Abstract
The identification of an effective and tolerable delivery method is a necessity for the success of DNA vaccines in the clinic. This article describes the development and validation of a multi-headed intradermal electroporation device which would be applicable for delivering multiple DNA vaccine plasmids simultaneously but spatially separated. Reporter gene plasmids expressing green and red fluorescent proteins were used to demonstrate the impact of spatial separation on DNA delivery to increase the number of transfected cells and avoid interference through visible expression patterns. To investigate the impact of plasmid interference on immunogenicity, a disease target was investigated where issues with multi-valent vaccines had been previously described. DNA-based Hantaan and Puumala virus vaccines were delivered separately or as a combination and the effect of multi-valence was determined by appropriate assays. While a negative impact was observed for both antigenic vaccines when delivered together, these effects were mitigated when the vaccine was delivered using the multi-head device. We also demonstrate how the multi-head device facilitates higher dose delivery to the skin resulting in improved immune responses. This new multi-head platform device is an efficient, tolerable and non-invasive method to deliver multiple plasmid DNA constructs simultaneously allowing the tailoring of delivery sites for combination vaccines. Additionally, this device would allow the delivery of multi-plasmid vaccine formulations without risk of impacted immune responses through interference. Such a low-cost, easy to use device platform for the delivery of multi-agent DNA vaccines would have direct applications by the military and healthcare sectors for mass vaccination purposes.
Keywords: dermal, DNA vaccine, electroporation, multi-agent, noninvasive, tolerable
Introduction
A major obstacle to plasmid vaccination is the requirement of the DNA vaccine to be effectively delivered intracellularly to the host cell nucleus. Outside of rodent models, the delivery of naked DNA through a standard intramuscular (IM) injection is notoriously inefficient. Historically, this has led to an inability to achieve robust immune responses in large mammals and humans.1,2 Many strategies have been developed to enhance the expression of DNA-based vaccines, such as codon-optimization3,4 and the development of optimized consensus sequences.5,6 The addition of co-delivered gene-based molecular adjuvants is another area where an augmentation of resulting immune responses frequently occurs.7 Despite the improvements in vector design and use of molecular adjuvants, there is still a clear requirement for an efficient method of administration of DNA vaccines that result in high level expression of the plasmid in the desired cell type of the desired tissue, most commonly muscle, tumor, or skin.
While vaccine delivery to the skin is associated with dose sparing immunization regimes, intradermal delivery is limited by the volume of vaccine that can be delivered at a single site. Whereas a standard volume for IM delivery in large mammals (pigs and non-human primates) and humans is 1–2 ml, ID delivery is usually limited to 100 μl. The 100 μl volume limitation spans small rodents such as guinea pigs and rats all the way up to non-human primates and humans and is a function of the de-lamination of epithelial tissue. Volumes much larger than 100 μl can be associated with tissue damage and a lack of tolerability in humans. As such, the dose delivered to a single site is significantly restricted. The use of a multiple head delivery device would in part alleviate this restriction, allowing for multiple sites to be treated simultaneously, therefore increasing the available dose 2–4 times. Treating multiple sites simultaneously would not only increase the systemic delivered dose but also significantly increase the number of cells directly transfected by the plasmid antigen of interest. This may have a significant impact on the immune response generated. Simultaneous multiple site treatment would also allow for the tailored delivery of combination vaccines. This could apply to the combination of plasmids within a single vaccine (e.g., Env, Gag, and Pol for HIV) or a combination of different target vaccines (e.g.,, Rabies, Ebola, Hanta for troops deployed in endemic regions).
The vast majority of published DNA vaccine studies report no negative impact on the magnitude or breadth of the generated immune response to the individual components when delivering combination vaccines.8-11 Indeed, combination DNA vaccines have now entered the clinic and have shown great promise.7,12 However, there are several published preclinical reports where interference was noted when delivering combination vaccines (Table 1). Interference between DNA vaccine components could occur at either the transcriptional or translational levels or be a function of delivery capability.
Table 1.
Summary of Documented Accounts of Studies Observing Interference when Delivering Multi-Valent DNA Vaccines
| Vaccine Target | Disease | Plasmid | Antigens | Delivery Methodology | Publications | |
|---|---|---|---|---|---|---|
| 1 | Hantaan virus (HTNV) and Andes virus (ANDV) | Hemorrhagic fever with renal syndrome (HFRS) | pWRG/HA-M | M gene products (G1 and G2 glycoproteins) | Gene Gun | Hooper, Custer, Smith and Wahl-Jensen, 200613 |
| 2 | Hantaan virus and Puumala virus | Hemorrhagic fever with renal syndrome | pWRG7077 | M segments of HTNV or PUUV | Gene Gun or IM EP | Spik, Badger, Mathiessen, Tjelle, Hooper and Schmaljohn, 200814 |
| 3 | Vaccinia Virus | Small pox | pWRG7077 | L1R and A33R proteins | Gene Gun | Hooper, Custer, Schmaljohn and Schmaljohn, 200015 |
| 4 | Human papillomavirus | Cervical dysplasia | pCDNA4-HPV16L1h-L2h/SV40ori | L1 proteins | IM | Gasparic, Rubio, Thones, Gissmann and Muller, 200716 |
| 5 | Human immune-deficiency virus type-1 | HIV/AIDS | pKCMV | Tat, nef, rev | IM | Kjerrstrom, Hinkula, Engstrom, Ovod, Krohn, Benthin and Wahren, 200117 |
| 6 | Hepatitis C virusHepatitis B virus | Chronic hepatic inflammation and liver disease | pHBsAg pC191 | Hepatitis B surface antigen and HCV core | IM EP | Zhu, Wu, Deng, Pei, Wang, Cao, Qin, Lu and Chen, 201218 |
| 7 | Human immune-deficiency virus type-1 | HIV/AIDS | pQL11 | Gag, Pol, Nef and Env | IM | Bockl, Wild, Bredl, Kindsmuller, Kostler and Wagner, 201219 |
One solution to the problem of interference would be to design a delivery device that would distribute each DNA vaccine component to spatially separate treatment sites. Such a delivery platform would allow each plasmid component a defined sub-population of target cells for the correct transcription, translation and ultimate presentation of the antigenic protein. As such, each component should function independently of each other and thus negate any immune interference issues.
Electroporation is a physical method used to temporarily increase skin permeability.20,21 This methodology involves the application of brief electrical pulses that result in the creation of aqueous pathways within the lipid bi-layer membranes of mammalian cells. This allows the passage of large molecules, including DNA, through the cell membrane, which would otherwise be less permeable. As such, electroporation increases the uptake, as well as the extent to which drugs and DNA are delivered to their target tissue.22-25
In the case of DNA vaccines, electroporation has been shown to quantitatively enhance immune responses, increase the breadth of those immune responses as well as improve the efficiency of dose.26,27 More recently, the DNA-EP platform has been successfully translated into the human clinical setting and has demonstrated significantly improved immune responses in several vaccine studies.7,12,28 The many advantages of skin delivery, most notably the presence of a variety of immune relevant cells, easy clinical accessibility as an immunization target organ, and the minimal depth of delivery (minimally invasive) prompted us to develop a dermal electroporation device that would be considered tolerable, user-friendly, and easily amenable to mass production, while continuing to achieve high transfection rates resulting in robust immune responses. The surface intradermal EP (SEP) device29 differs from other invasive platforms in that the electrodes are minimally invasive (make direct contact with the skin surface but do not penetrate) and operate at significantly lower voltages (20–25 V) compared with other dermal devices that routinely operate at 50–100 V (nominal voltage). The distinctive electrode configuration is designed to better achieve threshold electric fields over a wider area at lower applied voltages. Our previous work with this device demonstrated the effective uptake and expression of DNA plasmids resulting in robust humoral and cellular immune responses.29-33 However, the published version of the SEP device was only able to deliver multi-antigen formulations to a single site at any given time.
To increase the number of immunologically active cells at each immunization, provide the option to increase the available dose and mitigate any potential immune interference issues, the SEP device was re-designed and engineered to have the capability of delivering multiple vaccine components to spatially distinct sites simultaneously. This new multi-agent device (multi SEP or mSEP) would be particularly useful for combination vaccines that are rapidly formulated (and thus obviate the need for testing for interference) such as in response to emerging infectious disease threats or pandemics. In addition, such a multi-head device could be used to overcome the limited dosing issues often associated with intradermal delivery. Since this device design is modular in nature, multiple heads can be powered at once, allowing for a tailored delivery platform customized to the needs of each multi-valent vaccine.
The work detailed here outlines the development of the multi-head platform device and its application as a delivery modality in guinea pig and hamster skin. We investigate the effective transfection and expression of plasmid DNA using this device and demonstrate its ability to produce robust immunity which would be otherwise be impacted by cocktail delivery and most importantly, demonstrate the plasticity of the device to increase the dose of vaccine delivered.
Materials and Methods
Animals
Animals were housed at Biotox Sciences. All procedures complied with the Animal Welfare Act (AWA) and Institutional Animal Care and Use Committees (IACUC). Thirty female Golden Syrian hamsters (Charles River) and 25 Female Hartley guinea pigs (Charles River) between 3–4 wk of age were allowed to acclimate for 2 wk prior to initiating the study.
Devices
The SEP array was designed to contact the skin without penetrating the tissue and is therefore considered a minimally invasive surface electrode device. The single head array is comprised of 16 stainless steel needle electrodes with trocar grinds at 1.5 mm spacing in a 4 × 4 configuration.29 The multi-head device (mSEP) was designed to incorporate 2 (m2SEP) or 4 (m4SEP) of the single head arrays into a single hand-piece. The spacing between the heads was 5 mm. The current device was designed to work in conjunction with the ELGEN1000 (Inovio Pharmaceuticals) pulse generator. The electrical parameters for the device is 3 25 V pulses of 100 ms duration with 200 ms delay between pulses.
Treatments
All injections were performed using the Mantoux injection technique with a 29 gauge tuberculin syringe delivering plasmids formulated in PBS buffer. EP treatments were conducted immediately following injection by placing either the SEP device or mSEP device on top of the bleb(s) created by the injection and applying pressure during the entire EP procedure to ensure good contact of the electrodes.
Hamsters
Hamsters were divided into 3 groups of 10. A total of 3 treatments were performed at days 0, 21, and 42. Treatment regimen was as follows: group 1 received 2 50 μl injections of 50 μg of Hantaan virus (HTNV) and 50 μg of Puumala (PUUV) DNA vaccines with each injection immediately followed by EP using the SEP array; group 2 received one 50 μl injection of a cocktail mixture containing 50 μg each of HTNV and PUUV DNA vaccines immediately followed by EP using the SEP array; group 3 received 2 50 μl injections of 50 μg of HTNV and 50 μg of PUUV DNA vaccines immediately followed by EP using the m2SEP.
Guinea pigs
Guinea pigs were divided into 2 groups of 5 per group. A total of 3 treatments were performed at days 0, 14, and 21. Treatment regimen was as follows: group 1 received one 50 μl injections of 25 μg of H5HA DNA vaccine with the injection immediately followed by EP using the SEP array; group 2 received 4 50 μl injections of 25 μg each of H5HA DNA vaccine totaling 100 μg total dose immediately followed by EP using the m4SEP array.
Sample collection
Peripheral blood
Hamster and guinea pig blood collection was conducted every 3 wk beginning at day 0 and up to week 12. For hamsters collection consisted of inserting a 29 gauge 1 ml tuberculin syringe via jugular vein and placing the blood collected in serum separation microtainer tubes. Guinea pigs required the insertion of a 28 gauge ½ inch needle connected to a 3 ml syringe via jugular vein and placing the blood collected into a 3 ml serum separation tube. The blood was then centrifuged (Eppendorf 5417R or Rotanta 460) at 3540 rcf for 10 min. The serum was transferred into a 1.7 mL microcentrifuge tube and placed at −20°C until testing.
Tissue collection
Guinea pig skin was shaved and depilated one day prior to treatment. The skin was then excised 72 h post treatment and stored at −20°C for gross imaging using Olympus OV100 imaging system (AntiCancer Inc..) at 480 nm.
ELISA
Guinea pig
Antibody responses against H5 were evaluated by ELISA using sera. 96-well plates (Costar) were coated with 0.3 μg/ml H5(H5N1) (A/Vietnam/1203/2004) (Immune Tech) at 4°C overnight. The next day, using BioTek 96 well automated plate washer, the plates were washed 4 times then blocked with 200 μl of nonspecific binding solution (1 × PBS with 0.5% BSA) and incubated for one hour at 37°C. After incubation, the washing step was repeated. Samples were run in triplicates and added to row A at a 1:50 dilution using a dilution buffer solution (PBS with 0.2% BSA and 0.05% Tween-20). From row A, 50 uL of sample was taken and serially diluted 1:3 in the corresponding rows up to G; row H was used as a negative control background measurement. After two hours of incubation at 37°C, the plates were washed and 100 μL of goat anti-guinea pig IgG-HRP (Sigma) diluted at 1:10 000 using dilution buffer solution was added to each well and incubated for one hour at 37°C. The wash step was repeated a final time and 100 μl of TMB 2-component Microwell Peroxidase System (KPL) was added and developed for 6 min at room temperature. Development was stopped by adding 50 μl of TMB Stop Solution (KPL). The plates were read on a Molecular Devices SpectraMax 384 plate reader at an OD of 450 nm. A positive titer was calculated by subtracting 2 times the average background OD from the average sample OD. Positive titers were plotted as end-point titers.
PRNT assay
Neutralization assays were performed essentially as previously described (Chu et al., 1995; Hooper et al., 1999). Sera from vaccinated hamsters were incubated at 56°C for 30 min to destroy complement, then diluted 1:20–1:5120 in cEMEM with 10% heat inactivated fetal bovine serum (FBS, Invitrogen), 10 mM HEPES (Sigma Aldrich), 2 mM L-glutamine (Thermo Scientific), 1% non-essential amino acids (NEAA, Sigma Aldrich), 100 I.U. penicillin/100 μg/ml streptomycin (Cellgro), and 0.25 ug amphotericin B (Life Technologies). A viral stock of known titer was then diluted to 1 × 103 plaque forming units (pfu)/ml in EMEM with 10% FBS, HEPES, NEAA, penicillin/streptomycin, amphotericin B, and 10% guinea pig complement (Cedarlane). An equal volume of diluted virus was then added to each serum dilution tube and also to a cEMEM-only control. The tubes were incubated at 4°C overnight. The following day, 170 μl of the virus/serum mixture was added to duplicate wells containing 7-d-old Vero E6 monolayers in 6-well plates. The plates were incubated for 60 min at 37°C/5% CO2 with gentle rocking and shaking every 15 min to distribute the inoculum over the monolayer. At the end of the incubation period, 3 ml of a primary overlay mixture consisting of 2 × EMEM, 8 mM L-glutamine, 1% NEAA, 100 I.U. penicillin/100 μg/ml streptomycin, 0.25 ug amphotericin B, and 0.6% SeaKem ME agarose (Lonza) was added to the wells. The plates were then incubated for 7 d at 37°C/5% CO2. At this time, the HTNV plates had 2 ml of a secondary overlay added to each well, and the plates were incubated for an additional 3 d. This secondary overlay was identical to the primary overlay with the exception that 5% FBS and 5% of neutral red solution (Gibco) were added. The PUUV plates had 1 ml of the primary agarose overlay added and were incubated an additional 3 d, after which they received 2 mls of the secondary overlay and were incubated an additional 3 d. At the end of the incubation, plates were placed at room temperature in the dark. Plaques that appeared during the next 2–4 d were counted, and the neutralizing antibody titers were calculated. The 50% PRNT titer (PRNT50 titer) is the highest serum dilution that reduces the number of plaques by 50% relative to the average number of plaques in the control wells that received medium alone.
Statistical analysis
Data presented as the mean ± s.d. calculated from triplicate wells of pooled lymphocytes from each experimental group. Where appropriate, the statistical difference between immunization groups was assesses using a 2-tailed, paired Student t test that yielded a specific P value for each experimental group. Comparisons between samples with a P value < 0.05 were considered to be statistically different and therefore significant.
Results and Discussion
Development of a concept multi-head surface electroporation device
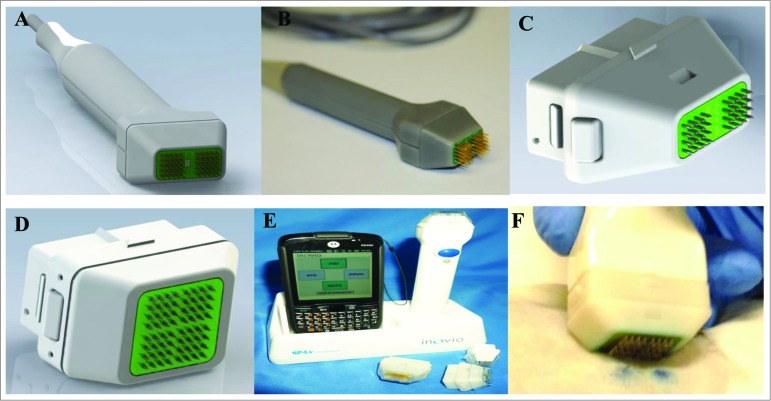
In a bid to build on an electroporation platform in current research use and to allow the tailored delivery of combination vaccines, we designed a multi-head surface electroporation device. The new EP applicator consists of a 2- or 4-head array (Fig. 1A–D). The electrode arrays (16 pins at a 4 × 4 orientation, 1.5 mm spacing between electrodes) are captured in individual sockets within the plastic handle, allowing each array to be individually addressed if necessary. The device is designed to only make contact with the surface of the skin and not directly penetrate the tissue. The prototype applicator was originally built as a tethered device which connected to the ELGEN 1000 pulse generator (Fig. 1A and B). However, later iterations are wireless, battery-powered devices in which the module is programmed through a hand-held tablet (Fig. 1E). The tablet also allows for real-time analysis of pulse data.
Figure 1.
Multi-head device. (A) CAD concept drawing of multi-head hand piece. (B) Working prototype of 4 × 4 array with 1.5 mm spacing. (C) Close up of CAD concept disposable 2-head array (m2SEP). (D) Close up of CAD concept 4-disposable head array (m4SEP). (E) Wireless device in base station with Pocket PC displaying the user interface. (F) The m4SEP in use in a guinea pig model.
Reporter gene expression allows visualization of spatial separation immunization using the multi-head electroporation device
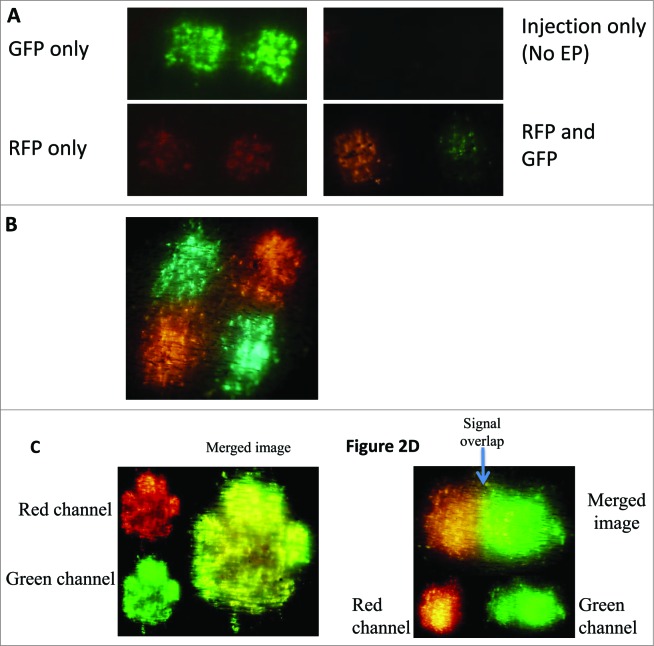
To demonstrate the ability of the multi-head device to deliver spatially separated DNA plasmids, separate skin sites on the flank of a guinea pig were injected with either 50 μl of 1 mg/ml GFP plasmid or 50 μl of 1 mg/ml RFP plasmid and immediately pulsed using the multi-head device (Fig. 2A). Robust and reproducible GFP and RFP transfection was visible on the skin surface 6 h following treatment and peaked between 24–48 h.33 Minimal or no reporter gene transfection was detectible following GFP or RFP plasmid injection alone (Fig. 2A). The expression patterns of both reporter genes were spatially separated when delivered by the m2SEP (Fig. 2A) and m4 SEP (Fig. 2B) devices and there was no evidence of signal overlap. To mimic delivery of a combination vaccine, GFP and RFP plasmids were mixed and delivered as a cocktail (Fig. 2C) using the single head SEP device. Since multiple transfected cells took up and expressed both reporter genes, the majority of the resulting signal is yellow. To demonstrate the possible need for a defined positioning and separation of the delivered plasmids, 2 reporter gene treatments were performed intentionally close to one another to demonstrate the overlap of expression resulting from the co-localization of the 2 reporter signals (Fig. 2D).
Figure 2.
Overlap of reporter gene expression can be overcome by using the multi-head EP device. GFP and RFP reporter gene expression is used as a surrogate for visualizing the spatial separation of DNA plasmid vaccines. (A) Reporter gene plasmid (GFP and RFP) was delivered using the dual-head EP (m2SEP) device to spatially separate the 2 plasmids. (B) Reporter gene plasmid (GFP and RFP) was delivered using the quad-head EP (m4SEP) device to spatially separate the 2 plasmids. (C) GFP and RFP plasmid were mixed to mimic a cocktail vaccine and delivered using the single head EP device. (D) Intradermal injections of RFP and GFP plasmid were intentionally performed in close proximity of each other. The overlap of signal can be seen in the yellow band section.
Multi-head delivery avoids immune interference between Hantaan virus and Puumala virus DNA vaccines
Hemorrhagic fever with renal syndrome (HFRS) caused by hantaviral infection is a significant disease burden in the areas where the viruses are endemic as well as a significant threat to military personnel in the field. Previous work from this group demonstrated that vaccination with DNA constructs expressing the M segment of HTNV, which produces the 2 viral glycoproteins, protect animals against 3 (Hantaan, Seoul and Dobrava viruses) of the 4 hantaviruses known to cause HFRS, but not against Puumala virus (PUUV). Therefore, to be a universally protective vaccine against HFRS, a combined HTNV and PUUV vaccine would be required. Both vaccines have excellent efficacy and provide 100% protection when delivered alone. However, when delivered as a combination, the efficacy of the HTNV vaccine drops off considerably.14 As a model of immune interference, we used this vaccine platform to evaluate the efficacy of our multi-head device. The M segment HTNV and PUUV DNA vaccines were delivered to Golden Syrian hamsters via either the multi-head device, as a combination at a single site, or separately at 2 sites using our SEP device. Hamsters were vaccinated 3 times (50 μg DNA per dose) through an intradermal injection followed immediately by electroporation. Plaque reduction neutralization tests (PRNT) were performed on sera from immunized animals and compared with titers from historical samples allowing assessment of protective antibody levels (Table 2).
Table 2.
Average Antibody (PRNT50 GMT) Responses to DNA Vaccines Delivered by Multi-Head Electroporation Following 3 Vaccinations
| Device | HTNV titers | PUUV titers |
|---|---|---|
| Multi-head (HTNV and PUUV DNA delivered separately) | 538 | 226 |
| SEP (HTNV and PUUV DNA delivered as a combination) | 148 | 63 |
| SEP (HTNV and PUUV DNA delivered separately) | 296 | 187 |
Multi-head delivery increases number of cells transfected and allows for increased dose delivery
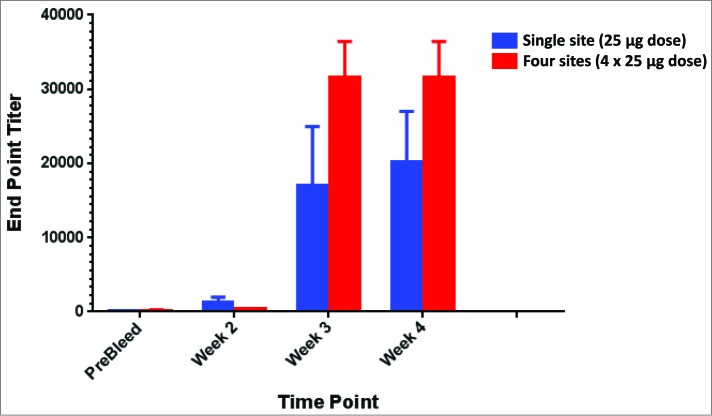
A significant limitation of ID delivery is the available volume of vaccine delivered. Single site ID immunizations are typically limited to 100 μl while IM volumes can reach between 1–2 mls. The ability to increase the volume of vaccine (and therefore the resulting dose) delivered to the skin may impact the resulting immunogenicity. The multi-head device offers a solution to increase the delivered vaccine volume from 100 μl to either 200 or 400 μl. As such, this also allows either a doubling or quadrupling of the delivered dose. To investigate this effect, we immunized guinea pigs either at a single site using SEP or at 4 sites using the m4SEP platform to evaluate the efficacy of our multi-head device to increase dosing in the skin. The H5 (pGX2001 SynConTM vaccine) construct encodes a consensus sequence of hemagglutinin (HA) from H5N1 viruses. DNA vaccines were delivered to Hartley guinea pigs via either the multi-head device at 4 sites or at a single site using our SEP device. Guinea pigs were immunized 3 times (25 μg DNA plasmid using the SEP or 100 μg total (4 × 25 μg) DNA plasmid using the m4SEP) through an intradermal injection followed immediately by electroporation. ELISA assays were performed on serum from immunized animals. Antibody titers generated from vaccination with the high dose 4 site multi-head (m4SEP) device were higher than the single lower dose using the SEP (Fig. 3).
Figure 3.
Improved antibody titers are generated through the use of higher doses facilitated by delivery with the multi-head EP device. Higher magnitude antibody titers are generated in guinea pigs immunized with higher doses of influenza plasmids facilitated by the use of the multi-head EP (m4SEP) device. Endpoint titers for ELISA against H5HA delivered either as a single 25 μg dose with the SEP or as a 100 μg dose with the m4SEP.
Conclusions
Intradermal electroporation is a platform technology which offers a solution to the tolerable delivery of DNA vaccines in the clinic for prophylactic immunization. Multiple examples of published literature demonstrate the ability of the ID-EP platform to elicit robust immune responses in a spectrum of animal models30,34-39 and in the clinic.28 The use of combination vaccine strategies has a number of clinical benefits. Such vaccine combinations can help to simplify the current immunization schedule, decreased anxiety associated with a perceived reduction in pain through fewer vaccinations, an increase in patient compliance, improved convenience and decreased cost for the patient as a result of fewer office visits. In the future, combined vaccination strategies for military personnel in the field could be envisaged where a soldier is rapidly immunized against disease endemic in the area of deployment. Specifically, in the case of DNA vaccines, due to their ease and rapidity of manufacture, multiple vaccines could be generated in a short period of time and deployed to troops and the general public when a response to a pandemic disease outbreak or bioterrorism attack is critical. Under these circumstances, the use of the multi-head delivery device could allow for the rapid deployment of the vaccination campaign without the need for pre-screening of the combination vaccine.
While ID delivery is associated with dose sparing, a limit to the volume/dose of vaccine delivered to skin may negatively impact its use as a target tissue for vaccination in the clinic. Additionally, a literature search confirmed that there were several noted incidences of DNA vaccine interference which impacted the immunogenicity of a variety of combination vaccine targets. To mitigate this risk of interference and increase delivered dose, we investigated the feasibility of developing a device designed to mitigate immune interference issues through spatial separation delivery. This study demonstrates through expression of reporter gene constructs and immunogenicity studies with multiple vaccine combinations that delivery via a multi-head surface EP device allows for higher dose immunization strategies, site tailored vaccine delivery and delivery that alleviates the negative impact of immune interference.
The device design for this delivery platform was an extension of our SEP device which had previously shown the ability to successfully deliver nucleotides to skin in a number of animal models.29-31,40 The 2 array and 4 array version of the mSEP devices (m2SEP and m4SEP respectively), were developed and manufactured using the same engineering processes as the SEP device. For example, the electrode arrays are built using printed circuit board (PCB) technology to both locate and hold the individual electrodes as well as to also connect the electrical stimuli to each electrode. Untethered units were have recently been developed to incorporate a wireless hand-piece design (Fig. 1E).The wireless iteration has a quick connect array mounting system allowing for the use of disposable arrays (Fig. 1C and D). The PCB circuit was designed to deliver the electrical pulses to each array simultaneously. To allow for distinct separation of vaccine delivery, the smallest theoretical gap between 2 cells expressing different plasmid constructs would be a single cell. Technically however, this would be hard to achieve from a device design perspective. Here we chose a spacing of 5 mm between the 2 heads allowing for optimal fluid delivery of the vaccine while still maintaining the required pressure to ensure appropriate conductivity.
The animal model of choice for many dermatological applications is the guinea pig. This is primarily due to the similarity in skin physiology between these rodents and humans. For this reason, the reporter gene studies and the influenza immunity study were performed in the Hartley guinea pig model. The hantavirus vaccine immunity study was performed in Syrian Golden hamsters, which is an infection model for HFRS- causing hantaviruses.
To demonstrate that the multi-head device was able to successfully deliver plasmid to the skin and be at least as effective a delivery device as its single-head predecessor, we used reporter gene transfection as a read-out. The resulting transfection patterns from the multi-head device were equivalent in size, shape and level of expression to the single head SEP device (Fig. 2A and B). To visually demonstrate the ability of the device to spatially separate 2 plasmids, RFP and GFP were injected and EPed with the multi-head device (Fig. 2A). The expression of both plasmids was robust and showed no evidence of overlap of the signal. In an attempt to visually demonstrate the impact of separation, we intentionally delivered a bolus of both GFP and RFP plasmid close together and EPed with the single head SEP device (Fig. 2C). While the extremes of the transfection areas were true to the expression of the single plasmid, there was an obvious area of overlap in the center where some cells had been transfected with both RFP and GFP plasmids and so the resulting signal appeared yellow. While this overlap of expression had no negative effects on the output of these reporter genes, the degree to which this overlap occurs could significantly impact the expression and immunogenicity of 2 plasmids which do interfere. To mimic a combination vaccine delivered at a single site, GFP and RFP plasmid were mixed at equal doses and delivered to skin using the single head SEP device (Fig. 2B). Using the red and green channels of the microscope, the expression pattern of each individual plasmid can be visualized clearly. When the images are merged, it becomes apparent that while some cells are exclusively RFP or GFP positive, the majority express both plasmids, suggesting that single cells are taking up both plasmids. This has wide ranging implications for combination vaccines. If single cells are capable of being transfected by multiple plasmids, then interfering plasmids could obliterate the protective ability of a vaccine.
To extend this study past expression, we investigated the impact of plasmid interference on immunogenicity and the ability of the mSEP device to mitigate this effect. Here we show a DNA combination vaccine where interference in a specific animal model is an issue, specifically a vaccine against Hantavirus. In the case of a DNA vaccine against Hantavirus, when HTNV and PUUV DNA are delivered as a combination, the efficacy of the HTNV DNA vaccine drops off considerably.14 It appears that the interference is at the mRNA level (Schmaljohn, personal communication). Using the multi-head device, we were able to entirely restore the potency of both vaccine components and observed robust PRNT titers against both antigens (Table 2).
In the context of plasmid interference, it is important to note that issues with interference can be animal model specific. This is supported by work from USAMRIID13 where they demonstrated that plasmid interference was animal model specific. It would therefore be prudent to evaluate combination vaccines in more than one animal species to ensure efficacy.
While it is intuitive that increasing a low dose of vaccine is likely to result in improved generation of immunity, the ability to deliver DNA vaccine doses closer to those delivered through IM EP may be an important tool for the success of ID EP vaccine delivery in the clinic. The multi-head device is a solution to dose limiting in the skin and we attempted to demonstrate this through the immune study in Figure 3. In a small animal model such as the guinea pig, it is easy to generate maximal immune responses. However, upon scale up in larger animals or humans, the plasticity to significantly increase the delivered dose may be paramount to clinical success. While maintaining combination vaccine efficacy through separation is vital, developing a device that is clinically acceptable is a critical requirement. It would technically be feasible to deliver all the components of a multi-agent vaccine separately in series, one after the other; however, from both a patient and clinicians perspective, this is far from ideal. We recognize that in principle the higher dose could be delivered by repeated administration of the SEP device on 4 separate ID injection sites. However, the m4SEP device allows for the single administration of EP pulses to the 4 injection sites. We are in the process of automating the vaccine injection step instead of the manual Mantoux method to make it easier to deliver 4 × dose via the m4SEP. The proof of principle data presented here supports the further development of the multi-head EP device as a means to both deliver a larger dose via ID administration, but also importantly, delivery up to 4 independent antigens to spatially distinct injection sites. With this in mind, our multi-head device has been developed to spatially separate the components of the vaccine but deliver them simultaneously. From the perspective of the patient, we believe they would be unable to differentiate between receiving a single dose of vaccine or multiple vaccine treatments.
A major constraint to ID delivery is a limitation to the volume of vaccine that can be delivered to the skin. For a single injection, generally, volumes delivered directly to the skin cannot exceed 100–150 μl due to issues with dermal delamination and pain. Since DNA is limited in the concentration that it can be manufactured to, with 10 mg/ml being the approximate upper limit,41 the volume restrictions constrain the resulting dose. However, the use of the multi-array device would lift this constraint, allowing for single injection volumes of 100–150 μl at multiple sites simultaneously at a single treatment. From a procedural perspective, this device would allow delivery of significantly higher doses during a single treatment without any added discomfort to the patient. The ability to deliver higher doses could have significant effects on the resulting immune responses for all delivered vaccines, combination or single component.
When designing clinical protocols involving combination DNA vaccines, the ability to ensure full efficacy and potency of each component is paramount. Where higher dosing strategies are required or the ability to tailor the positioning of vaccines is important, the use of the multi-head delivery device could provide a solution and preserve immunity while maintaining a tolerable and patient acceptable procedure.
Acknowledgments
We would like to thank Maria Yang and Sayed Sadat for plasmid preparation and Katherine Schultheis for manuscript preparation assistance.
Disclosure of Potential Conflicts of Interest
K.E.B., N.Y.S., J.B.M., A.G., and J.M.M. are employees of Inovio Pharmaceuticals and as such receive compensation in the form of salary, stock options and bonuses. C.S.S., K.W.S., and C.B. are employees of the US government and so do not declare any conflicts.
Funding
This work was supported in part by a Department of Defense SBIR grant (Phase I and Phase II) number W81XWH-11-C-0051.
References
- 1. Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421-9; PMID:21530212; http://dx.doi.org/ 10.1016/j.coi.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiner DB. DNA vaccines: crossing a line in the sand. Introduction to special issue. Vaccine 2008; 26:5073-4; PMID:18652867; http://dx.doi.org/ 10.1016/j.vaccine.2008.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. André S, Seed B, Eberle J, Schraut W, Bültmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol 1998; 72:1497-503; PMID:9445053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R, Wolf H, Wagner R. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J Virol 2001; 75:10991-1001; PMID:11602739; http://dx.doi.org/ 10.1128/JVI.75.22.10991-11001.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, Boyer JD, Weiner DB. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology 2003; 314:134-46; PMID:14517067; http://dx.doi.org/ 10.1016/S0042-6822(03)00459-8 [DOI] [PubMed] [Google Scholar]
- 6. Yang JS, Kim JJ, Hwang D, Choo AY, Dang K, Maguire H, Kudchodkar S, Ramanathan MP, Weiner DB. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999). J Infect Dis 2001; 184:809-16; PMID:11550123; http://dx.doi.org/ 10.1086/323395 [DOI] [PubMed] [Google Scholar]
- 7. Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al.; NIAID HIV Vaccine Trials Network. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818-29; PMID:23840043; http://dx.doi.org/ 10.1093/infdis/jit236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, Wu L, Hammarlund E, Khan AS, Babas T, Rhodes L, et al. . Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis 2011; 203:95-102; PMID:21148501; http://dx.doi.org/ 10.1093/infdis/jiq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konishi E, Kosugi S, Imoto J. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine 2006; 24:2200-7; PMID:16316713; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 10. Laddy DJ, Yan J, Kutzler M, Kobasa D, Kobinger GP, Khan AS, Greenhouse J, Sardesai NY, Draghia-Akli R, Weiner DB. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One 2008; 3:e2517; PMID:18575608; http://dx.doi.org/ 10.1371/journal.pone.0002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Musacchio A, Rodriguez EG, Herrera AM, Quintana D, Muzio V. Multivalent DNA-based immunization against hepatitis B virus with plasmids encoding surface and core antigens. Biochem Biophys Res Commun 2001; 282:442-6; PMID:11401479; http://dx.doi.org/ 10.1006/bbrc.2001.4580 [DOI] [PubMed] [Google Scholar]
- 12. Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et al. . Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012; 4:ra138; PMID:23052295; http://dx.doi.org/ 10.1126/scitranslmed.3004414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hooper JW, Custer DM, Smith J, Wahl-Jensen V. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology 2006; 347:208-16; PMID:16378630; http://dx.doi.org/ 10.1016/j.virol.2005.11.035 [DOI] [PubMed] [Google Scholar]
- 14. Spik KW, Badger C, Mathiessen I, Tjelle T, Hooper JW, Schmaljohn C. Mixing of M segment DNA vaccines to Hantaan virus and Puumala virus reduces their immunogenicity in hamsters. Vaccine 2008; 26:5177-81; PMID:18482782; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.097 [DOI] [PubMed] [Google Scholar]
- 15. Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 2000; 266:329-39; PMID:10639319; http://dx.doi.org/ 10.1006/viro.1999.0096 [DOI] [PubMed] [Google Scholar]
- 16. Gasparić M, Rubio I, Thönes N, Gissmann L, Müller M. Prophylactic DNA immunization against multiple papillomavirus types. Vaccine 2007; 25:4540-53; PMID:17485151; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 17. Kjerrström A, Hinkula J, Engström G, Ovod V, Krohn K, Benthin R, Wahren B. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology 2001; 284:46-61; PMID:11352667; http://dx.doi.org/ 10.1006/viro.2001.0905 [DOI] [PubMed] [Google Scholar]
- 18. Zhu W, Wu C, Deng W, Pei R, Wang Y, Cao L, Qin B, Lu M, Chen X. Inhibition of the HCV core protein on the immune response to HBV surface antigen and on HBV gene expression and replication in vivo. PLoS One 2012; 7:e45146; PMID:23024803; http://dx.doi.org/ 10.1371/journal.pone.0045146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Böckl K, Wild J, Bredl S, Kindsmüller K, Köstler J, Wagner R. Altering an artificial Gagpolnef polyprotein and mode of ENV co-administration affects the immunogenicity of a clade C HIV DNA vaccine. PLoS One 2012; 7:e34723; PMID:22509350; http://dx.doi.org/ 10.1371/journal.pone.0034723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugar IP, Förster W, Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys Chem 1987; 26:321-35; PMID:3607233; http://dx.doi.org/ 10.1016/0301-4622(87)80033-9 [DOI] [PubMed] [Google Scholar]
- 21. Sugar IP, Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem 1984; 19:211-25; PMID:6722274; http://dx.doi.org/ 10.1016/0301-4622(84)87003-9 [DOI] [PubMed] [Google Scholar]
- 22. Bråve A, Ljungberg K, Boberg A, Rollman E, Isaguliants M, Lundgren B, Blomberg P, Hinkula J, Wahren B. Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol Ther 2005; 12:1197-205; PMID:16112909; http://dx.doi.org/ 10.1016/j.ymthe.2005.06.473 [DOI] [PubMed] [Google Scholar]
- 23. Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, O’Hagan D, Donnelly J, Widera G, Rabussay D, et al. . Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004; 22:2489-93; PMID:15193413; http://dx.doi.org/ 10.1016/j.vaccine.2003.11.073 [DOI] [PubMed] [Google Scholar]
- 24. Prud’homme GJ, Draghia-Akli R, Wang Q. Plasmid-based gene therapy of diabetes mellitus. Gene Ther 2007; 14:553-64; PMID:17215847; http://dx.doi.org/ 10.1038/sj.gt.3302907 [DOI] [PubMed] [Google Scholar]
- 25. Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, et al. . Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000; 164:4635-40; PMID:10779767; http://dx.doi.org/ 10.4049/jimmunol.164.9.4635 [DOI] [PubMed] [Google Scholar]
- 26. Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 1999; 6:508-14; PMID:10476210; http://dx.doi.org/ 10.1038/sj.gt.3300847 [DOI] [PubMed] [Google Scholar]
- 27. Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J, Rabussay D, Barnett S, Ulmer JB. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine 2006; 24:4503-9; PMID:16181711; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 28. El-Kamary SS, Billington M, Deitz S, Colby E, Rhinehart H, Wu Y, Blackwelder W, Edelman R, Lee A, King A. Safety and tolerability of the Easy Vax™ clinical epidermal electroporation system in healthy adults. Mol Ther 2012; 20:214-20; PMID:22068424; http://dx.doi.org/ 10.1038/mt.2011.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broderick KE, Shen X, Soderholm J, Lin F, McCoy J, Khan AS, Yan J, Morrow MP, Patel A, Kobinger GP, et al. . Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther 2011; 18:258-65; PMID:20962869; http://dx.doi.org/ 10.1038/gt.2010.137 [DOI] [PubMed] [Google Scholar]
- 30. Broderick KE, Chan A, Lin F, Shen X, Kichaev G, Khan AS, Aubin J, Zimmermann TS, Sardesai NY. Optimized in vivo transfer of small interfering RNA targeting dermal tissue using in vivo surface electroporation. Mol Ther Nucleic Acids 2012; 1:e11; PMID:23344722; http://dx.doi.org/ 10.1038/mtna.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen X, Söderholm J, Lin F, Kobinger G, Bello A, Gregg DA, Broderick KE, Sardesai NY. Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model. Vaccine 2012; 30:6946-54; PMID:22406460; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.071 [DOI] [PubMed] [Google Scholar]
- 32. Lin F, Shen X, Kichaev G, Mendoza JM, Yang M, Armendi P, Yan J, Kobinger GP, Bello AJ, Khan A, et al. . Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum Gene Ther Methods 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendoza J, Amante D, Kichaev G, Knott C, Kiosses W, Smith T, Sardesai N, Broderick K. Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation. Vaccines 2013; 1:384-97; http://dx.doi.org/ 10.3390/vaccines1030384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bråve A, Gudmundsdotter L, Sandström E, Haller BK, Hallengärd D, Maltais AK, King AD, Stout RR, Blomberg P, Höglund U, et al. . Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine 2010; 28:8203-9; PMID:20951666; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donate A, Coppola D, Cruz Y, Heller R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS One 2011; 6:e19181; PMID:21559474; http://dx.doi.org/ 10.1371/journal.pone.0019181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eriksson F, Tötterman T, Maltais AK, Pisa P, Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 2013; 31:3843-8; PMID:23831327; http://dx.doi.org/ 10.1016/j.vaccine.2013.06.063 [DOI] [PubMed] [Google Scholar]
- 37. Gothelf A, Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum Vaccin Immunother 2012; 8:1694-702; PMID:23111168; http://dx.doi.org/ 10.4161/hv.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hallengärd D, Bråve A, Isaguliants M, Blomberg P, Enger J, Stout R, King A, Wahren B. A combination of intradermal jet-injection and electroporation overcomes in vivo dose restriction of DNA vaccines. Genet Vaccines Ther 2012; 10:5; PMID:22873174; http://dx.doi.org/ 10.1186/1479-0556-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krotova O, Starodubova E, Petkov S, Kostic L, Agapkina J, Hallengärd D, Viklund A, Latyshev O, Gelius E, Dillenbeck T, et al. . Consensus HIV-1 FSU-A integrase gene variants electroporated into mice induce polyfunctional antigen-specific CD4+ and CD8+ T cells. PLoS One 2013; 8:e62720; PMID:23667513; http://dx.doi.org/ 10.1371/journal.pone.0062720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cu Y, Broderick K, Banerjee K, Hickman J, Otten G, Barnett S, Kichaev G, Sardesai N, Ulmer J, Geall A. Enhanced Delivery and Potency of Self-Amplifying mRNA Vaccines by Electroporation in Situ. Vaccines 2013; 1:367-83; http://dx.doi.org/ 10.3390/vaccines1030367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai Y, Rodriguez S, Rameswaran R, Draghia-Akli R, Juba RJ, Jr., Hebel H. Production of pharmaceutical-grade plasmids at high concentration and high supercoiled percentage. Vaccine 2010; 28:2046-52; PMID:19896448; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.057 [DOI] [PubMed] [Google Scholar]