimulated with rYbgF was significantly higher (P < 0.05) than that seen in CD4+ T cells from uninfected mice. Secretion of interleukin (IL)-6 and IL-10 from T cells was not dramatically different between infected and uninfected mice (P > 0.05). Secretion of IFN-γ (P < 0.05), IL-6 (P < 0.001), and IL-10 (P < 0.001) by CD4+ T cells from infected mice was significantly greater compared with those from uninfected mice. The secretion levels of cytokines by CD4+ T cells were not significantly different between infected and uninfected mice stimulated with ConA (P > 0.05).
Table 1.
Cytokine secretion and proliferation of CD4+ or CD8+ T cells after antigen stimulation. CD4+ or CD8+ T cells isolated from mice injected with R. rickettsii or PBS were stimulated with rYbgF, rTolC, WCA of R. rickettsii, ConA, or PBS. TNF-α, IFN-γ, IL-6, and IL-10 in supernatants of CD4+ T cells or TNF-α, IFN-γ, IL-6, and IL-10 in supernatants of CD8+ T cells were detected using a BD Cytometric Bead Array and Mouse Th1/Th2 cytokine kit. Proliferation of CD4+or CD8+ T cells was measured using Cell Counting Kit-8 and expressed as a percent of the control group (stimulated with PBS). The data are expressed as the mean ± standard deviation. Significant differences between infected and uninfected mice were analyzed by the T test or Wilcoxon Two-Sample Test based on their normality and equality of variances and are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significance
| Cytokines | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T cells | Stimulations | Groups | TNF (pg/ml) | IFN-γ (pg/ml) | IL-6 (pg/ml) | IL-10 (pg/ml) | Proliferation (%) | |||||
| CD4+ | ConA | Uninfected | 1045.6 ± 582.79 | ns | 2677 ± 490.73 | ns | 45.48 ± 4.45 | ns | 579.8 ± 49.22 | ns | 533.5 ± 35.22 | ns |
| Infected | 317.33 ± 12.6 | 5462.3 ± 106.36 | 533.39 ± 391.99 | 1138.55 ± 588.25 | 673.34 ± 155.37 | |||||||
| WCA | Uninfected | 1287.83 ± 49.4 | ns | 21.7 ± 2.71 | * | 302.64 ± 33.33 | *** | 144.34 ± 21.59 | *** | 172.63 ± 19.65 | ns | |
| Infected | 1380.93 ± 65.49 | 1805.53 ± 49.65 | 602.76 ± 31.75 | 422.44 ± 28.95 | 195.48 ± 26.1 | |||||||
| rYbgF | Uninfected | 110.8 ± 1.94 | * | 2.1 ± 0.37 | * | 181.06 ± 102.95 | ns | 260.05 ± 152.79 | ns | 221.32 ± 11.68 | ** | |
| Infected | 128.67 ± 8.36 | 18.97 ± 4.66 | 221.35 ± 16.47 | 431.63 ± 23.72 | 416.13 ± 55.91 | |||||||
| rTolC | Uninfected | 391 ± 82.13 | ns | 1.2 ± 0.22 | ns | 54.12 ± 13.35 | ns | 47.46 ± 10.62 | ns | 56.8 ± 4.93 | ns | |
| Infected | 361.23 ± 16.43 | 11.1 ± 12.59 | 78.36 ± 9.5 | 68.44 ± 8.57 | 92.26 ± 18.22 | |||||||
| PBS | Uninfected | 0.22 ± 0.31 | ns | 0.03 ± 0.05 | ns | 4.18 ± 0.25 | ns | 10.54 ± 0.96 | ns | 97.94 ± 14.63 | ns | |
| Infected | 0.16 ± 0.22 | 1.5 ± 2.12 | 4.59 ± 0.15 | 11.84 ± 0.61 | 117.18 ± 18.78 | |||||||
| CD8+ | ConA | Uninfected | 346.77 ± 31.7 | *** | 3348.33 ± 184.58 | *** | 51.5 ± 6.99 | ns | 253.73 ± 43.55 | ns | 510.38 ± 10.08 | ns |
| Infected | 588.63 ± 12.3 | 5497.9 ± 110.62 | 65.58 ± 4.68 | 292.54 ± 45.88 | 693.03 ± 67.81 | |||||||
| WCA | Uninfected | 971.67 ± 68.51 | ** | 12.37 ± 3.36 | ** | 321.33 ± 68.13 | *** | 273.47 ± 41.75 | *** | 246.96 ± 17.82 | ns | |
| Infected | 1529.27 ± 125.81 | 1716.77 ± 170 | 810.8 ± 77.98 | 565.02 ± 78.27 | 235.82 ± 32.57 | |||||||
| rYbgF | Uninfected | 303.27 ± 10.23 | ns | 1.8 ± 0.17 | * | 165.22 ± 14.45 | ns | 457.19 ± 61.63 | * | 291.17 ± 24.34 | ** | |
| Infected | 327.13 ± 68.81 | 15.77 ± 2.48 | 196.75 ± 23.52 | 312.19 ± 29.93 | 472 ± 19.77 | |||||||
| rTolC | Uninfected | 363.77 ± 24.16 | ns | 0.93 ± 0.16 | ns | 56.35 ± 7.91 | ns | 94.45 ± 19.63 | ns | 152.08 ± 17.88 | ns | |
| Infected | 455.33 ± 84.25 | 11.73 ± 2.59 | 97.05 ± 3.78 | 131.72 ± 10.98 | 135.94 ± 7.85 | |||||||
| PBS | Uninfected | 0.09 ± 0.06 | ns | 0.11 ± 0.16 | ns | 3.97 ± 0.17 | ns | 10.93 ± 0.61 | ns | 100.81 ± 1.53 | ns | |
| Infected | 0.39 ± 0.55 | 0.64 ± 0.91 | 5.51 ± 0.41 | 15.33 ± 1.22 | 106.42 ± 13.9 | |||||||
The secretion of IFN-γ by CD8+ T cells from infected mice and stimulated with rYbgF was significantly higher (P < 0.05) compared with those from uninfected mice. Secretion of TNF-α or IL-6 by CD8+ T cells from infected mice was not significantly different (P > 0.05) compared with those from uninfected mice. Secretion of IL-10 by CD8+ T cells from infected mice was lower (P < 0.05) compared with those from uninfected mice. After stimulation with R. rickettsii WCA, cytokine secretion by CD8+ T cells from infected mice was greater than that from the CD8+ T cells of uninfected mice (P < 0.05). There was no significant difference in the secretion of these cytokines from CD4+ and CD8+ T cells between infected and uninfected mice after rTolC stimulation. Additionally, the secretion of IL-2, IL-4, and IL-5 was too low to be detected except after stimulation with ConA (data not shown).
Discussion
Two abundant and high-molecular-weight outer membrane proteins, OmpA and OmpB, of the SFG rickettsiae are well-known protective antigens. Passively administered antibodies against OmpA or OmpB protect guinea pigs from R. rickettsii infection, and protect severe combined immunodeficient mice from lethal infection by Rickettsia conorii, which is closely related to R. rickettsii. These findings suggest that antibodies against OmpA or OmpB are important in host defense against rickettsial infection.15 OmpA- and OmpB-induced protective immunity involves both complement-mediated killing in mammalian blood 12 and the activation of antigen-specific memory CD4+ and CD8+ T cells.13
YbgF and TolC have been previously identified as major SEPs in rickettsiae. YbgF, a periplasmic protein, is an abundant surface protein and major sero-reactive antigen of rickettsiae.16,17 It is thought to function in maintaining the cell envelope integrity of Gram-negative bacteria.18 TolC is thought to be a bioactive transport molecule that couples with different inner membrane/periplasmic proteins. These are associated with virulence or antimicrobial resistance in Gram-negative bacteria.19 From our TEM analysis, YbgF and TolC were shown to be associated with both the inner and outer membranes of R. rickettsii, suggesting that specific antibodies could prevent R. rickettsii from interacting with host cells. To validate this, sera from mice immunized with rYbgF or rTolC were used to neutralize R. rickettsii in vitro. Our findings showed that both sera could significantly reduce R. rickettsii invasion of vascular endothelial cells, suggesting that YbgF and TolC are both factors that mediate the interaction of R. rickettsii with vascular endothelial cells. Further studies regarding their receptors on vascular endothelial cells are warranted.
For the protective efficacy assay, our results showed that the rickettsial load in the lungs, spleens, or livers of mice immunized with rYbgF were significantly lower than those in mock-immunized mice. Additionally, organ impairment in rYbgF-immunized mice was lower than in rTolC- or mock-immunized mice. These results demonstrate that YbgF is a protective antigen of R. rickettsii, while rTolC is not. In a previous study, YbgF was also recognized as a protective antigen of R. heilongjiangensis.14 A comparison of the amino acid sequences of YbgF from the 2 species showed that only 6 of 245 amino acids were different (Fig. S1), suggesting that YbgF is a conservative antigen, at least among the SFG rickettsiae.
To explore the potential protective mechanisms of YbgF, the immune response elicited by rYbgF was compared with that elicited by rTolC. Our results showed that high levels of specific antibodies were produced after immunization with both rYbgF and rTolC. This indicates that both proteins could elicit a specific humoral response. Results from the in vitro neutralization tests indicated that rYbgF- and rTolC-immunized sera could significantly reduce the number of R. rickettsii in vascular endothelial cells, suggesting that these sera contained neutralizing antibodies that could efficiently counteract R. rickettsii invasion of host cells. R. rickettsii WCA-immunized sera were able to enhance the number of R. rickettsii in host cells. This was observed in a previous neutralization assay with R. heilongjiangensis WCA-immunized sera.14 The reason for this might be that antibodies against numerous rickettsia surface molecules in WCA-immunized sera interact with multiple Fc receptors expressed by vascular endothelial cells, thereby promoting entry of rickettsia.14 Our results showed that rYbgF induced a more rapid increase in IgG2a levels compared with IgG1 levels during immunization. For rTolC, the ratio of IgG2a to IgG1 levels decreased during the intermediate and final phases of immunization. The IgG2a molecule has been proven to be involved in the elimination of intracellular pathogens from hosts via Fc and complement receptors of macrophages.20 It has also been shown to mediate antibody-dependent cell-mediated cytotoxicity 21 and opsonophagocytosis of macrophages.22 A high level of specific IgG2a has been shown to be involved in T-helper cell type 1 (Th1)-immune responses and enhanced efficacy of protein vaccination.23 Eliciting high levels of specific IgG2a could be considered an important property of rYbgF in inducing protection against R. rickettsii infections.
Although specific antibodies can counteract rickettsial invasion of host cells, cell-mediated immunity plays a critical role in killing intracellular rickettsiae.24 Our results showed that rYbgF but not rTolC could elicit antigen-specific CD4+ and CD8+ T cells to rapidly proliferate and differentiate into IFN-γ-producing CD4+ Th1 cells and CD8+ cytotoxic T lymphocytes (Tcl) cells, respectively. Antigen-specific CD4+ Th1 cells and CD8+ Tcl cells are well known to play key effector functions in the control of intracellular bacterial infection.25 Antigen-specific CD8+ Tcl cells are critical for the clearance of intracellular bacteria.26 During Toxoplasma gondii infections, IFN-γ-producing CD8+ T cells are the major effectors, and IFN-γ-producing CD4+ T cells play a synergistic role in protection against this pathogen.27
Previous studies have also suggested that cytokine-activated intracellular killing of rickettsiae within endothelial cells, the major target cells of rickettsiae, could occur via nitric oxide- and hydrogen peroxide-mediated killing. These are induced by a combination of IFN- γ, TNF-α, IL-1, and CCL5.28-30 Our results showed that rYbgF could elicit antigen-specific CD4+ T cells to produce high levels of IFN-γ and TNF-α, while antigen-specific CD8+ T cells produced high levels of IFN-γ. These findings suggest that the protective antigen, rYbgF, could efficiently activate antigen-specific CD4+ and CD8+ T cells to rapidly produce and secrete large quantities of TNF-α and/or IFN-γ, which function synergistically to activate endothelial cells and other target cells to kill intracellular rickettsiae.31 In addition, secretion of TNF-α by infected and uninfected CD8+ T cells was not significantly different following stimulation with rYbgF or rTolC, but was dramatically different after stimulation with WCA of R. rickettsii, suggesting that there were other components in WCA of R. rickettsii that could efficiently stimulate the antigen-specific CD8+ T cells to generate high levels of TNF-α.
In conclusion, YbgF and TolC are surface proteins of R. rickettsii associated with the inner and outer membrane. YbgF is a protective antigen, and its use in a vaccine dramatically reduces the severity of R. rickettsii infection in mice. This protection is mainly dependent upon rYbgF-induced, Th1-type cell-mediated immune responses, manifesting as rapid proliferation and high levels of IFN-γ secretion from YbgF-specific CD4+ and CD8+ cells. The rYbgF-induced Th1-type cell-mediated immune response also mediates the rise of YbgF-specific IgG2a levels, synergistically activating and opsonizing host cells to kill intracellular rickettsiae.
Materials and Methods
Mice
Female C3H/HeN mice at 6 to 7 weeks of age were purchased from Vital River Laboratories (Beijing, China). The experiments were carried out according to the guidelines of the authors' institution. The protocol was approved by the Institute of Animal Care and Use Committee at the Academy of Military Medical Sciences (AMMS). All facilities were accredited by the AMMS Animal Care and Ethics Committee, and all efforts were made to minimize mice suffering.
Bacterial strains and plasmids
We cultured R. rickettsii (Sheila Smith strain) in Vero cells (ATCC). Rickettsia were purified from cells using isopycnic density gradient centrifugation as described previously.32 The quantity and viability of organisms was measured by qPCR assays specific for R. rickettsii 33 and plaque assays,34 respectively. Purified rickettsiae were inactivated at 90°C for 20 min 32 and then used as WCA. The prokaryotic expression plasmid pET32a(+) (Novagen) was used to construct recombinant plasmids containing R. rickettsia genes of interest. Recombinant plasmids were transformed into Escherichia coli BL21 cells (Novagen).17
Preparation of recombinant proteins and immunoblotting
The open reading frames of ybgF (GenBank Accession Number: ABV75922.1) and tolC (ABV75915.1) were amplified by PCR from the genomic DNA of R. rickettsii with specific primers (5′-GGG GAT CCA TGA AAC TGA TTG TCT TA-3′ and 5′-GGC TCG AGT TTA ATC TTA GCA TCT TC-3′ for ybgF with respective BamHI and XhoI sites underlined; and 5′-CCG GAT CCA CTG AAG GGT ATA AGA A-3′ and 5′-CCG TCG ACA AAC TCT TCT TCA GGA CTA-3′ for tolC with respective BamHI and SalI sites underlined). Recombinant YbgF and TolC proteins were tagged with histidine and purified as described previously.14 Purified proteins were analyzed by immunoblotting from pooled sera that had been collected from C3H/HeN mice on day 28 after R. rickettsii infection, as described previously.35 Endotoxins in purified recombinant protein fractions were removed with Toxin Eraser™ (GenScript).14
Evaluation of protective efficacy
We subcutaneously injected 8 mice with 30 μg of rYbgF, rTolC, or R. rickettsii WCA (positive control) in 200 μL of phosphate-buffered saline (PBS), or PBS mixed with complete Freund's adjuvant (negative control; Sigma-Aldrich). Mice were intraperitoneally (i.p.) boosted with 20 μg of cognate antigen in 200 μL of PBS or PBS mixed with incomplete Freund's adjuvant 28 and 42 days after the primary immunization. On day 14 after the last immunization, each mouse was challenged i.p. with 6 × 105 plaque forming units (PFU) of R. rickettsii. Six days after challenge, 2 mice were sacrificed and their spleens, livers, and lungs collected for analysis. Tissues were fixed with formalin and embedded in paraffin. Sections were routinely stained with hematoxylin and eosin and examined by light microscopy. For the other 6 mice, each spleen was weighed and the rickettsial load in 20 mg of spleen, liver, or lung was determined using qPCR assays.33
Humoral immune analysis
Levels of IgG1 and IgG2a were determined by indirect enzyme-linked immunosorbent assays (ELISAs) using cognate antigens.14 Blood samples were collected from the tail veins of 6 mice per group on day 0 and then days 14, 28, 42, and 56 after primary immunization. Each serum sample was serially diluted 2-fold for the determination of specific antibody titers by ELISA.17 The cutoff value was set according to a previous study.14
Serum neutralization of R. rickettsii
The human endothelial hybrid cell line EA.hy926 was used as host cells of R. rickettsii. Cells were previously cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone) containing 1% heat-inactivated fetal bovine serum (FBS; Hyclone) at 33°C/5% CO2. At 14 days after the last immunization, the sera from 6 mice were collected and the pooled sera inactivated at 56°C for 30 min and filter sterilized.36 We mixed 150 μL of sera with R. rickettsii cells in 150 μL of DMEM (1.0 × 106 PFU/mL) at room temperature for 60 min. This mixture was added to 9.7 × 105 cells in 2.7 mL of DMEM containing 1% heat-inactivated FBS; the mixture was then aliquoted into 3 wells of a 24-well plate (Corning) and cultured at 33°C for 6 h.36 The supernatant was removed and DNA was extracted from the remaining cells using a DNeasy Blood & Tissue Kit (Qiagen). The purified DNA samples were then subjected to R. rickettsii-specific qPCR assays.33
Proliferation and cytokine production of T cells
Fifteen mice were injected i.p. with a sublethal dose (5 × 106 PFU/mouse) of R. rickettsii (infected mice) or PBS (uninfected mice). Fifteen days after injection, the mice were sacrificed and their spleens collected for isolation of CD4+ or CD8+ T cells using mouse CD4+ or CD8+ T cell isolation kits, respectively (Miltenyi). Mononuclear cells isolated from 5 naïve mouse spleens were used as antigen-presenting cells.37 Isolated CD4+ or CD8+ T cells were suspended in DMEM; 6 × 105 T cells and 3 × 105 mononuclear cells were added to each well of 24-well plates. Cells in each well were stimulated with 10 μg of each antigen or 2.5 μg of concanavalin A (ConA; positive control), with 3 replicate wells used per sample. After a 48-h incubation at 37°C/5% CO2, cytokines TNF-α, IFN-γ, IL-2, -4, -5, -6, and -10 in 500 μL of supernatant aspirated from each well were measured using a CBA Mouse Th1/Th2 cytokine kit (BD PharMingen). The remaining cells in each well were used to detect the proliferation of CD4+ or CD8+ T cells using a Cell Counting Kit-8 (CCK-8; Dojindo).
Microscopic detection of YbgF and TolC in R. rickettsii
R. rickettsii were cultured in Vero cells, and immune sera were prepared in C3H/HeN mice as previously described.17 YbgF and TolC on the surface of R. rickettsii were detected using IFA as previously described.17
For TEM analysis, R. rickettsii cultured in Vero cells were fixed (0.5% glutaraldehyde, 4% paraformaldehyde, 0.1 M sodium cacodylate, 0.3% picric acid, pH 7.4) for 4 h on ice. After four washes in 0.1 M PBS, fixed cells were dehydrated with alcohol and permeabilized with a mix of LR White (Spi Supplies) and alcohol. Cells were then embedded in SPI-Pon 812 resin (Spi Supplies) and transferred to a 200-mesh nickel gird (BeiJingZhongXingBaiRui Technology Co., Ltd.) following standard methods 38. Grids were incubated with sera (diluted 1:100) from mice immunized with rYbgF, rTolC, or PBS (negative control) for 2 h. Grids were then incubated with goat anti-mouse IgG conjugated to 10-nm colloidal gold particles (1:20; Aurion; EMS) for 2 h at room temperature. The grids were fixed in 1% glutaraldehyde for 10 min and then stained with uranyl acetate (Spi Supplies) and lead citrate (Spi Supplies) according to standard methods.38 Grids were examined using an electron microscope (Hitachi H-7650; Hitachi Chemical Co., Ltd.) at 80 kV.
Statistical analysis
All statistics were computed using SAS statistical software (version 9.1, SAS Institute Inc.). The statistical significances of the differences in rickettsial numbers produced by qPCR and the levels of proliferation or cytokine secretion of T cells were assayed using the T test or Wilcoxon 2-sample test according to their normality and homogeneity of variance. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Basic Research Program of China (Grants 2010CB530200/2010CB530205), the National Natural Science Foundation of China (Grants 81371767 and 31170161), and the Natural Science and Technology Major Project of China (Grant 2013ZX10004803).
References
- 1. Walker DH. The realities of biodefense vaccines against Rickettsia. Vaccine 2009; 27:D52-D5; PMID:19837287; http://dx.doi.org/ 10.1016/j.vaccine.2009.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrell GT, Aikawa JK. Pathogenesis of circulatory failure in Rocky Mountain spotted fever: alterations in the blood volume and the thiocyanate space at various stages of the disease. Arch Int Med 1949; 83:331; PMID:18112395; http://dx.doi.org/ 10.1001/archinte.1949.00220320085007 [DOI] [PubMed] [Google Scholar]
- 3. Walker DH. Biology of rickettsial diseases. Vol. I and Vol. II CRC Press, Inc., 1988. [Google Scholar]
- 4. Parker R. Rocky Mountain spotted fever: results of fifteen years' prophylactic vaccination. Am J Trop Med Hygiene 1941; 1:369-83. [Google Scholar]
- 5. Cox HR. Cultivation of Rickettsiae of the Rocky Mountain Spotted Fever, typhus and Q Fever groups in the embryonic tissues of developing chicks. Science (New York, NY) 1941; 94:399. [DOI] [PubMed] [Google Scholar]
- 6. Kenyon RH, Pedersen C. Preparation of Rocky Mountain spotted fever vaccine suitable for human immunization. J Clin Microbiol 1975; 1:500-3; PMID:809483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clements M, Wisseman C, Woodward T, Fiset P, Dumler J, McNamee W, Black RE, Rooney J, Hughes TP, Levine MM. Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J Infect Dis 1983; 148:922-30; PMID:6415182; http://dx.doi.org/ 10.1093/infdis/148.5.922 [DOI] [PubMed] [Google Scholar]
- 8. DuPont H, Hornick R, Dawkins A, Heiner G, Fabrikant I, Wisseman CL, Jr, Woodward TE. Rocky Mountain spotted fever: a comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii. J Infect Dis 1973; 128:340-4; PMID:4199563 [DOI] [PubMed] [Google Scholar]
- 9. Li H, Walker DH. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb Pathog 1998; 24:289-98; PMID:9600861 [DOI] [PubMed] [Google Scholar]
- 10. Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YG, Martinez JJ. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect Immun 2010; 78:1895-904; PMID:20176791; http://dx.doi.org/ 10.1128/IAI.01165-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park H, Lee JH, Gouin E, Cossart P, Izard T. The Rickettsia surface cell antigen 4 applies mimicry to bind to and activate vinculin. J Biolog Chem 2011; 286:35096-103; PMID:21841197; http://dx.doi.org/ 10.1074/jbc.M111.263855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan YG-Y, Riley SP, Chen E, Martinez JJ. Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect Immun 2011; 79:2303-13; PMID:21444665; http://dx.doi.org/ 10.1128/IAI.01324-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crocquet-Valdes P, Dıaz-Montero C, Feng H, Li H, Barrett A, Walker D. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 2001; 20:979-88; PMID:11738766; http://dx.doi.org/ 10.1016/S0264-410X(01)00377-2 [DOI] [PubMed] [Google Scholar]
- 14. Qi Y, Xiong X, Duan C, Jiao J, Gong W, Wen B. Recombinant protein YbgF induces protective immunity against Rickettsia heilongjiangensis infection in C3H/HeN mice. Vaccine 2013; 31:5643-50; PMID:24113261; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.064 [DOI] [PubMed] [Google Scholar]
- 15. Feng H-M, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect Immun 2004; 72:2222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deringer JR, Chen C, Samuel JE, Brown WC. Immunoreactive Coxiella burnetii Nine Mile proteins separated by 2D electrophoresis and identified by tandem mass spectrometry. Microbiology 2011; 157:526-42; PMID:21030434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi Y, Xiong X, Wang X, Duan C, Jia Y, Jiao J, Gong W, Wen B. Proteome analysis and serological characterization of surface-exposed proteins of Rickettsia heilongjiangensis. PLoS One 2013; 8:e70440; PMID:23894656; http://dx.doi.org/ 10.1371/journal.pone.0070440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubuisson J-F, Vianney A, Hugouvieux-Cotte-Pattat N, Lazzaroni JC. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology 2005; 151:3337-47; PMID:16207916 [DOI] [PubMed] [Google Scholar]
- 19. Zgurskaya HI, Krishnamoorthy G, Ntreh A, Lu S. Mechanism and function of the outer membrane channel TolC in multidrug resistance and physiology of enterobacteria. Front Microbiol 2011; 2:189; PMID:21954395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. David MM, Xia Z. Measuring opsonic phagocytosis via Fcγ receptors and complement receptors on macrophages. Curr Protoc Immunol 2011; Chapter:Unit-14.27; PMID:22048802; http://dx.doi.org/ 10.1002/0471142735.im1427s95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kipps TJ, Parham P, Punt J, Herzenberg L. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med 1985; 161:1-17; PMID:3918141; http://dx.doi.org/ 10.1084/jem.161.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell 1994; 76:519-29; PMID:8313472; http://dx.doi.org/ 10.1016/0092-8674(94)90115-5 [DOI] [PubMed] [Google Scholar]
- 23. Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 2006; 13:981-90; PMID:16960108; http://dx.doi.org/ 10.1128/CVI.00156-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong X, Meng Y, Wang X, Qi Y, Li J, Duan C, Wen B. Mice immunized with bone marrow-derived dendritic cells stimulated with recombinant Coxiella burnetii Com1 and Mip demonstrate enhanced bacterial clearance in association with a Th1 immune response. Vaccine 2012; 30:6809-15; PMID:23000126; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 25. Crocquet-Valdes PA, Thirumalapura NR, Ismail N, Yu X, Saito TB, Stevenson HL, Pietzsch CA, Thomas S, Walker DH. Immunization with Ehrlichia P28 Outer Membrane Proteins Confers Protection in a Mouse Model of Ehrlichiosis. Clin Vaccine Immunol 2011; 18:2018-25; PMID:22030371; http://dx.doi.org/ 10.1128/CVI.05292-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker DH, Olano JP, Feng H-M. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect Immun 2001; 69:1841-6; PMID:11179362; http://dx.doi.org/ 10.1128/IAI.69.3.1841-1846.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gazzinelli R, Hakim F, Hieny S, Shearer G, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 1991; 146:286-92; PMID:1670604 [PubMed] [Google Scholar]
- 28. Feng H-M, Walker DH. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect Immun 2000; 68:6729-36; PMID:11083788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng H-M, Popov VL, Walker DH. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun 1994; 62:1952-60; PMID:8168962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Sousa R, Ismail N, Dória NS, França A, Amaro M, Anes M, Poças J, Coelho R, Torgal J, Bacellar F, et al. Intralesional expression of mRNA of interferon-γ, tumor necrosis factor-α, interleukin-10, nitric oxide synthase, indoleamine-2, 3-dioxygenase, and RANTES is a major immune effector in Mediterranean spotted fever rickettsiosis. J Infect Dis 2007; 196:770-81; PMID:17674321; http://dx.doi.org/ 10.1086/519739 [DOI] [PubMed] [Google Scholar]
- 31. Feng H, Walker DH. Interferon-gamma and tumor necrosis factor-alpha exert their antirickettsial effect via induction of synthesis of nitric oxide. Am J Pathol 1993; 143:1016; PMID:8213997 [PMC free article] [PubMed] [Google Scholar]
- 32. Ammerman NC, Beier-Sexton M, Azad AF. Laboratory maintenance of Rickettsia rickettsii. Curr Protoc Microbiol 2008; Chapter 3:Unit 3A.5.; PMID:19016440; http://dx.doi.org/ 10.1002/9780471729259.mc03a05s11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niu DS CM, Wen BH, Li QF, Qiu L, Zhang JB. Development of quantitative PCR specific for Ricekttsia rickettsii. Chinese J Epidemiol 2006. 6:526-9 [Google Scholar]
- 34. Weinberg EH, Stakebake JR, Gerone PJ. Plaque assay for Rickettsia rickettsii. J Bacteriol 1969; 98:398-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balraj P, Nappez C, Raoult D, Renesto P. Western-blot detection of RickA within spotted fever group rickettsiae using a specific monoclonal antibody. FEMS Microbiol Lett 2008; 286:257-62; PMID:18657112; http://dx.doi.org/ 10.1111/j.1574-6968.2008.01283.x [DOI] [PubMed] [Google Scholar]
- 36. Hanson BA. Effect of immune serum on infectivity of Rickettsia tsutsugamushi. Infect Immun 1983; 42:341-9; PMID:6137458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kruisbeek AM. Isolation of mouse mononuclear cells. Current Protocols in Immunology 2001: [chapter 3:Unit 3.1]. doi: 10.1002/0471142735.im0301s39 [DOI] [PubMed] [Google Scholar]
- 38. Vellaiswamy M, Campagna B, Raoult D. Transmission electron microscopy as a tool for exploring bacterial proteins: model of RickA in Rickettsia conorii. New Microbiologica 2011; 34:209-18; PMID:21617834 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.