Abstract
This randomized, blinded study evaluated the immunogenicity and safety of a booster dose of Gardasil (qHPV) or Cervarix (bHPV) when administered to 12–13 year-old girls who were vaccinated at the age of 9–10 with 2 doses of qHPV (0–6 months). 366 out of 416 eligible girls participated in this follow-up study. Antibody titers were measured just before and one month post-booster. A Luminex Total IgG assay was used for antibody assessment and results are presented in Liminex Units (LU). Three years post-primary vaccination, 99–100% of subjects had detectable antibodies to 4HPV genotypes included in the qHPV with GMTs varying from 50 to 322 LU depending on genotype. After a booster dose of qHPV, a ≥4 fold increase of antibody titers to genotypes included in the vaccine was observed in 88–98% of subjects. Post-booster GMTs varied from 1666 to 4536 LU depending on genotype. These GMTs were 1.1 to 1.8-fold higher when compared to those observed one month post-second dose. After a booster of bHPV, a ≥4 fold increase of antibody titers to HPV16 and HPV18 was observed in 93–99% of subjects. The anti-HPV16 and HPV18 GMTs were 5458 and 2665 LU, respectively. These GMTs were 1.2 and 1.8 higher than those observed in the qHPV group (both P < 0.01). In bHPV group a 1.4–1.6-fold increase of antibody GMTs to HPV6 and HPV11was also observed (P < 0.001). The safety profile was acceptable for both vaccines. Both qHPV and bHPV increase antibody titers when given as a booster to girls previously vaccinated with 2 doses of qHPV. The magnitude of the immune response after booster is vaccine-dependent and has the same pattern as that reported after primary vaccination with qHPV or bHPV. When given as a booster, both vaccines have an acceptable safety profile. Longer follow-up studies are warranted to assess the need of booster doses.
Keywords: HPV vaccines, two dose schedule, booster dose
Introduction
Human papillomavirus (HPV) vaccines have been available for almost a decade and are extensively used in pre-adolescent and adolescent girls. The clinical efficacy of HPV vaccines have been demonstrated in 16–45 years-old.1-3 The approval of HPV vaccines use in pre-adolescents and young adolescents was based exclusively on bridging immunogenicity data.4,5 In pre-licensure clinical trials conducted by vaccine manufacturers, the 0, 1–2 and 6 months schedule was used4,5 and only a few post-licensure clinical trials assessed the immunogenicity and efficacy of alternative vaccination schedules including a 0, 6 month schedule in 9–13 year-old girls.6-10 Existing data show that 2 doses of HPV vaccine administered to pre-adolescents induce an immune response which is similar to and even higher than the immune response observed after 3 doses given to 16–26 year-old females.11 These observations raised the question regarding the number of doses needed when vaccinating pre-adolescent girls. We have previously reported the immunogenicity results when using qHPV (quadrivalent vaccine Gardasil® contains HPV6, HPV11, HPV16 and HPV18) according to a 0–6 month schedule in 9–10 year-old girls.10 The results showed that 6 months post-first dose 94–100% of vaccinated girls have detectable antibodies to 4 HPV genotypes included in the vaccine and a 55–99-fold increase in antibody titers was observed post-second dose administration. However, little data on persistence of immunity exist when using qHPV in a 2-dose schedule and no data has been reported about the magnitude of the immune response to a booster third dose of vaccine when given a few years post-second dose.
Additionally, despite a relatively long period of time since the beginning of clinical use of qHPV and bHPV (bivalent vaccine Cervarix® contains HPV16 and HPV18), no evidence-based data on the interchangeable use of these 2 vaccines in humans is available.
The main objective of this study was to assess the effect of a booster dose of qHPV or bHPV when administered 3 y post-primary vaccination with 2 doses of qHPV according to 0–6 month schedule. The immunogenicity and safety profile of the 2 HPV vaccines were assessed.
Methods
Population and study design
Girls who participated in 2008–2009 in the clinical trial with 2 doses of qHPV were invited to continue their participation in this follow-up phase of the study. Subjects’ socio-demographic characteristics and immunogenicity and safety results post-first and post-second dose were reported elsewhere.10,12 In 2011–2012 subjects were 12–13 year-old. Those who accepted to continue their participation were randomized 1:1 (SAS Institute software) to receive either a dose of qHPV (Group qHPV) or a dose of bHPV (Group bHPV). Subjects and their parents were blinded to which group they were assigned for the 4 week period during which the safety profile was assessed.
This study is registered with ClinicalTrials.gov, NCT01456715.
Vaccines administration and blood sampling
A lot of qHPV and a lot of bHPV commercially available in Canada were used. Dosages recommended by the manufacturers were followed.4,5 Both vaccines were administered intramuscularly in the deltoid. Blood samples were collected just before and one month post-booster administration. Ten milliliters of blood were collected at each study time point.
Safety assessment
Data on injection site (pain, swelling, or redness at the injection site) and systemic adverse events (fatigue, headache, nausea/gastrointestinal upset, fever, rash, myalgia, arthralgia, and urticaria) were collected on standardized diary cards for 5 d after vaccine administration. The maximal severity of injection site reactions and systemic adverse events were recorded. Parents and subjects were invited to report any serious adverse event or any significant new chronic condition arising within 12 months of vaccine administration.
Laboratory procedures
Like in the previous phase of the study, laboratory assays were done at the Laboratoire de santé publique du Québec (LSPQ). HPV antibody titers were measured by a Luminex Total IgG assay.13,14 HPV antibody titers are presented in Luminex Units (LU).
Data analysis
We assessed the proportion of subjects with detectable anti-HPV in the 2 study groups and because there is no consensus for seroprotective titers for anti-HPV we used also the threshold of 3 LU and 10 LU in our analyses. The threshold of 3 LU correlates the best with positive results obtained with other assays used during assay validation.13 We arbitrarily decided that the threshold of 10 LU would be also helpful in understanding the anti-HPV titers distribution pre- and post-third dose. An anamnestic response was defined a priory as an antibody titer increase of ≥4-fold (the most often used criterion for other vaccines).15 Log transformed titers were used for geometrical mean titers (GMTs) calculation. To allow GMTs calculation, samples with undetectable antibodies were assigned the arbitrary value of 1 LU. Fisher's exact test was used for the comparison of proportions, Wilcoxon test for continuous variables and Kolmogorov-Smirnov test for comparison of titers distribution. All statistics were 2-tailed. P values of 0.05 or less were considered significant. SAS Institute software version 9.2 (Cary, NC, USA) was used for statistical analysis.
Results
A total of 366 (88%) subjects out of 416 who participated in the 2008–2009 phase of the study accepted to continue their participation. The 366 subjects who received a booster dose of vaccine were included in the safety assessment. The immunogenicity analysis included 363 participants as 3 subjects had only one blood sample collected (pre- or post-booster dose) and were excluded.
Antibody persistence and GMTs pre-booster
Thirty six months post-second dose administration all but 2 subjects randomized to Group qHPV and 2 randomized to Group bHPV had detectable antibodies to HPV18 (99%) and all (100%) had detectable antibodies to HPV6, HPV11 and HPV16. In both study groups 97–100% of subjects had an anti-HPV ≥3 LU and 89–100% had an anti-HPV titer ≥10 LU. GMTs varied from 50 to 332 LU depending on HPV genotype (Table 1). Similar proportions of seropositivity and GMTs were observed in 2 study groups pre-booster (all p > 0.3).
Table 1.
Proportion of subjects with detectable anti-HPV, ≥3 LU and ≥10 LU anti-HPV and GMTs in 2 study groups pre- and post-booster administration
| Group qHPV pre-booster | Group bHPV pre-booster | Group qHPV 1 month post-booster | Group bHPV 1 month post-booster | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 181 | N = 182 | N = 181 | N = 182 | |||||||||||||
| Results | % ≥1 LU | % ≥3 LU | % ≥10 LU | GMT 95%CI | % ≥1 LU | % ≥3 LU | % ≥10 LU | GMT 95%CI | % ≥1 LU | % ≥3 LU | % ≥10 LU | GMT 95%CI | % ≥1 LU | % ≥3 LU | % ≥10 LU | GMT 95%CI |
| HPV 6 | 100 | 100 | 96 | 82 | 100 | 99 | 94 | 81 | 100 | 100 | 100 | 1666 | 100 | 100 | 99 | 127 |
| 70–95 | 68–96 | 1506–1844 | 112–145 | |||||||||||||
| HPV 11 | 100 | 100 | 100 | 312 | 100 | 100 | 100 | 332 | 100 | 100 | 100 | 4419 | 100 | 100 | 100 | 474 |
| 271–359 | 289–382 | 4064–4805 | 421–534 | |||||||||||||
| HPV 16 | 100 | 100 | 100 | 322 | 100 | 100 | 99 | 289 | 100 | 100 | 100 | 4536 | 100 | 100 | 100 | 5458 |
| 278–374 | 246–340 | 4168–4937 | 4989–5971 | |||||||||||||
| HPV 18 | 99 | 97 | 89 | 50 | 99 | 98 | 91 | 54 | 100 | 100 | 100 | 1710 | 100 | 100 | 100 | 2665 |
| 42–61 | 44–65 | 1523–1919 | 2364–3006 | |||||||||||||
HPV immunogenicity results 1 month post-booster
All subjects in both study groups had an antibody titer ≥3 LU to all 4 HPV genotypes included in qHPV and only one subject in Group bHPV did not reach an antibody titer ≥10 LU (to HPV 6) (Table 1).
In Group qHPV, the booster dose administration was followed by an increase of GMTs to all 4 types included in the vaccine (Table 1). A ≥4-fold antibody increase post/pre-booster was observed in 94%, 89%, 88%, and 98% of subjects for HPV6, 11, 16 and 18, respectively. For GMTs, there was a 20-fold increase to HPV6, a 14-fold increase to HPV11 and HPV16, and a 34-fold increase to HPV18 (all P < 0.0001).
In Group bHPV after the booster administration a ≥4-fold antibody increase for HPV16 and HPV18 was observed in 93 and 99% of subjects, respectively. For the GMTs there was a 1.6-fold increase for HPV6 (P < 0.0001), a 1.4-fold increase for HPV11 (p = 0.0002), a 19-fold increase for HPV16 (P < 0.0001) and a 49-fold increase for HPV18 (P < 0.0001).
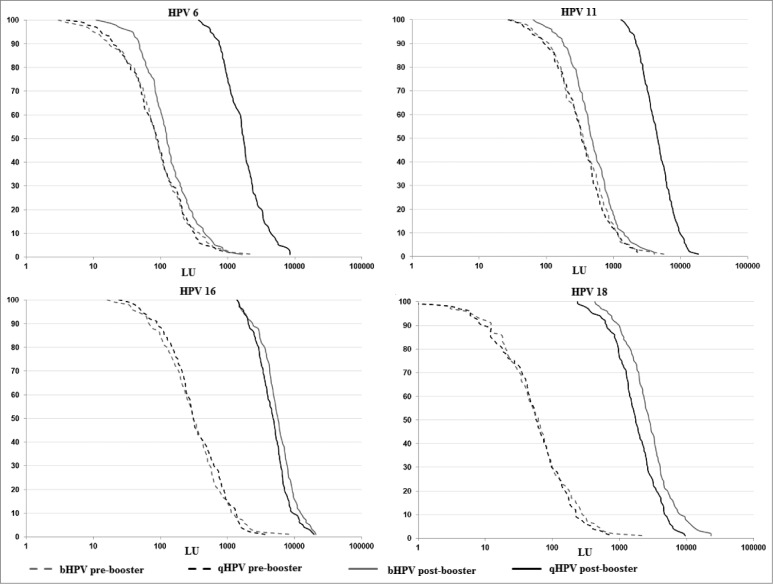
There were significant differences between bHPV and qHPV in the distribution of antibody titers after the booster dose (Fig. 1). The GMTs to HPV16 and HPV18 in Group bHPV were significantly higher than those observed in Group qHPV (p = 0.002 for HPV16 and P < 0.0001 for HPV18). However, the GMTs to HPV6 and HPV11 were about 10 times lower in Group bHPV than in Group qHPV (0.08 for HPV6 and 0.11 for HPV11; both P < 0.0001). The comparison of the distribution of antibody titers to 4 HPV genotypes included in the qHPV vaccine post-booster with qHPV or bHPV confirmed statistical significant difference for HPV6, HPV11, HPV18 (P < 0.0001) and for HPV16 (p = 0.01).
Figure 1.
Anti-HPV titers distribution pre- and post-booster.
Safety assessment
Eighty one percent of subjects in Group qHPV and 91% in Group bHPV reported at least one local site adverse event (p = 0.004), and 59% and 58% a systemic adverse event (p = 0.83), respectively.
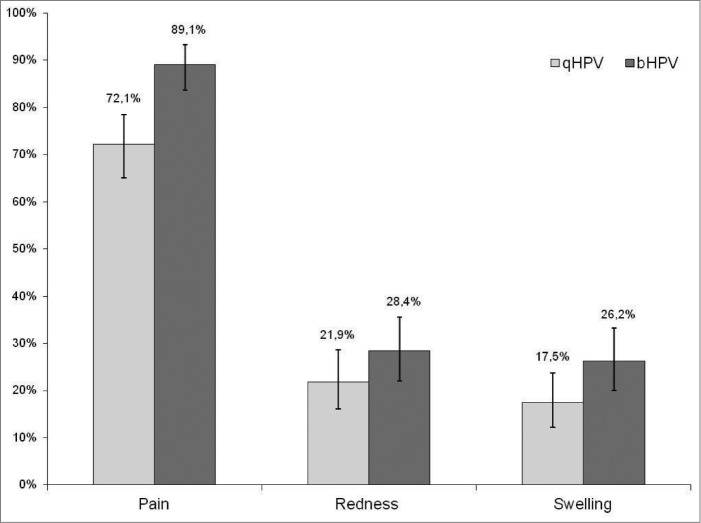
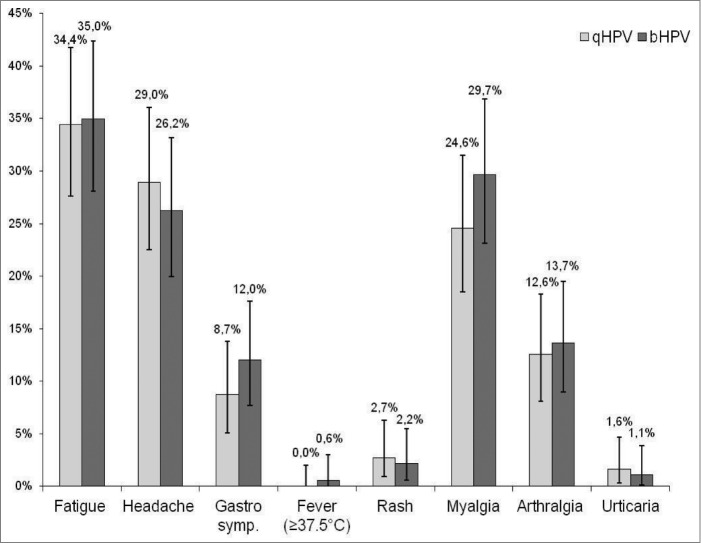
In both groups, pain at the injection site was the most often reported local adverse event. A higher proportion of subjects in Group bHPV than in Group qHPV reported pain 89% vs. 72% (P < 0.0001) and swelling 26% vs. 18% (p = 0.04), respectively (Fig. 2). Grade 3 pain at the injection site was also more often reported after bHPV than after qHPV (9% vs. 2%; P = 0.003). Redness and swelling of > 50 mm were reported in 0–1.6% of subjects with no significant difference between 2 study groups. In both study groups similar proportions of subjects reported various systemic adverse events (Fig. 3). No adverse event required medical intervention and no serious adverse event was reported during the follow-up period.
Figure 2.
Proportion of subjects who reported different local adverse events.
Figure 3.
Proportion of subjects who reported different systemic events.
Discussion
To our knowledge this is the first study to compare the effect of the 2 available HPV vaccines given as a booster dose to girls previously vaccinated with 2 doses of qHPV. In this study, 3 y after primary vaccination with 2 doses of qHPV nearly all subjects had detectable antibodies to HPV types included in the qHPV vaccine and had an increase in antibody titers post-booster. This is similar to the effect of a booster dose administered 5 y after primary vaccination of young females with a 3 dose schedule (0, 1–2, 6 months)5,16,17 and that despite of differences in age at time of vaccination and laboratory assays used. The great majority of subjects also showed an anamnestic response which was defined as at least a 4-fold increase in antibody titers against HPV genotypes included in the vaccines. However, differently from studies with other recombinant vaccines like hepatitis B, where a 10–16 fold increase of GMTs were reported one month post-booster dose when compared to one month post-primary vaccination18,19 in this study GMTs increase was much lower. In fact, in Group qHPV the GMTs ratios 1 month post-booster dose versus 1 month post 2nd dose were 1.4, 1.1, 1.3, and 1.8 for HPV 6, 11, 16 and 18, respectively (data not shown). Previously reported by Olsson et al. GMTs ratios post-booster dose vs. post-third dose of qHPV (first 3 doses given at the age of 16–23 and the booster dose at the age of 21–28) were in the same range.16 These observations might indicate that antibody titers obtained after 2 doses in pre-adolescents or 3 doses in young females are close to maximal values.
There was a significantly higher antibody response to HPV16 and HPV18 after administration of a booster dose of bHPV than qHPV. This is consistent with the higher immunogenicity of bHPV compared to qHPV after the primary vaccination with 3 doses given according to 0, 1–2 and 6 months schedule.20,21 As previously suggested this difference may be due to different adjuvants used in 2 HPV vaccines.20
As expected, anti-HPV6 and anti-HPV11 antibody titers were higher after the qHPV than after bHPV booster. However, the 1.4–1.6 increase in anti-HPV6 and HPV11 after bHPV administration is interesting. Potential mechanisms for this observation include the presence of type common antibodies22 or the presence of L1 sequences that are highly conserved between genotypes and are interspersed among segments of conserved regions.23 Our observation may explain at least partially previously reported moderate efficacy of bHPV against persistent infections with HPV 6/11 (VE-34.5%) and HPV74 (VE-49.5%)24 and the observed decline (−20.8%) in external genital warts in young women in England a few years after the implementation of an immunization program with bHPV.25 These observations warrant further investigation.
The results indicate that both vaccines have a clinically acceptable safety profile. The great majority of reported adverse events were minor and none required medical intervention. Consistently with previously published data20,26 a higher proportion of subjects who received bHPV reported local reactions and the main reported local reaction was pain at the injection site. No significant difference was observed when comparing the reported systemic adverse events in the 2 study groups. These results indicate that the regimen used in this study is safe.
In our opinion, the immunogenicity and safety results indicate that the qHPv and bHPV can be given to the same vaccinees. This knowledge is important when no information is available on which HPV vaccine was previously given to an individual, in case of manufacturing problems with a given vaccine and when important differences in vaccine price makes the interchangeable use of vaccines more attractive from cost-effectiveness perspective. Additionally, presently available data do not allow excluding that the use of one dose of qHPV and one dose of bHPV might be an alternative approach when vaccinating pre-adolescents.
This study has some limitations. First, 12% of subjects who were initially enrolled did not participate in this follow-up phase of the study. However, socio-demographic characteristics and immunogenicity results observed post-second dose of vaccine were similar in study subjects participating and not participating in this phase of the study. Thus, it is highly plausible that no different persistence of immunity or response to a booster dose would occur in non-participating subjects. Second, as previously reported10 we used relatively strict enrollment criteria in 2008–2009 and immunogenicity results presented here should not be extrapolated to individuals with underlying comorbidities, especially to the immunocompromised individuals. Third, we did not measure the antibody avidity or cellular immune response and cannot exclude some differences in the 2 study groups. In a previous Canadian study, the age of the recipient significantly impacted generation of HPV-18-specific B memory cells, while the number of doses displayed a significant effect on the development of HPV-specific T memory cells.27 In another recent publication it is mentioned that the anamnestic response typically shows the presence of memory B cells with the capacity to provide rapid antibody production following a booster dose of a virus challenge.28 However, the role of B and/or T cell memory as well as the level of antibody avidity and neutralization potential in protection against clinical HPV infections remain unknown and need further investigation. Additionally, the response to a booster dose of HPV vaccine cannot be directly extrapolated to the response arising from exposure to the virus which generally represents a localized infection of the mucosa. Fourth, we assessed the immunogenicity and tolerability profile when giving qHPV or bHPV boosters to girls who were previously primed with qHPV. We cannot exclude that different results might be observed when priming with bHPV which is known to induce a higher immune response to high risk HPV16 and HPV18.20,26 Our hypothesis is that higher antibody titers will be observed to HPV16 and HPV18 when priming with Cervarix, however with such a scenario we cannot exclude lower antibody titers to HPV6 and HPV11. To note also that the need and importance of the presence of high antibody titers post-vaccination in protection against clinical disease has not been demonstrated. Finally, we assessed the immune response only one month post-challenge dose of vaccine given at the time when an antibody plateau is expected (18–36 months post-second dose as reported in a previous study11) and long-term data would be helpful when deciding about the effect and persistence of immunity after a booster dose of qHPV or bHPV.
The main novelty of the study is the generation of first immunogenicity and safety data on interchangeable use of 2 presently available HPV vaccines as well as the assessment of the magnitude of immune response to a booster dose given to girls previously vaccinated with 2 doses of qHPV. The main strength of the study is that it was conducted in an age group which is targeted by most presently in place publicly funded HPV immunization programs. This helps with extrapolation of results to such programs.
In summary, both qHPV and bHPV increase antibody titers when given as a booster to girls previously vaccinated with 2 doses of qHPV. The magnitude of the immune response after booster is vaccine-dependent and has the same pattern as that reported after primary vaccination with qHPV or bHPV. When given as a booster, both vaccines have an acceptable safety profile.
Our results do not support the need of booster doses when vaccinating pre-adolescent girls with 2 doses of qHPV vaccine according to 0–6 month schedule. Longer follow-up studies are warranted to assess the need of booster doses after primary vaccination with 2 as well as with 3 doses. However, because of unknowns regarding the importance of post-vaccination antibodies persistence and the role of immune memory in protection against clinical disease the need of boosters should be based mainly on the results of monitoring of potential breakthrough cases in individuals vaccinated before sexual debut.
Acknowledgments
We are grateful to all study participants, to our research nurses, coordinators and research technicians.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was financially supported by the Quebec Ministry of Health and Social Services. No private company was involved in any way in study designing, conducting or data analysis and results interpretation.
References
- 1. Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on All HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102(5):325-39; PMID:20139221; http://dx.doi.org/ 10.1093/jnci/djp534 [DOI] [PubMed] [Google Scholar]
- 2. Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010; 341:c3493; PMID 20647284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374(9706):1975-85; PMID 19962185 [DOI] [PubMed] [Google Scholar]
- 4. Merck Frosst Canada ltée GARDASIL - Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine. Kirkland: Merck Canada Inc; August 26, 2011 2011. [Google Scholar]
- 5. GlaxoSmithKline Inc. CERVARIX - Human Papillomavirus vaccine Types 16 and 18 (Recombinant, AS04-adjuvanted). Ontario: GlaxoSmithKline Inc; May 02, 2013 2013. [Google Scholar]
- 6. Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103(19):1444-51; PMID:21908768; http://dx.doi.org/ 10.1093/jnci/djr319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GlaxoSmithKline Cervarix - Efficacity and Immunogenicity. GSKVaccinesDirect.com 2013; https://www.gskvaccines.ca/gsk.ca/CA/htdocs/products/CERVARIX/product_info_efficacy.htm. Accessed July 10th, 2013 [Google Scholar]
- 8. LaMontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. Immunogenicity of quadrivalent HPV vaccine among girls aged 11-13 years vaccinated using alternative dosing schedules: results 32 months after third dose. Oral presentation 28th International Papillomavirus Conference, San Juan, Puerto Rico, November 30 - December 6, 2012. [Google Scholar]
- 9. Safaejan M, Mugisha E, Pan Y, Kumakech E, Kemp T, Cover J, Pinto L, LaMontagne DS. Immunogenicity of the bivalent HPV vaccine among partially vaccinated young girls in Uganda. Oral presentation 28th International Papillomavirus Conference, San Juan, Puerto Rico, November 30 - December 6, 2012. [Google Scholar]
- 10. Gilca V, Sauvageau C, Boulianne N, De Serres, G, Couillard, M, Krajden, M, Ouakki, M, Murphy, D, Trevisan, A, Dionne, M. Immunogenicity of quadrivalent HPV and combined hepatitis A and B vaccine when co-administered or administered one month apart to 9-10 year-old girls according to 0-6 month schedule. Hum Vaccin Immunother 2014; 10(8):2438-45; PMID 25424952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309(17):1793-802; PMID:23632723; http://dx.doi.org/ 10.1001/jama.2013.1625 [DOI] [PubMed] [Google Scholar]
- 12. Gilca V, Dionne M, Sauvageau C, De Serres G, Boulianne N, Ouakki M. Gardasil and Twinrix co-administration: preliminary safety data. Poster presentation 25th International Papillomavirus Conference, May 8-14, Malmö, Sweden: 2009. [Google Scholar]
- 13. Gilca V., Sauvageau C., Krajden M., Cook, D., Dagenais, C., Therrien, C., Trevisan, A., Couillard, M. Comparison of different immunoassays used for HPV antibody assessment in vaccinated and unvaccinated individuals. Poster presentation 29th Annual International Papillomavirus Conference and Public Health & Clinical Workshops, Seattle, USA, 20-25 August, 2014. [Google Scholar]
- 14. Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, McNeil S, Money D, Dionne M, Palefsky J, et al. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014; 32(5):624-30; PMID:24055350; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 15. Plotkin SA, Orenstein WA, Offit PA. Vaccines. Sixth Edition ed. Philadelphia: Saunders Elsevier; 2012. [Google Scholar]
- 16. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Høye J, Steinwall M, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007;25(26):4931-39; PMID:17499406 [DOI] [PubMed] [Google Scholar]
- 17. Moscicki AB, Wheeler CM, Romanowski B, Hedrick J, Gall S, Ferris D, Poncelet S, Zahaf T, Moris P, Geeraerts B, et al. Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012;31(1):234-41; PMID:23063422 [DOI] [PubMed] [Google Scholar]
- 18. Gilca V, De Serres G, Boulianne N, De Wals P, Murphy D, Trudeau G, Massé R, Duval B, et al. Antibody kinetics among 8-10 years old respondents to hepatitis B vaccination in a low endemic country and the effect of a booster dose given five or ten years later. Vaccine 2009; 27(43):6048-53; PMID:19683086 [DOI] [PubMed] [Google Scholar]
- 19. Gilca V, De Serres G, Boulianne N, De Wals P, Murphy D, Trudeau G, Deceuninck G, Massé R, Duval B. Antibody and immune memory persistence after vaccination of preadolescents with low doses of recombinant hepatitis B vaccine. Hum Vaccin 2010; 6(2):212-8; PMID:19946212 [DOI] [PubMed] [Google Scholar]
- 20. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5(10):705-19; PMID:19684472 [DOI] [PubMed] [Google Scholar]
- 21. Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12-15 year old girls. PloS one 2013; 8(5):e61825; PMID:23650505; http://dx.doi.org/ 10.1371/journal.pone.0061825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin 2008; 4(2):134-42; PMID:18388490 [DOI] [PubMed] [Google Scholar]
- 23. Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cel 2000; 5(3):557-67. [DOI] [PubMed] [Google Scholar]
- 24. Szarewski A, Skinner SR, Garland SM, Romanowski B, Schwarz TF, Apter D, Chow SN, Paavonen J, Del Rosario-Raymundo MR, Teixeira JC, et al. Efficacy of the HPV-16/18 AS04-Adjuvanted Vaccine Against Low-Risk HPV Types (PATRICIA Randomized Trial): An Unexpected Observation. J Infect Dis 2013; 208(9):1391-6; ; http://dx.doi.org/ 10.1093/infdis/jit360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howell-Jones R, Soldan K, Wetten S, Mesher D, Williams T, Gill ON, Hughes G. Declining genital warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis 2013; 208(9):1397-103; PMID:24092908; http://dx.doi.org/ 10.1093/infdis/jit361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: Follow-up from Months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7(12):1343-58; PMID:22048173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES, 3rd, Kollmann TR. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30(24):3572-9; PMID:22469863 [DOI] [PubMed] [Google Scholar]
- 28. Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol 2010; 118(1 Suppl):S2-7; PMID:20494220; http://dx.doi.org/ 10.1016/j.ygyno.2010.04.002 [DOI] [PubMed] [Google Scholar]