Abstract
The ongoing influenza epidemic is characterized by intense activity with most influenza infections due to the A (H3N2) viruses. Using the screening method, mid-season vaccine effectiveness (VE) in preventing influenza-like illness in primary care was estimated to 32% (95% CI; 23 to 40) among risk groups and was 11% (95% CI; −4 to 23) among the elderly (≥ 65 y). The VE in ≥ 65 y was the lowest estimate regarding the 4 previous seasonal influenza epidemics.
Keywords: France, influenza-like illness, influenza, primary care, vaccine effectiveness
Introduction
In France, the influenza vaccination strategy targets the following 2 main at risk groups: persons aged 65 y and above and persons below 65 y with certain chronic illness.1
In the northern hemisphere, the ongoing influenza season was dominated by the A(H3N2) sub-type.2-5 The A(H3N2) viruses are known to cause more severe illness with potential for complications especially in the elderly and other risk groups targeted for vaccination than A(H1N1)pdm09 and/or B viruses.6,7 During the 2014/15 influenza season, a significant proportion of the A(H3N2) viruses characterized antigenically and genetically has demonstrated antigenic drift from the northern hemisphere vaccine component resulting in reduced vaccine effectiveness (VE).2-5 Early VE estimates reported from United States (US)3 United Kingdom (UK)4 and Canada,5 were low compared with previous seasons when circulating viruses and vaccine viruses were well-matched.
None of these studies provide specific early VE for high-risk population targeted for influenza vaccination. Thus, we estimated here early estimates of influenza VE in the prevention of influenza-like illness (ILI) among target groups in primary care, using the screening method.8
Methods
Study ILI population
The French Sentinelles Network is a surveillance system based on approximately 2% of all French General Practitioners (GPs)9 combining epidemiological and virological data. ILI cases were reported by sentinel GPs in metropolitan France, as part of routine surveillance using the following definition, “sudden onset of fever >39°C (102°F) with respiratory signs and myalgia”.10 The following information was collected for each ILI patient by their GP: date of consultation, age, sex, vaccine status for current seasonal trivalent vaccine, time since vaccination (more or less than 3 weeks) and presence of risk factors (chronic illness). Nasopharyngeal swabs were also collected by GPs in a randomized sample of patients presenting with ILI according to the Sentinelles case definition.11
Study period
Influenza VE against ILI was estimated over 5 influenza epidemic periods (seasons 2010/11 to 2014/15) identified by the French Sentinelles Network (http://www.sentiweb.fr).12 In order to estimate 2014/15 early VE, the study period ran from week 3 (12th to 18th January 2015), which was the beginning of the influenza epidemic as declared by the French Sentinelles Network, to week 8 (16th to 22nd February 2015).
The screening method
We estimated VE using the screening method, a “case-base” design13 able to provide early estimates of influenza VE.14,15 VE is calculated using the following equation:
where PVC is the proportion of vaccinated among ILI cases (not laboratory confirmed) and PV is the proportion of vaccinated among the population. PV was obtained from robust administrative sources (CNAMTS - Caisse Nationale d'Assurance Maladie des Travailleurs Salariés, the main National Health Insurance System, covering about 85% of the French population) for the 2 risk groups: <65 y with chronic illness and ≥65 y.16 Since influenza vaccines are not given to children under 6 months old they were excluded from the study. Individuals with missing age or vaccination status were also excluded. Vaccination status was reported by GPs, based on GPs records or patient's declaration. Vaccines were considered as potentially effective if administrated at least 3 weeks prior to the onset of symptoms. Patients whose vaccination occurred <3 weeks prior to symptom onset were considered as not vaccinated.
Estimation of vaccine effectiveness
VE estimates were stratified according to age as proposed by Farrington.17 In practice, VE for all risk groups was estimated with a logistic regression model allowing a different offset in each age strata (2 strata: <65 y with chronic illness; ≥65 y). Analyses were performed using the R software (version 2.15.3).
Ethical statement
The protocol was conducted in agreement with the Helsinki declaration. Authorization was obtained from the French Data Protection Agency (CNIL, registration number #471393).
Results
Description of the ongoing influenza epidemic in France
During the 2014/15 winter, ILI incidence crossed the epidemic threshold in week 3 (from 12th to 18th January 2015), increased during the next 4 weeks from 239 cases per 100,000 inhabitants to 827 cases per 100,000 inhabitants (week 3 to week 6) and then decreased afterwards (from 802 per 100,000 inhabitants in week 7 to 723 per 100,000 inhabitants in week 8). Cumulated incidence rates during the beginning of this 2014/15 influenza epidemic (3,754 per 100,000 inhabitants) were already higher than the overall 2011/12 (2,276 per 100,000 inhabitants), 2010/11 (3,491 per 100,000 inhabitants) and 2013/14 (1,450 per 100,000 inhabitants) epidemics (data available on http://www.sentiweb.fr).
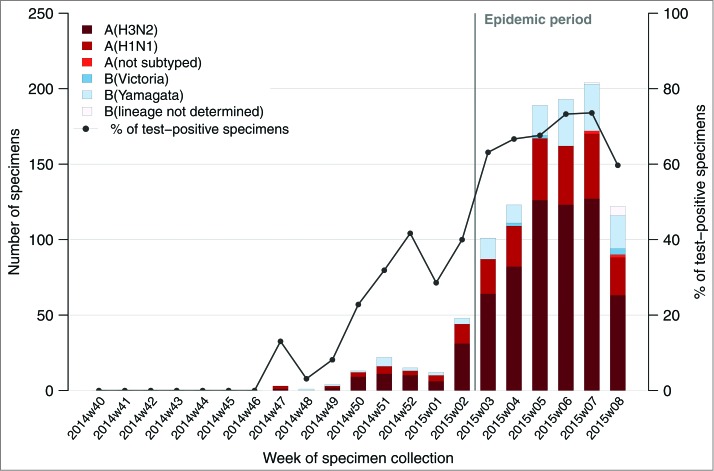
During the first weeks of this ongoing influenza epidemic - from week 3 to 8 of 2015, 10,730 ILI cases were reported by sentinel GPs. The positivity rate of at least one influenza virus for the ILI patients enrolled and sampled by GPs during the study period ranged from 63% (101/160; week 3) to 60% (120/201; week 8) and peaked at 73% (193/263; week 6). Positivity rate for A (H3N2) viruses among influenza laboratory confirmed ILI cases ranged from 63% (64/101; week 3) to 53% (63/120; week 8) and peaked at 64% (123/263; week 6) (Fig. 1).
Figure 1.
Number of positive influenza-like illness patients swabbed by general practitioners who tested positive to at least one influenza virus by types/subtypes and proportion of laboratory confirmed influenza patients swabbed by week, French Sentinelles surveillance Network, 29 September 2014 – 22 February 2015 (n = 1,923).
Vaccine effectiveness
To estimate early VE of the ongoing influenza epidemic, the analysis was based on the 1,060 ILI cases reported by sentinel GPs belonging to the groups targeted for vaccination who did not have missing information concerning age, risk factors and vaccination status. Among all target groups, 400 ILI patients (37.7%) were vaccinated with the 2014/15 trivalent seasonal vaccine (Table 1).
Table 1.
Description of ILI cases included in the study, French Sentinelles surveillance network
| Epidemic season | Period | Groups (age, ys) | Total described | Total vaccinated n (%) | Vaccine coverage for the whole population (%) a |
|---|---|---|---|---|---|
| (Early) 2014/15 | 201503 – 201508 | 6m-64y with chronic illness | 396 | 74 (18.7) | 38.3 b |
| (Early) 2014/15 | 201503 – 201508 | ≥65 y | 664 | 326 (49.1) | 51.9 b |
| (Early) 2014/15 | 201503 – 201508 | Overall at risk | 1060 | 400 (37.7) | 48.9 b |
| 2013/14 | 201405 – 201409 | 6m-64y with chronic illness | 72 | 9 (12.5) | 38.3 |
| 2013/14 | 201405 – 201409 | ≥65 y | 111 | 35 (31.5) | 51.9 |
| 2013/14 | 201405 – 201409 | Overall at risk | 183 | 44 (24.0) | 48.9 |
| 2012/13 | 201251 – 201311 | 6m-64y with chronic illness | 286 | 40 (14.0) | 39.1 |
| 2012/13 | 201251 – 201311 | ≥65 y | 552 | 180 (32.6) | 53.1 |
| 2012/13 | 201251 – 201311 | Overall at risk | 838 | 220 (26.3) | 50.1 |
| 2011/12 | 201205 – 201212 | 6m-64y with chronic illness | 157 | 38 (24.2) | 39.5 |
| 2011/12 | 201205 – 201212 | ≥65 y | 411 | 189 (46.0) | 55.2 |
| 2011/12 | 201205 – 201212 | Overall at risk | 568 | 227 (40.0) | 51.7 |
| 2010/11 | 201051 – 201107 | 6m-64 y.o. with chronic illness | 211 | 24 (11.4) | 37.2 |
| 2010/11 | 201051 – 201107 | ≥65 y | 214 | 78 (34.4) | 56.2 |
| 2010/11 | 201051 – 201107 | Overall at risk | 425 | 102 (24.0) | 51.8 |
data from CNAMTS (French National Health Insurance System).
For 2014/15 influenza season, vaccine coverage of 2013/14 influenza season were used.
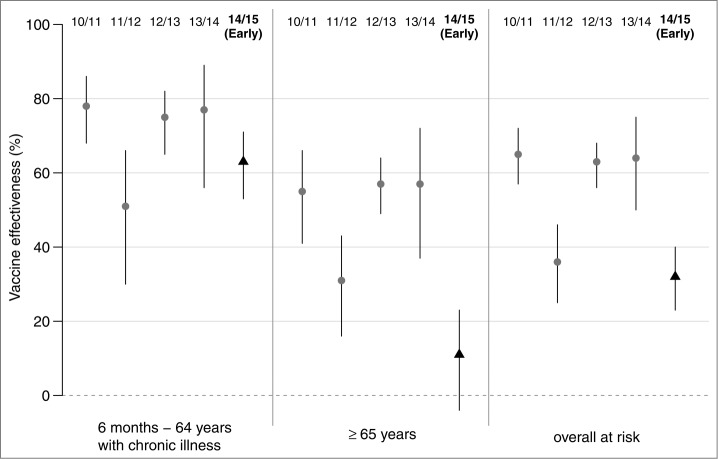
Estimated VE in preventing ILI according to age group and risk factors using administrative data for the 5 last influenza epidemics are detailed in Table 2. The early VE of the 2014/15 influenza vaccine in preventing ILI was estimated to 32% for all target groups (95% confidence interval (CI): 23 to 40); 63% for patients aged <65 y with chronic illness (95% CI: 53 to 71) and 11% for patients aged of ≥ 65 y (95% CI: −4 to 23).
Table 2.
Estimated vaccine effectiveness in preventing ILI for at-risk groups (6 months–64 y with chronic illness, over 65 y, and overall at risk), during 5 influenza epidemics between 2010/11 and 2014/15 and mismatch between dominant circulating strains and vaccine strains
| Epidemic season | Groups (age, ys) | VE (%) | 95% CI | Considered viral circulation b |
|---|---|---|---|---|
| (Early) 2014/15a | 6m-64y with chronic illness | 63 | 53 to 71 | A(H3N2) * |
| (Early) 2014/15a | ≥65 y | 11 | −4 to 23 | |
| (Early) 2014/15a | Overall at risk | 32 | 23 to 40 | |
| 2013/14 | 6m-64y with chronic illness | 77 | 56 to 89 | A(H1N1)pdm09 + A(H3N2) |
| 2013/14 | ≥65 y | 57 | 37 to 72 | |
| 2013/14 | Overall at risk | 64 | 50 to 75 | |
| 2012/13 | 6m-64y with chronic illness | 75 | 65 to 82 | A(H1N1)pdm09 + B |
| 2012/13 | ≥65 y | 57 | 49 to 64 | |
| 2012/13 | Overall at risk | 63 | 56 to 68 | |
| 2011/12 | 6m-64y with chronic illness | 51 | 30 to 66 | A(H3N2) * |
| 2011/12 | ≥65 y | 31 | 16 to 43 | |
| 2011/12 | Overall at risk | 36 | 25 to 46 | |
| 2010/11 | 6m-64y with chronic illness | 78 | 68 to 86 | A(H1N1)pdm09 + B |
| 2010/11 | ≥65 y | 55 | 41 to 66 | |
| 2010/11 | Overall at risk | 65 | 57 to 72 |
from week 3 to week 8 of 2015.
From Flunet database (http://www.who.int/influenza/gisrs_laboratory/flunet/); Indicate the viral dominant type or subtype;
Indicate when the circulating strains differs from the vaccine's ones
When considering all target groups, the VE estimated during the beginning of the 2014/15 influenza epidemic was lower than the VE value of the 2010/11, 2012/13 and 2013/14 influenza epidemics and close to the VE value of the previous A(H3N2) epidemic (2011/12). The VE estimate among patients aged ≥ 65 y was the lowest value estimated during the study period (Table 2; Fig. 2).
Figure 2.
Effectiveness of trivalent seasonal influenza vaccine for 5 influenza epidemics (2010/11 to 2014/15), for at-risk groups (6 months–64 y with chronic illness, over 65 y, and overall at risk) estimated by the French Sentinelles surveillance Network; segments delimitate the 95% confidence intervals of the point estimates. For the 2014/15 influenza epidemic early vaccine effectiveness is reported (from week 3 to week 8 of 2015).
Discussion
Our analysis shows that the 2014/15 influenza vaccine did not offer the expected protection against the circulating viruses, particularly among elderly. The estimated early VE for the prevention of ILI in primary care among the ≥65 y was the lowest estimate regarding the 4 previous seasonal influenza epidemics. Among the A (H3N2) viruses characterized from swabbed patients, a significant proportion were antigenically drifted from the vaccine component.2-5 This low VE could be explained by concomitant A(H3N2) vaccine mismatch and by the immunosenescence process.
Overall, early VE estimated here among all target groups (32%; 95% CI: 23 to 40) and among the elderly (11%; 95% CI −4 to 23) are in agreement with the interim VE recently reported by other countries against laboratory-confirmed influenza cases.3-5 The US reported a low VE against laboratory-confirmed influenza cases (all influenza viruses) in primary care of 23% (95% CI; 8 to 36),3 similarly the UK reported a VE of 3.4% (95% CI; −44.8 to 35.5)4 and Canada observed a VE of −1% (95% CI; −40 to 28).5 The early VE estimated in our study among the <65 y with chronic illness (63% (95% CI: 53 to 71) may seem high, but could be affected by confounding because selective rather than universal vaccination is recommended for this population.18,19
The screening method based on ILI cases used in this study allows estimating early VE for high-risk population targeted for vaccination over several influenza epidemics14,15 which was underlined as a current issue to guide policy decisions.20
Considering ILI as an outcome leads to set up large enough sample database available in real time and standardized over the years.21 Samples based on laboratory-confirmed influenza cases are limited in size and not as quickly updated, especially for individual descriptions in our case. The use of a non-specific influenza outcome can bias VE estimates downward since only a portion of ILI cases may be due to influenza virus infection.8 However, considering ILI cases with a very specific definition,10 and only during the epidemic period - where influenza positivity rates of ILI were higher, allows to reduce this bias.22
Moreover, as recently reported,23 the screening method using laboratory-confirmed influenza cases allows to provide similar estimates among the elderly as the test-negative design, that has been advocated as a valid method to estimate almost unbiased influenza VE. As the screening method using ILI cases (with a very specific definition) or laboratory-confirmed influenza cases provide VE estimates very close,22 estimation of VE by the screening method using ILI cases would be similar to those estimated by the test-negative design.
Consistency between proportions of vaccinated among ILI cases (PVC) and proportion of vaccinated among the population (PV) were controlled by using robust administrative data covering more than 85% of the French population to assess PV.22 In order to minimize confounding factors analyses were restricted to high risk groups and stratified by age groups.17
Finally, the stability of data and method used in our study allows estimating and comparing VE over several influenza epidemics even if values could be slightly biased. We assumed that if weak bias did occur, it should have affected similarly the results during the 5 influenza seasons here compared.
The low VE reported by several countries for this ongoing influenza epidemic shows the importance to estimate early VE during epidemics, especially among risk groups as elderly, to inform public health policy makers and remind specific recommendations as preventive actions (hand cleaning, masks) or influenza antiviral prescriptions for high-risk populations.
Acknowledgments
The authors wish to thank all the participating general practitioners of the French Sentinelles Network.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
CS, CT: data management, data analysis; AF: draft of the manuscript; CS, TB, IB, DLB, SB, VE, MV, MB, CT, LC, VR, TH: interpreted data and reviewed the manuscript. All authors reviewed and approved the final draft of the manuscript.
References
- 1.Ministère des Affaires sociales de la Santé et des Droits des femmes (Paris). [2014 vaccination schedule and recommendations]. Paris: The institute; 2014. [Google Scholar]
- 2.Broberg E, Snacken R, Adlhoch C, Beaute J, Galinska M, Pereyaslov D, Brown C, Penttinen P; WHO European Region and the European Influenza Surveillance Network . Start of the 2014/15 influenza season in Europe: drifted influenza A(H3N2) viruses circulate as dominant subtype. Euro Surveill 2015; 20(4):pii: 21023; PMID:25655052 [DOI] [PubMed] [Google Scholar]
- 3.Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Petrie JG, McLean HQ, Belongia EA, et al.. Early estimates of seasonal influenza vaccine effectiveness - United States, january 2015. MMWR Morb Mortal Wkly Rep 2015. January 16; 64(1):10-5; PMID:25590680 [PMC free article] [PubMed] [Google Scholar]
- 4.Pebody RG WF, Ellis J, Andrews N, Thompson C, von Wissmann B, Green HK, Cottrell S, Johnston J, de Lusignan S, Moore C, et al.. Low effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 mid–season results. Euro Surveill 2015; 2015; 20(5):pii=21025 [PubMed] [Google Scholar]
- 5.Skowronski D, Chambers C, Sabaiduc S, De Serres G, Dickinson J, Winter A, Drews SJ, Fonseca K, Charest H, Gubbay JB, et al.. Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada s Sentinel Physician Surveillance Network, January 2015. Euro Surveill 2015; 20(4):pii: 21022; PMID:25655053 [DOI] [PubMed] [Google Scholar]
- 6.Lee CS, Lee JH. Dynamics of clinical symptoms in patients with pandemic influenza A (H1N1). Clin Microbiol Infect 2010 Apr; 16(4):389-90; PMID:20222893 [DOI] [PubMed] [Google Scholar]
- 7.Turbelin C, Pelat C, Boelle PY, Levy-Bruhl D, Carrat F, Blanchon T, Hanslik T. Early estimates of 2009 pandemic influenza A(H1N1) virus activity in general practice in France: incidence of influenza-like illness and age distribution of reported cases. Euro Surveill 2009; 14(39):pii: 19341; PMID:19814965 [DOI] [PubMed] [Google Scholar]
- 8.Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, Orenstein WA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007 Jun; 36(3):623-31; PMID:17403908; http://dx.doi.org/ 10.1093/ije/dym021 [DOI] [PubMed] [Google Scholar]
- 9.Flahault A, Blanchon T, Dorleans Y, Toubiana L, Vibert JF, Valleron AJ. Virtual surveillance of communicable diseases: a 20-y experience in France. Stat Methods Med Res 2006. October; 15(5):413-21; PMID:17089946 [DOI] [PubMed] [Google Scholar]
- 10.Carrat F, Tachet A, Rouzioux C, Housset B, Valleron AJ. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995–1996 epidemic in France. Clin Infect Dis 1999. February; 28(2):283-90; PMID:10064245; http://dx.doi.org/ 10.1086/515117 [DOI] [PubMed] [Google Scholar]
- 11.Falchi A, Turbelin C, Andreoletti L, Arena C, Blanchon T, Bonmarin I, Hanslik T, Leruez-Ville M, De Lamballerie X, Carrat F. Nationwide surveillance of 18 respiratory viruses in patients with influenza-like illnesses: a pilot feasibility study in the French Sentinel Network. J Med Virol 2011. August; 83(8):1451-7; PMID:21638286; http://dx.doi.org/ 10.1002/jmv.22113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costagliola D, Flahault A, Galinec D, Garnerin P, Menares J, Valleron AJ. A routine tool for detection and assessment of epidemics of influenza-like syndromes in France. Am J Public Health 1991. January; 81(1):97-9; PMID:1983924; http://dx.doi.org/ 10.2105/AJPH.81.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field. Further observations. Epidemiol Rev 1988; 10:212-41; PMID:3066628 [DOI] [PubMed] [Google Scholar]
- 14.Minodier L, Blanchon T, Souty C, Turbelin C, Leccia F, Varesi L, Falchi A. Influenza vaccine effectiveness: best practice and current limitations of the screening method and their implications for the clinic. Expert Rev Vaccines 2014. August; 13(8):1039-48; PMID:24946796; http://dx.doi.org/ 10.1586/14760584.2014.930666 [DOI] [PubMed] [Google Scholar]
- 15.Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine 2006. November 10; 24(44–46):6605-11; PMID:16806607; http://dx.doi.org/ 10.1016/j.vaccine.2006.05.063 [DOI] [PubMed] [Google Scholar]
- 16.Tuppin P, Choukroun S, Samson S, Weill A, Ricordeau P, Allemand H. Vaccination contre la grippe saisonnière en France en 2010 et 2011 : diminution des taux de couverture et facteurs associés. La Presse Médicale 2012; 41(11):e568-e76; http://dx.doi.org/ 10.1016/j.lpm.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 17.Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol 1993. August; 22(4):742-6; PMID:8225751; http://dx.doi.org/ 10.1093/ije/22.4.742 [DOI] [PubMed] [Google Scholar]
- 18.Thomas HL, Andrews N, Green HK, Boddington NL, Zhao H, Reynolds A, McMenamin J, Pebody RG. Estimating vaccine effectiveness against severe influenza in England and Scotland 2011/2012: applying the screening method to data from intensive care surveillance systems. Epidemiol Infect 2014. January; 142(1):126-33; PMID:23591102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonmarin I, Belchior E, Le Strat Y, Levy-Bruhl D. First estimates of influenza vaccine effectiveness among severe influenza cases, France, 2011/12. Euro Surveill 2012; 17(18):pii: 20163; PMID:22587956 [DOI] [PubMed] [Google Scholar]
- 20.Neuzil KM, Victor JC. Editorial Commentary: Annual Studies of Influenza Vaccine Effectiveness: Evaluating Performance, Informing Policy, and Generating New Questions. Clinical Infectious Diseases 2014. February 1, 2014; 58(3):328-9; http://dx.doi.org/ 10.1093/cid/cit739 [DOI] [PubMed] [Google Scholar]
- 21.Turbelin C, Boëlle P-Y. Improving general practice based epidemiologic surveillance using desktop clients: the French Sentinel Network experience. Stud Health Technol Inform. 2010; 160(Pt 1):442; PMID:20841725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falchi A, Souty C, Grisoni ML, Mosnier A, Hanslik T, Daviaud I, Varesi L, Kerneis S, Carrat F, Blanchon T. Field seasonal influenza vaccine effectiveness: evaluation of the screening method using different sources of data during the 2010/2011 French influenza season. Hum Vaccin Immunother 2013. November; 9(11):2453-9; PMID:23811610; http://dx.doi.org/ 10.4161/hv.25513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remschmidt C, Rieck T, Bödeker B, Wichmann O. Application of the screening method to monitor influenza vaccine effectiveness among the elderly in Germany. BMC Infectious Diseases 2015; 15(1):137; PMID:25887460; http://dx.doi.org/ 10.1186/s12879-015-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]