Abstract
Background
A growing body of evidence indicates that in patients after anterior cruciate ligament reconstruction (ACLR) with a combined semitendinosus and gracilis (STGR) graft there are large deficits in the internal rotation strength, which has led some authors to recommend harvest of only ST tendon whenever possible. The purpose of this study was to assess the isometric (IT) and peak torque (PT) of the muscles responsible for shin rotation in patients after ACLR with an ST or with an STGR graft.
Material/Methods
Twenty patients with an ST graft and 20 patients with a combined STGR graft underwent a 6-month postoperative rehabilitation program after ACLR. At the end of the rehabilitation program, the IT and PT of the muscles responsible for internal (IR) and external rotation (ER) of the shin were measured. The results were compared to the results of a control group. Additionally, to determine the reliability of the dynamometer for clinical research, a test-retest assessment was performed.
Results
There were no statistically significant differences between the 3 groups of participants. Nevertheless, in the STGR group there was a statistically significant difference between the IT of muscles internally rotating the shin in the involved knee and uninvolved knee at 25° of the internal shin rotation.
Conclusions
Comparison of IT and PT measurements performed after 24 weeks of postoperative rehabilitation generally showed no differences between patients after ACLR with the use of ST graft and patients who received a combination graft consisting of STGR. Nevertheless, there was an influence of GR harvest on internal shin rotation torque at a deep internal rotation angle.
Keywords: Anterior Cruciate Ligament Reconstruction, Isometric Contraction, Knee Joint
Background
Reconstruction of the anterior cruciate ligament (ACL) is recommended for prevention of instability, further intraarticular disease, and recurrent injury in the ACL-deficient knee [1–3]. A semitendinosus (ST) or combined ST and gracilis (STGR) tendon graft is commonly used for reconstruction of the ruptured ACL [4,5]. Evaluation of patient muscle strength after ACL reconstruction is used to determine whether the patient can safely return to pre-injury activity level [6–10]. Most of the studies that have evaluated autologous ST and STGR grafts have focused on postoperative tendon regeneration and knee flexor strength [11–13]. Studies examining the knee flexion strength of patients after ACL reconstruction have noted very small or no deficits in peak torque after ST or STGR harvest [14–16], but some authors have reported a persistent deficiency in flexor strength after surgery [17–19]. There have also been reports evaluating the rotation torque of the knee [20–25]. A growing body of evidence indicates that there are large deficits in the internal rotation strength, a significant weakness of hamstring muscle strength at high knee flexion angles, and a significantly lower standing knee flexion angle after STGR harvest, which has led some authors to recommend harvest of only ST tendon whenever possible [21,26–29]. Nevertheless, studies indicating large deficits in the internal rotation strength involved only isokinetic testing [28].
The purpose of this study was to evaluate the isometric and peak torques of the muscles responsible for shin rotation in the knee, and to compare these in patients with an ST graft and patients with a combined STGR graft.
Material and Methods
This study protocol was approved by the local ethics committee and written consent forms were signed prior to participation. The study was conducted according to the ethics guidelines and principles of the Declaration of Helsinki.
The initial sample population comprised 46 males with ACL tears who underwent ACL reconstruction using ST or STGR autologous grafts from the ipsilateral leg followed by a standardized rehabilitation program [14]. Patients were excluded if they met any of the exclusion criteria (Figure 1). The remaining 40 males were divided into 2 groups according to the type of tendon graft used for their ACL reconstruction; patients in the ST group (n=20) received an ST tendon graft and patients in the STGR group (n=20) received a combination graft consisting of STGR tendons. The evaluation was performed 6 months after ACL reconstruction. The interval between injury and reconstruction was 3–6 months. The results of males after ACLR were compared to the results of 20 male volunteers without known cardiovascular or orthopedic problems (control group). The physical characteristics of the study participants are presented in Table 1. The frequency of sports participation in the control group and in the ST and STGR groups before injury was similar. Preoperatively, the patients after ACLR performed recreational activity an average of 2–3 times a week.
Figure 1.
Flow chart of eligibility selection. ACLR – anterior cruciate ligament reconstruction; Group ST – patients who underwent ACL reconstruction with a semitendinosus graft; Group STGR – patients who underwent ACL reconstruction with a combined semitendinosus and gracilis graft; Control group – healthy male volunteers; IT – isometric torque; PT – peak torque.
Table 1.
The physical characteristics of the study participants.
| Age (years) | Body mass (kg) | Body height (cm) | ||||
|---|---|---|---|---|---|---|
| x | SD | x | SD | x | SD | |
| Group ST (n=20) | 26.65 | 9.71 | 80.70 | 8.34 | 181.25 | 6.29 |
| Group STGR (n=20) | 29.55 | 8.23 | 80.30 | 11.72 | 181.80 | 8.61 |
| Control group (n=20) | 24.90 | 3.11 | 80.75 | 7.77 | 182.10 | 5.70 |
| p | 0.16 | 0.99 | 0.93 | |||
| Group T-R (n=12) | 24.42 | 1.93 | 79.50 | 13.18 | 183.83 | 10.79 |
Group ST – patients who underwent ACL reconstruction with a semitendinosus graft; Group STGR – patients who underwent ACL reconstruction with a combined semitendinosus and gracilis graft; Group T-R – males who participated in the test-retest analysis; p – significance level; x – mean; SD – standard deviation.
Surgical technique
All of the ACL reconstructions were performed or supervised by the same surgeon. Diagnostic arthroscopy was performed through standard anteromedial (AM) and anterolateral (AL) portals. The ST and GR tendons were harvested through a 3-cm oblique incision over the ‘pes anserinus’. Patients in the ST group underwent ACL reconstruction with doubled autologous ST grafts, while patients in the STGR group underwent ACL reconstruction with combined STGR grafts. The same fixation devices were used for both types of reconstruction.
Rehabilitation protocol
Participants in both the ST and the STGR groups underwent a 4-stage, postoperative rehabilitation program that lasted on average 6 months [14]. The mean frequency of participation in the rehabilitation program was 4 days per week for 2 hours each day.
Stage I (1st–5th postoperative weeks)
During the initial postoperative period, ice packs were used to reduce swelling. After several days these were replaced with local cryotherapy. Continuous passive motion (CPM) knee exercises and mobilization of the patellofemoral joint were performed. The electrostimulation of vastus medialis and a magnetic field were applied. Closed kinetic chain (CKC) proprioceptive exercises were added to the exercise regimen. Isometric tensioning of the quadriceps and flexor muscles around the involved knee, followed by isometric exercises with manually dosed resistance from muscle groups distant to the affected area, including the uninvolved lower extremity, the upper extremities, and the trunk, were performed.
Stage II (6th–12th postoperative weeks)
Treadmill walking was added. The CPM exercises were replaced with exercises on a cycloergometer. Proprioceptive exercises progressed to being performed on a soft surface. Step-up exercises, 1- and 2-legged squats on a collapsible surface, and concentric and eccentric exercises for the ischiotibial muscles of the operated leg in the sagittal and transverse planes (especially internal shin rotation) were gradually added.
Stage III (13th–20th postoperative weeks)
Isometric exercises with partial resistance from the extensor muscles of the involved knee and treadmill running were introduced. Discipline-specific exercises, plyometric exercises, functional training with movement pattern corrections, and core exercises were added to the rehabilitation program. From the 16th postoperative week, strength training under isokinetic conditions was performed.
Stage IV (21st postoperative week to 6–8 postoperative months)
Exercises performed during the 3rd stage were continued. Patients also performed exercises with a jump rope on different surfaces and practiced jumping obstacles and controlled slides with the physiotherapist’s assistance. The main goal of this stage of rehabilitation was restoration of the speed, power, agility, and field orientation specific to the patient’s specific sport or job. Swimming was recommended once a week.
Isometric torque (IT) measurement under static conditions and peak torque (PT) measurement under isokinetic conditions of the muscles responsible for shin rotation within the knee
The torque measurements of the muscles responsible for internal rotation (IR) and external rotation (ER) of the shin in the knee were performed using the Humac Norm Testing & Rehabilitation System (Cybex dynamometer) [23]. A warm-up on a cycle ergometer preceded all measurements. The IT and PT measurements were taken with the participant lying in a supine position. The participant was stabilized with belts and had arms crossed over the chest. The knee being examined was stabilized at 80° flexion on a support and the hip-flexion angle was 80°. The angle between the shin and the foot was 90°.
The IT measurements were performed at 6 shin rotation angles relative to the thigh: at 30°, 20° and 10° ER, in neutral position (NP; a rotation angle of 0°), and at 10° and 25° IR (Figure 2). At each position the participant performed 1 maximal isometric contraction of the internal shin rotator muscles and then, after a 45-second break, the participant performed 1 maximal isometric contraction of the external shin rotator muscles. The duration of a single contraction was 6 seconds and the participants were given verbal encouragement by the experimenter. After the measurements were taken in 1 position, the leg rotation angle was changed. The break between measurement under static conditions and measurement under isokinetic conditions was 20–30 minutes.
Figure 2.
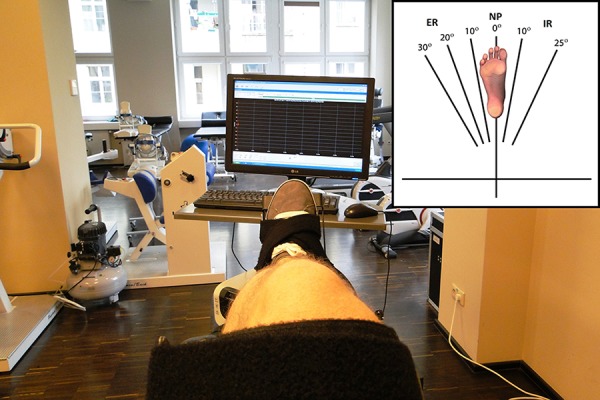
A participant’s leg held in a position of neutral position (NP) of the shin relative to the thigh. The inset diagram shows the 6 different shin rotation angles used for IT measurements.
The PT measurements were taken with an angular velocity of 60°/s (5 repetitions of alternating internal and external shin rotation relative to the thigh at maximal speed) and 180°/s (8 repetitions of alternating internal and external shin rotation relative to the thigh at maximal speed). There was a 90-second break between the exercise sets. The measurements were performed through the full range of motion of each participant.
All participants were tested bilaterally, starting with the uninvolved leg in the ST and STGR groups and with the right leg in the control group.
The test-retest
The test-retest reliability of the IT and PT measurements using the Humac Norm Testing and Rehabilitation System dynamometer was evaluated to validate the use of the dynamometer in clinical research. Twelve male recreational athletes (group T-R) without known cardiovascular or orthopedic problems were recruited to participate in the validation of the IT and PT measurements from the student population of the college where the study was conducted. Their physical characteristics are presented in Table 1. They were instructed to maintain their regular training regimens throughout the experimental period and not to take part in any vigorous physical activity for 2 days prior to the day of the measurement. Testing was conducted in 2 identical sessions held 3 days apart at the same time of day. All measurements were performed by the same researcher. During sessions, torque measurements of the muscles responsible for internal and external shin rotation in the knee were performed under both static and isokinetic conditions [23,30]. Both knees of the group T-R participants were tested, starting with the right knee.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics 20. The mean values (x) and standard deviations (SD) of the measured variables were calculated. For the ST, STGR, and control groups, the IT and PT values were normalized to body mass (BM) and expressed as Nm*kg−1 BM. Data distributions were tested for normality using the Shapiro-Wilk test [31]. Comparisons between the involved legs in the ST and STGR groups and the control group (the right leg and the left leg) were made using the 1-way analysis of variance (ANOVA). Differences were considered significant if p<0.05. Additionally, side-to-side differences in the ST and STGR groups based on mean values were tested with the dependent t-test (paired t-test). Intraclass correlation coefficients (ICC; Shrout and Fleiss model 2) were calculated to compare the data between sessions in the test-retest assessment [32]. The following guidelines, described by Cicchetti and Sparrow [33], were used to assess reliability coefficients: less than 0.40 was considered poor, 0.40 to 0.59 was considered fair, 0.60 to 0.74 was considered good, and 0.75 or greater was considered excellent.
Results
Table 2 shows the normalized IT values of the ST, STGR, and control groups. The highest normalized IT values for IR muscles in the three groups occurred at 30° ER. As the IR angle increased, normalized IT values decreased until they reached their lowest value at 25° IR. The normalized PT values of the muscles responsible for IR and ER of the shin are shown in Table 3. There were no statistically significant differences between the 3 groups of participants.
Table 2.
Normalized isometric torque of the muscles responsible for IR and ER of the shin in the ST, STGR and control groups.
| Normalized isometric torque (Nm·kg−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studied muscle group | Shin rotation angle | Group ST involved leg | Group STGR involved leg | Control group right leg | p | Control group left leg | p | ||||
| x | SD | x | SD | x | SD | x | SD | ||||
| IR muscles | ER 30° | 0.60 | 0.12 | 0.61 | 0.09 | 0.58 | 0.11 | 0.77 | 0.56 | 0.10 | 0.34 |
| ER 20° | 0.54 | 0.11 | 0.54 | 0.08 | 0.51 | 0.10 | 0.66 | 0.48 | 0.09 | 0.13 | |
| ER 10° | 0.47 | 0.10 | 0.47 | 0.08 | 0.45 | 0.09 | 0.22 | 0.43 | 0.09 | 0.34 | |
| NP 0° | 0.42 | 0.09 | 0.42 | 0.08 | 0.39 | 0.07 | 0.49 | 0.37 | 0.07 | 0.19 | |
| IR 10° | 0.37 | 0.11 | 0.35 | 0.07 | 0.34 | 0.09 | 0.95 | 0.32 | 0.07 | 0.10 | |
| IR 25° | 0.26 | 0.08 | 0.26 | 0.07 | 0.24 | 0.07 | 0.41 | 0.24 | 0.06 | 0.46 | |
| ER muscles | ER 30° | 0.35 | 0.07 | 0.30 | 0.11 | 0.30 | 0.07 | 0.13 | 0.30 | 0.08 | 0.12 |
| ER 20° | 0.42 | 0.08 | 0.38 | 0.11 | 0.37 | 0.09 | 0.21 | 0.38 | 0.07 | 0.25 | |
| ER 10° | 0.45 | 0.08 | 0.43 | 0.10 | 0.43 | 0.08 | 0.59 | 0.43 | 0.07 | 0.56 | |
| NP 0° | 0.48 | 0.10 | 0.47 | 0.10 | 0.46 | 0.08 | 0.77 | 0.47 | 0.08 | 0.97 | |
| IR 10° | 0.49 | 0.10 | 0.49 | 0.10 | 0.48 | 0.09 | 0.85 | 0.49 | 0.09 | 0.99 | |
| IR 25° | 0.50 | 0.11 | 0.51 | 0.09 | 0.50 | 0.10 | 0.89 | 0.51 | 0.09 | 0.89 | |
Group ST – patients who underwent ACL reconstruction with a semitendinosus graft; Group STGR – patients who underwent ACL reconstruction with a combined semitendinosus and gracilis graft; IR – internal shin rotation; ER – external shin rotation; NP – neutral position; p – significance level; x – mean; SD – standard deviation.
Table 3.
Normalized PT of the muscles responsible for IR and ER of the shin in the ST, STGR and control groups.
| Normalized peak torque (Nm·kg−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studied muscle group | Angular velocity | Group ST involved leg | Group STGR involved leg | Control group right leg | p | Control group left leg | p | ||||
| x | SD | x | SD | x | SD | x | SD | ||||
| IR muscles | 60°/s | 0.49 | 0.11 | 0.48 | 0.06 | 0.46 | 0.10 | 0.63 | 0.45 | 0.09 | 0.44 |
| 180°/s | 0.41 | 0.09 | 0.39 | 0.39 | 0.39 | 0.08 | 0.26 | 0.39 | 0.07 | 0.80 | |
| ER muscles | 60°/s | 0.47 | 0.07 | 0.46 | 0.46 | 0.46 | 0.07 | 0.78 | 0.45 | 0.08 | 0.78 |
| 180°/s | 0.40 | 0.07 | 0.38 | 0.38 | 0.41 | 0.06 | 0.29 | 0.38 | 0.06 | 0.57 | |
Group ST – patients who underwent ACL reconstruction with a semitendinosus graft; Group STGR – patients who underwent ACL reconstruction with a combined semitendinosus and gracilis graft; IR – internal shin rotation; ER – external shin rotation; p – significance level; x – mean; SD – standard deviation.
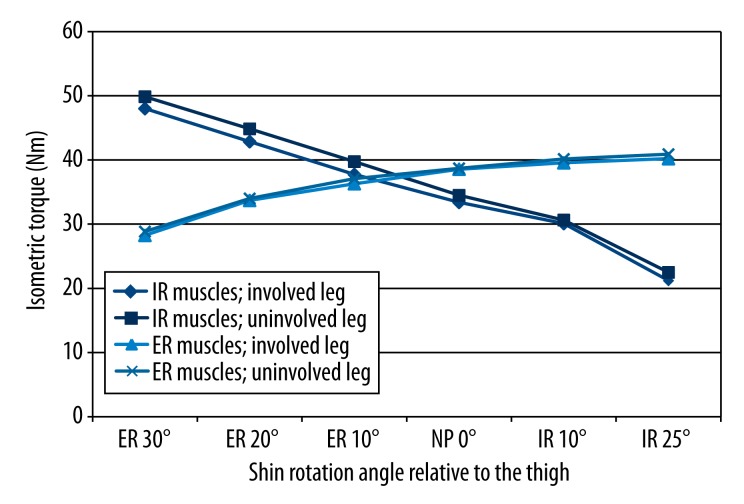
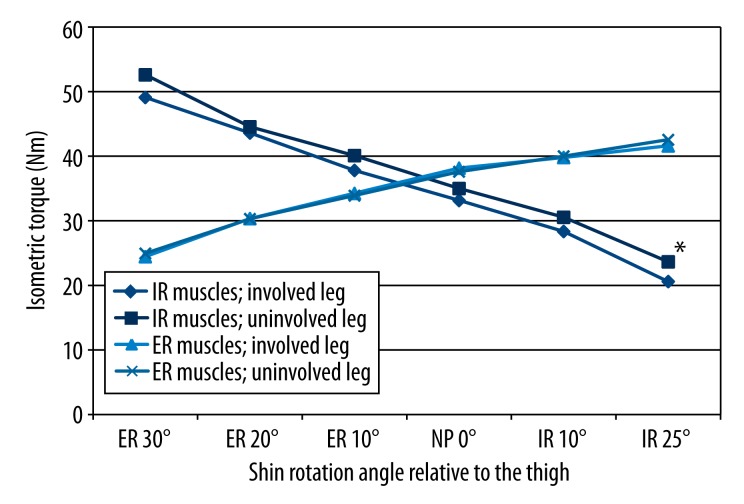
Figures 3 and 4 present side-to-side differences of the IT in the ST group (Figure 3) and STGR group (Figure 4). We found no statistically significant differences in the IT of IR and ER muscles between the involved and uninvolved legs in the ST group. We also found no side-to-side differences in 5 of 6 measurement positions of the shin towards the thigh in the STGR group. Nevertheless, there was a statistically significant difference between the IT of muscles internally rotating the shin in the involved knee and uninvolved knee at 25° of the internal shin rotation (Figure 4).
Figure 3.
Comparison of IT of the muscles responsible for internal rotation and external rotation of the shin obtained from the involved and uninvolved legs in the group of patients who underwent ACL reconstruction with a semitendinosus graft. IR – internal shin rotation; ER – external shin rotation; NP – neutral position; * significance level <0.05.
Figure 4.
Comparison of IT of the muscles responsible for internal rotation and external rotation of the shin obtained from the involved and uninvolved legs in the group of patients who underwent ACL reconstruction with a combined semitendinosus and graft. IR – internal shin rotation; ER – external shin rotation; NP – neutral position; * significance level <0.05.
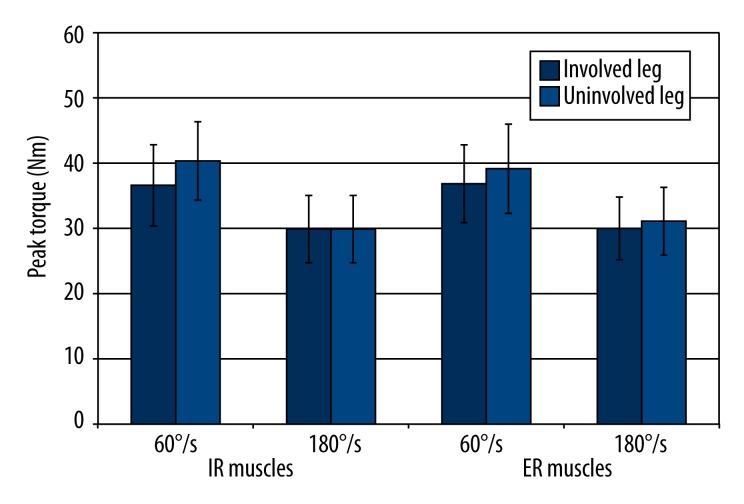
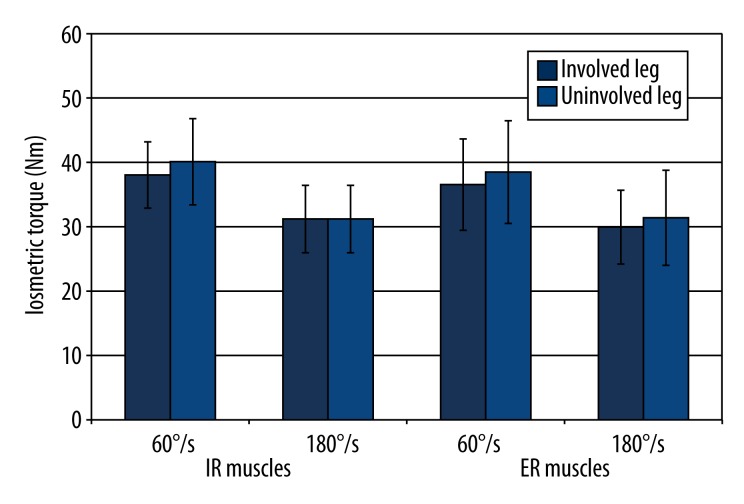
There were no side-to-side differences in the PT in the ST group (Figure 5) and STGR group (Figure 6).
Figure 5.
Comparison of PT of the muscles responsible for internal rotation and external rotation of the shin obtained from the involved and uninvolved legs in the group of patients who underwent ACL reconstruction with a semitendinosus graft. IR – internal shin rotation; ER – external shin rotation; * significance level<0.05.
Figure 6.
Comparison of IT of the muscles responsible for internal rotation and external rotation of the shin obtained from the involved and uninvolved legs in the group of patients who underwent ACL reconstruction with a combined semitendinosus and graft.
Excellent reliability values were obtained in the test-retest analysis (ICC = 0.83–0.95) of IT measurement of the muscles responsible for IR (Table 4). There was also strong agreement between the values obtained in each session for measurement of IT of the muscles responsible for ER (ICC=0.70–0.96; Table 4). The test-retest values for PT measurement also indicated excellent reliability (ICC=0.84–0.97) for the muscles responsible for both IR and ER (Table 5).
Table 4.
Comparison of IT of the muscles responsible for IR and ER of the shin obtained from the right and left legs during two separate measurements (the test-retest).
| Measurement | Isometric torque (Nm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR muscles | ER muscles | |||||||||||||
| Right leg | Left leg | Right leg | Left leg | |||||||||||
| 1st | 2nd | ICC | 1st | 2nd | ICC | 1st | 2nd | ICC | 1st | 2nd | ICC | |||
| Shin rotation angle | ER 30° | x | 52.42 | 52.67 | 0.93 | 45.50 | 48.25 | 0.92 | 24.83 | 25.58 | 0.70 | 24.17 | 27.00 | 0.74 |
| SD | 11.52 | 10.80 | 10.19 | 11.77 | 7.28 | 7.40 | 5.04 | 6.25 | ||||||
| ER 20° | x | 45.42 | 46.42 | 0.95 | 40.42 | 42.58 | 0.90 | 30.58 | 31.83 | 0.93 | 30.58 | 32.08 | 0.75 | |
| SD | 10.84 | 11.53 | 7.89 | 12.75 | 6.88 | 7.28 | 4.50 | 7.23 | ||||||
| ER 10° | x | 40.42 | 40.50 | 0.95 | 34.92 | 37.33 | 0.87 | 35.58 | 34.50 | 0.90 | 33.83 | 35.00 | 0.86 | |
| SD | 10.55 | 11.38 | 8.08 | 11.58 | 7.12 | 8.07 | 4.90 | 7.97 | ||||||
| NP 0° | x | 36.08 | 35.17 | 0.93 | 28.58 | 33.67 | 0.83 | 38.92 | 39.58 | 0.94 | 36.67 | 37.42 | 0.91 | |
| SD | 9.28 | 9.44 | 8.34 | 10.21 | 6.57 | 9.07 | 6.44 | 8.71 | ||||||
| IR 10° | x | 31.00 | 30.42 | 0.94 | 24.42 | 28.50 | 0.87 | 40.08 | 41.33 | 0.76 | 38.42 | 39.17 | 0.92 | |
| SD | 8.10 | 9.43 | 7.43 | 9.41 | 5.81 | 10.26 | 8.35 | 9.27 | ||||||
| IR 25° | x | 22.50 | 22.67 | 0.94 | 18.42 | 21.75 | 0.89 | 43.33 | 42.58 | 0.93 | 39.83 | 40.92 | 0.96 | |
| SD | 7.13 | 8.05 | 6.49 | 8.65 | 8.96 | 11.66 | 10.70 | 11.75 | ||||||
Group T-R – males who participated in the test-retest assessment; x – mean; SD – standard deviation; IR – internal shin rotation; ER – external shin rotation; NP – neutral position; ICC – intraclass correlation coefficient.
Table 5.
Comparison of PT of the muscles responsible for IR and ER of the shin obtained from the right and left legs during two separate measurements (the test-retest).
| Measurement | Peak torque (Nm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR muscles | ER muscles | |||||||||||||
| Right leg | Left leg | Right leg | Left leg | |||||||||||
| 1st | 2nd | ICC | 1st | 2nd | ICC | 1st | 2nd | ICC | 1st | 2nd | ICC | |||
| AV | 60°/s | x | 41.50 | 40.08 | 0.97 | 37.17 | 40.67 | 0.91 | 37.92 | 38.25 | 0.88 | 34.42 | 37.58 | 0.92 |
| SD | 9.72 | 9.39 | 7.30 | 10.63 | 5.60 | 8.11 | 7.20 | 9.02 | ||||||
| 180°/s | x | 33.58 | 34.33 | 0.97 | 29.67 | 31.25 | 0.85 | 32.17 | 33.58 | 0.84 | 29.50 | 31.00 | 0.87 | |
| SD | 8.48 | 8.39 | 6.67 | 6.80 | 4.86 | 6.93 | 5.27 | 6.71 | ||||||
AV – angular velocity; Group T-R – males who participated in the test-retest assessment; x – mean; SD – standard deviation; IR – internal shin rotation; ER – external shin rotation; NP – neutral position; ICC – intraclass correlation coefficient.
Discussion
Our results indicate that after 24 weeks of postoperative rehabilitation there were no significant differences in the IT and PT of the muscles responsible for internal shin rotation between patients who underwent ACL reconstruction using an ST graft and patients who underwent reconstruction using a combined STGR graft. There were also no differences in the IT and PT of the muscles responsible for external shin rotation between the 2 groups of patients. The comparison between the 2 groups of patients and the control group also did not show any statistically significant differences. Nevertheless, in the STGR group there was a side-to-side difference in IT mean value of muscles internally rotating the shin at 1 of the measurement positions of the shin towards the femur (IR 25°).
It is highly important to restore muscle strength after ACL reconstruction, because the motion and stability of the knee are controlled not only by static stabilizers, including ligaments, but also by dynamic stabilizers such as muscles [34]. Most studies that have investigated ST and GR strength after ACL reconstruction have concentrated on knee flexor strength [35]. Czamara and Tomaszewski [14] reported no statistically significant side-to-side differences in the IT of the knee flexors during the 21st week of postoperative physiotherapy, and in PT during the 24th week of postoperative physiotherapy after ACL reconstruction using a hamstring graft. Lipscomb and Johnston [36] reported no significant hamstring weakness, as indicated by mean PT values, 2 years after ACL reconstruction using an STGR graft. Simonian and Harrison [37] evaluated the effect of harvesting both the ST and GR tendons for ACL reconstruction on knee function, and knee extension and flexion strength, over a minimum 3-year follow-up period. Their results suggest that harvesting both the ST and GR tendons does not significantly reduce the function and strength of the operated knee. In contrast, Coombs and Cochrane [18] reported a deficit in knee flexor strength lasting at least 12 months post-surgery after ACL reconstruction with a combined ST and GR tendon graft, even after a full rehabilitation protocol was followed. Other studies have also shown a significant side-to-side difference in flexor muscle strength after ACL reconstruction with the use of hamstring tendon grafts [27,38]. Ardern and Webster [39] showed hamstring strength deficits persisted for a mean of 32.5 months after ACL reconstruction, despite completion of a rehabilitation program. However, they did not find significant differences between the STGR and ST groups in any of the measures used in their study (IT and PT of knee flexors and standing knee flexion angle). Inagaki et al. (2013), based on an evaluation of knee stability and clinical outcome, found no differences between the ST and STGR groups 2 years after ACLR reconstruction [40]. Yosmaoglu and Baltaci [41] compared the results of a multi-joint lower-limb tracking-trajectory test, the PT of knee extensors and flexors at 60°/s and 180°/s, and anterior tibial translation in patients who received ST and STGR grafts 12 months after ACL reconstruction. They found that the side-to-side differences in flexor peak torque at 60°/s were significantly higher in the STGR group than in the ST group. The other side-to-side differences did not significantly differ, suggesting that preservation of the GR might improve postoperative athletic function [41].
The ST and GR muscles also contribute to internal shin rotation; thus, it has been suggested that harvest of these tendons can result in internal shin rotation weakness. Although GR is not truly a hamstring muscle, and is considered to be a muscles of the medial compartment of the thigh, which is a muscle that flexes and medially rotates the shin at the knee [42]. Interestingly, Ahlen and Liden [43], evaluating a group of patients with a mean time since ACL reconstruction (STGR) of 8.5 years, demonstrated significant side-to-side differences in flexion PT but no significant PT deficit in internal shin rotation. Because of significant weakness in deep knee flexion, the authors suggested avoiding STGR autografts for athletes who depend on strength in deep flexion [43]. Other authors have reported an association between lack of weakness in the muscles responsible for internal shin rotation and completion of a systematic physiotherapy program [23]. The internal shin rotation torques under static and isokinetic conditions in the operated legs of patients 21 weeks after ACL reconstruction with an STGR graft were similar to the values obtained from their uninvolved legs, and to the results from healthy participants; however, these values were significantly higher than those obtained from males who did not participate in any systematic physiotherapy program after ACL reconstruction [23]. The results that we obtained for external shin rotation torque were similar to those reported by Viola et al.; they showed no statistically significant differences in PT between reconstructed and contralateral knees at any angular velocities [20]. The IT of the muscles responsible for external rotation also did not significantly differ between limbs in our study. However, our analysis of side-to-side differences in the PT of internal shin rotation differed from the findings of Viola and Sterett [20]; we obtained similar values in each leg. Our results also differed from those of Segawa and Omori [21], who noted that the internal shin rotation PT of the involved limb was decreased in the STGR group, but not in the ST group at 1 year after surgery. Thus, they recommended harvesting the ST tendon only to minimize harvesting morbidity [21]. The side-to-side differences in PT reported in the current study were lower than the deficits in internal rotation strength that were found in the involved extremity as compared with the uninvolved side by Armour and Forwell [24] 2 years after ACL reconstruction using STGR grafts. Nevertheless, our analysis in the STGR group showed a side-to-side difference in IT values of muscles internally rotating the shin at one of the angular positions of the shin towards the femur (IR 25°). This indicates that 24 weeks of physiotherapy may not be enough to correct between-limb differences in internal shin rotation strength, and the physiotherapy program may need to be extended in this group of patients because the loss of muscle strength at deep internal rotation and flexion angles can influence performance in various sports activities. The possibility of increased risk of reinjury due to internal shin rotational weakness, and that internal shin rotation deficits could be clinically significant in sports requiring shin rotation, has also been discussed by Armour and Forwell [24].
Information about the reliability of a measurement when using a specified test protocol in a given participant or patient group is needed for these measurements to be useful [44–46]. A high degree of reliability allows us to evaluate whether the change in torque output that occurs following physical therapy or a clinical intervention is a true change. The protocols that we used to measure internal and external shin rotation torque under static and isokinetic conditions have very high reliability. The test-retest performed under isokinetic conditions confirmed the results of other studies
This study evaluated the IT and PT of the muscles responsible for internal shin rotation in patients that had undergone ACL reconstruction with or without GR tendon harvesting. The results showed no differences in IT and PT between the groups of patients and the control group. However, at deep internal rotation, side-to-side differences in IT of muscles internally rotating the shin occurred in STGR group. This suggests that rehabilitation in a group of patients after ACLR with ipsilateral autologous ST tendon graft with additional harvest of GR tendon should focus on muscle strengthening in deep internal rotation and flexion. On the other hand, it might suggest harvesting of only ST tendon whenever possible.
Conclusions
Comparison of IT and PT measurements performed after 24 weeks of postoperative rehabilitation generally showed no differences between patients who underwent ACL reconstruction with ipsilateral hamstring autograft with the use of semitendinosus tendon graft and patients who received a combination graft consisting of semitendinosus and gracilis tendons. Nevertheless, there was an influence of gracilis tendon harvest on internal shin rotation isometric torque at deep internal rotation angle.
Footnotes
Source of support: Departmental sources
Statement
The study was conducted at the Centre of Rehabilitation and Medical Education in Wroclaw, Poland for the Department of Physiotherapy of the College of Physiotherapy in Wroclaw, Poland.
There is no conflict of interest because all of the expenses were covered by the College of Physiotherapy in Wroclaw, Poland and Andrzej Czamara.
References
- 1.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33(4):621–36. v. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 2.Fithian DC, Paxton EW, Stone ML, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33(3):335–46. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 3.Zaffagnini S, Marcacci M, Lo Presti M, et al. Prospective and randomized evaluation of ACL reconstruction with three techniques: a clinical and radiographic evaluation at 5 years follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1060–69. doi: 10.1007/s00167-006-0130-x. [DOI] [PubMed] [Google Scholar]
- 4.Marumo K, Saito M, Yamagishi T, Fujii K, et al. The “ligamentization” process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: a biochemical study. Am J Sports Med. 2005;33(8):1166–73. doi: 10.1177/0363546504271973. [DOI] [PubMed] [Google Scholar]
- 5.Goradia VK, Rochat MC, Kida M, Grana WA, et al. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40–46. doi: 10.1177/03635465000280011901. [DOI] [PubMed] [Google Scholar]
- 6.Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2010;40(3):141–54. doi: 10.2519/jospt.2010.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myer GD, Schmitt LC, Brent JL, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377–87. doi: 10.2519/jospt.2011.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–59. doi: 10.2519/jospt.2012.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentz TA, Zeppieri G, Jr, Tillman SM, et al. Return to preinjury sports participation following anterior cruciate ligament reconstruction: contributions of demographic, knee impairment, and self-report measures. J Orthop Sports Phys Ther. 2012;42(11):893–901. doi: 10.2519/jospt.2012.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czamara A. Functional benchmarking of rehabilitation outcomes following anterior cruciate ligament reconstruction. Ortop Traumatol Rehabil. 2010;12(6):519–33. [PubMed] [Google Scholar]
- 11.Kondo E, Yasuda K, Katsura T, et al. Biomechanical and histological evaluations of the doubled semitendinosus tendon autograft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2012;40(2):315–24. doi: 10.1177/0363546511426417. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH. Graft healing in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rehabil Ther Technol. 2009;1(1):21. doi: 10.1186/1758-2555-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen RP, van der Wijk J, Fiedler A, et al. Remodelling of human hamstring autografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1299–306. doi: 10.1007/s00167-011-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czamara A, Tomaszewski W, Bober T, Lubarski B. The effect of physiotherapy on knee joint extensor and flexor muscle strength after anterior cruciate ligament reconstruction using hamstring tendon. Med Sci Monit. 2011;17(1):CR35–41. doi: 10.12659/MSM.881327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkoshi Y, Inoue C, Yamane S, et al. Changes in muscle strength properties caused by harvesting of autogenous semitendinosus tendon for reconstruction of contralateral anterior cruciate ligament. Arthroscopy. 1998;14(6):580–84. doi: 10.1016/s0749-8063(98)70053-2. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda K, Tsujino J, Ohkoshi Y, et al. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am J Sports Med. 1995;23(6):706–14. doi: 10.1177/036354659502300613. [DOI] [PubMed] [Google Scholar]
- 17.Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478–84. doi: 10.1177/036354659101900510. [DOI] [PubMed] [Google Scholar]
- 18.Coombs R, Cochrane T. Knee flexor strength following anterior cruciate ligament reconstruction with the semitendinosus and gracilis tendons. Int J Sports Med. 2001;22(8):618–22. doi: 10.1055/s-2001-18528. [DOI] [PubMed] [Google Scholar]
- 19.Barenius B, Webster WK, McClelland J, Feller J. Hamstring tendon anterior cruciate ligament reconstruction: does gracilis tendon harvest matter? Int Orthop. 2013;37(2):207–12. doi: 10.1007/s00264-012-1672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viola RW, Sterett WI, Newfield D, et al. Internal and external tibial rotation strength after anterior cruciate ligament reconstruction using ipsilateral semitendinosus and gracilis tendon autografts. Am J Sports Med. 2000;28(4):552–55. doi: 10.1177/03635465000280041801. [DOI] [PubMed] [Google Scholar]
- 21.Segawa H, Omori G, Koga Y, et al. Rotational muscle strength of the limb after anterior cruciate ligament reconstruction using semitendinosus and gracilis tendon. Arthroscopy. 2002;18(2):177–82. doi: 10.1053/jars.2002.29894. [DOI] [PubMed] [Google Scholar]
- 22.Hrycyna M, Zielinski J. Torque of the shank rotating muscles in patients with knee joint injuries. Acta Bioeng Biomech. 2011;13(4):77–83. [PubMed] [Google Scholar]
- 23.Czamara A, Szuba Ł, Krzemińska A, et al. Effect of physiotherapy on the strength of tibial internal rotator muscles in males after anterior cruciate ligament reconstruction (ACLR) Med Sci Monit. 2011;17(9):CR523–31. doi: 10.12659/MSM.881940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armour T, Forwell L, Litchfield R, et al. Isokinetic evaluation of internal/external tibial rotation strength after the use of hamstring tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(7):1639–43. doi: 10.1177/0363546504263405. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti A, Vadalà A, De Carli A, et al. Minimizing internal rotation strength deficit after use of semitendinosus for anterior cruciate ligament reconstruction: a modified harvesting technique. Arthroscopy. 2008;24(7):786–95. doi: 10.1016/j.arthro.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Adachi N, Ochi M, Uchio Y, et al. Harvesting hamstring tendons for ACL reconstruction influences postoperative hamstring muscle performance. Arch Orthop Trauma Surg. 2003;123(9):460–65. doi: 10.1007/s00402-003-0572-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura N, Horibe S, Sasaki S, et al. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2002;18(6):598–602. doi: 10.1053/jars.2002.32868. [DOI] [PubMed] [Google Scholar]
- 28.Gobbi A, Domzalski M, Pascual J, Zanazzo M. Hamstring anterior cruciate ligament reconstruction: is it necessary to sacrifice the gracilis? Arthroscopy. 2005;21(3):275–80. doi: 10.1016/j.arthro.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro T, Kurosawa H, Kawakami A, et al. Influence of medial hamstring tendon harvest on knee flexor strength after anterior cruciate ligament reconstruction. A detailed evaluation with comparison of single- and double-tendon harvest. Am J Sports Med. 2003;31(4):522–29. doi: 10.1177/31.4.522. [DOI] [PubMed] [Google Scholar]
- 30.Czamara A, Królikowska A, Szuba Ł, et al. Single- versus double-bundle anterior cruciate ligament reconstruction: a new aspect of knee assessment during activities involving dynamic knee rotation. J Strength Cond Res. 2015;29(2):489–99. doi: 10.1519/JSC.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 31.Royston P, Remark AS. R94: A Remark on Algorithm AS 181: The W-test for Normality. Journal of the Royal Statistical Society Series C (Applied Statistics) 1995;44(4):547–51. [Google Scholar]
- 32.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–28. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 33.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86(2):127–37. [PubMed] [Google Scholar]
- 34.Insall JN, Scott WN. Insall and Scott surgery of the knee. Churchill Livingstone/Elsevier; 2006. [Google Scholar]
- 35.Buchner M, Schmeer T, Schmitt H. Anterior cruciate ligament reconstruction with quadrupled semitendinosus tendon – minimum 6 year clinical and radiological follow-up. Knee. 2007;14(4):321–27. doi: 10.1016/j.knee.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Lipscomb AB, Johnston RK, Snyder RB, et al. Evaluation of hamstring strength following use of semitendinosus and gracilis tendons to reconstruct the anterior cruciate ligament. Am J Sports Med. 1982;10(6):340–42. doi: 10.1177/036354658201000603. [DOI] [PubMed] [Google Scholar]
- 37.Simonian PT, Harrison SD, Cooley VJ, et al. Assessment of morbidity of semitendinosus and gracilis tendon harvest for ACL reconstruction. Am J Knee Surg. 1997;10(2):54–59. [PubMed] [Google Scholar]
- 38.Aune AK, Holm I, Risberg MA, et al. Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction. A randomized study with two-year follow-up. Am J Sports Med. 2001;29(6):722–28. doi: 10.1177/03635465010290060901. [DOI] [PubMed] [Google Scholar]
- 39.Ardern CL, Webster KE, Taylor NF, Feller JA. Hamstring strength recovery after hamstring tendon harvest for anterior cruciate ligament reconstruction: a comparison between graft types. Arthroscopy. 2010;26(4):462–69. doi: 10.1016/j.arthro.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Inagaki Y, Kondo E, Kitamura N, et al. Prospective clinical comparisons of semitendinosus versus semitendinosus and gracilis tendon autografts for anatomic double-bundle anterior cruciate ligament reconstruction. J Orthop Sci. 2013;18(5):754–61. doi: 10.1007/s00776-013-0427-9. [DOI] [PubMed] [Google Scholar]
- 41.Yosmaoglu HB, Baltaci G, Ozer H, Atay A. Effects of additional gracilis tendon harvest on muscle torque, motor coordination, and knee laxity in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1287–92. doi: 10.1007/s00167-011-1412-5. [DOI] [PubMed] [Google Scholar]
- 42.Palastanga N, Field D, Soames R. Anatomy and human movement: structure and function. Butterworth-Heinemann; 2002. [Google Scholar]
- 43.Åhlén M, Lidén M, Bovaller Å, et al. Bilateral magnetic resonance imaging and functional assessment of the semitendinosus and gracilis tendons a minimum of 6 years after ipsilateral harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(8):1735–41. doi: 10.1177/0363546512449611. [DOI] [PubMed] [Google Scholar]
- 44.Mackenzie SJ, Evans DB. Validity and reliability of a new method for measuring putting stroke kinematics using the TOMI® system. J Sports Sci. 2010;28(8):891–99. doi: 10.1080/02640411003792711. [DOI] [PubMed] [Google Scholar]
- 45.Boddington MK, Lambert MI, St Clair Gibson A, Noakes TD. Reliability of a 5-m multiple shuttle test. J Sports Sci. 2001;19(3):223–28. doi: 10.1080/026404101750095394. [DOI] [PubMed] [Google Scholar]
- 46.Rota S, Rogowski I, Champely S, Hautier C. Reliability of EMG normalisation methods for upper-limb muscles. J Sports Sci. 2013;31(15):1696–704. doi: 10.1080/02640414.2013.796063. [DOI] [PubMed] [Google Scholar]