Abstract
Purpose
This study aimed to compare dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with T2-weighted imaging (T2WI) for the preoperative staging of early endometrial carcinoma.
Methods
This retrospective study included 22 subjects with early endometrial carcinoma who underwent 3.0 T MRI examination prior to hysterectomy. DCE-MRI and T2WI were evaluated for the preoperative staging of endometrial carcinoma. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of DCE-MRI and T2WI were assessed and compared using the revised International Federation of Gynecology and Obstetrics surgical staging guidelines (2009) as the reference standard.
Results
Out of the 22 cases of endometrial carcinoma, the use of the reference standard method led to the staging of 14 as IA and eight as IB. The sensitivity, specificity, PPV, NPV, and accuracy of DCE-MRI for preoperative staging were 100% (95% confidence interval: 0.73–1.0), 62.5% (95% CI: 0.26–0.90), 82.4% (95% CI: 0.56–0.95), 100% (95% CI: 0.46–1.0), and 86.4%, respectively, and these values were 85.7% (95% CI: 0.56–0.97), 75% (95% CI: 0.36–0.96), 85.7% (95% CI: 0.56–0.97), 75% (95% CI: 0.36–0.96), and 81.8%, respectively, for T2WI. Thus, the sensitivity and accuracy of DCE-MRI were greater than those of T2WI for preoperative endometrial carcinoma staging.
Conclusion
DCE-MRI was more sensitive but less specific than T2WI for the preoperative staging of early endometrial carcinoma. DCE-MRI may serve as a useful and reliable tool for the preoperative assessment of endometrial carcinoma.
Keywords: endometrial carcinoma, dynamic contrast-enhanced magnetic resonance imaging, T2-weighted imaging, preoperative staging
Introduction
Endometrial carcinoma is the most common gynecological cancer in the US and the fourth most common malignancy among females worldwide.1,2 The stage of endometrial carcinoma is closely related to treatment planning and patient survival and prognosis.3–6 Presurgical staging helps to determine the extent of surgery, such as lymphadenectomy, that should be performed. For example, patients with stage IA endometrial carcinoma display nodal metastases in less than 1% of cases, and these patients require only simple hysterectomy. However, patients with endometrial carcinoma that has advanced beyond stage IB require pelvic lymph node dissection and other therapies.7,8 Additionally, studies have demonstrated that the overall 5-year survival rate of endometrial carcinoma dramatically increases from 20% to 90% with decreasing cancer stage.3,4 However, the traditional and reliable staging of endometrial carcinoma relies on the surgical staging system, which was first used in 1988 and was revised in 2009. Surgical staging is performed after an operation; thus, this system cannot provide useful information prior to surgery.4,9 Therefore, there is a clinical need for noninvasive imaging modalities that can provide accurate preoperative staging information for endometrial carcinoma.
Magnetic resonance imaging (MRI) has been used as an imaging modality for endometrial carcinoma for several decades.4,10–15 Due to its unique characteristics of noninvasiveness, high soft tissue resolution, and multiple imaging parameters, MRI noninvasively provides anatomical and functional information about endometrial carcinoma and is playing an increasing role in clinical decisions regarding endometrial carcinoma.4,10–15 T2-weighted imaging (T2WI) can be used to identify the depth of myometrial invasion, and diffusion-weighted MRI (DWI) differentiates between benign and malignant or metastatic lesions. In addition, T2WI with or without contrast-enhanced MRI (CE-MRI) can be used to stage endometrial carcinoma.4,10–17 Although clinicians and radiologists currently agree that MRI is superior to transvaginal sonography and computed tomography,18–20 no consensus has been achieved regarding the optimal MRI protocols for staging endometrial carcinoma. Several studies using different MRI protocols, including unenhanced MRI, CE-MRI, and dynamic CE-MRI (DCE-MRI), have reported that the accuracy, sensitivity, and specificity for myometrial invasion range from 47% to 100%, from 33% to 100%, and from 50% to 100%, respectively.4,21–23 Therefore, the depth of myometrial invasion (ie, the cancer stage) strongly correlates with metastasis and prognosis; thus, determining the optimal MRI protocol is of critical importance for assessing the endometrial cancer stage.
This study aimed to compare DCE-MRI and T2WI for the preoperative staging of early endometrial carcinoma.
Materials and methods
The institutional review board approved this retrospective study and waived the requirement for informed consent.
Patient selection
From March 2007 to December 2009, 31 consecutive subjects underwent hysterectomy for the treatment of early endometrial carcinoma, and histopathological diagnoses were established for the tumors at our hospital. All of these subjects received pelvic MRI, including conventional MRI (T1-weighted imaging [T1WI] or T2WI) and DCE-MRI, prior to hysterectomy. Seven out of the 31 subjects were excluded due to missing T2WI, DCE-MRI, or pathological data. In addition, two of the remaining 24 subjects were excluded due to the lack of positive findings on MRI. Finally, the remaining 22 patients (mean age, 60.1 years; range, 37–81 years), including 16 postmenopausal women (mean age, 63.2 years; range, 49–81 years) and six premenopausal women (mean age, 51.8 years; range, 37–58 years), were included in this study (Table 1).
Table 1.
Summary of the characteristics of the 22 subjects
| Case number | Age (years) | Menopausal status | Concomitant disease | Histopathological results
|
MRI findings
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2WI
|
DCE-MRI
|
|
|||||||||||
| Subtype | Stagea | Signal | Stagea | Signal | Stagea | Detectable phase
|
|||||||
| EAP | LAP | PP | DP | ||||||||||
| 1 | 57 | Postmenopausal | Fibroids | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | + | + |
| 2 | 71 | Postmenopausal | N/A | Adenosquamous carcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | ||
| 3 | 72 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | ||
| 4 | 53 | Postmenopausal | N/A | Clear cell carcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | + | + |
| 5 | 63 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | ||
| 6 | 49 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | + | |
| 7 | 64 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Iso-intense | IB | Hypo-intense | IA | + | + | ||
| 8 | 60 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Mildly hyper-intense | IA | Hypo-intense | IA | + | |||
| 9 | 52 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | + | |
| 10 | 37 | Premenopausal | N/A | Endometrioid adenocarcinoma | IA | Mildly hyper-intense | IA | Hypo-intense | IA | + | |||
| 11 | 52 | Premenopausal | Endometriosis | Clear cell carcinoma | IA | Mildly hyper-intense | IB | Hypo-intense | IA | + | + | ||
| 12 | 54 | Premenopausal | Right ovarian tumor | Endometrioid adenocarcinoma | IA | Iso-intense | IA | Hypo-intense | IA | + | + | + | |
| 13 | 55 | Premenopausal | Fibroids, cervical cancer | Endometrioid adenocarcinoma | IA | Mildly hyper-intense | IA | Hypo-intense | IA | + | + | ||
| 14 | 58 | Premenopausal | Adenomyosis | Endometrioid adenocarcinoma | IA | Mildly hyper-intense | IA | Hypo-intense | IA | + | + | ||
| Total | 14 | Total | 2 | 5 | 12 | 14 | |||||||
| 15 | 57 | Postmenopausal | Fibroids | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IB | Hypo-intense | IA | + | + | + | |
| 16 | 58 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IB | Hypo-intense | IB | + | + | + | |
| 17 | 68 | Postmenopausal | Fibroids | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IA | Hypo-intense | IA | + | + | + | + |
| 18 | 66 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IB | Iso-intense | IB | Hypo-intense | IB | + | + | + | |
| 19 | 64 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IB | Hypo-intense | IA | + | + | ||
| 20 | 76 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IA | Hypo-intense | IB | + | + | + | |
| 21 | 81 | Postmenopausal | N/A | Endometrioid adenocarcinoma | IB | Iso-intense | IB | Hypo-intense | IB | + | + | + | + |
| 22 | 55 | Premenopausal | N/A | Endometrioid adenocarcinoma | IB | Mildly hyper-intense | IB | Hypo-intense | IB | + | + | + | |
| Total | 8 | Total | 2 | 7 | 8 | 8 | |||||||
Note:
Revised International Federation of Gynecology and Obstetrics surgical staging guidelines (2009).4,9,25
Abbreviations: MRI, magnetic resonance imaging; T2WI, T2-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; EAP, early arterial phase; LAP, late arterial phase; PP, parenchymal phase; DP, delayed phase; +, detectable; N/A, not applicable.
MRI protocol
All MRI studies were performed using a 3.0 T magnet (Signa Excite HD 3.0 T System, GE Medical Systems, Milwaukee, WI, USA) equipped with an eight-channel HD cardiac coil. The basic MRI protocol included the following sequences: a) two-dimensional (2D) axial T1WI fast spin-echo (FSE), b) 2D T2WI FSE, and c) breath-hold three-dimensional fat-suppressed gadolinium-enhanced DCE-MRI with ultra-fast spoiled gradient-echo (liver acquisition with volume acquisition [LAVA]).15,24 The parameters used in the MRI protocol are summarized in Table 2. DCE-MRI scans were acquired after an intravenous bolus injection of 0.1 mmol/kg gadolinium-diethylenetriamine pentaacetic acid (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) using an automatic power injector at a 4 mL/s flow rate followed by a flush with 30 mL of 0.9% sterile saline solution. The LAVA imaging delays for the early arterial phase, late arterial phase, parenchymal phase, and delayed phase were 15 seconds, 30 seconds, 60 seconds, and 90 seconds, respectively, after arrival of the contrast agent in the iliac artery.
Table 2.
Summary of the parameters used in the MRI protocol
| Parameter | T1WI | T2WI | DCE-MRI |
|---|---|---|---|
| Sequence | 2D FSE | 2D FSE | 3D GRE (LAVA) |
| Acquisition plane | Axial | Axial, sagittal | Axial, sagittal |
| TR/TE (ms) | 440/7 | 3,060/137 | 3.8/1.7 |
| FOV (cm2) | 30×24 | 30×24 | 30×27 |
| Matrix | 384×224 | 384×224 | 288×256 |
| FA | 15° | 90° | 15° |
| Slice thickness (mm) | 5 | 5 | 3 |
| Intersection gap (mm) | 1 | 1 | 1 |
| Bandwidth (kHz/pixel) | 244.1 | 244.1 | 244.1 |
| NEX | 2 | 2 | 0.75 |
Abbreviations: MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; 2D, two-dimensional; FSE, fast spin-echo; 3D, three-dimensional; GRE, gradient-echo; LAVA, liver acquisition with volume acquisition; TR, repetition time; TE, echo time; FOV, field of view; FA, flip angle; NEX, number of acquisitions.
Image analysis
The magnetic resonance (MR) images were interpreted by two senior MR radiologists (with 15 years and 20 years of experience in MRI) who were blinded to the pathologic findings. First, each radiologist evaluated the neoplasm location, size, contour, signal intensity, and enhancement characteristics on T1WI, T2WI, and DCE-MRI. Next, 10 days after the first evaluation, we divided the T2WI and DCE-MRI scans into two separate files to which each reading radiologist was blinded. Each reader was initially randomly assigned to evaluate the cancer stage using either the T2WI or DCE-MRI data, and then, he or she examined the data from the other imaging modality. For the T2WI or DCE-MRI scans, identical staging criteria were used to classify the tumor stage as IA (<50% myometrial invasion) or IB (≥50% myometrial invasion), according to the revised International Federation of Gynecology and Obstetrics (FIGO) surgical staging guidelines (2009).4,9,25 For either T2WI- or DCE-MRI-based staging, if the two readers agreed on the stage for the same set of images for the same subject, the result was recorded as the final stage. If the two readers reported discrepant findings, a consensus among the readers was achieved by interpreting the images together at a separate time point, and the consensus result was recorded as the final stage.
Reference standard
All tumor samples were stained with hematoxylin and eosin. All histopathological characteristics of the endometrial carcinoma samples were assessed and staged by two experienced pathologists in gynecological oncology (with 20 years and 6 years of experience) according to the revised FIGO surgical staging guidelines (2009).9
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of DCE-MRI and T2WI were calculated. Kappa statistics were measured to assess the agreement between T2WI- or DCE-MRI-based staging and pathology-based staging according to previous literature.12 All analyses were conducted using a web-based statistical tool (http://vassarstats.net/).
Results
Out of the 22 endometrial carcinoma patients, seven (7/22) presented with one or two concomitant diseases (three [3/22] with uterine fibroids, one [1/22] with endometriosis, one [1/22] with a right ovarian tumor, one [1/22] with uterine fibroids and cervical cancer, and one [1/22] with adenomyosis). The histopathological results indicated that 19 patients (19/22) had endometrioid adenocarcinoma, one (1/22) had adenosquamous carcinoma, and two (2/22) had clear cell carcinoma (Table 1). Using the revised FIGO surgical staging guideline (2009) reference standard, histology indicated that 14 patients (14/22) had stage IA cancer, and eight (8/22) had stage IB cancer (Tables 1 and 3).
Table 3.
Comparisons of DCE-MRI- and T2WI-based staging with histopathological findings according to the revised FIGO surgical staging guidelines (2009) (n=22)
| MRI findings | Histopathological results based on FIGO
|
Total | |
|---|---|---|---|
| IA | IB | ||
| T2WI | |||
| IA | 12 | 2 | 14 |
| IB | 2 | 6 | 8 |
| Total | 14 | 8 | 22 |
| DCE-MRI | |||
| IA | 14 | 3 | 17 |
| IB | 0 | 5 | 5 |
| Total | 14 | 8 | 22 |
Abbreviations: DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; T2WI, T2-weighted imaging; FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging.
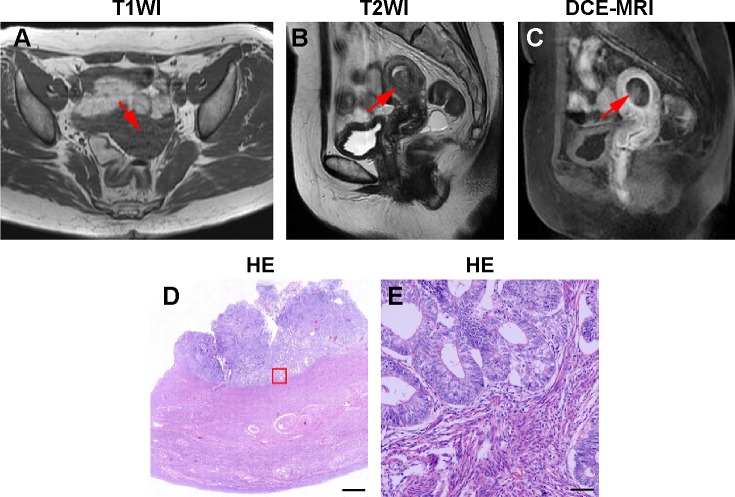
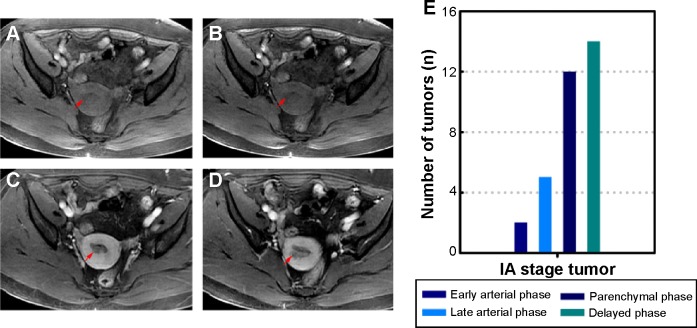
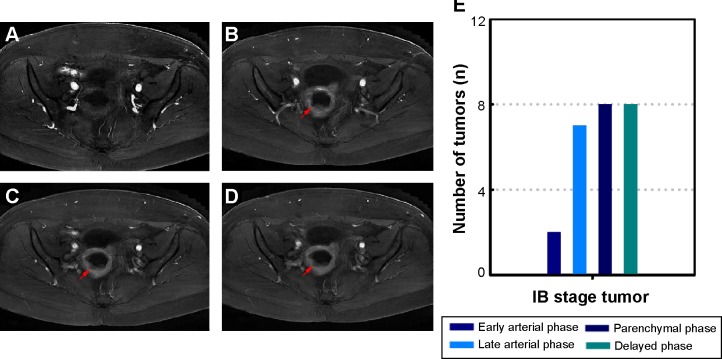
Figures 1 and 2 depict representative MR and pathological images from one stage IA patient and one stage IB patient. The stage IA tumors presented as iso-intense on T1WI compared to the myometrium (Figure 1A) and as iso- or mildly hyper-intense on T2WI compared to the myometrium (Figure 1B). On DCE-MRI, the stage IA tumors appeared as a hypo-intense mass compared to the adjacent myometrium (Figure 1C). The stage IB tumors presented as iso-intense on T1WI compared to the myometrium (Zheng et al, unpublished data, 2015) and as iso- or mildly hyper-intense on T2WI compared to the myometrium (Figure 2A). On DCE-MRI, the stage IB tumors appeared as a hypo-intense mass compared to the adjacent myometrium (Figure 2B). Representative images of the stage IA and IB tumor masses on different phases of DCE-MRI are displayed in Figures 3A–D and 4A–D, respectively. We detected two, five, 12, and 14 stage IA tumors (Table 1 and Figure 3E) and two, seven, eight, and eight stage IB tumors on early arterial phase, late arterial phase, parenchymal phase, and delayed phase DCE-MRI, respectively (Table 1 and Figure 4E).
Figure 1.
Representative MR and histopathological images of stage IA EC.
Notes: (A) Axial T1WI of stage IA EC. (B) Sagittal T2WI of stage IA EC. (C) Sagittal DCE-MRI of stage IA EC. (D) Hematoxylin and eosin (HE) staining of stage IA EC. (E) Magnified image of the box shown in (D). The arrows in (A–C) indicate the tumor. The scale bar is 1,000 µm in (D) and 60 µm in (E).
Abbreviations: MR, magnetic resonance; EC, endometrial cancer; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging.
Figure 2.
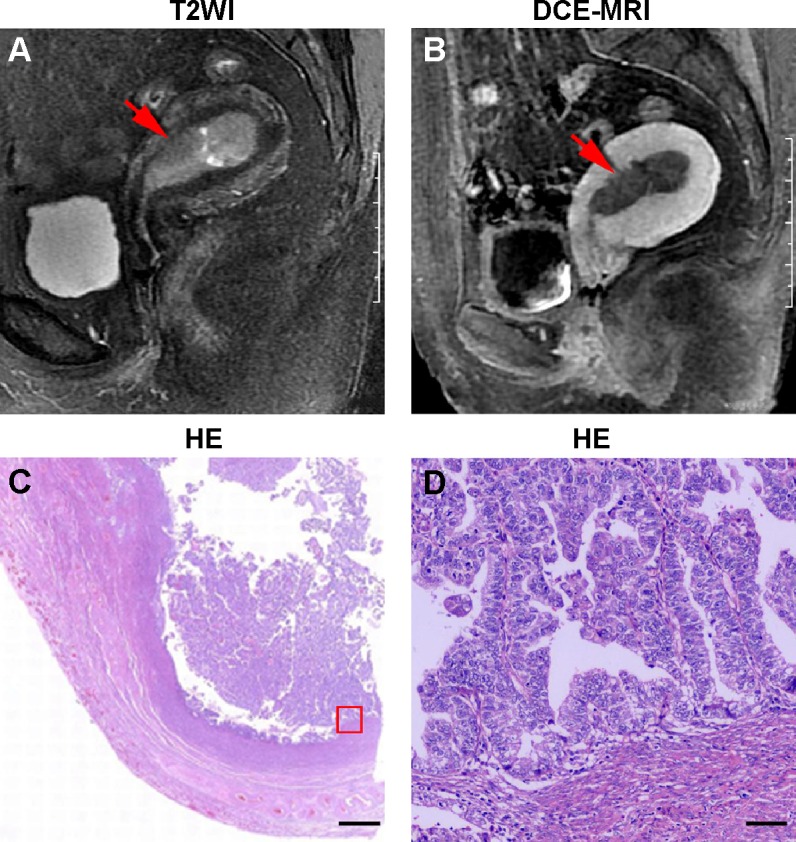
Representative MR and histopathological images of stage IB EC.
Notes: (A) Sagittal T2WI of stage IB EC. (B) Sagittal DCE-MRI of stage IB EC. (C) Hematoxylin and eosin (HE) staining of stage IB EC. (D) Magnified image of the box shown in (C). The arrows in (A and B) indicate the tumor. The scale bar is 1,000 µm in (C) and 60 µm in (D).
Abbreviations: MR, magnetic resonance; EC, endometrial cancer; T2WI, T2-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging.
Figure 3.
Representative four-phase DCE-MRI of detectable stage IA tumors.
Notes: (A) Early arterial phase MRI. (B) Late arterial phase MRI. (C) Parenchymal phase MRI. (D) Delayed phase MRI. The images reveal a mass with relative hypo-intensity compared with the myometrium. The arrows in (A–D) indicate the tumor. (E) The number of detectable tumors at different phases.
Abbreviations: DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; MRI, magnetic resonance imaging.
Figure 4.
Representative four-phase DCE-MRI of detectable stage IB tumors.
Notes: (A) Early arterial phase MRI. (B) Late arterial phase MRI. (C) Parenchymal phase MRI. (D) Delayed phase MRI. The tumor was clearly detected in the parenchymal and delayed phases and was observed as relative hypo-intensity compared to the myometrium. The arrows in (B–D) indicate the tumor. (E) The number of detectable tumors at different phases.
Abbreviations: DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; MRI, magnetic resonance imaging.
In all the 14 (14/14) stage IA patients, the staging based on DCE-MRI and histopathology was equivalent. Among the stage IB patients, five out of eight (5/8) tumors were correctly staged by DCE-MRI according to histopathology (Tables 1 and 3). When DCE-MRI was used to preoperatively stage endometrial carcinoma, the sensitivity, specificity, PPV, NPV, and accuracy were 100% (95% confidence interval: 0.73–1.0), 62.5% (0.26–0.90), 82.4% (0.56–0.95), 100% (0.46–1.0), and 86.4%, respectively, with a kappa of 0.68 (Table 4). For preoperative staging of endometrial carcinoma using T2WI, 12 out of the 14 stage IA tumors and six out of the eight stage IB tumors were correctly staged (Tables 1 and 3). The sensitivity, specificity, PPV, NPV, and accuracy of T2WI for endometrial carcinoma staging were 85.7% (0.56–0.97), 75% (0.36–0.96), 85.7% (0.56–0.97), 75% (0.36–0.96), and 81.8%, respectively, with a kappa of 0.61 (Table 4). The sensitivity and accuracy of DCE-MRI for the preoperative staging of endometrial carcinomas were superior to those of T2WI.
Table 4.
Performance of MRI for the staging of endometrial cancer
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy | Kappa | |
|---|---|---|---|---|---|---|
| DCE-MRI | 100% (0.73–1.0) | 62.5% (0.26–0.90) | 82.4% (0.56–0.95) | 100% (0.46–1.0) | 86.4% | 0.68 |
| T2WI | 85.7% (0.56–0.97) | 75% (0.36–0.96) | 85.7% (0.56–0.97) | 75% (0.36–0.96) | 81.8% | 0.61 |
Abbreviations: MRI, magnetic resonance imaging; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; T2WI, T2-weighted imaging.
Discussion
Staging endometrial carcinoma is a crucial process that affects prognosis and treatment selection. One of the advantages of discriminating stage IA from IB disease in this group of patients is the greater likelihood of lymph node involvement and the possibility of simultaneous lymph node dissection during hysterectomy. Therefore, it is important for clinicians to determine the pathological stage using MRI prior to surgery. In this retrospective study, DCE-MRI was more sensitive but less specific than T2WI for the preoperative staging of early endometrial carcinoma. These results potentially impact the diagnosis and management of patients in daily clinical practice.
Accurate pretreatment staging of endometrial carcinoma is the primary requirement for tailoring the best surgical treatment. For example, for patients with stage IA tumors, the standard treatment approach is surgery. However, for patients with stage ≥IB tumors, surgery followed by adjuvant chemotherapy with or without radiation therapy is recommended.5,8,26 In recent decades, an increasing number of studies have used MRI to stage endometrial carcinoma.4,10,12,14,24,27–30 Commonly used MRI protocols for staging endometrial malignancy include CE- or DCE-MRI, T2WI, DWI, and diffusion tensor imaging (DTI) and various combinations of these protocols using either a 1.5 T or 3.0 T scanner.4,10–12,14,15,17,24,27–31 Although a recent systematic review and meta-analysis by Andreano et al32 have reported no differences between 1.5 T and 3.0 T scanners, controversy remains regarding the optimal selection of MRI protocols for staging endometrial cancer. Some investigators have maintained that T2WI in combination with CE- or DCE-MRI is the standard strategy for the staging of endometrial cancer.4,25,30,31,33 Beddy et al24 have reported that DWI exhibits superior staging accuracy to DCE-MRI for assessing myometrial invasion. Bonatti et al31 have reported that T2WI combined with DWI may be reliably used in place of T2WI combined with CE-T1WI for local endometrial carcinoma staging. However, two recently published literature analyses by Wu et al33 and Andreano et al32 have concluded that CE-MRI is superior to T2WI for assessing endometrial cancer stage and that DCE-MRI and DWI do not differ in sensitivity or specificity. Given that T2WI and DCE-MRI are considered as the reference standards for evaluating the endometrial tumor stage in most studies,4,25,30–35 we compared their diagnostic performances.
In the present study, we used DCE-MRI to stage early endometrial carcinomas. Various previous CE-MRI studies demonstrated that the contrast peak difference between the tumor and the myometrium after gadolinium injection was due to differences in vascularity.16,34 The DCE-MRI technique used here was the LAVA sequence. This technique provides enhanced image contrast and uniform fat suppression by optimizing the inversion pulse and applying a novel fat suppression technique followed by dynamic imaging in multiple phases using the array spatial sensitivity-encoding technique with partial data filling and a shorter repetition time and echo time than T2WI.24,36 Using DCE-MRI, endometrial neoplasms were detected at different phases, and the accuracy of staging endometrial carcinomas was increased compared to using T2WI (86.4% vs 81.8%). These results are consistent with previous studies by Joja et al37 and Manfredi et al30 and were confirmed by an updated meta-analysis published by Wu et al.33 The interobserver agreement between DCE-MRI and the revised FIGO staging was moderate (kappa=0.68), which was higher than that of T2WI and was consistent with the results of Haldorsen et al38 suggesting that DCE-MRI is a reproducible method for evaluating the endometrial carcinoma stage. Although T2WI is commonly used in everyday clinical practice, our results indicate that DCE-MRI using the LAVA protocol is superior to T2WI for detecting the depth of myometrial invasion of endometrial cancer, thereby providing important clinical information to gynecologists.
The present study has some limitations. First, only a small number of subjects were included. Large-scale multicenter studies using the same methods are needed to increase the power of this study. Second, our study group included more postmenopausal than premenopausal women. In a recent study, Wu et al have demonstrated that the accuracy of preoperative evaluation of deep myometrial invasion using MRI was higher among premenopausal women than that among postmenopausal women (88.57% vs 74.19%). They attributed this discrepancy to the different myometrial thicknesses between the premenopausal and postmenopausal women.14 Additionally, other studies have suggested that CE-MRI more accurately assesses the depth of myome-trial tumor invasion in postmenopausal patients with early endometrial carcinoma but that T2WI is more suitable for the staging of endometrial carcinoma in premenopausal patients.39 Stratification analysis of premenopausal and postmenopausal subjects will minimize confounding factors and strengthen the value of the present results. Third, we did not perform staging using other functional MRI techniques, such as DWI and DTI. Recently, reports in the literature of the potential application of DWI and DTI for determining myometrial invasion have increased.24,27 The combined use of these techniques may be more accurate and valuable for further studies of the preoperative staging of endometrial carcinoma. Finally, we did not perform perfusion curve analysis and did not determine which phases are optimal for different assessments of endometrial carcinoma using pelvic MRI in women. Additionally, although the LAVA sequence was initially used to evaluate liver tumors, its reliability and reproducibility for assessing endometrial tumors should be examined. These studies must investigate a variety of clinical applications to optimally tailor DCE-MRI with the LAVA sequence for use in patients.
In summary, DCE-MRI is more sensitive and less specific than T2WI for the preoperative staging of early endometrial carcinoma; thus, it can serve as a useful and reliable tool for the preoperative assessment of endometrial carcinoma.
Acknowledgments
The authors would like to thank Yunsheng Hu from the Department of Radiology, Shanghai First People’s Hospital, Shanghai Jiao Tong University for MRI technical support, the pathologists from the Department of Pathology, Shanghai First People’s Hospital, Shanghai Jiao Tong University, and Lesi Xie from the Department of Pathology, Hangzhou First People’s Hospital for reviewing the pathological data. Linfeng Zheng is supported by the State Scholarship Fund provided by the China Scholarship Council and the “The Best Youth Medical Scholars” award from Shanghai First People’s Hospital.
Footnotes
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26(1):1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Messiou C, Spencer JA, Swift SE. MR staging of endometrial carcinoma. Clin Radiol. 2006;61(10):822–832. doi: 10.1016/j.crad.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol. 2012;67(1):2–12. doi: 10.1016/j.crad.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N, Preti E, Landoni F, et al. ESMO Guidelines Working Group Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 6.Cancer.gov [homepage on the Internet] Endometrial Cancer. 2015. [Accessed May 17, 2015]. Available from: http://www.cancer.gov/types/uterine.
- 7.Koyama T, Tamai K, Togashi K. Staging of carcinoma of the uterine cervix and endometrium. Eur Radiol. 2007;17(8):2009–2019. doi: 10.1007/s00330-006-0555-0. [DOI] [PubMed] [Google Scholar]
- 8.Esmo.org [homepage on the Internet] Endometrial Cancer: A Guide for Patients – Information Based on ESMO Clinical Practice Guidelines – v. 2012.1. European Society for Medical Oncology; 2015. [Accessed May 17, 2015]. Available from: http://www.esmo.org/content/download/6604/115031/file/ESMO-ACF-Endometrial-Cancer-Guide-for-Patients.pdf. [Google Scholar]
- 9.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Powell MC, Womack C, Buckley J, Worthington BS, Symonds EM. Pre-operative magnetic resonance imaging of stage 1 endometrial adenocarcinoma. Br J Obstet Gynaecol. 1986;93(4):353–360. [PubMed] [Google Scholar]
- 11.Zahra MA, Martin CW. Commentary on: staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol. 2012;67(1):13–14. doi: 10.1016/j.crad.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 12.He H, Bhosale P, Wei W, Ramalingam P, Iyer R. MRI is highly specific in determining primary cervical versus endometrial cancer when biopsy results are inconclusive. Clin Radiol. 2013;68(11):1107–1113. doi: 10.1016/j.crad.2013.05.095. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Pan CS, Zhang HG, et al. Diffusion-weighted MRI of the normal adult pancreas: the effect of age on apparent diffusion coefficient values. Clin Radiol. 2013;68(10):e532–e537. doi: 10.1016/j.crad.2013.05.100. [DOI] [PubMed] [Google Scholar]
- 14.Wu WJ, Yu MS, Su HY, Lin KS, Lu KL, Hwang KS. The accuracy of magnetic resonance imaging for preoperative deep myometrium assessment in endometrial cancer. Taiwan J Obstet Gynecol. 2013;52(2):210–214. doi: 10.1016/j.tjog.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Zhang GF, He ZY, Li ZY, Zhang GX. Prospective evaluation of 3T MRI findings for primary adnexal lesions and comparison with the final histological diagnosis. Arch Gynecol Obstet. 2014;289(2):357–364. doi: 10.1007/s00404-013-2990-x. [DOI] [PubMed] [Google Scholar]
- 16.Frei KA, Kinkel K, Bonel HM, Lu Y, Zaloudek C, Hricak H. Prediction of deep myometrial invasion in patients with endometrial cancer: clinical utility of contrast-enhanced MR imaging-a meta-analysis and Bayesian analysis. Radiology. 2000;216(2):444–449. doi: 10.1148/radiology.216.2.r00au17444. [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Kim T, Onishi H, et al. Endometrial cancer: preoperative staging using three-dimensional T2-weighted turbo spin-echo and diffusion-weighted MR imaging at 3.0 T: a prospective comparative study. Eur Radiol. 2013;23(8):2296–2305. doi: 10.1007/s00330-013-2815-0. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir S, Celik C, Emlik D, Kiresi D, Esen H. Assessment of myometrial invasion in endometrial cancer by transvaginal sonography, Doppler ultrasonography, magnetic resonance imaging and frozen section. Int J Gynecol Cancer. 2009;19(6):1085–1090. doi: 10.1111/IGC.0b013e3181ad3eb6. [DOI] [PubMed] [Google Scholar]
- 19.SGO Clinical Practice Endometrial Cancer Working Group; Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol. 2014;134(2):385–392. doi: 10.1016/j.ygyno.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Mizutani H, Torashima M, et al. Assessment of myometrial invasion by endometrial carcinoma: transvaginal sonography vs contrast-enhanced MR imaging. AJR Am J Roentgenol. 1993;161(3):595–599. doi: 10.2214/ajr.161.3.8352114. [DOI] [PubMed] [Google Scholar]
- 21.Chung HH, Kang SB, Cho JY, et al. Accuracy of MR imaging for the prediction of myometrial invasion of endometrial carcinoma. Gynecol Oncol. 2007;104(3):654–659. doi: 10.1016/j.ygyno.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Rieck GC, Bulman J, Whitaker R, Leeson SC. A retrospective review of magnetic resonance imaging in assessing the extent of myometrial infiltration for patients with endometrial carcinoma. J Obstet Gynaecol. 2005;25(8):765–768. doi: 10.1080/01443610500327951. [DOI] [PubMed] [Google Scholar]
- 23.Akaeda T, Isaka K, Takayama M, Kakizaki D, Abe K. Myometrial invasion and cervical invasion by endometrial carcinoma: evaluation by CO2-volumetric interpolated breathhold examination (VIBE) J Magn Reson Imaging. 2005;21(2):166–171. doi: 10.1002/jmri.20243. [DOI] [PubMed] [Google Scholar]
- 24.Beddy P, Moyle P, Kataoka M, et al. Evaluation of depth of myome-trial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2012;262(2):530–537. doi: 10.1148/radiol.11110984. [DOI] [PubMed] [Google Scholar]
- 25.Sala E, Crawford R, Senior E, et al. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int J Gynecol Cancer. 2009;19(1):141–146. doi: 10.1111/IGC.0b013e3181995fd9. [DOI] [PubMed] [Google Scholar]
- 26.Kupets R, Le T, Le T, et al. SOGC-GOC-SCC Policy and Practice Guidelines Committee The role of adjuvant therapy in endometrial cancer. J Obstet Gynaecol Can. 2013;35(4):375–379. doi: 10.1016/S1701-2163(15)30968-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Liu A, Zhang T, Song Q, Wei Q, Wang H. Use of diffusion tensor imaging in assessing superficial myometrial invasion by endometrial carcinoma: a preliminary study. Acta Radiol. 2014 doi: 10.1177/0284185114552293. [DOI] [PubMed] [Google Scholar]
- 28.Seki H, Kimura M, Sakai K. Myometrial invasion of endometrial carcinoma: assessment with dynamic MR and contrast-enhanced T1-weighted images. Clin Radiol. 1997;52(1):18–23. doi: 10.1016/s0009-9260(97)80300-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee EJ, Byun JY, Kim BS, Koong SE, Shinn KS. Staging of early endometrial carcinoma: assessment with T2-weighted and gadolinium-enhanced T1-weighted MR imaging. Radiographics. 1999;19(4):937–945. doi: 10.1148/radiographics.19.4.g99jl06937. discussion 946–937. [DOI] [PubMed] [Google Scholar]
- 30.Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology. 2004;231(2):372–378. doi: 10.1148/radiol.2312021184. [DOI] [PubMed] [Google Scholar]
- 31.Bonatti M, Stuefer J, Oberhofer N, et al. MRI for local staging of endometrial carcinoma: is endovenous contrast medium administration still needed? Eur J Radiol. 2015;84(2):208–214. doi: 10.1016/j.ejrad.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S. MR diffusion imaging for preoperative staging of myo-metrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol. 2014;24(6):1327–1338. doi: 10.1007/s00330-014-3139-4. [DOI] [PubMed] [Google Scholar]
- 33.Wu LM, Xu JR, Gu HY, Hua J, Haacke EM, Hu J. Predictive value of T2-weighted imaging and contrast-enhanced MR imaging in assessing myometrial invasion in endometrial cancer: a pooled analysis of prospective studies. Eur Radiol. 2013;23(2):435–449. doi: 10.1007/s00330-012-2609-9. [DOI] [PubMed] [Google Scholar]
- 34.Kinkel K, Forstner R, Danza FM, et al. European Society of Urogenital Imaging Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19(7):1565–1574. doi: 10.1007/s00330-009-1309-6. [DOI] [PubMed] [Google Scholar]
- 35.Rauch GM, Kaur H, Choi H, et al. Optimization of MR imaging for pretreatment evaluation of patients with endometrial and cervical cancer. Radiographics. 2014;34(4):1082–1098. doi: 10.1148/rg.344140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rofsky NM, Lee VS, Laub G, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212(3):876–884. doi: 10.1148/radiology.212.3.r99se34876. [DOI] [PubMed] [Google Scholar]
- 37.Joja I, Asakawa M, Asakawa T, et al. Endometrial carcinoma: dynamic MRI with turbo-FLASH technique. J Comput Assist Tomogr. 1996;20(6):878–887. doi: 10.1097/00004728-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Haldorsen IS, Husby JA, Werner HM, et al. Standard 1.5-T MRI of endometrial carcinomas: modest agreement between radiologists. Eur Radiol. 2012;22(7):1601–1611. doi: 10.1007/s00330-012-2400-y. [DOI] [PubMed] [Google Scholar]
- 39.Rockall AG, Meroni R, Sohaib SA, et al. Evaluation of endometrial carcinoma on magnetic resonance imaging. Int J Gynecol Cancer. 2007;17(1):188–196. doi: 10.1111/j.1525-1438.2007.00805.x. [DOI] [PubMed] [Google Scholar]