Abstract
Despite improvements in anti-allergy medication, the prevalence of allergic airway inflammation remains high, affecting up to 40% of the population worldwide. Allergen immunotherapy is effective for inducing tolerance but has the adverse effect of severe allergic reaction. This can be avoided by denaturing with urea. In this study, we demonstrated that the serum level of allergen-specific IgE in mice sensitized with native Dermatophagoides pteronyssinus (Der p) crude extract after receiving local nasal immunotherapy (LNIT) with urea-denatured Der p crude extract (DN-Dp) significantly decreased compared to that in the normal saline (NS) treatment group. Expressions of IL-4 were significantly reduced in lung tissues after treatment. Inflammation around the bronchial epithelium improved and airway hypersensitivity was down-regulated. LNIT with DN-Dp can down-regulate IL-1b, IL-6 and TNF-a expression and then decrease Der p-induced allergic airway inflammation. This therapeutic modality may be used as an alternative treatment for airway allergic diseases.
Keywords: Allergic airway inflammation, allergy, Dermatophagoides pteronyssinus, local nasal immunotherapy, urea denatured allergen
Abbreviations
- Der p
Dermatophagoides pteronyssinus
- LNIT
Local nasal immunotherapy
- DN-Dp
Urea denatured Dermatophagoides pteronyssinus crude extract
- DEX
dexamethasone
Introduction
Allergic airway inflammation is caused by allergen-induced immune response that can lead to asthma and allergic rhinoconjunctivitis.1 Both diseases are treated with antihistamines, leukotriene receptor antagonists, and glucocorticoids. However, these medications are used only to relieve symptoms and suppress inflammation.2 Despite substantial improvements in treatments, the diseases still affect 10–40% of the population worldwide.3 Successful treatment depends on the identification of the allergen for avoidance and immunotherapy. Allergen-specific immunotherapy is the only available treatment for allergic disease that can induce long-term specific allergen tolerance.4
Local nasal immunotherapy (LNIT) has been reported to be effective for allergen-induced asthma and allergic rhinitis. In a previous study, LNIT regulated the production of IgE immunologic response and modulated both the systemic immune system and local airway inflammation.5,6 The secretion of local salivary IgA and systemic serum IgG2a was up-regulated after LNIT in a mouse asthma model.7,8 Our previous study showed that LNIT with Dermatophagoides pteronyssinus (Der p)-coated strips was effective for treating patients with allergic rhinitis and Der p allergy after LNIT.3
Der p-specific IgE and IgG1 can down-regulate and up-regulate Der p-specific IgG4 in the sera. However, some patients have transient nasal symptoms while receiving LNIT. LNIT has been reported to frequently cause local adverse reactions; the percentage of unpleasant symptoms is 56.6% and the withdrawal rate is 43.9%. Thus, it is feasible to reduce allergenicity to an allergen as treatment.3
To avoid IgE-mediated allergic reaction, the use of hypo-allergenic materials has been recommended.9 There is a strong rationale for developing biological immune response modifiers using denatured allergens.10 Denatured ovalbumin (DN-OVA) has been reported to markedly minimize allergenicity. A previous study has also demonstrated that oral administration of DN-OVA to ovalbumin-sensitized guinea pigs can improve OVA-induced airway hypersensitivity with decreased pulmonary resistance and significantly increased OVA-specific IgG.11 Furthermore, treatment of ragweed hay fever with urea-denatured antigen E has been reported.12 Thus, the aim of this study was to investigate the effects of urea-denatured Der p crude extract (DN-Dp) on Der p-sensitized mice.
Results
Effects of LNIT with DN-Dp on Der p-induced immune responses
The results showed that Der p-specific IgE was upregulated in the NS group. In the DN-Dp group, allergen-specific IgE expression was significantly downregulated compared to that in the NS group (P < 0.05) (Fig. 1A). There was a similar finding in the DEX group. However, there was no difference between the DN-Dp group and the NS group in Der p specific IgG2a (Fig. 1B). After animal sacrifice, mRNA of lung tissue was immediately extracted without any stimulation and qPCR was used to evaluate cytokine mRNA expression of Th cells. The results showed that IL-4 expression was up-regulated and IFN-g expression was down-regulated in the NS group compared to the naïve group (Fig. 2). Similarly, LNIT with DN-Dp significantly downregulated IL-4 expression but expressions of IFN-g and IL-17 were only slightly upregulated and down-regulated, respectively, compared to that in the NS group (Fig. 2). There was no difference in expression of IL-10 between these 2 groups.
Figure 1.
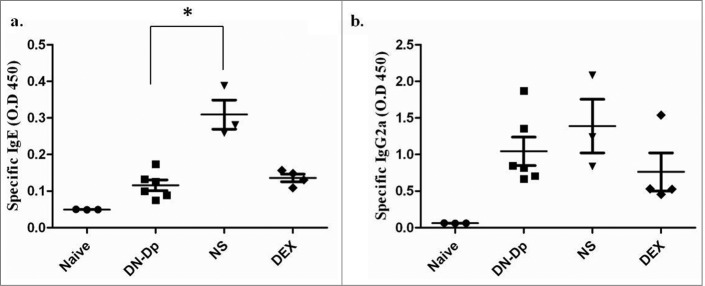
Effects of LNIT with DN-Dp on systemic immune responses. Serum concentrations of antigen-specific IgE (a) and IgG2a (b) were measured by ELISA. Values are expressed as mean±SEM of optical density (O.D.) at 450 nm of mice in each group. *P<0.05, compared to the denatured Der p and non-treatment groups.
Figure 3.
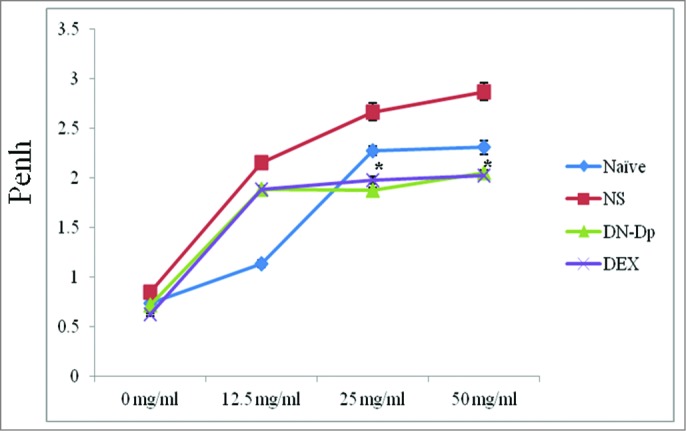
Effects of LNIT with DN-Dp on pulmonary function of mice. The allergen challenge was conducted with Der p crude extract. Airway hypersensitivity to methacholine was measured 30 min after the second allergen IT challenge. *P<0.05, compared to the denatured Der p and non-treatment groups.
Figure 4.
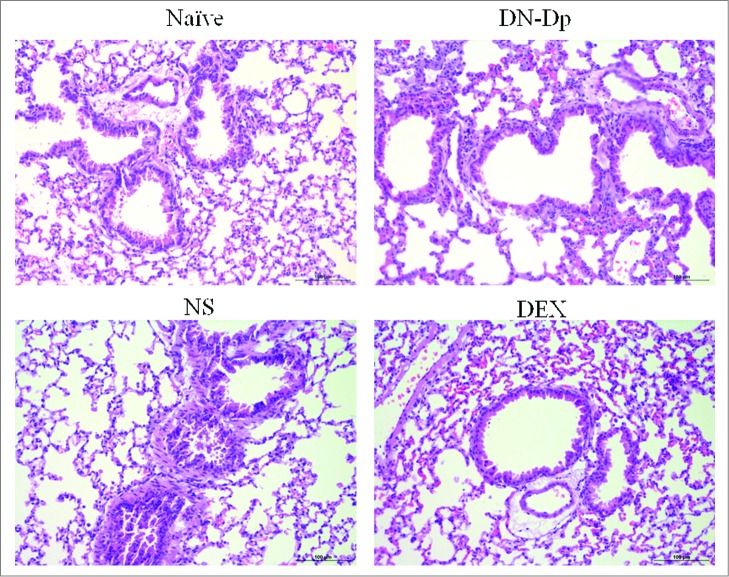
Lung pathology after LNIT with DN-Dp. Lung tissues were obtained 2 d after the second IT exposure to Der p crude extract in Der p-sensitized mice and examined by H and E staining (magnification 200x).
Effects of LNIT with DN-Dp on Der p-induced airway hypersensitivity
Der p-sensitized mice were IT challenged with 3 ug/30 ul of Der p crude extract on day 28 and day 35. After 30 min of the second challenge, airway hypersensitivity was measured by plethysmography and later compared among groups. Airway hypersensitivity was significantly reduced after LNIT with DN-Dp (Penh: 1.88 ± 0.03; 2.04 ± 0.04) compared to that in the NS group (Penh: 2.67 ± 0.09; 2.87 ± 0.07) at methacholine 25 and 50 mg/ml, respectively, P < 0.05 (Fig. 3). Similar findings were observed in the DEX treatment group.
Figure 8.
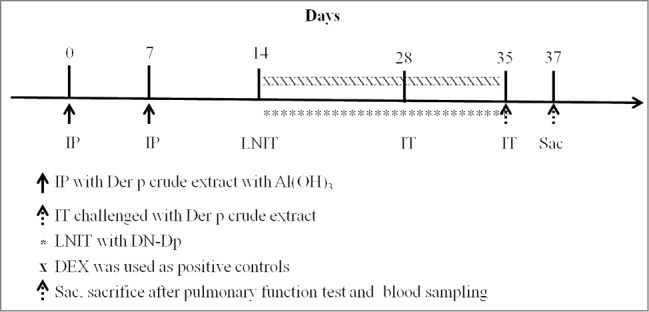
Experimental schedule for IP sensitization and the therapeutic procedure. BALB/c mice were IP-sensitized with 4 ug of HDM crude extract on days 0 and 7. From days 14 to 35 the mice received LNIT with NS 5ul/mouse/day, DN-Dp 3ug/5ul/mouse/day or DEX 10ug/5ul/mouse/day. IT challenge with HDM crude extract of 3ug/30ul was conducted on day 28 and day 35. Mice were sacrificed on day 37.
Pathology of different groups of mice after LNIT
Inflammatory cell infiltration around the bronchial epithelium was evaluated by hematoxylin and eosin staining and compared among groups. Increased inflammatory cells infiltration were observed in the Der p sensitized mice. These cells decreased in the DN-Dp group and DEX group in comparison with NS group (Fig. 4).
The effects of LNIT with DN-Dp on Der p induced proinflammation cytokine expression
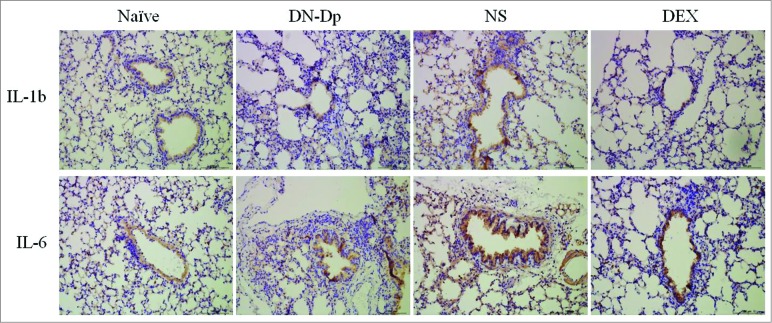
The expressions of proinflammatory cytokines were analyzed. Expressions of IL-1b, IL-6 and TNF-a from lung tissues were detected by qPCR. The results showed that the increased expression of IL-1b, IL-6 and TNF-a were observed in Der p sensitized mice. These proinflammatory cytokines expression were significantly downregulated after LNIT with DN-Dp, P < 0.05 (Fig. 5). The protein expression of IL-1b and IL-6 were determined by immunohistochemistry (IHC). The results showed that IL-1b and IL-6 expression in the Der p sensitized mice were increased in comparison with naïve group which were decreased in groups of DN-Dp and DEX (Fig. 6).
Figure 5.
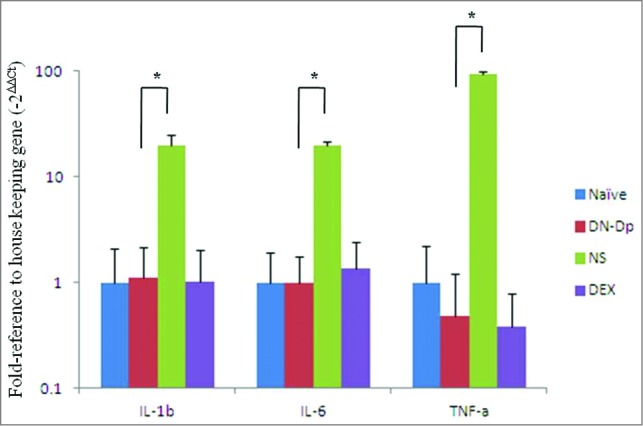
Effects of LNIT with DN-Dp on proinflammatory cytokine expression. Proinflammatory cytokine mRNA of lung tissue was measured by qPCR. IL-1b, IL-6 and TNF-a were expressed as relative mRNA expression using the 2(-△△CT) calculation method. Data represent the mean relative mRNA expression (±SD) from 3 mice in each group. *P<0.05, compared to the denatured Der p and non-treatment groups.
Figure 6.
IHC analysis of proinflammatory cytokine expression in lung tissues. Lung tissues were obtained 2 d after the second IT exposure to Der p crude extract in Der p-sensitized mice (magnification 200x).
Figure 7.
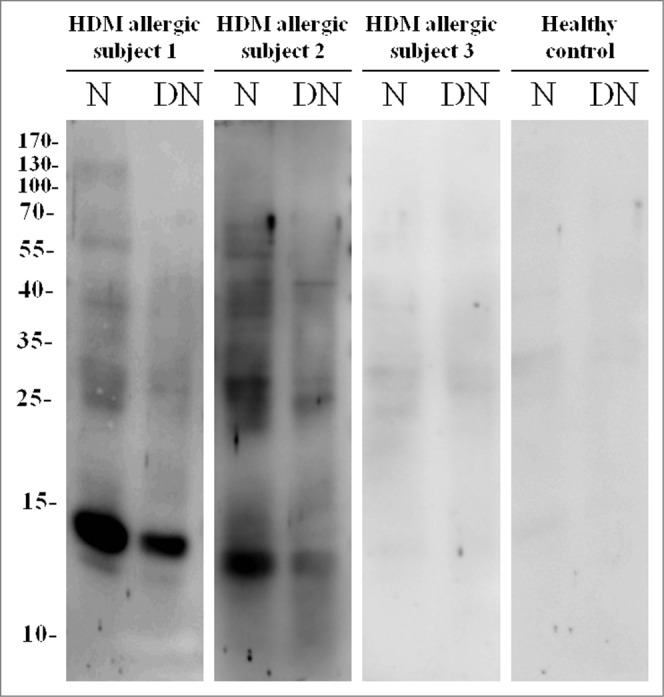
IgE binding capacity of DN-Dp. N: Native Der p crude extract; DN: DN-Dp dialyzed in PBS buffer. Protein extracts were separated on 12% SDS-PAGE. After electrophoresis, protein extracts were transferred to nitrocellulose membranes. Sera derived from HDM allergic subjects and healthy controls were hybridized and signals were detected with nitro-blue tetrazolium and 5-bromo-4-chloro-3'-indolyl phosphate. Levels of HDM specific IgE: subject 1: >100 kU/L; subject 2: 3.97 kU/L; subject 3: 0.50 kU/L.
Discussion
This study demonstrates that Der p induces airway inflammation and hypersensitivity to methacholine can be modulated by LNIT with DN-Dp. Der p-specific IgE, IL-4 and IL-17 decrease after treatment. Urea-denatured ragweed antigen E (UDE) has been reported to exert notable effects on T cells although it is non-allergenic.12 In animals immunized with ragweed antigen followed by weekly injections of UDE, IgE synthesis was suppressed. Similarly, Der p-specific IgE was suppressed after LNIT with DN-Dp.
As there is evidence that allergen subcutaneous immunotherapy (SCIT) may offer the possibility of a cure for allergic airway inflammation,13,14 many researches have concentrated on reducing the side effects caused by allergens. Urea-denatured antigen has lost its major allergenicity and can elicit trace IgE production and stimulate T cells, leading to a suppression of IgE response.12 This finding suggests that denatured antigen can be suitable for therapeutic use. In the present study, LNIT with DN-Dp was proven to be effective in reducing the hypersensitivity caused by Der p. Thus, it may be used as an alternative allergen immunotherapy.
LNIT offers advantages in treating allergies, such as no risk of life-threatening reactions, and more convenient allergen delivery. Furthermore, the effects on the target organ can be monitored directly by evaluating the nasal reactivity with a nasal allergen-specific provocation test (NPT). This test can be used to establish the dose to be delivered, just below the provocative threshold, i.e. to further improve the efficacy/safety ratio, mainly in special populations such as children.15 Compared to sublingual immunotherapy (SLIT), LNIT uses a lower dose to achieve a therapeutic effect and hence is more cost-effective. In this study, LNIT with DN-Dp provided benefits of a low dose, a fixed dose, low allergenicity DN-Dp and a convenient way to administrate DN-Dp to the host. Thus, LNIT is a safe and useful way to administer allergy treatment.
Our previous report demonstrated that LNIT in allergic rhinitis using strips of Der p crude extract could down-regulate Der p-specific IgE in the sera.3 Other reports showed that allergen specific immunotherapy could down-regulate Th2 response and allergen-specific IgE expression.4,16 According to our previous animal model and other reports, IgE was elevated in mice sera before allergen sensitization and was decreased after treatment.8,17 Similar findings were observed after LNIT with DN-Dp. Significantly, decreased Der p-specific IgE may be due to decreased production of IL-4 in Th2 cells. LNIT with DN-Dp can reduce levels of circulating specific IgE, further decreasing the binding of its receptors (FcϵRI) on mast cells, basophils, and B lymphocytes. This further leads to the down-regulation of the release of mediators such as histamines, leukotrienes, and pro-inflammatory cytokines from mast cells.18,19 Similar findings were observed in this study, the expressions of proinflammatory cytokines were significantly downregulated in the lung tissue after LNIT with DN-Dp.
In the present study, allergen-specific IgG2a was not significantly increased and expression of IFN-g was only slightly increased, which may not be adequate to induce IgG2a up-regulation. It has been reported that B cell class switching to IgG2a requires increased levels of Th1 cytokine-IFN-g to induce a response.20 Whether the production of IFN-g can be up-regulated by increasing the duration of LNIT with DN-Dp warrants further investigation.
Effective allergen-specific immunotherapy can also down-regulate Th17 cell activation to reduce allergic inflammation.21 In the present study, IL-17 expression was downregulated in lung tissue after LNIT with DN-Dp, suggesting that LNIT with DN-Dp can reduce inflammation by down-regulating IL-17 production in lung tissue. Thus, inflammation around the bronchial epithelium can be significantly improved.
Bronchial asthma is characterized by airway inflammation with clinical features of airway hypersensitivity.22,23 In the present study, airway hypersensitivity to methacholine was decreased in association with reduction of airway inflammation, suggesting that LNIT with DN-Dp may be applicable as treatment for allergic bronchial asthma.
In conclusion, this is the first report in the English literature on the efficacy of DN-Dp use for LNIT. LNIT with DN-Dp can down-regulate the production of Der p-specific IgE and expressions of IL-4 and IL-17 in lung tissues. Furthermore, airway hypersensitivity and bronchial airway inflammation are improved. These results suggest that LNIT with DN-Dp can be used as a therapeutic modality of Der p-induced airway inflammation. However, LNIT with a low dose of DN-Dp takes longer to achieve its therapeutic effects, which may delay remission and also cause poor compliance. Thus, LNIT with a higher dose of DN-DP and compared the therapeutic effect of DN-Dp with SLIT will be of interest and worth to be further investigation.
Materials and Methods
Patient selection
Base on GINA guidelines 2014, asthma is characterized by chronic airway inflammation and defined by history of respiratory syndromes such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation. In this study, patients recruited in this study were defined as a history of reversible obstructive airway disease associated with exacerbation resulting from allergen exposure and concomitant skin reactivity to exacerbating allergens. All patients were sensitive to mites and had mite-specific IgE as determined using the Phadia CAP system (Thermo Fisher Scientific, Uppsala, Sweden). Healthy subject was defined as subjects without HDM-specific IgE in serum and no history of allergic symptoms. All subjects were selected from the clinic of the Division of Allergy, Immunology and rheumatology of Taichung Veterans General Hospital.
Animals
Female BALB/c mice (specific pathogen-free environment, 5–8 weeks) were purchased from the National Laboratory Breeding Research Center in Taiwan and housed at Taichung Veterans General Hospital (TCVGH), Taichung, Taiwan, in a barrier environment. Mice were kept at a constant temperature of 23 ± 1°C, relative humidity of 55 ± 5% and under a regular cycle (light: dark = 12 h : 12 h). Twenty-one mice were divided randomly into 4 groups, and mice in each group were kept in a cage. All mice were housed in standard cages for one week before the experiments began.
Ethics statement
The Institutional Review Board and Institutional Animal Care and Use Committee of TCVGH approved all study protocols involving humans and mice (TCVGH-CF12009; La-1021128). All of the subjects provided informed consent.
DN-Dp crude extracts preparation
Purified house dust mites were purchased from Allergon (Ängelholm, Sweden). The urea denaturation procedure was performed as previously described.11 Briefly, mites were ground and sonicated in 8M of urea dissolved with PBS buffer and the resulting extract was placed on ice for 30 min. The DN-Dp was further dialyzed against phosphate buffer, pH 7.4, and concentrated by ultra-filtration. The reduction of allergenicity was further confirmed by IgE binding using western blotting.
Western blotting
Der p crude extract or DN-Dp at a concentration of 10 ug/lane was placed on 12% polyacrylamide gel before electrophoresis. The separated proteins on the gel were transferred onto nitrocellulose membranes (Millipore, Maharashtra, India) and blocked with 5% skim milk for 1 hour, followed by overnight incubation with 1:10 serum derived from an HDM allergic patient.
After incubation, the membranes were washed 3 times (10 min per time) in TBS with 0.1% Tween 20. The membranes were then incubated with alkaline phosphatase-conjugated goat-anti human IgE (Bethyl, Montgomery, TX, USA) 1:3000 for 1 hour. Detection was performed with nitro-blue tetrazolium and 5-bromo-4-chloro-3′-indolyl phosphate (Thermo Fisher Scientific, Rockford, IL, USA). Sera from 3 HDM allergic asthmatic patients were used to detect Der p-specific IgE binding and the allerginicity of DN-Dp was decreased compared to that of native Der p crude extract (Fig. 7).
Induction of airway inflammation and LNIT with DN-Dp
Mice were immunized by intra-peritoneal (IP) injection of 4 ug of Der p crude extract adsorbed in Al(OH)3 gel (Sigma, St Louis, MO, USA) on day 0 and 7 (Fig. 8). One week after the second immunization, the mice received LNIT with normal saline (NS), DN-Dp or dexamethasone (DEX) daily from days 14 to 35 (NS 5ul/mouse/day; DN-Dp 3ug/5ul/mouse/day; positive treatment control was LNIT with DEX 10ug/5ul/mouse/day).
On days 28 and 35, the mice were intratracheally (IT) challenged with 3ug/30 ul of Der p crude extract. Two days after the second IT challenge, the mice underwent pulmonary function measurement and then were sacrificed.
Determination of antigen-specific immunoglobulin in sera
Der p-specific IgG2a and IgE antibodies in sera were detected by ELISA. Briefly, each sample was diluted at 1:50 for IgG2a and 1:10 for IgE in diluent (50 mM Tris, 0.14 M NaCl, 1% BSA, and 0.05% Tween 20). One hundred micro-liters of the sample solution were added to ELISA plates (Nunc, MA, USA) coated with Der p crude extract (0.5 ug/well) and incubated overnight at 4°C. The plates were washed 4 times with PBS-T. For antibody detection, horseradish peroxidase-conjugated goat anti-mouse IgG2a and IgE antibodies were diluted at 1:50,000 for IgG2a and 1:75,000 for IgE (Bethyl, Montgomery, TX, USA) and then added separately to each assay before incubation for 1 h at room temperature.
Bound enzyme substrate was detected using 3,3′,5,5′-tetramethylbenzidine (BioLegend, London, UK). The reaction was stopped after 30 min and the optical density was measured at 405 nm with a TECAN Sunrise ELISA reader. The results were expressed as ELISA units.
Quantification of cytokine mRNA expression by qPCR
After LNIT with DN-Dp or DEX, the lung tissues of mice were collected and total RNA was extracted using TRIZOL reagent (Invitrogen, New York, USA) according to the manufacturer's protocol. The RNA quality and quantity were determined by Nanodrop, based on the A260/A280 ratio (1.8–2.0 in all samples). Reverse transcription of RNA into cDNA was carried out using a Thermo Reverse Transcriptase Kit and an Oligo(dT) primer (Thermo, New York, USA) in a thermocycler (G-Storm, Life Science Research, UK). qPCR was performed in ABI StepOne plus (ABI, New York, USA) using Qiagen SYBR Green Master Mix (Qiagen, Manchester, UK). The thermal cycler was programmed as follows: initial set up for 10 min at 95°C, 40 cycles of denaturation at 95°C for 15 s each and thereafter annealing/extension at 60°C for 1 min.
The sequences of the primers used were: 5′ GGCCATCAGCAACAACATAAGCGT 3′ and 5′ TGGGTTGTTGACCTCAAACTTGGC 3′ for IFN-g; 5′ AGCCATATCCACGGATG CGACAAA 3′ and 5′ AATATGCGAAGCACCTTGGAAGCC 3′ for IL-4; 5′ GTCATCGATTTCTCCCCTGT 3′ and 5′ ACGAGTTTTCCAAGGAGTT 3′ for IL-10; 5′ CCAGGGAGAGCTTCATCTGT 3′ and 5′ AGGAAGTCCTTGGCCTCAGT 3′ for IL-17; 5′ CAACCAACAAGTGATATTCTCC ATG 3′ and 5′ GATCCACACTCTCCAGCTGCA 3′ for IL-1b; 5′ CCGCTATGAAGTTCCTCTC 3′ and 5′ GGTATCC TCTGTGAAGTCTC 3′ for IL-6; 5′ CTGTAGCCCACGTC GTAGC 3′ and 5′ TTGAGATCCATGCCGTTG 3′ for TNF-a; 5′ CGGTTCCGATGCCCTGAGGCTCTT 3′ and 5′ CGTCACACTTCATGATGGAATTGA 3′ for b-actin; 5′ AAA ATGGTGAAGGTCGGTGT 3′ and 5′ AGATGATGACCCTT TTGGCT 3′ for GAPDH.
Lung tissue staining
Lung tissues of mice from the experimental and control groups were immersed in formalin after the animals were sacrificed. The tissue sections were stained with hematoxylin-eosin and the slides were mounted with cover slips.
Immunohistochemistry
3 μm thick sections were prepared from formalin-fixed paraffin-embedded mice lung tissue blocks and were dried in a 60°C oven one hour. The sections were placed in a Bond Max Automated Immunohistochemistry Vision Biosystem (Leica Biosystems, Breckland, UK) according to the following protocol. Tissues were deparaffinized and pre-treated with the Epitope Retrieval Solution 2 (EDTA-buffer pH9.0) at 100°C for 20 min. After washing steps, peroxidase blocking was carried out for 10 min using the Bond Polymer Refine Detection Kit DS9800 (Leica Biosystems, Breckland, UK). Tissues were again washed and then incubated with the rabbit anti-mouse IL-1b (Santa Cruz, Heidelberg, Germany) and rabbit anti-mouse IL-6 (Abcam, MA, USA) for 30 min. Subsequently, tissues were incubated with polymer for 8 min and developed with 3,3′-Diaminobenzidine tetrahydrochloride (DAB) chromogen for 5 min. Slides were counterstained with hematoxylin and mounted with coverslips.
Pulmonary function measurement
Pulmonary function of mice was measured by a plethysmograph (Buxco Electronics, New York, USA) as previously described.8 Briefly, aerosol was generated by placing 200 ul of saline or different doses of methacholine (0, 12.5, 25, and 50 mg/ml; Sigma) solution in the cup of an ultrasonic nebulizer. It was delivered via a connecting tube and a 3-way connector to the animal chamber of the plethysmograph. Each mouse inhaled saline aerosol for 3 min. The saline aerosol was then replaced with aerosol of methacholine solution for 3 min. In both the saline and methacholine experiments, the aerosol in the chamber was cleared immediately after exposure. The respiratory parameters were then measured for 3 min following inhalation of methacholine, with a half-hour interval between exposures. Pulmonary resistance was expressed as enhanced pause (Penh). The value of Penh obtained from each dose of methacholine was presented as mean ± SEM.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM) in Figures 1 and 3 and mean ± standard deviation (SD) in Figure 2. P-values ≤0.05 were considered statistically significant. The nonparametric Mann-Whitney test and pair student's t test were used to determine the statistical differences between groups.
Figure 2.
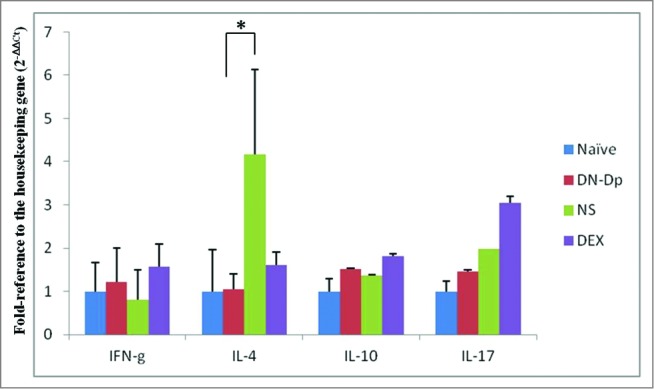
Effects of LNIT with DN-Dp on Th cell-related cytokine expression. Th cell-related cytokine mRNA of lung tissue was measured by qPCR. IFN-g, IL-4, IL-10, and IL-17 were expressed as relative mRNA expression using the 2(-△△CT) calculation method. Data represent the mean relative mRNA expression (±SD ) from 3 mice in each group. *P <0.05, compared to the denatured Der p and non-treatment groups.
Acknowledgements
The authors sincerely appreciate the assistance of the Center for Translational Medicine of Taichung Veterans General Hospital, Taichung, Taiwan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from Taichung Veterans General Hospital, Taiwan (TCVGH-1033803C).
References
- 1.Bielory L, Skoner DP, Blaiss MS, Leatherman B, Dykewicz MS, Smith N, Ortiz G, Hadley JA, Walstein N, Craig TJ, et al.. Ocular and nasal allergy symptom burden in America: the Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Allergy Asthma Proc (2014); 35:211-8; PMID:24801463; http://dx.doi.org/ 10.2500/aap.2014.35.3750 [DOI] [PubMed] [Google Scholar]
- 2.Nelson HS. Advances in upper airway diseases and allergen immunotherapy. J Allergy Clin Immunol (2005); 115:676-84; PMID:15805984; http://dx.doi.org/ 10.1016/j.jaci.2005.01.037 [DOI] [PubMed] [Google Scholar]
- 3.Tsai JJ, Liao EC, Tsai FH, Hsieh CC, Lee MF. The effect of local nasal immunotherapy in allergic rhinitis: using strips of the allergen dermatophagoides pteronyssinus. J Asthma (2009); 46:165-70; PMID:19253124; http://dx.doi.org/ 10.1080/02770900802553110 [DOI] [PubMed] [Google Scholar]
- 4.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol (2014); 133:621-31; PMID:24581429; http://dx.doi.org/ 10.1016/j.jaci.2013.12.1088 [DOI] [PubMed] [Google Scholar]
- 5.Georgitis JW, Reisman RE, Clayton WF, Mueller UR, Wypych JI, Arbesman CE. Local intranasal immunotherapy for grass-allergic rhinitis. J Allergy Clin Immunol (1983); 71:71-6; PMID:6337198; http://dx.doi.org/ 10.1016/0091-6749(83)90549-3 [DOI] [PubMed] [Google Scholar]
- 6.Georgitis JW, Clayton WF, Wypych JI, Barde SH, Reisman RE. Further evaluation of local intranasal immunotherapy with aqueous and allergoid grass extracts. J Allergy Clin Immunol (1984); 74:694-700; PMID:6389648; http://dx.doi.org/ 10.1016/0091-6749(84)90232-X [DOI] [PubMed] [Google Scholar]
- 7.Liu YH, Kao MC, Lai YL, Tsai JJ. Efficacy of local nasal immunotherapy for Dp2-induced airway inflammation in mice: Using Dp2 peptide and fungal immunomodulatory peptide. J Allergy Clin Immunol (2003); 112:301-10; PMID:12897735; http://dx.doi.org/ 10.1067/mai.2003.1619 [DOI] [PubMed] [Google Scholar]
- 8.Liu YH, Tsai JJ. Production of salivary immunoglobulin A and suppression of Dermatophagoides pteronyssinus-induced airway inflammation by local nasal immunotherapy. Int Arch Allergy Immunol (2005); 138:161-8; PMID:16192741; http://dx.doi.org/ 10.1159/000088438 [DOI] [PubMed] [Google Scholar]
- 9.McEldowney SJ, Bush RK. Pollen immunotherapy: selection,prevention, and future directions. Curr Allergy Asthma Rep (2006); 6:420-6; PMID:16899205; http://dx.doi.org/ 10.1007/s11882-996-0016-5 [DOI] [PubMed] [Google Scholar]
- 10.Di Gioacchino M, Cavallucci E, Ballone E, Cervone M, Di Rocco P, Piunti E, Filardo GS, Turi MC, Mangifesta R, Quecchia C, et al.. Dose-dependent clinical and immunological efficacy of sublingual immunotherapy with mite monomeric allergoid. Int J Immunopathol Pharmacol (2012); 25:671-9; PMID:23058017 [DOI] [PubMed] [Google Scholar]
- 11.Tsai JJ, Peng HJ, Han SH. Airway hyperreactivity modulated by immunotherapy with denatured ovalbumin in ovalbumin-sensitized guinea pigs. J Formos Med Assoc (1998); 97:626-32; PMID:9795531 [PubMed] [Google Scholar]
- 12.Norman PS, Ishizaka K, Lichtenstein LM, Adkinson NF Jr. Treatment of ragweed hay fever with urea-denatured antigen E. J Allergy Clin Immunol (1980); 66:336-41; PMID:6158532; http://dx.doi.org/ 10.1016/0091-6749(80)90030-5 [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Kim YK, Kim SH, Park HW, Min KU, Kim YY, Cho SH. Murine subcutaneous immunotherapy models with beneficial immunological and physiological effects. Asia Pac Allergy (2013); 3:50-8; PMID:23403956; http://dx.doi.org/ 10.5415/apallergy.2013.3.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taher YA, Henricks PA,van Oosterhout AJ. Allergen-specific subcutaneous immunotherapy in allergic asthma: Immunologic mechanisms and improvement. Libyan J Med (2010); 5; PMID:21483568; http://dx.doi.org/ 10.3402/ljm.v5i0.5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcucci F, Sensi LG, Caffarelli C, Cavagni G, Bernardini R, Tiri A, Riva G, Novembre E. Low-dose local nasal immunotherapy in children with perennial allergic rhinitis due to Dermatophagoides. Allergy (2002); 57:23-8; PMID:11991284 [PubMed] [Google Scholar]
- 16.Maggi E, Vultaggio A, Matucci A. T-cell responses during allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol (2012); 12:1-6; PMID:22157159; http://dx.doi.org/ 10.1097/ACI.0b013e32834ecc9a [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Guo D, Liang Q, Ding S, Wu B, Zhang L, Li Q. The efficacy of sublingual immunotherapy with Dermatophagoides farinae vaccine in a murine atopic dermatitis model. Clin Exp Allergy (2014); PMID:25258013; http://dx.doi.org/ 10.1111/cea.12417 [DOI] [PubMed] [Google Scholar]
- 18.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol (2010); 125:S73-80; PMID:20176269; http://dx.doi.org/ 10.1016/j.jaci.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platzer B, Baker K, Vera MP, Singer K, Panduro M, Lexmond WS, Turner D, Vargas SO, Kinet JP, Maurer D, et al.. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol (2014); PMID:25227985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegmann M, Hauber HP. Experimental approaches towards allergic asthma therapy-murine asthma models. Recent Pat Inflamm Allergy Drug Discov (2010); 4:37-53; PMID:19891606; http://dx.doi.org/ 10.2174/187221310789895612 [DOI] [PubMed] [Google Scholar]
- 21.Blaser K. [Immunological principles of allergen-specific immune therapy]. HNO (2008); 56:759-63; PMID:18648758; http://dx.doi.org/ 10.1007/s00106-008-1731-0 [DOI] [PubMed] [Google Scholar]
- 22.Higgins BG, Francis HC, Yates C, Warburton CJ, Fletcher AM, Pickering CA, Woodcock AA. Environmental exposure to air pollution and allergens and peak flow changes. Eur Respir J (2000); 16:61-6; PMID:10933086; http://dx.doi.org/ 10.1034/j.1399-3003.2000.16a11.x [DOI] [PubMed] [Google Scholar]
- 23.Tochigi R. [Clinical studies on bronchial hypersensitivity to methacholine in bronchial asthma of children]. Arerugi (1972); 21:28-39; PMID:5025148 [PubMed] [Google Scholar]