Abstract
Intradermal immunization has become a forefront of vaccine improvement, both scientifically and commercially. Newer technologies are being developed to address the need to reduce the dose required for vaccination and to improve the reliability and ease of injection, which have been major hurdles in expanding the number of approved vaccines using this route of administration. In this review, 7 y of clinical experience with a novel intradermal delivery device, the MicronJet600, which is a registered hollow microneedle that simplifies the delivery of liquid vaccines, are summarized. This device has demonstrated both significant dose-sparing and superior immunogenicity in various vaccine categories, as well as in diverse subject populations and age groups. These studies have shown that intradermal delivery using this device is safe, effective, and preferred by the subjects. Comparison with other intradermal devices and potential new applications for intradermal delivery that could be pursued in the future are also discussed.
Keywords: dose-sparing, immunogenicity, influenza vaccine, intradermal, Mantoux, microneedles, vaccine delivery, vaccine device
Abbreviations
- ID
intradermal
- BCG
Bacillus Calmette–Guérin
- PPD
Purified protein derivative
- HBV
hepatitis B virus
- WHO
World Health Organization
- SAGE
Strategic Advisory Group of Experts
- IPV
inactivated polio vaccine
- SQ
subcutaneous
- IM
Intramuscular
- BD
Becton Dickinson
- GMT
geometric mean titer
- HIV
Human immunodeficiency virus
- DTP
diphtheria, pertussis and tetanus
- HPV
human papilloma virus
- MEMS
Micro Electro Mechanical System
- FDA
Food and Drug Administration
- EMEA
European Medicines Agency
- HA
hemagglutinin
- AE
adverse event
- CDC
Center of Disease Control
- icddr,b
International Center for Diarrheal Disease Research, Bangladesh
A Brief History of Intradermal Vaccination
Intradermal (ID) immunization dates back to the advent of vaccines. Variolation (applying scabs or fluids from infected smallpox lesions onto healthy individuals) was practiced in many areas of the world for hundreds of years before the pioneering work of Edward Jenner, who used cowpox scarification for smallpox at the turn of the 19th century.1-6
Further major milestones were achieved over a century later by Calmette and Guérin,7 who developed the BCG vaccine for tuberculosis circa 1921. Tuberculin (PPD) and the Mantoux technique8 of intradermal injection, which typically uses a standard 25G-27G, 5/8-1.0 (16-25mm) needle for shallow (5–15 degrees) injection into the skin, were developed around the same time.
Importantly, the uptake of the standard ID Mantoux technique is still limited, some hundred years later, to a very narrow list of vaccines (Table 1). The Mantoux technique is neither simple nor reliable9-11 and very often delivers the antigen too deep or it leaks out, failing on occasion to produce the typical 6–10 mm white bleb,12 thereby limiting adoption of perhaps the most natural and physiological route of delivery of vaccines.
Table 1.
Approved and pipeline vaccines delivered intradermally
| Approved for ID delivery | Positive Clinical Data | Mixed Results |
|---|---|---|
| *BCG13 | Hepatitis A14,15 | HBV16-21 |
| Rabies22-26 | Pandemic influenza27 | Measles28-30 |
| Influenza31-36 | Yellow Fever37-39 | Inactivated Polio40-43 |
| Tick-Borne encephalitis44 | ||
| Smallpox45 |
* Intradermal delivery is the standard route for delivery for BCG.
Benefits of ID Vaccination
ID vaccination has primarily been explored for its ability to generate equivalent antibody responses at lower doses, a phenomenon typically described as “dose-sparing”.46 The importance of dose-sparing is most evident in high-surge situations, such as in pandemic47 and seasonal flu,48,49 where large populations are at risk and a new set of strains can be required each year.50 Dose sparing is also important in increasing capacity and reducing the expense of a vaccine dose, especially in cost-sensitive global-health indications where the price of the vaccine limits its use and coverage, as in the case of polio.51,52 Exploring the intradermal approach was recommended at a recent meeting of the World Health Organization Strategic Advisory Group of Experts (SAGE),53 as a means to reduce dose prices to make injectable polio vaccines (IPV) affordable for successful eradication of the disease in the Polio End Game54 A limitation of many of the studies, however, lies in the fact that they have not evaluated equivalent low-dose IM or SQ vaccination groups.55
The most recently registered indication for intradermal vaccination is influenza, where the ID approach has actually been pursued since the 1930's.56,57 This vaccine (Intanza®, Sanofi Pasteur), is a 5-fold concentrated form of Fluzone58 (inactivated influenza split-virus vaccine) delivered with an intradermal prefilled syringe (BD Soluvia™ Micro Injection System, Becton Dickinson and Company) that uses a 1.5 mm needle to provide a lower (9 μg HA/strain) or a standard dose (15 μg HA/strain), depending on the population and approved indication.35,58 Another example of an ID vaccine is rabies. Rabies is a zoonosis that occurs in over 100 countries and is invariably fatal once symptomatic. The cost of a full-dose rabies vaccine limits its widespread use in many areas. ID administration of the vaccine offers an equally safe and immunogenic alternative that requires only 20% of the dose for post-exposure prophylaxis, which could reduce the direct cost of the vaccine by 60–80%. ID regimens have been successfully introduced for post-exposure rabies prophylaxis in India, the Philippines, Sri Lanka and Thailand.59
Despite limited clinical data, ID vaccination also holds the promise to enhance immune responses using equivalent, rather than fractional, doses. Efforts have been made to improve influenza immunization by concentrating the formulation and delivering an equivalent dose of 15 μg HA/strain. A Phase II study administering ID with the BD 30-gauge 1.5 mm short needle60,61 demonstrated that an equivalent dose of 15 μg in elderly patients above 60 induced GMT ratios about 1.5-1.7-fold higher, compared with the same dose IM. This study was later confirmed in a Phase III study,62 demonstrating that equivalent dose (15 μg HA/strain) given ID can produce superior GMT's and seroprotection at 21 d post-vaccination. However, Intanza15 has not yet been shown to have superior clinical efficacy in terms of reducing mortality and morbidity, although a large retrospective study suggests a reduction in influenza related hospitalizations.63,64
Improving immunogenicity of various vaccines in immunocompromised hosts via the intradermal route is extremely important. Hepatitis B virus (HBV) vaccine has a 3–5% failure rate of non-seroconversion and there is a significant improvement in this after ID injection.65 Studies have demonstrated that in patients on dialysis or in patients with HIV, the intradermal route was more immunogenic than standard intramuscular delivery with the HBV vaccine. ID vaccine recipients had significantly better seroconversion rates compared with the standard dose intramuscular group,66 which was also demonstrated in ID HBV vaccination of dialysis patients.67
Adverse Effects of ID Vaccination
Overall, intradermal vaccination has been demonstrated to be very safe. Studies have shown that ID vaccination may be associated with a greater incidence of local reactogenicity, including primarily mild pain, swelling, and redness, but not systemic adverse events. These events typically resolve quickly, as was noted in a meta-analysis68 comparing the safety and immunogenicity of a large number of intradermal versus intramuscular influenza vaccines. ID vaccination was not associated with a greater incidence of any systemic adverse events examined and was associated with a lower incidence of myalgia. There was evidence of heterogeneity for most adverse events.
Devices for ID Vaccination
To address the unmet clinical and usability needs, various devices have been developed over the years. These are conceptually grouped into liquid delivery devices, including needles, mini-needles, and hollow microneedles, as well as needle adaptors and jet injectors, and solid delivery devices, such as solid microneedles, particle-injectors, and patches with coated micro-projections or dissolvable needles (Table 2).
Table 2.
Devices for ID delivery of vaccines69
| Type of delivery | Type of device | Vaccine fields evaluated clinically |
| Liquid administration | Needle and syringe (Mantoux) | Flu46,70-74, Rabies22-26,59, BCG13, Polio75,76 |
| Hollow mini and microneedles | Flu36,62, Rabies77, Anthrax78, Japanese encephalitis, DNA-encoding reporter genes (preclinical only)79-83 | |
| Tattoo devices | HPV84-89 | |
| Jet injectors | Smallpox99, BCG90, DTP91, Polio43,92, Tetanus93, Typhoid94-96, Rabies97, Influenza98, Yellow Fever99 | |
| Solid administration | Solid arrays | HPV100 |
| Dissolvable patches | Flu101 |
The most clinically advanced approach is the mini-needle technology, represented by the Intanza-Soluvia influenza vaccine combination (Sanofi Pasteur), which is commercially available. In its Intanza9 version, the 1.5 mm mini-needle demonstrated relative dose-sparing, at least non-inferior immunogenicity to standard unadjuvanted influenza vaccines, and high acceptability.102-104 Another licensed ID vaccine delivery device that may have been used with the largest number of vaccine types is a disposable hollow microneedle (<1 mm) device known as the MicronJet600™, which is the focus of this review.
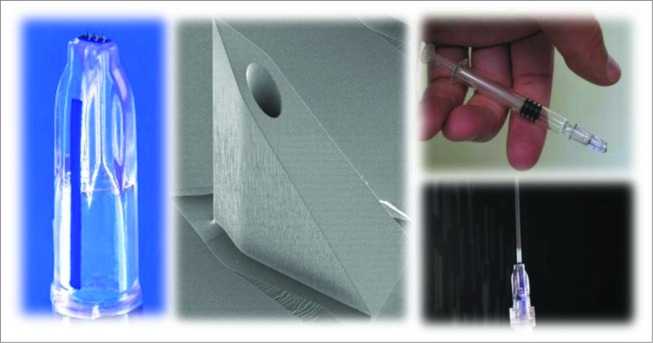
The MicronJet600 has 3 pyramid-shaped microneedles of 0.6 mm (600 μm) length (Fig. 1) and the device can be attached to any standard luer tip or luer-lock syringe. The needles are fabricated as a single 3-dimensional crystal silicon chip105 that is etched in a pattern to produce micropyramid-shaped microneedles, each having a very sharp tip that penetrates the epidermis followed by a conduit or through-channel for liquid delivery, and is produced using Micro Electro Mechanical System (MEMS) fabrication technology106 in a semiconductor fabrication house. The microneedle chip is integrated with a plastic hub or female luer that attaches to any male luer tip or luer lock syringe, thereby enabling the delivery of liquid formulations from any standard (prefilled or disposable) syringe directly into the skin. The integrated device forms a direct fluid channel from the syringe or container through the microneedles, in order to deliver a vaccine where the dendritic cells are most prevalent in the superficial dermis of the skin. Injection with the MicronJet600 is characterized by an intradermal bleb or wheal, which is the hallmark of an acceptable ID injection.107 It is registered with the US FDA (510 k), the EMEA (CE Mark), Canada, Hong Kong, and in other countries.
Figure 1.
The MicronJet600 microneedle and attachment to a standard syringe. Left: Close-up of the MicronJet600; Middle: SEM picture (∼×100) of a single microneedle prior to dicing, on wafer; Top Right: needle attached to a prefilled syringe; Bottom Right: direction of injection flow.
Prior to the development and commercialization of the MicronJet600, an older (original) version of the MicronJet was used in the clinical trials conducted in 2007–2008. This device included 4 microneedles that were 450 μm in length, made in a very similar design. The performance characteristics of the original model were similar, but the insertion technique was less intuitive, requiring insertion at about 60 degrees and lowering the syringe while in skin to about 30 degrees. The MicronJet600 was developed to improve ease of use, requiring insertion at a more natural angle of about 45 degrees with no subsequent adjustment of position.
The Past: Clinical Results with the Original MicronJet Device
The MicronJet device was tested both in immune-competent healthy adults and in an elderly population that was considered to be relatively immunocompromised. A first-in-man study was conducted to demonstrate effective dose-sparing, safety, and user preference, using a commercially available influenza vaccine, Fluarix 2006/2007 (GSK, Belgium)33 in healthy adults. This Phase I/II study used the original model MicronJet microneedle, described above. Groups received intradermal doses with 20% (3 μg HA/strain) or 40% (6 μg HA/strain) of the usual dose using the MicronJet device, or a 100% dose (15 μg HA/strain) given IM with a standard 26 G needle and syringe. Local reactogenicity was more frequent with ID vaccination, but was generally mild and transient. The low-dose ID groups had immune responses that were similar to those in the IM control group, demonstrating the potential for up to 5-fold dose-sparing. The regulatory criteria for re-licensure of seasonal influenza vaccines were met in full in all study groups. Recipient acceptance and discomfort was assessed using a questionnaire and demonstrated less pain and intimidation with the device compared to the IM injection (data on file).
A second study had a similar design using the A/2009/H1N1 strain and was the first intradermal vaccination study of pandemic influenza.27 The study, which was conducted mostly in the elderly population in Hong Kong, demonstrated 5-fold dose-sparing as well, with a safety profile that was comparable to the previous study. There was a similar incidence of systemic adverse events (AEs) such as fever and arthralgia, and a higher incidence of local AEs such as erythema and edema, which is consistent with other ID influenza vaccine studies.60,61,68
A study in the elderly compared fractional-dose ID delivery to the full IM dose of the unadjuvanted influenza vaccine (Fluvirin™, Novartis), as well as to MF59-adjuvanted formulations with various antigen and adjuvant doses.108 This study showed that the unadjuvanted ID approach yielded significantly higher immunogenicity at 6 μg HA/strain than unadjuvanted IM formulations at 15 μg or 30 μg HA/strain, in at least the A/H1N1 strain, with non-inferior GMTs in the other strains. One study arm (12 μg HA/strain ID) was also higher with the A/H3N2 strain compared to the unadjuvanted IM formulations. In addition, the study showed that formulations adjuvanted with MF59 yielded significantly higher GMTs than the unadjuvanted ID formulation in the A/H1N1 and B strains, but not for A/H3N2. However, the adjuvanted formulation included 15 μg HA/strain (and 30 μg HA for A/H3N2), which was 2.5-fold higher than the unadjuvanted ID groups, so a direct dose-for-dose comparison of ID (unadjuvanted) with IM (MF-59-adjuvanted) was not established.
Another seasonal influenza study evaluated various ID or IM doses of a virosomal influenza vaccine (Inflexal V™, Crucell, BV).36 This study was unique in that it included a head to head comparison of the use of the MicronJet device with the same formulation and dose using a 25 G 16 mm (5/8 in.) length needle and syringe with the Mantoux technique (typically using a 15 degree injection angle). This study showed that ID delivery of the low dose virosomal vaccine (3 μg HA/strain) with the MicronJet achieved statistically significant higher GMT fold-increases for the H1N1 and B strains as compared with the same dose ID using Mantoux (84.2 vs. 37.8 [P < 0.05] and 28.5 vs. 6.9 [P < 0.01], respectively). Superior immunogenicity was also demonstrated for the H3N2 strain compared to IM delivery of the full dose (15 μg HA/strain) vaccine, despite using 1/5th of the dose (39.9 vs. 16.9 [P < 0.05]). The improved immunogenicity results observed with the MicronJet600 could potentially be due to the consistent delivery of the influenza vaccine primarily to the superficial dermis and the epidermis, where Dendritic Cells (DCs), and Langerhans cells, (LCs) are respectively abundant. Injection site for all influenza studies was the deltoid area.
The Present: Demonstrating Improved Immunogenicity with the MicronJet600
Improving the immunogenicity of vaccines is an important unmet clinical need that might even be more important than mere dose-sparing. Theoretically, using higher or equivalent doses of an antigen intradermally (instead of reducing the dose due to volume constraints) may enhance such immunogenicity, and with it, potentially, vaccine efficacy. Intradermal delivery of high doses of the antigens may require concentration, which may result in some additional manufacturing costs.
A Phase II clinical study was conducted in 2010 at Hong Kong University to evaluate the ability of ID delivery to enhance the immunogenicity of seasonal influenza vaccines with Intanza9 2009/2010 as the source of antigen.109 The study included 2 experimental ID groups using the MicronJet device to give either 20% (3 μg HA/strain) or 60% (9 μg HA/strain) of the usual IM dose and 2 control arms dosed ID with either Intanza9 (9 μg HA/strain) or IM with Fluzone (15 μg HA/strain). The doses selected for the study were based on the available vaccines on the market. A direct comparison between 3 μg using the MicronJet to the same dose with Intanza was not done, as this dose was not tested for Intanza and the 6 μg dose did not show non-inferiority in previous studies. The study demonstrated that the typical reduction in immunogenicity of the 2009 H1N1 strain could be overcome and was significantly higher with ID vaccination when compared with the IM vaccination, with the highest seroprotection rate and GMT fold increase value generated by the lowest dose of 3 ug (20%) HA vaccine delivered by the MicronJet600. The H3N2 strain seroconversion rates were also significantly higher in the ID groups compared with the IM group. There was no significant difference in immune response between the ID groups.
Additional promising results demonstrating very significant dose-sparing, as well as improved immunogenicity, have been recently released by Merck & Co, for live attenuated herpes zoster vaccine (NCT01385566). Further detailed information is pending publication.
Table 3 outlines various published clinical studies using the MicronJet device models for the delivery of vaccines, along with a summary of results, benefits and references.
Table 3.
Published clinical studies using the MicronJet and MicronJet600
| Field | Study ID | Phase | N | Device used | Benefit demonstrated* |
|---|---|---|---|---|---|
| Seasonal Influenza | EudraCT number 2007-001160-77 | Pilot | 180 | MicronJet | Dose sparing33 |
| Seasonal Influenza | ISRCTN 33950739 | Phase II | 280 | MicronJet | Dose sparing and superior immunogenicity36 |
| Seasonal Influenza | NCT00848848 | Phase I | 450 | MicronJet | Superior immunogenicity108 |
| Pandemic Influenza | NCT01049490 | Phase I | 37 | MicronJet600 | Dose sparing27 |
| Seasonal Influenza | NCT01304563 | Phase II | 282 | MicronJet600 | Dose sparing and superior immunogenicity109 |
*Compared to a standard dose of the unadjuvanted vaccine
The Future of ID Delivery of Vaccines and Immunotherapeutics: Promise and Challenges
Despite many years of clinical development and the very promising early-stage trials described above, there are still significant challenges facing the ID delivery approach, for the MicronJet600 or any other device. For instance, late stage clinical trials are still required to validate superior immunogenicity and vaccine efficacy, especially in challenging populations like the elderly.110 In addition to having a low response to vaccination at a young age (below 6 months),111 the pediatric population also poses specific mechanical challenges, due to their thin skin, making them unsuitable for immunization with certain delivery technologies.112 However, the MicronJet600 device was recently utilized in a large Phase III inactivated polio vaccine (IPV) study in 6–14 week-old infants sponsored by the US CDC and the International Center for Diarrheal Disease Research, Bangladesh (icddr,b) (NCT01813604). The device performed very well in this setting (publication in preparation). Additional validation of ID delivery is required in order to expand the list of applicable vaccines beyond BCG, PPD, rabies, and influenza. Another phase I of ID iPV using Micronjet600 was conducted in HIV positive adults (NCT01686503).113
The use of ID delivery with immunotherapeutics holds future promise, coupled with unique challenges, in the settings of allergy (in Phase III clinical trials),114 cancer immunotherapy, and Type 1 Diabetes (in preclinical studies).115 Of most interest perhaps, is antigen-specific cancer immunotherapy, which despite past failures116,117 is still the most vibrant vaccine field to undergo clinical evaluation of the ID approach. There are over 30 clinical programs today with ID delivery of cancer vaccines (at least one of which with the MicronJet600) and likely many more to come. The ability to enhance the skin's potent immune system with ID immunization, to directly target its Dendritic and Langerhans cells,118,119 and to potentiate the response against cancer cells, remains one of the great challenges and promises of the 21st century.120
Disclosure of Potential Conflicts of Interest
Dr. Yotam Levin and Dr. Efrat Kochba are permanent employees of NanoPass Technologies, Ltd.
References
- 1. Gross CP, Sepkowitz KA. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. IJID 1998; 3:54-60; PMID:9831677 http://www.ijidonline.com/article/S1201-9712(98)90096-0/pdf [DOI] [PubMed] [Google Scholar]
- 2. Millar JD, Foege W.H. Status of eradication of smallpox (and control of measles) in West and Central Africa. J Infect Dis 1969; 120:725-32; PMID:5374972; http://dx.doi.org/ 10.1093/infdis/120.6.725 [DOI] [PubMed] [Google Scholar]
- 3. Jenner E. An Inquiry into the Causes and Effects of the Variolae Vaccinae: A Disease Discovered in Some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of the Cow Pox. London: Sampson Low; 1798, 1-2 [Google Scholar]
- 4. Jenner E. Two cases of small-pox infection communicated to the foetus in utero under peculiar circumstances, with additional remarks. Med Chir Trans 1809; 1:271-7; PMID:20895118 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2128795/pdf/medcht00073-0301.pdf [PMC free article] [PubMed] [Google Scholar]
- 5. Payette PJ, Davis HL. History of vaccines and positioning of current trends. Curr Drug Targets Infect Disord 2001; 1:241; PMID:12455398; http://dx.doi.org/ 10.2174/1568005014606017 [DOI] [PubMed] [Google Scholar]
- 6. Jenner E, Razzell PE. The history of a medical myth. Med Hist 1965; 9:216-29. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1033502/pdf/medhist00154-0020.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med 1931; 24:1481 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2182232/pdf/procrsmed00808-0023.pdf [PMC free article] [PubMed] [Google Scholar]
- 8. Mantoux C. L’intradermo-reaction a la tuberculine et son interpretation clinique. Presse Med 1910; 18:10-3 [Google Scholar]
- 9. Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest 1994; 106:1463-65; PMID:7956403; http://dx.doi.org/ 10.1378/chest.106.5.1463 [DOI] [PubMed] [Google Scholar]
- 10. Tarnow K, King N. Intradermal injections: traditional bevel up versus bevel down. ANR 2004; 17:275-82; PMID:15573336; http://dx.doi.org/ 10.1016/j.apnr.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 11. Lambert PH, Laurent PE. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008; 26:3197-208; PMID:18486285; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.095 [DOI] [PubMed] [Google Scholar]
- 12. Dacso CC. Skin Testing for Tuberculosis. In: Walker HK, Hall WD, Hurst JW, editors Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 47. Available from: http://www.ncbi.nlm.nih.gov/books/NBK369/ [PubMed] [Google Scholar]
- 13. Salisbury DM, Begg NT.(Eds.). 1996. Immunisation against infectious disease: the green book. Department of Health, London. [Google Scholar]
- 14. Frösner G, Steffen R, Herzog C. Virosomal hepatitis A vaccine: Comparing intradermal and subcutaneous with intramuscular administration. J Travel Med 2009; 16:413-9; PMID:19930383; http://dx.doi.org/ 10.1111/j.1708-8305.2009.00351.x [DOI] [PubMed] [Google Scholar]
- 15. Pancharoen C, Mekmullica J, Thisyakorn U, Kasempimolporn S, Wilde H, Herzog C. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. CID 2005; 41:1537-40; PMID:16231271; http://dx.doi.org/ 10.1086/497266 [DOI] [PubMed] [Google Scholar]
- 16. Chen W, Gluud C. Vaccines for preventing hepatitis B in health-care workers. Cochrane Database of Systematic Reviews 2005; Issue 4. Art. No.: CD000100. DOI: 10.1002/14651858.CD000100.pub3 [DOI] [PubMed] [Google Scholar]
- 17. Sangare L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: A systematic review and meta-analysis. Vaccine 2009; 27:1777-86; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.043 [DOI] [PubMed] [Google Scholar]
- 18. Braddick MR. Intradermal hepatitis B vaccine. Lancet 1989; 1:957-8; http://dx.doi.org/ 10.1016/S0140-6736(89)92533-6 [DOI] [PubMed] [Google Scholar]
- 19. Redfield RR, Innis BL, Scott RM, Cannon HG, Bancroft WH. Clinical evaluation of low-dose intradermally administered hepatitis B virus vaccine—A cost reduction strategy. JAMA 1985; 254:3203-6; PMID:2933535; http://dx.doi.org/ 10.1001/jama.1985.03360220069031 [DOI] [PubMed] [Google Scholar]
- 20. Wistrom J. Intramuscular vs. intradermal hepatitis B vaccination: A 6-year follow-up. JAMA 1995; 273:1835-6; PMID:7776497; http://dx.doi.org/ 10.1001/jama.1995.03520470043027 [DOI] [PubMed] [Google Scholar]
- 21. Kyi KP, Oo KM, Htun MM, Tun WM, Aye KK, Oo SS, Lwin KO, Nyunt S. Clinical trial of the intradermal administration of hepatitis B vaccine produced at the Department of Medical Research, Myanmar. Vaccine 2002; 20:1649-52; PMID:11858874; http://dx.doi.org/ 10.1016/S0264-410X(01)00468-6 [DOI] [PubMed] [Google Scholar]
- 22. Fishbein DB, Pacer RE, Holmes DF, Ley AB, Yager P, Tong TC. Rabies pre exposure prophylaxis with human diploid cell rabies vaccine: A dose–response study. J Infect Dis 1987; 156:50-5; http://dx.doi.org/ 10.1093/infdis/156.1.50 [DOI] [PubMed] [Google Scholar]
- 23. Warrell MJ, Warrell DA, Nicholson KG, Suntharasamai P, Chanthavanich P, Viravan C, Udomsakdi D. Economical multiple-site intradermal immunisation with human diploid-cell-strain vaccine is effective for post-exposure rabies prophylaxis. Lancet 1985; 325:1059-62; PMID:2860284; http://dx.doi.org/ 10.1016/S0140-6736(85)92367-0 [DOI] [PubMed] [Google Scholar]
- 24. Quimbao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerczyk C. Reducing the cost of post-exposure rabies prophylaxis: Efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine 2005; 23:1709-14; PMID:15705476; http://dx.doi.org/ 10.1016/j.vaccine.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 25. Khawplod P, Wilde H, Sirikwin S, Benjawonqkulchai M, Limusanno S, Jaijaroensab W, Chiraguna N, Supich C, Wangroongsarb Y, Sitprija V. Revision of the Thai Red Cross intradermal rabies post-exposure regimen by eliminating the 90-day booster injection. Vaccine 2006; 24:3084-6; PMID:16494972; http://dx.doi.org/ 10.1016/j.vaccine.2006.01.051 [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization Rabies vaccines. WHO position paper. Wkly Epi demiol Rec 2010; 85:309-20. http://www.who.int/wer/2010/wer8532.pdf?ua=1 [Google Scholar]
- 27. Hung IF, Levin Y, To KK. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine 2012; 30:707-2708; PMID:22210225; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.069 [DOI] [PubMed] [Google Scholar]
- 28. Whittle HC, Rowland MG, Mann GF, Lamb WH, Lewis RA. Immunisation of 4–6 month old Gambian infants with Edmonston-Zagreb measles vaccine. Lancet 1984; 2:834-7; PMID:6148572; http://dx.doi.org/ 10.1016/S0140-6736(84)90873-0 [DOI] [PubMed] [Google Scholar]
- 29. Edens C, Collins ML, Ayers J, Rota PA, Prausnit MR. Measles vaccination using a microneedle patch. Vaccine 2013; 31:3403-9; PMID:23044406; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burland WL. Measles vaccination by the intradermal route. Postgrad Med J 1969; 45:323-6; http://dx.doi.org/ 10.1136/pgmj.45.523.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by SoluviaTM licensed micro injection system. Hum Vaccin Immunother 2012; 8:67-75; PMID:22293531; http://dx.doi.org/ 10.4161/hv.8.1.18419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: Randomized, controlled, phase III trial. Hum Vaccine 2010; 6:346-54; PMID:20372053; http://dx.doi.org/ 10.4161/hv.6.4.10961 [DOI] [PubMed] [Google Scholar]
- 33. Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intra-dermal influenza vaccination in healthy adults. Vaccine 2009; 27:454-9; PMID:19022318; http://dx.doi.org/ 10.1016/j.vaccine.2008.10.077 [DOI] [PubMed] [Google Scholar]
- 34. Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 2007; 25:6755-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, Diaz-Granados C, Landolfi V. Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: A randomized, controlled, phase II trial. Vaccine 2014; 32:2507-17; PMID:24120672; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.074 [DOI] [PubMed] [Google Scholar]
- 36. Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: Are all delivery methods the same? Vaccine 2014; 32:4249-52; PMID:24930715; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 37. Roukens AH, Vossen AC, Bredenbeek PJ, Van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS One 2008; 3:e1993; PMID:18431480; http://dx.doi.org/ 10.1371/journal.pone.0001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roukens AH, Vossen AC, Van Dissel JT, Visser LG. Reduced intradermal test dose 240 of yellow fever vaccine induces protective immunity in individuals with egg 241 allergy. Vaccine 2009; 27:2408-9; PMID:19368780; http://dx.doi.org/ 10.1016/j.vaccine.2009.02.049 [DOI] [PubMed] [Google Scholar]
- 39. Hickling J, Jones R. Yellow fever vaccination: The potential of dose-sparing to increase vaccine supply and availability. PATH report. April 18, 2013. http://www.path.org/publications/files/TS_vtg_yf_rpt.pdf. Retrieved August 1st, 2014 [Google Scholar]
- 40. Mohammed AJ, AlAwaidy S, Bawikar S, Kurup PJ, Elamir E, Shaban M, Sharif SM, Van Der Avoort H, Pallansch MA, Malankar P, et al. . Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med 2010; 362:2351-9; PMID:20573923; http://dx.doi.org/ 10.1056/NEJMoa0909383 [DOI] [PubMed] [Google Scholar]
- 41. Resik S, Tejeda A, Lago PM, Diaz M, Carmenates A, Sarmiento L, Alemañi N, Galindo B, Burton A, Friede M, et al. . Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis 2010; 201:1344-52; PMID:20350164; http://dx.doi.org/ 10.1086/651611 [DOI] [PubMed] [Google Scholar]
- 42. Nirmal S, Cherian T, Samuel BU, Rajasinght J, Raghupathy P, Jacob JT. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine 1998; 16:928-31; http://dx.doi.org/ 10.1016/S0264-410X(97)00293-4 [DOI] [PubMed] [Google Scholar]
- 43. Estívariz CF, Jafari H, Sutter RW, John TJ, Jain V, Agarwal A, Verma H, Pallansch MA, Singh AP, Guirguis S, et al. . Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: A community-based, randomised controlled trial. Lancet Infect Dis 2012; 12:128-35; PMID:22071249; http://dx.doi.org/ 10.1016/S1473-3099(11)70190-6 [DOI] [PubMed] [Google Scholar]
- 44. Zoulek G, Roggendorf M, Deinhardt F, Kunz C. Different immune responses after intradermal and intramuscular administration of vaccine against tick borne encephalitis virus. J Med Virol 1986; 19:55-61; PMID:3701303; http://dx.doi.org/ 10.1002/jmv.1890190109 [DOI] [PubMed] [Google Scholar]
- 45. Baxby D. Smallpox vaccination techniques; from knives and forks to needles and pins. Vaccine 2002; 20:2140-9; PMID:11972983; http://dx.doi.org/ 10.1016/S0264-410X(02)00028-2 [DOI] [PubMed] [Google Scholar]
- 46. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/ 10.1056/NEJMoa043540 [DOI] [PubMed] [Google Scholar]
- 47. Brown LE. The role of adjuvants in vaccines for seasonal and pandemic influenza. Vaccine 2010; 28:8043-5; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 48. Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, Rush B, Safirstein B, Wheeler D, Nichol KL. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: Data from 3 health plans. J Infect Dis 2001; 184:665-70; http://dx.doi.org/ 10.1086/323085 [DOI] [PubMed] [Google Scholar]
- 49. Gill HS, Kang SM, Quan FS, Compans RW. Cutaneous immunization: An evolving paradigm in influenza vaccines. Expert Opin Drug Deliv 2014; 11:615-27; PMID:24521050; http://dx.doi.org/ 10.1517/17425247.2014.885947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994; 331:778-84; PMID:8065407; http://dx.doi.org/ 10.1056/NEJM199409223311206 [DOI] [PubMed] [Google Scholar]
- 51. Resik S, Tejeda A, Sutter RW, Diaz M, Sarmiento L, Alemañi N, Aylward RB. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368:416-24; PMID:23363495; http://dx.doi.org/ 10.1056/NEJMoa1202541 [DOI] [PubMed] [Google Scholar]
- 52. Hickling J, Jones R, Nundy N. Improving the affordability of inactivated poliovirus vaccines (IPV) for use in low-and middle-income countries: PATH (Program for Appropriate Technology in Health), 2010. http://www.path.org/publications/files/TS_IPV_econ_analysis.pdf [Google Scholar]
- 53. Meeting of the Strategic Advisory Group of Experts on immunization, April 2012–—Conclusions and recommendations. 25 May 2012, vol. 87, 21 (pp 201-16).http://www.who.int/wer. Retrieved on August 1, 2014 [PubMed] [Google Scholar]
- 54. Polio Eradication and Endgame Strategic Plan 2013–2018 http://www.polioeradication.org/resourcelibrary/strategyandwork.aspx#sthash.IF1yZB1Z. Retrieved on August 1, 2014 [Google Scholar]
- 55. Zehrung D. Kristensen D. Intradermal Delivery of Vaccines: A Review of the Literature and the Potential for Development for Use in Low- and Middle-Income Countries. August 27, 2009. http://www.path.org/publications/detail.php?i=1746. [Google Scholar]
- 56. Tuft L. The skin as an immunological organ with results of experimental investigations and review of the literature. J Immunol 1931; 21:85-100. http://www.jimmunol.org/content/21/2/85.abstract [Google Scholar]
- 57. Francis T, Magill TP. The antibody response of human subjects vaccinated with the virus of human influenza. J Exp Med 1937; 65:251-9; PMID:19870599; http://dx.doi.org/ 10.1084/jem.65.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fluzone Prescribing Information" Sanofi Pasteur. April 2013 [Google Scholar]
- 59. World Health Organization : WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. WHO/EMC/ZOO.96.6. 1997. http://whqlibdoc.who.int/hq/1996/WHO_EMC_ZOO_96.6.pdf [Google Scholar]
- 60. Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, et al. . Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J Infect Dis 2008; 198:650-8; PMID:18652550; http://dx.doi.org/ 10.1086/590434 [DOI] [PubMed] [Google Scholar]
- 61. Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 2007; 25:8833-42; PMID:18023942; http://dx.doi.org/ 10.1016/j.vaccine.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 62. Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine 2009; 27:7304-12; PMID:19849996; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.033 [DOI] [PubMed] [Google Scholar]
- 63.Intanza–Seasonal Influenza Vaccine For Intradermal Use–NHS July 2012 http://hertsvalleysccg.nhs.uk/uploads/file/Pharmacy/Local%20Decisions/Intanza%20intradermal%20influenza%20vaccine%20201207(HMMC).pdf [Google Scholar]
- 64. Puig-Barberà J, Natividad-Sancho A, Calabuig-Pérez J, Lluch-Rodrigo JA, Pastor-Villalba E, Martínez-Úbeda S, Díez-Domingo J. Intradermal and virosomal influenza vaccines for preventing influenza hospitalization in the elderly during the 2011–2012 influenza season: A comparative effectiveness study using the Valencia health care information system. Vaccine 2014; 32:5447-54; http://dx.doi.org/ 10.1016/j.vaccine.2014.07.095 [DOI] [PubMed] [Google Scholar]
- 65. Filippelli M., Lionetti E., Gennaro A., Lanzafame A., Arrigo T., Salpietro C., La Rosa M., Salvatore L. Hepatitis B vaccine by intradermal route in non responder patients: An update. World J Gastroenterol 2014; 20(30):10383-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Launay O, Van der Vliet D, Rosenberg AR, Michel ML, Piroth L, Rey D, de Verdière NC, Slama L, Martin K, Lortholary O, et al. . Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard Hepatitis B vaccine regimen in adults with HIV-1. JAMA 2011; 305:1432-40; PMID:21486976; http://dx.doi.org/ 10.1001/jama.2011.351 [DOI] [PubMed] [Google Scholar]
- 67. Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: A meta-analysis of randomized trials. J Viral Hepat 2011; 18:730-7; PMID:20819147; http://dx.doi.org/ 10.1111/j.1365-2893.2010.01354.x [DOI] [PubMed] [Google Scholar]
- 68. Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses 2013; 7:584-603; PMID:22974174 10.1111/irv.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kis EE, Winter G, Myschik J. Devices for intradermal vaccination. Vaccine 2012; 30:523-38; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 70. Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 2007; 25:659-63; PMID:17011678; http://dx.doi.org/ 10.1016/j.vaccine.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 71. Brown H, Kasel JA, Freeman DM, Moise LD, Grose NP, Couch RB. The immunizing effect of influenza A/New Jersey/76 (Hsw1N1) virus vaccine administered intradermally and intramuscularly to adults. J Infect Dis 1977; 136 :S466-S471; PMID:606768; http://dx.doi.org/ 10.1093/infdis/136.Supplement_3.S466 [DOI] [PubMed] [Google Scholar]
- 72. Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics 2007; 119:1076-108; PMID:17545373; http://dx.doi.org/ 10.1542/peds.2006-3176 [DOI] [PubMed] [Google Scholar]
- 73. Halperin W, Weiss WI, Altman R, Diamond MA, Black KJ, Iaci AW, Black HC, Goldfield M. A comparison of the intradermal and subcutaneous routes of influenza vaccination with A/New Jersey/76 (swine flu) and A/Victoria/75: Report of a study and review of the literature. Am J Public Health 1979; 69:1247-51; PMID:507256; http://dx.doi.org/ 10.2105/AJPH.69.12.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Herbert FA, Larke RP, Markstad EL. Comparison of responses to influenza A/New Jersey/76-A/Victoria/75 virus vaccine administered intradermally or subcutaneously to adults with chronic respiratory disease. J Infect Dis 1979; 140:234-8; PMID:479641; http://dx.doi.org/ 10.1093/infdis/140.2.234b [DOI] [PubMed] [Google Scholar]
- 75. Samuel BU, Cherian T, Sridharan G, Mukundan P, John TJ. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet 1991; 338:343-4; PMID:1677699; http://dx.doi.org/ 10.1016/0140-6736(91)90480-D [DOI] [PubMed] [Google Scholar]
- 76. Samuel BU, Cherian T, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to inactivated poliovirus vaccine injected intradermally. Vaccine 1992; 10:135; PMID:1311491; http://dx.doi.org/ 10.1016/0264-410X(92)90039-M [DOI] [PubMed] [Google Scholar]
- 77. Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine 2010; 28:5850-6; PMID:20600481; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.062 [DOI] [PubMed] [Google Scholar]
- 78. Mikszta JA, Dekker JP, III, Harvey NG, Dean CH, Brittingham JM, Huang J, Sullivan VJ, Dyas B, Roy CJ, Ulrich RG. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun 2006; 74:6806-10; PMID:17030580; http://dx.doi.org/ 10.1128/IAI.01210-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McAllister DV, Wang PM, Davis SP, Park J-H, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. PNAS 2003; 100:13755-60; PMID:14623977; http://dx.doi.org/ 10.1073/pnas.2331316100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol 2006; 126:1080-7; PMID:16484988; http://dx.doi.org/ 10.1038/sj.jid.5700150 [DOI] [PubMed] [Google Scholar]
- 81. Daugimont L, Baron N, Vandermeulen G, Pavselj N, Miklavcic D, Jullien MC, Cabodevila G, Mir LM, Préat V. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J Membr Biol 2010; 236:117-25; PMID:20652559; http://dx.doi.org/ 10.1007/s00232-010-9283-0 [DOI] [PubMed] [Google Scholar]
- 82. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 2004; 56:581-7; http://dx.doi.org/ 10.1016/j.addr.2003.10.023 [DOI] [PubMed] [Google Scholar]
- 83. Vrdoljak A. Review of recent literature on microneedle vaccine delivery technologies. Vaccine 2013; 3:47-55; http://dx.doi.org/ 10.2147/VDT.S34682 [DOI] [Google Scholar]
- 84. Pokorná D, Poláková I, Kindlová M, Dusková M, Ludvíková V, Gabriel P, Kutinová L, Müller M, Šmahel M. Vaccination with human papillomavirus type 16-derived peptides using a tattoo device. Vaccine 2009; 27:3519-29; PMID:19464530; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.073 [DOI] [PubMed] [Google Scholar]
- 85. Pokorna D, Rubio I, Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet Vaccines 2008; 6:4; PMID:18257910; http://dx.doi.org/ 10.1186/1479-0556-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Van den Berg JH, Nuijen B, Beijnen JH, Vincent A, van Tinteren H, Kluge Jr, Woerdeman LA, Hennink WE, Storm G, Schumacher TN, et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum Gene Ther 2009; 20:181-9; PMID:19301471; http://dx.doi.org/ 10.1089/hgt.2008.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ciernik IF, Krayenbühl BH, Carbone DP. Puncture-mediated gene transfer to the skin. Hum Gene Ther 1996; 7:893-9; PMID:8727503; http://dx.doi.org/ 10.1089/hum.1996.7.8-893 [DOI] [PubMed] [Google Scholar]
- 88. Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TNM, Haanen JB. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med 2005; 11:899-904; PMID:15965482; http://dx.doi.org/ 10.1038/nm1264 [DOI] [PubMed] [Google Scholar]
- 89. Quaak SG, van den Berg JH, Oosterhuis K, Beijnen JH, Haanen JBAG, Nuijen B. DNA tattoo vaccination: effect on plasmid purity and transfection efficiency of different topoisoforms. J Control Release 2009; 139:153-9; PMID:19580829; http://dx.doi.org/ 10.1016/j.jconrel.2009.06.033 [DOI] [PubMed] [Google Scholar]
- 90. Ten Dam HG, Fillastre C, Conge G, Orssaud E, Gateff C, Tanaka A, Ramirez OO, Collas R, Wright J, Chambon L, et al. The use of jet-injectors in BCG vaccination. Bull WHO 1970; 43:707-20. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2427792/pdf/bullwho00209-0064.pdf [PMC free article] [PubMed] [Google Scholar]
- 91. Stanfield JP, Bracken PM, Waddell KM, Gall D. Diphtheria-Tetanus-Pertussis immunization by intradermal jet injection. Br Med J 1972; 2:197-9; PMID:5022729 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1787936/pdf/brmedj02200-0029.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Soonawala D, Verdijk P, Wijmenga-Monsuur AJ, Boog CJ, Koedam P, Visser LG, Rots NY. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013; 31:3688-94; PMID:23770332; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.104 [DOI] [PubMed] [Google Scholar]
- 93. Dimache G1, Stoean C, Durbacă S, Croitoru M, Ionescu M, Nedelcu IN, Corbu I. Study of specific immune response to unadsorbed concentrated tetanus vaccine administered by intradermal route to non-immunized persons in the last ten years. Arch Roum Pathol Exp Microbiol 1990; 49:51-62; PMID:2101203 [PubMed] [Google Scholar]
- 94. Dimache G, Croitoru M, Dimache V, Cuteanu I, Vasilescu T. Lyophilized typhoid vaccine for intradermal use. J Biol Stand 1983; 11:261-9; PMID:6643508; http://dx.doi.org/ 10.1016/S0092-1157(83)80014-6 [DOI] [PubMed] [Google Scholar]
- 95. Dimache G, Croitoru M, Nicolae IN. Subcutaneous versus intradermal typhoid vaccination by "jet-injector" apparatus. Arch Roum Pathol Exp Microbiol 1982; 41:259-64; PMID:7159215 [PubMed] [Google Scholar]
- 96. Dimache G, Dimache V, Ciudin L, Palade R, Croitoru M. Intradermal typhoid vaccination in men by jet-injector. Immunological estimation by laboratory tests. Arch Roum Pathol Exp Microbiol 1976; 36:227-32; PMID:617039 [PubMed] [Google Scholar]
- 97. Bernard KW, Roberts MA, Sumner J, Winkler WG, Mallonee J, Baer GM, Chaney R. Human diploid cell rabies vaccine. Effectiveness of immunization with small intradermal or subcutaneous doses. JAMA 1982; 247:1138-42; PMID:7057603; http://dx.doi.org/ 10.1001/jama.1982.03320330034022 [DOI] [PubMed] [Google Scholar]
- 98. McAllister L, Anderson J, Werth K, Cho I, Copeland K, Le Cam NB, Plant D, Mendelman PM, Cobb DK. Needle-free jet injection for administration of influenza vaccine: A randomised non-inferiority trial. Lancet 2014; 384:674-81; PMID:24881803; http://dx.doi.org/ 10.1016/S0140-6736(14)60524-9 [DOI] [PubMed] [Google Scholar]
- 99. Meyer Jr, Harry M, Daniel D., Hostetler Jr, Bernheim BC, Rogers NG, Lambin P, Chassary A, Labusquière R, Smadel JE. Response of Volta children to jet inoculation of combined live measles, smallpox and yellow fever vaccines. Bull World Health Organ 1964; 30:783-94; PMID:14215185 http://apps.who.int/iris/bitstream/10665/73714/1/bulletin_1964_30%286%29_783-794.pdf?ua=1 [PMC free article] [PubMed] [Google Scholar]
- 100. Kendall MAF. Needle-free vaccine injection. Drug Delivery Handbook Exp Pharmacol 2010; 197:193-219; http://dx.doi.org/ 10.1007/978-3-642-00477-3_7 [DOI] [PubMed] [Google Scholar]
- 101. Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014; 32:1856-62; PMID:24530146; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Leroux-Roels I, Weber F. Intanza® 9 μg intradermal seasonal influenza vaccine for adults 18 to 59 years of age. Hum Vaccines Immunother 2013; 9:115-21; PMID:23442585; http://dx.doi.org/ 10.4161/hv.22342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza® 9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther 2011; 28:640-9; PMID:21751080; http://dx.doi.org/ 10.1007/s12325-011-0042-0 [DOI] [PubMed] [Google Scholar]
- 104. Prymula R, Usluer G, Altinel S, Sichova R, Weber F. Acceptance and opinions of Intanza/IDflu intradermal influenza vaccine in the Czech Republic and Turkey. Adv Ther 2012; 29:41-52; PMID:22228256; http://dx.doi.org/ 10.1007/s12325-011-0090-5 [DOI] [PubMed] [Google Scholar]
- 105. Gardeniers HJ, Luttge R, Berenschot EJ, De Boer MJ, Yeshurun SY, Hefetz M, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst 2003; 12:855-62; http://dx.doi.org/ 10.1109/JMEMS.2003.820293 [DOI] [Google Scholar]
- 106. Rebeiz GM. RF MEMS: Theory, Design, and Technology. John Wiley & Sons; 2004 [Google Scholar]
- 107. Nadim Maluf. and Kirt Williams. An Introduction to Microelectromechanical Systems Engineering, Second Edition Hardcover - June 30, 2004. [Google Scholar]
- 108. Della Cioppa G, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, Galli G1, Groth N, Levin Y, Del Giudice G. A dose-range study in older adults to compare the safety and immunogenicity profiles of MF59®-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum Vaccines Immunother 2014; 10:6, 1-10; PMID:24732325; http://dx.doi.org/ 10.4161/hv.28618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 2012; 30:6427-35; PMID:22910287; http://dx.doi.org/ 10.1016/j.vaccine.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 110. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol 2011; 11:865-72; http://dx.doi.org/ 10.1038/nri3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chiu SS, Chan KH. Tu W, Lau YL, Peiris JS. Immunogenicity and safety of intradermal versus intramuscular route of influenza immunization in infants less than 6 months of age: A randomized controlled trial. Vaccine 2009; 27:4834-9; PMID:19523908; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.066 [DOI] [PubMed] [Google Scholar]
- 112. Ploin D, Schwarzenbach F, Dubray C, Nicolas JF, Goujon C, Trong MD, Laurent PE. Echographic measurement of skin thickness in sites suitable for intradermal vaccine injection in infants and children. Vaccine 2011; 29:8438-42; PMID:21821081; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.111 [DOI] [PubMed] [Google Scholar]
- 113. Stephany Troy, et al. Comparison of the Immunogenicity of Various Booster Doses of Inactivated Polio Vaccine Delivered Intradermally Versus Intramuscularly to HIV-Infected Adults. JID Advance Access published January 28, 2015; http://jid.oxfordjournals.org/content/early/2015/01/07/infdis.jiu841.full.pdf+html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. A new immunotherapy developed by the UK Company Circassia has the potential to make cat allergies an easily treatable condition. Human Vaccines Immunother 2013; 9:941-4; http://dx.doi.org/ 10.4161/hv.25213 [DOI] [Google Scholar]
- 115. Riedmann E. A vaccine for long-suffering cat allergy patients. Human Vaccines & Immunotherapeutics: News. Hum Vaccines Immunother 2013, 9:5, 941-944, DOI: 10.4161/hv.25213 [DOI] [PubMed] [Google Scholar]
- 116. Investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoints in MAGRIT, a phase III non-small cell lung cancer clinical trial. Issued: Thursday 20 March 2014, London UK; Retrieved June 23, 2014: http://www.gsk.com/media/press-releases/2014/investigational-MAGE-A3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoints-in-MAGRIT.html [Google Scholar]
- 117. Tyagi P, Beloo M. MAGRIT: The largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer 2009; 10:371-4; PMID:19808198; http://dx.doi.org/ 10.3816/CLC.2009.n.052 [DOI] [PubMed] [Google Scholar]
- 118. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137:1142-62; PMID:4573839; http://dx.doi.org/ 10.1084/jem.137.5.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Glenn GM, Kenney RT. Mass vaccination: Solutions in the skin. Curr Top Microbiol Immunol 2006; 304:247-68; PMID:16989274; http://dx.doi.org/ 10.1007/3-540-36583-4_14 [DOI] [PubMed] [Google Scholar]
- 120. Couzin-Frankel Cancer immunotherapy. Science 2013; 342:1432-3; http://dx.doi.org/ 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]