Abstract
H2N2 influenza viruses have not circulated in the human population since 1968, but they are still being regularly detected in the animal reservoir, suggesting their high pandemic potential. To prepare for a possible H2N2 pandemic, a number of H2N2 vaccine candidates have been generated and tested in preclinical and clinical studies. Here we describe the results of a randomized, double-blind placebo-controlled phase 1 clinical trial of an H2N2 live attenuated influenza vaccine (LAIV) candidate prepared from a human influenza virus isolated in 1966. The vaccine candidate was safe and well-tolerated by healthy adults, and did not cause serious adverse events or an increased rate of moderate or severe reactogenicities. The H2N2 vaccine virus was infectious for Humans. It was shed by 78.6% and 74.1% volunteers after the first and second dose, respectively, most probably due to the human origin of the virus. Importantly, no vaccine virus transmission to unvaccinated subjects was detected during the study. We employed multiple immunological tests to ensure the adequate assessment of the H2N2 pandemic LAIV candidate and demonstrated that the majority (92.6%) of the vaccinated subjects responded to the H2N2 LAIV in one or more immunological tests, including 85.2% of subjects with antibody responses and 55.6% volunteers with cell-mediated immune responses. In addition, we observed strong correlation between the H2N2 LAIV virus replication in the upper respiratory tract and the development of antibody responses.
Keywords: clinical trial, immunogenicity, influenza H2N2, live attenuated influenza vaccine, pandemic, safety, transmissibility, vaccine virus shedding
Introduction
H2N2 influenza viruses are considered as potentially pandemic for several reasons. First, this subtype is known to have caused previous pandemics in 1889 and 19571,2 with significant rates of morbidity and mortality among humans.3,4 Second, H2 subtype influenza viruses continue to circulate in avian reservoirs posing the threat of generating new H2 reassortants with some genes of currently circulating human influenza viruses, as it happened in 1950s causing the worldwide "Asian flu" pandemic.5-9 In addition, a natural infection with H2N3 influenza virus was recently detected in a swine host in the United States suggesting possible adaptation of avian H2 viruses to mammals.10 Finally, H2N2 influenza viruses disappeared from human circulation in 1968, and thus, people born after this year have no immunity against H2 influenza viruses and therefore will be vulnerable to the infection should this subtype return to circulation. A re-emergence of H1N1 influenza viruses in 1977, after 20 y of the absence in humans, is a striking example of such situation. It was noted that the new H1N1 epidemic was almost entirely restricted to persons younger than 25 y of age.11
Preventive vaccination remains the principal weapon against influenza. The main objective set by the World Health Organization (WHO) in its Global Action Plan to fight influenza and protect against pandemics is to create a collection of vaccine strains against potential pandemic viruses, which could be quickly ordered into production in the case of a pandemic.12,13 Moreover, in the event of an H2N2 pre-pandemic situation, some leading virologists recommend starting a vaccination campaign before the pandemic breaks out.14,15 In recent years, interest in a live cold-adapted reassortant influenza vaccine (LAIV) has grown considerably. This is partly because WHO has recognized the advantage of using LAIVs over inactivated vaccines if a pandemic breaks out.13 Thus, its higher yield in eggs, easier down-stream processing and faster scalability of production indicates that it is more likely to meet the demand for a vaccine at the beginning of a pandemic. In addition, LAIV is capable of inducing more cross-reactive immune responses and therefore will protect against antigenically drifted variants. Finally, its easy mode of nasal administration makes LAIV a particularly desirable vaccine for use in a pandemic setting.16-21In response to a potential H2N2 pandemic threat, the Russian Institute of Experimental Medicine (IEM) has developed and characterized in pre-clinical studies 2 H2N2 LAIV candidates based on human influenza viruses isolated in 1966 and 1967,22 an A/California/1/66 strain, and an A/Tokyo/3/67 strain. A ferret challenge study revealed superior immunogenicity and cross-reactivity of the LAIV candidate based on the A/California/1/66 strain. Therefore this was chosen for clinical testing in a phase I study in which we assessed the reactogenicity, safety, shedding, transmissibility and immunogenicity in healthy adult volunteers.
Results
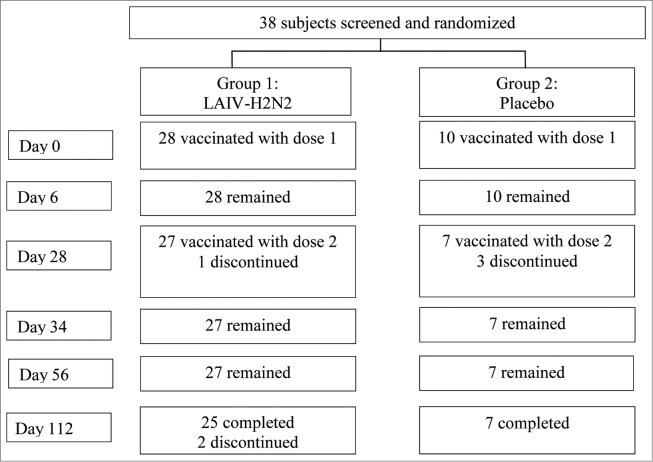
Thirty-eight healthy male and female adults aged 18 to 40 were randomized at a 3:1 ratio to receive the H2N2 LAIV or a placebo under blind. The demographic characteristics of the cohort are shown in Table 1. Two doses of the H2N2 LAIV or a matched placebo were administered intranasally 28 d apart. Volunteers were placed in an isolation unit for 6 d after receipt of each dose of the study vaccine or placebo. Subjects were closely monitored for adverse reactions occurring within 6 d after administration of each dose. Three subjects in the vaccine group and 3 in the placebo group discontinued participation prematurely due to non-trial related reasons (see study flow diagram, Fig. 1). Of them, 4 subjects (one in the vaccine group and 3 in the placebo group) did not receive the second dose due to: lacunar tonsillitis starting the day before the second vaccination in one; signs of acute respiratory disease 3-5 d before the second vaccination in 2; and personal reasons in one. All the volunteers were tested by PCR for influenza A and B viruses, as well as for other acute respiratory viral infections (adeno-, boca-, metapneumo-, corona-, RS, rhino- and parainfluenza viruses) on day 28. One subject was PCR positive for rhinovirus. Additionally, 2 subjects did not attend the visit set up for the last specimen collection (day 112).
Table 1.
Demographic characteristics of study participants
| Characteristics | Vaccine N = 28 (100%) | Placebo N = 10 (100%) |
|---|---|---|
| Gender | ||
| Male | 16 (57.1) | 7 (70) |
| Female | 12 (42.9) | 3 (30) |
| Race | ||
| White | 28 (100) | 10 (100) |
| Age (years) | ||
| Mean (SE) | 28.0 (1.26) | 26.2 (2.49) |
| Median | 27.0 | 24.5 |
| Range | 18.0– 40.0 | 18.0–40.0 |
Figure 1.
Study flow diagram.
Reactogenicity
The H2N2 LAIV was well tolerated with no clinically significant safety signals detected during the daily clinical evaluations or the metabolic and hematologic laboratory tests conducted a week post each vaccination. No adverse events were reported within the first 2 hours post vaccination. Over the 7 d post-vaccination period local reactions occurred in 4 (14.3%) of the vaccinated subjects after the first dose, but in none after the second (Table 2). Local reactions were also reported by 2 subjects (20.0%) in the placebo group after receipt of the first dose, but in none after the second. Solicited systemic reactions were recorded in similar frequencies for subjects in the vaccine group (46.4% after dose 1, 32.1% after dose 2) and placebo group (50% after dose 1, 20% after dose 2). These included mild events of sore throat, headache, vomiting, fatigue and fever. The most common systemic reaction observed among vaccine recipients was fever, which occurred in 12 subjects (42.9%) after dose 1 and in 4 subjects (14.3%) after dose 2. Among subjects who received placebo 1 (10%) had fever and 4 (40%) had sore throat after dose 1 (Table 2). Importantly, all temperature elevations observed in either treatment group were mild (37.0 to 37.5ºC). No moderate or severe solicited reactions were recorded in the course of the study.
Table 2.
Subjects with reactogenicity and adverse events following vaccination
| Post dose 1 n; % (95% CI) | Post dose 2 n; % (95% CI) | |||
|---|---|---|---|---|
| Outcomes | Vaccine N=28 | Placebo N=10 | Vaccine N=28 | Placebo N=10 |
| Within 2 hours post-vaccination | ||||
| Any adverse reaction | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Within 7 d post-vaccination | ||||
| Solicited reactions | ||||
| Local reaction | 4; 14.3 (5.7; 31.5) | 2; 20.0 (5.7; 51.0) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Systemic reaction | 13; 46.4 (29.5; 64.2) | 4; 40.0 (16.8; 68.7) | 9; 32.1 (17.9; 50.7) | 2; 20.0 (5.7; 51.0) |
| Any | 13; 46.4 (29.5; 64.2) | 5; 50.0 (23.7; 76.3) | 9; 32.1 (17.9; 50.7) | 2; 20.0 (5.7; 51.0) |
| Solicited local reactions | ||||
| Nose dryness | 4; 14.3 (5.7; 31.5) | 2; 20.0 (5.7; 51.0) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Solicited systemic reactions | ||||
| Fatigue/malaise | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Headache | 2; 7.1 (2.0; 22.6) | 0; 0.0 (0.0; 27.8) | 2; 7.1 (2.0; 22.6) | 1; 10.0 (1.8; 40.4) |
| Sore throat | 3; 10.7 (3.7; 27.2) | 4; 40.0 (16.8; 68.7) | 5; 17.9 (7.9; 35.6) | 1; 10.0 (1.8; 40.4) |
| Vomiting | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Temperature | 12; 42.9 (26.5; 60.9) | 1; 10.0 (1.8; 40.4) | 4; 14.3 (5.7; 31.5) | 0; 0.0 (0.0; 27.8) |
| Adverse events | ||||
| Worst grade – mild | 22; 78.6 (60.5; 89.8) | 9; 90.0 (59.6; 98.2) | 25; 89.3 (72.8; 96.3) | 7; 70.0 (39.7; 89.2) |
| Worst grade – moderate | 1; 3.6 (0.6; 17.7) | 1; 10.0 (1.8; 40.4) | 3; 10.7 (3.7; 27.2) | 0; 0.0 (0.0; 27.8) |
| Worst grade – severe | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Any event | 22; 78.6 (60.5; 89.8) | 9; 90.0 (59.6; 98.2) | 25; 89.3 (72.8; 96.3) | 7; 70.0 (39.7; 89.2) |
| Any treatment–related event | 16; 57.1 (39.1; 73.5) | 5; 50.0 (23.7; 76.3) | 19; 67.9 (49.3; 82.1) | 4; 40.0 (16.8; 68.7) |
| Any serious adverse event | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
CI, 95% confidence interval as calculated by Wilson test.
The most frequent unsolicited adverse events observed following vaccination consisted of mild laboratory chemistry abnormalities, which were generally similar in frequency between treatment groups, with the exception of moderate cases of increased alanine amino transferase (2 cases, one after each dose), bilirubin and glucose (one case each) in the vaccine group after dose 2 (Table 3). A single moderate case of bilirubin elevation was recorded in the placebo group after dose 1.
Table 3.
Subjects with adverse events following vaccination
| Post dose 1 n; % (95% CI) | Post dose 2 n; % (95% CI) | |||
|---|---|---|---|---|
| Outcomes | Vaccine N=28 | Placebo N=10 | Vaccine N= 28 | Placebo N=10 |
| Ear Congestion | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Fatigue | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) |
| Pyrexia | 2; 7.1 (2.0; 22.6) | 1; 10.0 (1.8; 40.4) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| Periodontitis | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
| ALT elevation | 1; 3.6 (0.6; 17.7)* | 0; 0.0 (0.0; 27.8) | 3; 10.7 (3.7; 27.2)* | 0; 0.0 (0.0; 27.8) |
| AST elevation | 1; 3.6 (0.6; 17.7) | 0; 0.0 (0.0; 27.8) | 2; 7.1 (2.0; 22.6) | 0; 0.0 (0.0; 27.8) |
| Bicarbonate elevation | 3; 10.7 (3.7; 27.2) | 2; 20.0 (5.7; 51.0) | 2; 7.1 (2.0; 22.6) | 0; 0.0 (0.0; 27.8) |
| Bilirubin elevation | 2; 7.1 (2.0; 22.6) | 1; 10.0 (1.8; 40.4)* | 2; 7.1 (2.0; 22.6)* | 1; 10.0 (1.8; 40.4) |
| Glucose elevation | 1; 3.6 (0.6; 17.7) | 1; 10.0 (1.8; 40.4) | 3; 10.7 (3.7; 27.2)* | 1; 10.0 (1.8; 40.4) |
| Calcium elevation | 2; 7.1 (2.0; 22.6) | 0; 0.0 (0.0; 27.8) | 2; 7.1 (2.0; 22.6) | 1; 10.0 (1.8; 40.4) |
| Lymphocyte count elevation | 12; 42.9 (26.5; 60.9) | 1; 10.0 (1.8; 40.4) | 19; 67.9 (49.3; 82.1) | 6; 60 (31.3; 83.2) |
| Monocyte count elevation | 5; 17.9 (7.9; 35.6) | 1; 10.0 (1.8; 40.4) | 6; 21.4 (10.2; 39.5) | 1; 10.0 (1.8; 40.4) |
| Neutrophil count decreased | 9; 32.1 (17.9; 50.7) | 5; 50.0 (23.7; 76.3) | 10; 35.7 (20.7; 54.2) | 4; 40.0 (16.8; 68.7) |
| RBC sedimentation rate increased | 4; 14.3 (5.7; 31.5) | 1; 10.0 (1.8; 40.4) | 0; 0.0 (0.0; 12.1) | 0; 0.0 (0.0; 27.8) |
*denotes moderate grade AE in one subject; all the other events were mild; CI, 95% confidence interval as calculated by Wilson test; ALT - Alanine aminotransferase; AST - Aspartate aminotransferase; RBCs – red blood cells.
Vaccine virus shedding, transmissibility and genetic stability of LAIV isolates
Nasal and throat swabs were collected daily while subjects were in isolation and tested by RNA PCR or culture in embryonated chicken eggs to assess the ability of the H2N2 LAIV virus to infect the vaccinated subjects, as well as the potential transmissibility to unvaccinated contacts. For safety reasons, participants were eligible for discharge on the 7th day post-vaccination only if PCR-diagnosis results confirmed that no influenza virus was present in nasal swabs for at least 2 consecutive days. This precaution was taken to reduce the risk of possible reassortment between the vaccine and a wild-type strain.
After dose 1, viral RNA was detected in nasal swabs in 22 of 28 vaccinated subjects on day 1 post vaccination (78.6%), whereas only 11 subjects were positive on day 2 (39.3%), 5 on day 3 (17.9%), and 3 on day 4 (10.7%) after the first dose. The detection of viral RNA in throat swabs was much lower. No subjects were positive on day 1 after vaccination, and virus replication was detected only in 3 subjects on day 2 (10.7%), one of whom shed the virus for 2 additional days (Table 4). No viral replication was detected in any subject beyond day 4 post-vaccination.
Table 4.
Detection of influenza H2N2 virus in subjects vaccinated with H2N2 LAIV
| Vaccination | Revaccination | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 dpv | 2 dpv | 3 dpv | 4 dpv | 5–6 dpv | 29 (1) dpv | 30 (2) dpv | 31–34 (3–6) dpv | ||||||||||||||||||
| ID# | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | PCR nose | PCR throat | Eggs | Virus detected |
| 02 | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | + |
| 04 | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 07 | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 09 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | − |
| 10 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 11 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 14 | + | − | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | + |
| 15 | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | ND | − | − | − | + |
| 18 | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 20 | + | − | + | + | + | − | + | + | − | + | + | − | − | − | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | + |
| 21 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | − |
| 23 | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 25 | + | − | − | + | − | − | + | − | − | + | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 26 | + | − | + | + | + | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 27 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 31 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | − |
| 32 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 36 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 37 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | ND | − | − | − | + |
| 38 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 39 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | ND | − | − | − | + |
| 41 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 42 | + | − | + | + | − | + | − | − | − | − | − | − | − | − | − | + | − | + | + | − | ND | − | − | − | + |
| 46 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 49 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | ND | − | − | − | + |
| 50 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ND | − | − | − | + |
| 52 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | − |
| 54 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | ND | − | − | − | + |
| Total (%) | 22 (78.6) | 0 (0.0) | 11 (39.3) | 11 (39.3) | 3 (10.7) | 1 (3.6) | 5 (17.9) | 1 (3.6) | 0 (0.0) | 3 (10.7) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (74.1) | 2 (7.4) | 8 (29.6) | 2 (7.4) | 1 (3.7) | ND | 0 (0.0) | 0 (0.0) | 0 (0.0) | 24 (85.7) |
dpv – days post vaccination; ND – not done.
Viral RNA was detected in nasal swabs in 21 of 27 vaccinated volunteers following the second dose. One subject was PCR negative in the nasal swab sample, but positive in the throat swab specimen collected on day 2 after revaccination (Table 4). Notably, only 3 subjects had detectable viral RNA on day 2 after revaccination and no virus was detected beyond this time point, highlighting a shorter period of vaccine virus shedding after dose 2 compared with dose 1.
Infectious virus was isolated by culture in eggs only from subjects who were positive for viral RNA by PCR. The majority of the isolates were recovered on day 1 after vaccination and revaccination, (11 of 28 and 8 of 27, respectively). There was only one isolate recovered on day 2 after the first dose, and no viable virus was isolated after day 2 after either dose. No viral RNA or infectious virus was detected in any subject in the placebo group by PCR or virus culture indicating the absence of the H2N2 vaccine virus transmission from immunized to unvaccinated contacts.
Altogether, 20 H2N2 LAIV isolates were recovered from vaccinated subjects by culturing nasal swabs in eggs. All these isolates were sequenced to assess the genetic stability of the vaccine virus after replication in humans. In addition, their ts/ca phenotype was assessed by titration in eggs at various temperatures. All twenty clinical isolates were shown to preserve all the attenuating mutations of the A/Leningrad/134/17/57 master donor virus. No spontaneous mutations were detected in HA or NA genes. In addition, the ts/ca phenotype of all isolates was essentially identical to that of the master donor virus (data not shown). These data suggest a high level of genetic stability after replication in humans.
Immunogenicity of H2N2 LAIV
Antibody responses to the vaccine were measured at day 0, 28 d after dose 1 (Day 28), 28 d after dose 2 (Day 56) and 84 d after dose 2 (Day 112). Cell-mediated immunity (CMI) was assessed at baseline, at days 6 and 28 after the first dose and at days 28 and 84 after the second dose.
All the subjects were seronegative prior to immunization. The proportion of subjects with ≥4 -fold rise in antibody titers varied depending on the assay employed. The majority of seroconversions were detected by hemagglutination-inhibition assay (HAI): 18.5%, 33.3%, and 60.0% of vaccinated subjects developed a response by days 28, 56 and 112, respectively, whereas only 2 (8.0%) volunteers had serum IgG responses measured by an - enzyme-linked immunosorbent assay (ELISA) by day 112 (Table 5). Microneutralization (MN) detected seroconversions in 8 (29.6%), 12 (44.4%) and 11 (44.0%) subjects on days 28, 56 and 112, respectively.
Table 5.
Proportion of subjects with ≥4-fold rise in antibody titers after vaccination with H2N2 LAIV or Placebo
| After 1 dose (Day 28) LAIV: N=27 placebo: N=9 | After 2 doses (Day 56) LAIV: N=27 placebo: N=7 | After 2 doses (Day 112) LAIV: N=25 placebo: N=7 | Cumulative conversions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay | Test article | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI |
| HAI: serum Ab | LAIV | 5 (18.5) | 8.2–36.7 | 9 (33.3) | 18.6–52.2 | 15 (60.0)1 | 40.7–76.6 | 16 (59.3) | 40.7–75.5 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–29.9 | |
| MN: serum Ab | LAIV | 8 (29.6) | 15.9–48.5 | 12 (44.4)2 | 27.6–62.7 | 12 (48.0) | 26.7–62.9 | 16 (59.3) | 40.7–75.5 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–29.9 | |
| ELISA: serum IgA | LAIV | 2 (7.4) | 2.1–23.4 | 14 (51.9)3 | 34.0–69.3 | 12 (48.0)4 | 30.0–66.5 | 14 (51.9) | 34.0–69.3 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–29.9 | |
| ELISA: serum IgG | LAIV | 0 | 0.0–12.5 | 1 (3.7) | 0.7–18.3 | 2 (8.0) | 2.2–25.0 | 2 (7.4) | 2.1–23.4 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–29.9 | |
| ELISA: nasal IgA | LAIV | 6 (22.2) | 10.6–40.8 | 11 (40.7) | 24.5–59.3 | n/a | n/a | 13 (48.1) | 30.7–66.0 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | n/a | n/a | 0 | 0.0–29.9 | |
| ELISA: salivary IgA | LAIV | 3 (11.1) | 3.9–28.1 | 9 (33.3) | 18.6–52.2 | n/a | n/a | 12† (44.4) | 27.6–62.7 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | n/a | n/a | 0 | 0.0–29.9 | |
| Cumulative conversions | LAIV | 15 (55.6)5 | 37.3–72.4 | 23 (85.2)6 | 67.5–94.1 | 21 (84.0)7 | 65.3–93.6 | 23 (85.2) | 67.5–94.1 |
| Placebo | 0 | 0.0–29.9 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–29.9 | |
CI, 95% confidence interval as calculated by Wilson test; n/a – not applicable (nasal swab and saliva samples were not collected at Day 112); HAI – hemagglutination inhibition assay; MN – microneutralization; ELISA - Enzyme-linked immunosorbent assay; Ab – antibody;
†2 subjects had 4-fold rises in salivary IgA antibody titer at Day 56 compared to Day 28, and only 2-fold rise at Day 56 compared to Day 0;
1percent of subjects with HAI Ab conversions 84 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.008);
2percent of subjects with MN Ab conversions 28 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.036);
3percent of subjects with serum IgA conversions 28 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.026);
4percent of subjects with serum IgA conversions 84 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.029);
5cumulative percent of subjects with Ab conversions is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.005);
6cumulative percent of subjects with Ab conversions 28 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.0001);
7cumulative percent of subjects with Ab conversions 56 d after 2nd vaccination is significantly higher in LAIV group than in Placebo group (Fisher exact (2–tailed) p = 0.0001);
In addition, 16 (59.3%) subjects had measurable responses in serum IgA antibody titers at any time. Importantly, intranasal administration of the H2N2 LAIV induced local antibody immune responses in 17 (63.0%) subjects, of which 5 (18.5%) subjects had ≥4 -fold increases of nasal IgA antibodies, 4 (14.8%) subjects had ≥4 -fold increases of salivary IgA antibodies, and 8 (29.6%) volunteers had responses measured by both assays (Tables 5, 9). Of note, 11 of 14 (78.6%) subjects with serum IgA responses had significant increases of local IgA titers detected by either of the assays (Table 9).
Table 9.
Summary on individual data on antibody and cellular immune responses in subjects vaccinated with H2N2 LAIV
| Antibody (Ab) responses | Cell mediated immune (CMI) responses | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID# | HAI | MN | serum IgG | serum IgA | nasal IgA | salivary IgA | Any | CD4+ | CD8+ | CD4 Tcm | CD4 Tem | CD8 Tcm | CD8 Tem | Any | Either Ab or CMI response |
| 02 | + | + | + | + | + | + | |||||||||
| 04 | + | + | + | + | + | + | + | + | |||||||
| 07 | + | + | + | + | + | + | + | + | + | + | + | ||||
| 09 | + | + | + | ||||||||||||
| 10 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| 11 | + | + | + | + | |||||||||||
| 14 | + | + | + | + | + | + | + | + | + | + | |||||
| 15 | + | + | + | + | + | + | |||||||||
| 18 | + | + | + | + | + | + | + | + | + | ||||||
| 21 | + | + | + | ||||||||||||
| 23 | + | + | + | + | |||||||||||
| 25 | + | + | + | + | + | + | |||||||||
| 26 | + | + | + | + | + | + | + | + | + | + | |||||
| 27 | + | + | + | ||||||||||||
| 31 | |||||||||||||||
| 32 | + | + | + | + | + | + | + | ||||||||
| 36 | + | + | + | + | + | ||||||||||
| 37 | + | + | + | + | |||||||||||
| 38 | + | + | + | + | + | + | + | + | |||||||
| 39 | + | + | + | + | + | + | + | ||||||||
| 41 | + | + | + | + | + | + | |||||||||
| 42 | + | + | + | + | + | + | + | + | |||||||
| 46 | + | + | + | + | + | + | + | + | + | ||||||
| 49 | + | + | + | + | + | + | + | + | + | + | |||||
| 50 | + | + | + | + | + | + | + | ||||||||
| 52 | |||||||||||||||
| 54 | + | + | + | + | + | ||||||||||
| Total | 16 | 16 | 2 | 14 | 13 | 12 | 23 | 6 | 6 | 4 | 4 | 4 | 12 | 15 | 25 |
| (59.3%) | (59.3%) | (7.4%) | (51.9%) | (48.1%) | (44.4%) | (85.2%) | (22.2%) | (22.2%) | (14.8%) | (14.8%) | (14.8%) | (44.4%) | (55.6%) | (92.6%) | |
GMT – geometric mean titer; HAI – hemagglutination inhibition assay; MN – microneutralization; Tcm – central memory T cells; Tem – effector memory T cells.
In summary, the majority of vaccinated subjects (23 of 27, 85.2%) developed antibody responses measured by any of the assays, though the geometric mean titers (GMT) of the antibodies were relatively low. Thus, the GMT of the MN antibodies among vaccines was only 1:15 at 84 d after the second vaccination, and the HAI antibody GMT was < 1:10 (Table 6). Nonetheless, the increases of HAI, MN and serum IgA antibody titers were statistically significant already after the first dose (p = 0 .028 for HAI, p = 0. 0002 for MN and p = 0.019 for serum IgA antibodies). Second vaccination resulted in 2.0 to 3.0 GMT fold rises of all antibodies, with the exception of serum IgG, for which significant GMT increase was noted only 84 d after the second dose (p = 0.019, Table 6). Notably, all antibody titers continued to rise over time, and GMTs measured at the latest time point (84 d after dose 2) were higher than those detected 28 d after the second dose, indicating a continuous development of immune responses after immunization with LAIV. Table 7 summarizes the GMTs of various antibodies from subjects exhibiting ≥4 -fold rises in antibody titers measured by the various assays employed. No antibody responses were observed in any of the placebo recipients.
Table 6.
Geometric mean titers of serum and local antibodies in volunteers vaccinated with H2N2 LAIV
| Reverse GMTs | GMT fold changes* | |||||||
|---|---|---|---|---|---|---|---|---|
| Assay | Test article | Day 0 LAIV: N=27 placebo: N=9 | Day 28 LAIV: N=27 placebo: N=9 | Day 56 LAIV: N=27 placebo: N=7 | Day 112 LAIV: N=25 placebo: N=7 | Day 28 / Day 0 | Day 56 / Day 0 | Day 112 / Day 0 |
| HAI: serum Ab | LAIV | 2.5 | 3.5 | 5.1 | 7.4 | 1.41 | 2.02 | 3.03 |
| Placebo | 2.5 | 2.5 | 3.0 | 2.8 | 1.0 | 1.2 | 1.1 | |
| MN: serum Ab | LAIV | 5.4 | 10.5 | 13.6 | 14.7 | 1.94 | 2.55 | 2.76 |
| Placebo | 6.8 | 8.6 | 8.2 | 9.0 | 1.3 | 1.2 | 1.3 | |
| ELISA: serum IgA | LAIV | 12.7 | 16.8 | 29.6 | 33.8 | 1.37 | 2.38 | 2.49 |
| Placebo | 13.7 | 16.0 | 14.5 | 13.1 | 1.2 | 0.9 | 0.8 | |
| ELISA: serum IgG | LAIV | 10.9 | 11.8 | 11.8 | 14.3 | 1.1 | 1.1 | 1.310 |
| Placebo | 12.7 | 13.7 | 13.1 | 14.5 | 1.1 | 1.0 | 1.1 | |
| ELISA: nasal IgA | LAIV | 3.5 | 4.8 | 8.6 | n/a | 1.4 | 2.511 | n/a |
| Placebo | 10.9 | 6.9 | 8.8 | n/a | 0.6 | 1.0 | n/a | |
| ELISA: salivary IgA | LAIV | 3.1 | 3.3 | 6.7 | n/a | 1.1 | 2.212 | n/a |
| Placebo | 3.7 | 3.2 | 4.4 | n/a | 0.9 | 1.2 | n/a | |
GMT – geometric mean titer; HAI – hemagglutination inhibition assay; MN – microneutralization; ELISA - Enzyme-linked immunosorbent assay; Ab – antibody;
*GMT fold changes are calculated only for those subjects who was available at indicated days;
1GMT of HAI Ab after 1st vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.028);
2GMT of HAI Ab 28 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.0007);
3GMT of HAI Ab 84 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.0002);
4GMT of MN Ab after 1st vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.0002);
5GMT of MN. Ab 28 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.00002);
6GMT of MN. Ab 84 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.00001);
7GMT of serum IgA after 1st vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.019);
8GMT of serum IgA 28 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.00002);
9GMT of serum IgA 84 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.0005);
10GMT of serum IgG 84 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.019);
11GMT of nasal IgA 28 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.003);
12GMT of salivary IgA 28 d after 2nd vaccination is significantly higher than GMT before vaccination (Wilcoxon Matched Pairs Test: p = 0.0003).
Table 7.
GMTs in subjects with ≥4 -fold rises in antibody titers after 2 doses of H2N2 LAIV
| After 2 doses, Day 56 | After 2 doses, Day 112 | |||||||
|---|---|---|---|---|---|---|---|---|
| Reverse GMTs | Reverse GMTs | |||||||
| Assay | n | Day 0 | Day 56 | GMT rise | n | Day 0 | Day 112 | GMT rise |
| HAI: serum Ab | 9 | 2.5 | 13.6 | 5.4 | 15 | 2.5 | 13.2 | 5.3 |
| MN: serum Ab | 12 | 5.0 | 20.0 | 4.0 | 12 | 5.0 | 20.0 | 4.0 |
| ELISA: serum IgA | 14 | 8.8 | 41.0 | 4.7 | 12 | 9.0 | 47.9 | 5.3 |
| ELISA: nasal IgA | 13 | 1.9 | 12.9 | 6.8 | n/a | n/a | n/a | n/a |
| ELISA: salivary IgA | 12 | 3.6 | 14.3 | 4.0 | n/a | n/a | n/a | n/a |
GMT – geometric mean titer; HAI – hemagglutination inhibition assay; MN – microneutralization; ELISA - Enzyme-linked immunosorbent assay; Ab – antibody; n/a – not applicable (nasal swab and saliva samples were not collected at Day 112).
T cell mediated immune responses were evaluated by the fold change (FC) in the number of H2N2 vaccine virus-specific CD4+ and CD8+ IFN-γ producing central memory T cells (Tcm) and effector memory T cells (Tem) induced by vaccination. The mean FC value for the placebo group plus 3 standard deviations (SD) was used as the cut-off line to consider a response to be positive. After the first dose only 3 vaccinated subjects (11.1%) on day 6 and 6 (22.2%) on day 28 had T cell immune responses, respectively (Table 8). While no increase in such responses were observed 28 d after the second vaccination, 11 of 25 (44.0%) vaccinees had demonstrable responses on day 84 after the second dose. Altogether, 15 (55.6%) vaccinated volunteers had significant increases in the virus-specific T cells at any time point.
Table 8.
T cell immune responses in volunteers vaccinated with H2N2 LAIV or placebo
| Percent of subjects with significant increases* in virus–specific memory T cells | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ | CD4+ Tcm** | CD4+ Tеm** | CD8+ | CD8+ Tcm | CD8+ Tеm | Total | |||||||||
| Time points | Test article | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI |
| Day 6 / Day 0 | LAIV (n = 27) | 1 (3.7) | 0.7–18.3 | 0 | 0.0–12.5 | 0 | 0.0–12.5 | 3 (11.1) | 3.9–28.1 | 0 | 0.0–12.5 | 0 | 0.0–12.5 | 3 (11.1) | 3.9–28.1 |
| Placebo (n = 10) | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | |
| Day 28 / Day 0 | LAIV (n = 27) | 2 (7.4) | 2.1–23.4 | 2 (7.4) | 2.1–23.4 | 3 (11.1) | 3.9–28.1 | 3 (11.1) | 3.9–28.1 | 2 (7.4) | 2.1–23.4 | 3 (11.1) | 3.9–28.1 | 6 (22.2) | 10.6–40.8 |
| Placebo (n = 10) | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | |
| Day 56 / Day 0 | LAIV (n = 27) | 3 (11.1) | 3.9–28.1 | 3 (11.1) | 3.9–28.1 | 1 (3.7) | 0.7–18.3 | 1 (3.7) | 0.7–18.3 | 1 (3.7) | 0.7–18.3 | 3 (11.1) | 3.9–28.1 | 6 (22.2) | 10.6–40.8 |
| Placebo (n = 7) | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | |
| Day 112 / Day 0 | LAIV (n = 25) | 5 (20.0) | 8.9–39.1 | 1 (4.0) | 0.7–19.5 | 2; (8.0) | 2.2–25.0 | 3 (12.0) | 4.2–30.0 | 3 (12.0) | 4.2–30.0 | 9 (36.0) | 20.2–55.5 | 11 (44.0) | 26.7–62.9 |
| Placebo (n = 7) | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | 0 | 0.0–34.5 | |
| TOTAL | LAIV (n = 27) | 6 (22.2) | 10.6–40.8 | 4 (14.8) | 5.9–32.5 | 4 (14.8) | 5.9–32.5 | 6 (22.2) | 10.6–40.8 | 4 (14.8) | 5.9–32.5 | 12 (44.4) | 27.6–62.7 | 15 (55.6) | 37.3–72.4 |
| Placebo (n = 10) | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | 0 | 0.0–27.8 | |
*More than Placebo mean value plus 3 Standard Deviations; CI, 95% confidence interval as calculated by Wilson test;
**Tcm – central memory T cells; Tem – effector memory T cells.
Table 9 summarizes individual data on the antibody and cellular immune responses by any assay employed for each vaccinated volunteer. Cumulative data shows that the majority of the volunteers (92.6%) responded to the H2N2 LAIV by the induction of virus-specific antibodies and/or T cells.
Discussion
Due to their persistence in animal reservoirs, the historic evidence of their ability to cause devastating pandemics and the absence of collective immunity in the population born after 1968, H2N2 influenza viruses have long been recognized as potentially pandemic.2,5,9,23,24 To be adequately prepared for an H2N2 pandemic, safe and effective vaccines should be developed and tested in clinical trials now, to shorten the time needed to provide the population with sufficient vaccine doses to start global campaigns as early as possible.
LAIVs are considered to be the preferred vaccine modality to use at the beginning of a pandemic given their induction of a broader range and specificity of immune responses as well as their ease of administration and the cheaper and faster production process.13 Multiple studies have directly compared inactivated influenza vaccines (IIV) with LAIVs in animal models and clinical trials have demonstrated a superior performance for LAIVs, especially with regards to breadth of responses and cross-protection against antigenically diverse strains.18,19,25-32
Two cold-adapted H2N2 LAIVs have been tested in clinical trials thus far: A/Leningrad/134/17/57 (Len/17) and caA/Ann Arbor/6/60 (AA/60). Both viruses have been used as backbones for the development of seasonal reassortant LAIVs in Russia and the United States for decades. In addition, the Len/17 strain was used back in the 1960s as a cold-adapted LAIV for immunization against Asian flu in the USSR, with a well-documented safety, immunogenicity and efficacy profile in controlled clinical trials.33 Results of a phase I clinical trial of AA/60 vaccine demonstrated low immunogenicity for healthy adults, most likely due to minimal viral replication, which was unexpected since the AA/60 virus was of human origin.34 A separate AA/60-based H2 LAIV strain prepared from a swine H2N3 virus isolated in 2006 was tested in pre-clinical studies and demonstrated promising immunogenic and cross-reactive potential.35 These results prompted the initiation of a phase I clinical trial in healthy adults (Clinicaltrials.gov identifier: NCT01175122), but no results from this trial have been published.
As a part of H2N2 pandemic preparedness we tested 3 LAIV candidates in preclinical studies, including Len/17 master donor virus and 2 reassortants based on H2N2 viruses of human origin isolated at the end of the H2N2 wave.36 Even though animal studies had demonstrated better cross-reactivity of avian H2 viruses than late human viruses,35 we selected the human viruses because of their expected better replication in the human upper respiratory tract, which in turn might result in stronger immune responses. Of the 3 candidates A/17/California/66/395 (H2N2 LAIV) based on A/California/1/66 virus demonstrated the best immunogenicity, cross-reactivity and cross-protection in ferrets, prompting the selection of this candidate for the phase I clinical trial described in this article. The clinical study was designed to allow an adequate assessment of not only safety, virus shedding, genetic stability and immunogenicity of the H2N2 vaccine, but also its potential transmissibility to unvaccinated close contacts.
Since the A/17/California/66/395 LAIV has HA and NA genes of human origin, we expected to see intensive virus replication in the human upper respiratory tract after vaccination. Indeed, the H2N2 LAIV was highly infectious: 22 of 28 (78.6%) and 20 of 27 (74.1%) volunteers shed the virus after the first and second dose, respectively (Table 4), and several subjects shed the virus for as long as 4 d after vaccination, which was in contrast to other pandemic LAIVs which were only detected on day 1 and, in some cases, on day 2 after vaccination.16,37 Infectious virus was isolated by egg culture from 20 nasal swab specimens, which gave us the opportunity to examine the genetic and phenotypic stability of the H2N2 LAIV strain after human passage. No reversions or other unwanted mutations were detected in any of the clinical isolates. In addition, although in a limited way, our study supports the absence of vaccine virus transmissibility to close unvaccinated contacts during the in-patient periods in this study. Absence of transmissibility of LAIV viruses has been observed not only with pandemic candidates but also with seasonal LAIVs. To our knowledge, thus far there was only a single documented case of type B vaccine virus transmission to an unvaccinated child in MedImmune's LAIV study in children.38
Our safety evaluation of the A/17/California/66/395 (H2N2) LAIV demonstrated that the vaccine was safe and well-tolerated by healthy adults, did not cause serious adverse events or an increased rate of moderate or severe reactogenicities. The few moderate self-limiting events observed were equally frequent in the vaccine and placebo groups. Similar observations have been made in clinical trials of other pandemic LAIVs subtypes H5N2 and H7N3 based on the Len/17 backbone, where the few solicited local and systemic reactions recorded were short in duration and with no consequences.37,39,40 Unfortunately, several earlier clinical studies of pandemic LAIVs based on AA/60 backbone excluded a placebo group, and therefore all adverse events (in 20–40% of vaccinees) were attributed to the vaccines.16
It has been previously shown that HAI antibody titers do not necessarily reflect the ability of influenza vaccines to protect against seasonal and pandemic influenza.41-43 This is particularly the case for LAIVs as applied to children, in whom no single correlate of protection has been identified.44 Several studies suggested that mucosal IgA antibody titers and cellular immune responses could be associated with protection by LAIV in children.45,46 Therefore, WHO recommends the assessment of immune responses to LAIV using a broad range of assays, such as HAI in combination with neutralization, local secretory antibody titers in upper respiratory tract secretions, as well as the evaluation of cellular immune responses with specific attention to antigen specific memory B- and T-cells.47 Following this strategy, we employed multiple immunological tests to ensure the adequate assessment of the H2N2 pandemic LAIV and demonstrated that the majority (92.6%) of the vaccinated subjects responded to the H2N2 LAIV in one or more immunological tests, including 85.2% of subjects with antibody responses and 55.6% volunteers with CMI responses (Table 9). Of note, there were only 4 subjects with no antibody responses detected, and these subjects did not shed virus at any time point. In contrast, all 24 subjects with detectable virus shedding presented one or more antibody responses (Tables 4 and 9). These data suggest a strong correlation between virus replication in the upper respiratory tract and the development of antibody responses, a correlation which would not have been detected if only HAI antibody responses had been measured.
Though the proportion of subjects with ≥4 -fold rises in antibody titers was relatively high, the antibody titers after 2 doses of the H2N2 LAIV remained significantly lower than those observed with seasonal LAIVs. Similar results were obtained in clinical trials of H7N3 and H5N2 pandemic LAIV candidates. A potential explanation for this is the absence of pre-existing immunity to pandemic viruses, whereas seasonal vaccines may act as booster vaccines given the presence of memory B-cells in most individuals resulting from multiple priming by natural exposure. In concordance with the results for the H2N2 LAIV presented here, a pandemic H2N2 inactivated influenza vaccine (IIV) also demonstrated markedly reduced immunogenicity when compared with seasonal IIVs.48 Even though the responses to pandemic LAIVs have been weak, they have been recently demonstrated to prime for strong immune responses after a one-dose booster with IIV.49 In that study, subjects vaccinated with pandemic LAIVs developed minimal HAI and MN antibody immune responses, but after a single immunization with an IIV several years later, they developed robust booster responses that included cross-reactive antibodies. It is likely that similar booster responses be observed after natural exposure with wild-type viruses. Since the Cal/66-based H2N2 LAIV tested in this study is by itself capable of inducing significant immune responses in naïve subjects after a primary immunization, it should be considered as an optimal candidate to prime populations, should the H2N2 influenza viruses re-emerge in humans. This is especially important for low income countries with a huge population density where the preferential use of LAIV is explained by several reasons, such as much easier scalability of the vaccine's production, the lower cost of a dose, the ease of intranasal administration and the ability to generate herd immunity.13,16,18
Methods
Study design and participants
This trial was a phase 1, double-blind, individually-randomized, placebo-controlled study of reactogenicity, safety and immunogenicity of a live monovalent A/17/California/66/395 (H2N2) influenza vaccine (H2N2 LAIV) and matched placebo conducted in an inpatient isolation unit operated by the Research Institute of Influenza (RII) in St Petersburg, Russia. Subjects were randomly distributed into 2 groups to receive either vaccine or placebo at a 3:1 vaccine/placebo ratio. The study used a "random permuted block" design to assure more equal spacing of the 2 treatments in the allocation sequence. The randomization scheme was generated according to procedures described in http://www.randomization.com. The study was approved by the Ministry of Health and Social Development of Russian Federation (Moscow, Russia), Research Institute of Influenza Ethics Committee (St Petersburg, Russia) and was conducted in compliance with the Declaration of Helsinki. The clinical trial was registered on http://www.clinicaltrials.gov/, under the identifier NCT01982331.
Two doses of the H2N2 LAIV or matched placebo were administered 28 d apart and all volunteers remained in the isolation unit for 6 d after receipt of each dose of study vaccine or placebo. The vaccine strain was a reassortant virus which contained the surface proteins hemagglutinin and neuraminidase genes from the human influenza virus A/California/1/66 (H2N2) and 6 internal gene proteins of the cold-adapted master donor virus A/Leningrad/134/17/57 (H2N2).22 Both LAIV and placebo were supplied by the manufacturer "Microgen" (Irkutsk, Russia). The LAIV was formulated to contain 107.5 EID50 per dose (0.5 ml).
Approximately one week prior immunization subjects were screened for eligibility through medical history review, physical examination, testing for serologic evidence of chronic viral infection [human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV)], routine biochemical and hematological blood tests and routine urinalysis. Women were tested for pregnancy using urine samples. All subjects underwent ear, nose and throat (ENT) examinations. A total of 38 seronegative subjects of both sexes and aged 18 through 40 y were eligible for the study, randomized and admitted to the isolation unit. According to the information obtained from the volunteers, none of them received seasonal influenza vaccine during 2 preceding seasons. For feasibility reasons and in order for an Independent Safety Monitor (ISM) to review safety data in a portion of subjects, the total cohort of 38 subjects was enrolled in 2 randomized sub-cohorts, a first cohort of 19 subjects (14 vaccine and 5 placebo), followed 2 weeks later by a second cohort of 19 subjects (14 vaccine and 5 placebo). After all volunteers of the first sub-cohort completed the first isolation period following receipt of dose one (Day 0 to Day 6), an interim safety review was performed by the ISM. The ISM reviewed all adverse events (AEs), including clinical laboratory evaluations (pre- and post-vaccination) and shedding data for all subjects and provided guidance to administer dose 2 to the first sub-cohort, and to enroll the additional 19 volunteers in the second sub-cohort.
At the time of admission to the isolation unit, nasal and throat swabs, saliva, and blood specimens were collected for virological and immunological testing. Blood specimens were also collected for routine biochemical and hematological blood tests. Subjects and investigators conducting assessments of safety were unaware of which allocation, the H2N2 LAIV or matched placebo, was received; study vaccine and placebo were masked. All subjects remained in the isolation unit for at least 6 d after receipt of the study vaccine or placebo and were carefully monitored for adverse reactions. Nasal and throat swabs were collected daily while subjects were in isolation to test for the presence of influenza virus shed in the upper respiratory tract. Any subject still exhibiting evidence of influenza virus shedding in the nasal or throat swab on Days 5 or 6 or Days 33 or 34 post-administration with each dose was supposed to be placed on influenza antiviral (oseltamivir) treatment at the standard dose for treatment of 75 mg (mg) twice a day for a course of 5 days; however such provision was not necessary, since none of the subjects was shedding virus after day 4. A final visit occurred at day 84 following the administration of dose 2 (or day 112 following the admission of dose 1) during which a final blood sample was collected for immunological testing.
Detection of shed virus by real-time RT-PCR
Nasal and throat swab specimens were tested for evidence of influenza virus using rRT-PCR. Specimen collection for this purpose occurred daily on Days 0–6 and Days 28–34, while the subjects were admitted to the isolation unit. Collection of the swab specimens was performed with sterile dry applicators (COPAN Diagnostics, Inc..) using standard procedure in accordance with MG 4.2.2136–06. Following collection of the material the applicator was placed into the sterile tube containing transport medium (COPAN). RNA was extracted from the swab specimens using a "RIBO–prep" Reagent Kit for RNA/DNA Isolation from Human Specimens (InterLabService, Moscow) followed by the first step of rRT-PCR testing performed using SuperScript III Platinum One–step qRT–PCR System (Invitrogen) and primers and probes for influenza virus A RNA isolation (CDC, Atlanta, USA). During the second step isolated RNA samples were subjected to "in–house" rRT-PCR testing using SuperScript III Platinum One–step qRT–PCR System (Invitrogen) and primers and probes for the H2 subtype HA of influenza A virus developed at the RII. Whenever the presence of other agents was suspected conventional tests were used to detect them.
Isolation of shed virus in chicken embryos
Samples from the nasal secretions obtained on days 1, 2, 3, 5, 6 after the first vaccination and on days 1 and 3 after the second vaccination, were tested for detection of viral shedding by inoculation into 10–11 day old embryonated chicken eggs ("Nazia" poultry plant, St Petersburg, Russia) and incubation at 32°C for 72 hours. The studies were conducted in accordance with the laws of the Russian Federation and complied with the official regulations: "Rules for working with experimental animals" approved on November 13, 1984 by Russian Ministry of Education. Eggs were chilled overnight before harvesting. The presence of an influenza virus was detected by standard hemagglutination (HA) test with 1% chicken red blood cells (RBCs) according to the WHO Manual.50 Allantoic fluids positive for HA were harvested and frozen. If no HA was present after the first passage, 2 additional passages were performed, before finally reporting whether the virus was isolated or not.
Genetic stability of vaccine viruses isolated from vaccinated volunteers
To evaluate genetic stability of H2N2 LAIV strain after replication in humans, viruses isolated from nasal specimens by culture in eggs were subjected to partial sequencing as described in.40 Briefly, viral RNA was extracted from virus-containing allantoic fluid followed by RT-PCR with segment-specific primer set. Nucleotide sequencing of the amplified regions was performed using an automated capillary sequencer 3130xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA) according to the instructions of the manufacturer. Multiple sequence alignment analysis was performed using Lasergene version 7.1 sequence analysis software. In addition, temperature sensitive and cold-adapted (ts/ca) phenotypes were determined for all clinical isolates by their titration in chicken embryos at various temperatures. Viruses were considered as ts if the titer at elevated temperatures over 39°C was ≤ 4.2 log10EID50/ml. Viruses were considered as having a ca phenotype if the titer at low temperature of 25°C was ≥ 5.7 log10EID50/ml.
Hemagglutination inhibition assay (HAI)
Serum samples were collected at Day 0, Day 28 after the first dose, Day 28 after the second dose (Day 56 of the study) and Day 84 after the second dose (Day 112 of the study). HAI tests were performed on serum samples with the conventional WHO-recommended assays.50 Sera were pretreated with receptor destroying enzyme (RDE, Denka Seiken, Japan) and tested against 4 HA units of the A/17/California/66/395 (H2N2) antigen. A 4-fold or greater antibody rise in titer was considered to be a seroconversion.50
Microneutralization assay (MN)
Serum specimens were tested for the presence of neutralizing antibodies against the A/17/California/66/395 (H2N2) influenza virus by MN assay using a culture of Madin–Darby Canine Kidney (MDCK) cells as described.51 Titers of neutralizing antibodies were expressed as reciprocal of the greatest dilution giving a neutralization of 50% of tissue cytopatic effects of the virus in the tissue culture (TCID50).
Secretory anti-influenza IgA
Nasal wick and saliva specimens were collected at Day 0, Day 28 after the first dose and Day 28 after the second dose (Day 56 of the study) and tested for the presence of IgA antibody against A/17/California/66/395 (H2N2) virus using Enzyme-linked immunosorbent assay (ELISA) according to.40 Sixteen HA units of the H2N2 vaccine virus were used as antigen.
Serum anti-influenza IgA and IgG
Serum samples collected at Days 0, 28, 56 and 112 were tested for the presence of A/17/California/66/395 virus-specific IgA and IgG antibodies by ELISA as described previously. Likewise, 16 HA units of the virus were used as antigen.
Quantification of IFN–γ producing CD4+ and CD8+ memory T–cells
Cellular immune responses were ascertained by a post–vaccination increase (%) of CD4 and CD8 Т–cell levels with respect to the mean counts in the placebo group. A higher than 3 standard deviations from a placebo mean was considered to be a response.40 The test was performed using peripheral blood mononuclear cells (PBMCs) collected at days 0, 6, 28, 56 and 112 and assessed in Beckman Coulter Navios flow cytofluorimeter (USA). Levels of the H2N2 vaccine virus-specific cells were measured by standard intracellular cytokine staining assay52 following in vitro stimulation with the vaccine strain A/17/California/66/395 (H2N2) at a dose of 6 MOI (multiplicity of infection). The following fluorescent markers were used for staining: Live/Dead stain (APC), CD4 (APC–AlexaFluor 750), CD8 (PC 5.5), CCR7 (FITC), CD45RA (ECD) and IFN–γ (PE).
Statistical analyses
Statistical analysis of the data was performed by Statistica 6 and GraphPad Prizm 5 software using the Wilcoxon Matched Pairs Test, Friedman ANOVA and Fisher exact test (2–tailed). All the protocol–specified analysis, including the results of the immunogenicity assays were conducted under blind. Unblinding of the study took place only after all the data had been locked.
Acknowledgments
We are thankful to Andrey Rekstin, Elena Doroshenko and Oleg Kiselev for the help in organizing this clinical trial; to Vera Krivitskaya from the Research Institute of Influenza for conducting microneutralization assays; to Vadim Tsvetnitsky, Kathy Neuzil, Yuxiao Tang, Kristin Bedell and Rick Bright from PATH for their collaboration on the development, preclinical and clinical trial of the H2N2 LAIV; and to all the volunteers who participated in the clinical trial. We are also thankful to Microgen Company for the production of the vaccine lot for the clinical trial and to PSI for their competent support in data management, statistical analysis and other operational aspects of the study.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The clinical trial was funded by PATH.
References
- 1. Mulder J, Masurel N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet 1958; 1:810-4; PMID:13526279; http://dx.doi.org/ 10.1016/S0140-6736(58)91738-0 [DOI] [PubMed] [Google Scholar]
- 2. Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, Webster RG. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology 1993; 194:781-8; PMID:7684877; http://dx.doi.org/ 10.1006/viro.1993.1319 [DOI] [PubMed] [Google Scholar]
- 3. Dunn FL. Pandemic influenza in 1957; review of international spread of new Asian strain. J Am Med Assoc 1958; 166:1140-8; PMID:13513331; http://dx.doi.org/ 10.1001/jama.1958.02990100028006 [DOI] [PubMed] [Google Scholar]
- 4. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998; 178:53-60; PMID:9652423; http://dx.doi.org/ 10.1086/515616 [DOI] [PubMed] [Google Scholar]
- 5. Shortridge KF. H2N2 influenza viruses in domestic ducks. Lancet 1979; 1:439; PMID:84284; http://dx.doi.org/ 10.1016/S0140-6736(79)90911-5 [DOI] [PubMed] [Google Scholar]
- 6. Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 1978; 87:13-20; PMID:664248; http://dx.doi.org/ 10.1016/0042-6822(78)90153-8 [DOI] [PubMed] [Google Scholar]
- 7. Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol 1989; 63:4603-8; PMID:2795713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makarova NV, Kaverin NV, Krauss S, Senne D, Webster RG. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J Gen Virol 1999; 80 ( Pt 12):3167-71; PMID:10567648 [DOI] [PubMed] [Google Scholar]
- 9. Glaser L, Zamarin D, Acland HM, Spackman E, Palese P, Garcia-Sastre A, Tewari D. Sequence analysis and receptor specificity of the hemagglutinin of a recent influenza H2N2 virus isolated from chicken in North America. Glycoconj J 2006; 23:93-9; PMID:16575526; http://dx.doi.org/ 10.1007/s10719-006-5441-0 [DOI] [PubMed] [Google Scholar]
- 10. Ma W, Vincent AL, Gramer MR, Brockwell CB, Lager KM, Janke BH, Gauger PC, Patnayak DP, Webby RJ, Richt JA. Identification of H2N3 influenza A viruses from swine in the United States. Proc Natl Acad Sci U S A 2007; 104:20949-54; PMID:18093945; http://dx.doi.org/ 10.1073/pnas.0710286104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis 2006; 12:9-14; PMID:16494710; http://dx.doi.org/ 10.3201/eid1201.051254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO WHO global influenza preparedness plan. WHO/CDS/CSR/GIP/2005.5 http://www.who.int/csr/resources/publications/influenza/en/WHO_CDS_CSR_GIP_2005_5.pdf. 2005. [DOI] [PubMed] [Google Scholar]
- 13. WHO Global pandemic influenza action plan to increase vaccine supply. Geneva, Belgium WHO/IVB/0613 WHO/ODS/EPR/GIP/20061 http://whqlibdocwhoint/hq/2006/WHO_IVB_0613_engpdf 2006. [Google Scholar]
- 14. Nabel GJ, Wei CJ, Ledgerwood JE. Vaccinate for the next H2N2 pandemic now. Nature 2011; 471:157-8; PMID:21390107; http://dx.doi.org/ 10.1038/471157a [DOI] [PubMed] [Google Scholar]
- 15. Stohr K. Vaccinate before the next pandemic? Nature 2010; 465:161; PMID:20463719; http://dx.doi.org/ 10.1038/465161a [DOI] [PubMed] [Google Scholar]
- 16. Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 2014; 13:855-71; PMID:24867587; http://dx.doi.org/ 10.1586/14760584.2014.922417 [DOI] [PubMed] [Google Scholar]
- 17. Rudenko L, van den Bosch H, Kiseleva I, Mironov A, Naikhin A, Larionova N, Bushmenkov D. Live attenuated pandemic influenza vaccine: clinical studies on A/17/California/2009/38 (H1N1) and licensing of the Russian-developed technology to WHO for pandemic influenza preparedness in developing countries. Vaccine 2011; 29 Suppl 1:A40-4; PMID:21684428; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.122 [DOI] [PubMed] [Google Scholar]
- 18. Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, Rekstin AR, Beljaev AL, Bragina VE, Cox N, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis 1993; 168:881-7; PMID:8376833; http://dx.doi.org/ 10.1093/infdis/168.4.881 [DOI] [PubMed] [Google Scholar]
- 19. Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JG, et al. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J Infect Dis 2011; 204:1491-9; PMID:21957153; http://dx.doi.org/ 10.1093/infdis/jir596 [DOI] [PubMed] [Google Scholar]
- 20. He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 2006; 80:11756-66; PMID:16971435; http://dx.doi.org/ 10.1128/JVI.01460-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudenko L, Isakova-Sivak I, Donina S. H7N3 live attenuated influenza vaccine has a potential to protect against new H7N9 avian influenza virus. Vaccine 2013; 31:4702-5; PMID:23988294; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.040 [DOI] [PubMed] [Google Scholar]
- 22. Isakova-Sivak I, de Jonge J, Smolonogina T, Rekstin A, van Amerongen G, van Dijken H, Mouthaan J, Roholl P, Kuznetsova V, Doroshenko E, et al. Development and pre-clinical evaluation of two LAIV strains against potentially pandemic H2N2 influenza virus. PloS one 2014; 9:e102339; PMID:25058039; http://dx.doi.org/ 10.1371/journal.pone.0102339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anonymous Development of a Clinical Trial Plan for Pandemic Influenza Vaccines. Department of Health and Human Services. National Institute of Allergy and Infection Diseases. September 22–23, 2003, Bethesda, Maryland. Meeting Summary. http://www.niaid.nih.gov/about/organization/dmid/Documents/pansummary.pdf. 2003. [Google Scholar]
- 24. Pyhala R. Antibody status to influenza A/Singapore/1/57(H2N2) in Finland during a period of outbreaks caused by H3N2 and H1N1 subtype viruses. J Hyg 1985; 95:437-45; PMID:4067298; http://dx.doi.org/ 10.1017/S0022172400062860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gambaryan AS, Lomakina NF, Boravleva EY, Kropotkina EA, Mashin VV, Krasilnikov IV, Klimov AI, Rudenko LG. Comparative safety, immunogenicity, and efficacy of several anti-H5N1 influenza experimental vaccines in a mouse and chicken models (Testing of killed and live H5 vaccine). Influenza Other Respir Viruses 2012; 6:188-95; PMID:21951678; http://dx.doi.org/ 10.1111/j.1750-2659.2011.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses 2011; 5:67-75; PMID:21306569; http://dx.doi.org/ 10.1111/j.1750-2659.2010.00183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ambrose CS, Walker RE, Connor EM. Live attenuated influenza vaccine in children. Semin Pediatr Infect Dis 2006; 17:206-12; PMID:17055372; http://dx.doi.org/ 10.1053/j.spid.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 28. Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J 2010; 29:806-11; PMID:20458256; http://dx.doi.org/ 10.1097/INF.0b013e3181e2872f [DOI] [PubMed] [Google Scholar]
- 29. Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM, Group C-TCES . Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685-96; PMID:17301299; http://dx.doi.org/ 10.1056/NEJMoa065368 [DOI] [PubMed] [Google Scholar]
- 30. Clements ML, Betts RF, Murphy BR. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet 1984; 1:705-8; PMID:6143042; http://dx.doi.org/ 10.1016/S0140-6736(84)92222-0 [DOI] [PubMed] [Google Scholar]
- 31. Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, Oymar K, Garcia ML, Krygier A, Costa H, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006; 25:860-9; PMID:17006278; http://dx.doi.org/ 10.1097/01.inf.0000237797.14283.cf [DOI] [PubMed] [Google Scholar]
- 32. Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011; 204:845-53; PMID:21846636; http://dx.doi.org/ 10.1093/infdis/jir436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aleksandrova GI, Mikutskaia BA, Sirotenko EA, Pleshanova RA, Shaposhnikova RP. [Results in the study of a special live influenza vaccine variant for the immunization of preschool and young school-age children]. Vestn Akad Med Nauk SSSR 1968; 23:41-5; PMID:5745694 [PubMed] [Google Scholar]
- 34. Talaat KR, Karron RA, Liang PH, McMahon BA, Luke CJ, Thumar B, Chen GL, Min JY, Lamirande EW, Jin H, et al. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respir Viruses 2012; 7:66-73; PMID:22417012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen GL, Lamirande EW, Yang CF, Jin H, Kemble G, Subbarao K. Evaluation of replication and cross-reactive antibody responses of H2 subtype influenza viruses in mice and ferrets. J Virol 2010; 84:7695-702; PMID:20504935; http://dx.doi.org/ 10.1128/JVI.00511-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindstrom SE, Cox NJ, Klimov A. Genetic analysis of human H2N2 and early H3N2 influenza viruses, 1957-1972: evidence for genetic divergence and multiple reassortment events. Virology 2004; 328:101-19; PMID:15380362; http://dx.doi.org/ 10.1016/j.virol.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 37. Rudenko L, Kiseleva I, Larionova N, Naykhin A, Donina S, Petukhova G, Dubrovina I, Bazhenova E, Stukova M, Erofeeva M, et al. Clinical Testing of Pre–pandemic Live Attenuated A/H5N2 Influenza Candidate Vaccine in Adult Volunteers. Vaccine 2015; In press. [DOI] [PubMed] [Google Scholar]
- 38. Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpaa R, Salmi A, Saville MK, Cho I, Razmpour A, Rappaport R, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590-5; PMID:16804427; http://dx.doi.org/ 10.1097/01.inf.0000220229.51531.47 [DOI] [PubMed] [Google Scholar]
- 39. Rudenko L, Desheva J, Korovkin S, Mironov A, Rekstin A, Grigorieva E, Donina S, Gambaryan A, Katlinsky A. Safety and immunogenicity of live attenuated influenza reassortant H5 vaccine (phase I-II clinical trials). Influenza Other Respir Viruses 2008; 2:203-9; PMID:19453396; http://dx.doi.org/ 10.1111/j.1750-2659.2008.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rudenko L, Kiseleva I, Naykhin AN, Erofeeva M, Stukova M, Donina S, Petukhova G, Pisareva M, Krivitskaya V, Grudinin M, et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PloS One 2014; 9:e87962; PMID:24533064; http://dx.doi.org/ 10.1371/journal.pone.0087962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879-85; PMID:21998477; http://dx.doi.org/ 10.1093/infdis/jir661 [DOI] [PubMed] [Google Scholar]
- 42. Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081-5; PMID:21983214; http://dx.doi.org/ 10.1097/INF.0b013e3182367662 [DOI] [PubMed] [Google Scholar]
- 43. Kulkarni PS, Agarkhedkar S, Lalwani S, Bavdekar AR, Jog S, Raut SK, Parulekar V, Agarkhedkar SS, Palkar S, Mangrule S. Effectiveness of an Indian-made Attenuated influenza A(H1N1pdm 2009 vaccine: A case control study. Hum Vaccin Immunother 2014; 10; 566-71; PMID:24406308; http://dx.doi.org/ 10.4161/hv.27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden FG, Bernstein DI, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis 2000; 181:1133-7; PMID:10720541; http://dx.doi.org/ 10.1086/315323 [DOI] [PubMed] [Google Scholar]
- 45. Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine 2012; 30:6794-801; PMID:23000125; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 46. Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol 2008; 15:1042-53; PMID:18448618; http://dx.doi.org/ 10.1128/CVI.00397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Girard MP, Katz JM, Pervikov Y, Hombach J, Tam JS. Report of the 7th meeting on Evaluation of Pandemic Influenza Vaccines in Clinical Trials, World Health Organization, Geneva, 17-18 February 2011. Vaccine 2011; 29:7579-86; PMID:21856358; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.031 [DOI] [PubMed] [Google Scholar]
- 48. Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol 2002; 191:203-8; PMID:12458361; http://dx.doi.org/ 10.1007/s00430-002-0147-9 [DOI] [PubMed] [Google Scholar]
- 49. Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014; 209:1860-9; PMID:24604819; http://dx.doi.org/ 10.1093/infdis/jiu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. WHO WHO Manual on animal influenza diagnosis and surveillance. Available from: http://www.bvsde.paho.org/bvsacd/cd52/animal.pdf accessed 12 August 2013 2002. [Google Scholar]
- 51. Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937-43; PMID:10074505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curr Protoc Immunol 2014; Chapter 3:Unit 3.12. PMID: 18432927; http://dx.doi.org/ 10.1002/0471142735.im0312s60. [DOI] [PubMed] [Google Scholar]