Abstract
Influenza virus (IFV) infection causes serious health problems and heavy financial burdens each year worldwide. The classical inactivated influenza virus vaccine (IIVV) and live attenuated influenza vaccine (LAIV) must be updated regularly to match the new strains that evolve due to antigenic drift and antigenic shift. However, with the discovery of broadly neutralizing antibodies that recognize conserved antigens, and the CD8+ T cell responses targeting viral internal proteins nucleoprotein (NP), matrix protein 1 (M1) and polymerase basic 1 (PB1), it is possible to develop a universal influenza vaccine based on the conserved hemagglutinin (HA) stem, NP, and matrix proteins. Recombinant adenovirus (rAd) is an ideal influenza vaccine vector because it has an ideal stability and safety profile, induces balanced humoral and cell-mediated immune responses due to activation of innate immunity, provides ‘self-adjuvanting’ activity, can mimic natural IFV infection, and confers seamless protection against mucosal pathogens. Moreover, this vector can be developed as a low-cost, rapid-response vaccine that can be quickly manufactured. Therefore, an adenovirus vector encoding conserved influenza antigens holds promise in the development of a universal influenza vaccine. This review will summarize the progress in adenovirus-vectored universal flu vaccines and discuss future novel approaches.
Keywords: adenovirus vector, broadly neutralizing antibodies, cellular immunity, hemagglutinin, humoral immunity, influenza, mucosal immunity, universal vaccine
Abbreviations
- flu
influenza
- IFV
Influenza virus
- HA
hemagglutinin
- NA
neuraminidase
- NP
nucleoprotein
- M1
matrix protein 1
- M2
matrix protein 2
- IIVV
inactivated influenza virus vaccine
- LAIV
live attenuated influenza vaccine
- Ad
adenovirus
- rAd
recombinant adenovirus
- ITRs
inverted terminal repeats
- HDAd
helper-dependent adenoviral
- HEK293
human embryonic kidney 293 cell
- RCA
replication competent adenovirus
- DVD
drug–vaccine duo
- HI
hemagglutination inhibition
- mAbs
monoclonal antibodies
- FcγRs
Fc receptors for IgG
- ADCC
antibody-dependent cell-mediated cytotoxicity
- IF-γ
interferon-γ
- IL-2
interleukin-2
- MHC-I
major histocompatibility complex class I
- HLA
human leukocyte antigen
- VAERD
vaccine-associated enhanced respiratory disease
- CTLs
cytotoxic T lymphocytes
- APC
antigen-presenting cell
- DC
lung dendritic cells
- CAR
Coxsackie-Adenovirus Receptor
Introduction
Influenza is an acute respiratory infectious disease that leads to serious health problems. Each year, influenza infects 5%–10% of adults and 20%–30% of children globally. Worldwide, 3 to 5 million cases of severe illness and approximately 250 000 to 500 000 deaths due to influenza are reported each year,1 and the newest statistical data show that influenza activity continues to increase in the southern hemisphere.2
Influenza is classified into 3 groups: A, B and C; however, influenza A is responsible for most seasonal influenza infections and all known pandemics.3 Influenza viruses are divided into 17 HA subtypes and 10 neuraminidase (NA) subtypes based on the expressed surface proteins HA and NA.4 Influenza evolves through antigenic drift and antigenic shift, resulting in the emergence of new strains; therefore, IIVV and LAIV cannot control emerging pandemic influenza virus threats. Furthermore, the production of a new vaccine cannot be achieved until 4 months after the identification of a pandemic strain5 because it is not easy to expand vaccine production capacity within a short time due to limited egg supplies. In general, both IIVV and LAIV have limited capacity to prevent and control pandemic influenza; therefore, identifying alternative vaccine strategies for influenza outbreaks is critical. Recent studies have led to progress in the development of a universal vaccine. rAd is a respiratory virus. An adenoviral vector can mimic natural infection6 and induce long-term cross-protective immunity toward influenza viruses,7,8 and many studies indicate that rAd induces effective transgene-specific humoral9 and cellular immune responses.10,11 Therefore, the adenovirus vector is one of the most promising types of vaccine vectors. This review describes the progress in adenoviral vectored universal flu vaccines and outlines novel future approaches.
Recombinant Adenoviral Vectors for Vaccines
Adenovirus was first isolated from human adenoid tissue culture nearly 60 y ago,12 and since then, additional adenoviruses have been isolated from a variety of animal species and humans.13 Human Ads are classified into 53 serotypes, which are grouped into 7 subgroups (A-G), based on serological properties and genome DNA sequences.14 Adenovirus is a non-enveloped, 70–100-nm diameter, icosahedron, DNA virus.15 The adenovirus capsid is composed of 3 major structural proteins (i.e., hexon, penton base and fiber) and several minor proteins.16 The viral genome is a linear, double-stranded DNA between 33 and 38 kb that is flanked by 2 inverted terminal repeats (ITRs); the upstream ITR is followed by a packaging signal (ψ).17 The Ad genes are classified into early transcription units (E1a, E1b, E2a, E2b, E3 and E4) and later transcription units (L1-L5).17,18
rAds have many advantages as vaccine delivery vectors. Many clinical and preclinical studies have demonstrated that rAds are safe, and rAd-vectored vaccines can be easily generated and cultured in suspension cells, such as PER.C6, at low cost.19 The rAd vaccine may retain activity for at least 1 y in lyophilized or liquid form,20 and new thermostabilization techniques enable the complete recovery of rAd titer and immunogenicity after storage at up to 45°C for 6 months and longer, with minimal losses.21 rAd vectored vaccines do not require classical adjuvants, which may result in unpredictable side effects,22 because the Ad hexon protein is a potent adjuvant for the activation of innate immunity.23 rAd can infect a variety of cells and tissues; therefore, rAd can be administered via nasal, aerosol and intramuscular vaccination.24,25
Ad5 has been widely studied, and we now have extensive knowledge of the structure of the virion, the mechanism of the virus-cell interaction, and the replication, transcription, expression and assembly of the virus.17 Ad5 is primarily used for gene/vaccine delivery vectors,26 and currently, rAd5 vectors are in at least the third generation of development. Progenitor vectors with the E1 gene deleted can be packaged and cultured in the human embryonic kidney 293 (HEK293) packaging cell line, which provides the E1 gene product in trans.27 Replication-deficient adenovirus can regain the deleted E1 gene and become replication-competent adenovirus (RCA) as a result of recombination.28 The appearance of RCA in an Ad vector population raised the possibility of undesired Ad infection. Furthermore, RCA also induces the host immune response, which may result in inflammation and tissue damage.29 An RCA-free Ad vector can be constructed using the PER.C6 cell line,30 which has permitted the production of adenovirus for clinical trials using good manufacturing practices.30,31
Furthermore, the second-generation rAd vector had the E2 and/or E4 as well as E1/E3 genes deleted from the vector backbone to reduce toxicity and increase the packaging size of the rAd vector.32,33 The third-generation rAd vector has nearly all capsid-coding sequences deleted, except for the essential cis-acting elements, including the ψ and ITRs. A helper virus provides the viral functions that are required for replication of the vector DNA, produces viral structural proteins and packages the vector DNA into virions. Therefore, third-generation rAd vectors are also called helper-dependent adenoviral (HDAd) vectors.34 The second- and third-generation rAd vectors avoid pre-existing anti-vector immunity and induce robust immune responses against the encoded transgenes.35 While major anti-Ad adaptive immune responses focus on the capsid proteins, antigen-presenting cells (APCs) infected by the Ad5 vector deleted for E1 and E2b may be less susceptible to attenuation by pre-existing anti-Ad immunity because deletion of E2b prevents expression of late gene products, including highly immunogenic proteins such as hexon. Thus, the infected DC are not cleared as rapidly by NK cells, allowing more time for immune responses to the antigen (Ag) to develop.35 However, the problems of manufacturing and purification remain unsolved.
Ad5 is the leading subtype of human adenovirus. Because of natural exposure to wild type Ad5, antibodies against Ad5 pre-exist in the majority of human populations,36,37 which may severely reduce the immune response to injected Ad5-vectored vaccines.38,39 Many researchers have attempted to resolve this potential problem by developing rare-serotype rAd vectors (Ad11, Ad26, Ad35, Ad48, Ad49 and Ad50),40-44 nonhuman rAd vectors (chimpanzee Ads,45,46 bovine Ad3,47 canine Ad2,48 porcine Ad349), and molecularly engineered Ad5 vectors.50,51 However, studies have shown that novel rAd vectors derived from rare serotypes and nonhuman rAd vectors are less potent than rAd5 vectors.43,52,53 Furthermore, pre-existing Ad5-specific T cells are cross-reactive with Ad vectors derived from rare serotypes.54 Hutnick et al. found that Ad-specific CD4+ and CD8+ T cell responses against chimpanzee-derived AdC6 and AdC7 were found in all 17 human subjects, indicating the commonality of cross-serotype reactivity of Ad-specific T cells.55 This cross-reactivity is due in part to epitopes recognized by Ad-specific T cells conserved across many adenovirus serotypes.54,56,57 The prevalence and cross reactivity of Ad-specific T-cells in humans may interfere with transgene product-specific immune responses by eliminating vector-infected cells even when rare serotype Ad vectors are employed.55
Because of these obstacles, researchers have designed molecularly engineered Ad vectors to induce lower immune responses than wild type Ad.58,59 These approaches include PEGylation of vectors, using fibers from other serotypes, modification of fibers, and using hexon proteins modified by ‘Antigen Capsid-Incorporation’.59 Because fiber proteins modified with polylysine residues target heparin sulfates on the cellular surface, an Ad in which the fiber protein is modified to contain 7 lysine residues, AdK7, shows reduced spleen distribution, which in turn decreases the production of inflammatory cytokines, compared with conventional Ad.60 Because fiber binding to Coxsackie-Adenovirus Receptor (CAR) plays a major role in inducing the production of cytokine in non-immune cells,61 another strategy for reducing innate immune responses is the substitution of Ad5 fiber with the fiber protein of other types of Ad vectors that do not bind to CAR, such as Ad7, Ad35 and Ad4.62,63 Ad vector modified with monomethoxypoly-ethylene glycol (MPEG) is another approach to avoid the innate immune responses. PEGylation reduces vector uptake in spleen, resulting in the decrease of cytokine production.64 ‘Antigen Capsid-Incorporation’ is another novel strategy to circumvent preexisting immunity. This strategy consists of incorporating antigenic peptides within the Ad capsid protein, and offers potential advantages: a strong humoral response against the given Ag similar to the response generated by native Ad capsid proteins, allowing boosting of the immune response against antigenic epitopes that are part of the Ad capsid50,51 Antigen capsid-incorporation display platforms based on Ad565,66 and Ad367 have been used for a variety of vaccines against infectious diseases, including virus infection66,67 and parasite infection65. The results show that this novel Ag capsid-incorporation approach may provide exciting opportunities to circumvent the major limitations associated with Ad vectors. Although some progress has occurred for molecularly engineered rAd, it is difficult to construct and manufacture these new vectors on a large scale. Furthermore, the safety of these new vectors remains unclear.68
Administration Route of Adenovirus-Vectored Vaccine
rAd can infect a variety of cells and tissues and can be administered via many delivery routes, such as nasal and aerosol vaccination.24,69-72 The route and dose of rAd administration impact the phenotype and quality of the transgene-specific immune response.73-76 The traditional intramuscular route induces robust humoral and cellular immune responses;77 however, pre-existing Ad5 immunity can weaken the immune responses of rAd vectors.78 Mucosal immunity may overcome pre-existing immunity against the rAd5 vector. Growing evidence shows that nasal vaccination can effectively avoid pre-existing Ad5 antibodies in mouse, rabbit and primate animal models, induce a potent antibody (Ab) effect against the encoded antigens and protect the vaccinated animal from pathogen challenge.9,79-81
Clinical research indicates that nasal vaccines are more potent than epicutaneous administration under adjuvant-free conditions. Nasal Ad5 vaccines induce strong immune responses, even when antibodies against Ad5 exist.70 Recent research has focused on mucosal immunity, including mucosal immunity in response to nasal and aerosol vaccinations, because there are many advantages to mucosal immunity. Mucosal administration is a pain-free and needle-free systemic delivery that can be performed by non-medical personnel82. Therefore, this type of vaccine may be suitable for mass vaccination programs during a crisis because nasal and aerosol administration is simple and economical and these vaccines are well tolerated.
Pre-existing S-IgA, IgG and CD8+ T cells are the keys to broad-spectrum cross-protection.83 Increasing evidence has shown that nasal vaccination with the rAd-vectored influenza vaccine induces robust antigen-specific IgAs and IgGs during respiratory illness. Compared to other administrations, mucosal rAd induced stronger IgA responses and more virus-specific activated T cells in the lung.84-86 Because mucosal vaccination can mimic natural infection, it is superior to parenteral administration for inducing cross-protection. Furthermore, mucosal vaccination can induce a cross-reactive IgA and IgG response, resulting in cross-protection against different subtypes of influenza viruses.87-89
Mucosal vaccination shows good safety. Nasal-vaccinated rAd seeds into the olfactorius bulbus and central nervous system (CNS),90-92 and no cytopathic effect (CPE) has been observed in the CNS due to this approach.90 Nasal administration of an Ad-vectored vaccine encoding influenza HA has also been shown to be safe and well tolerated in human volunteers.70
Intranasal spray is an efficacious delivery route for the rAd vector. However, many of the large droplets do not reach the target nasal airway tissues. Aerosol delivery may provide a strategy to improve vaccine efficacy93 because a fine aerosol regimen of rAd vector induced remarkably high and stable lung T-cell responses and humoral responses of both IgA and IgG isotypes in nonhuman primates.72 rAd5 encoding influenza HA protected ferrets against challenge with a lethal dose of H5N1 avian influenza via 4-μm aerosol immunization.72 To achieve better mucosal immunity induced by an aerosol rAd vector, Roy et al. characterized the dynamics of aerosolization and its effects on immune responses, including particle size, vector viability, and the actual delivered dose of the aerosolized adenoviral vector. Because of the clogging effect, a nebulizer can produce smaller aerosolized particles at high rAd concentrations. The particle diameter has an effect on the immune responses of rAd because the smaller particles can reach deep into the respiratory tract and induce robust biological responses.94
Mucosal vaccination with rAd5 rapidly induces an anti-influenza state, similar to a prophylactic drug, followed by the elicitation of sustained protective immunity, similar to a vaccine. Therefore, rAd5 confers seamless protection against mucosal pathogens when administered as a drug-vaccine duo (DVD) in a single package by mucosal vaccination.95 rAd vectors have been shown to activate innate immune responses and induce the production of inflammatory cytokines and chemokines in mouse models.96 Many factors have been shown to be involved in this process, including type I interferon (IFN-α and β),97 lung dendritic cells (DCs),98 natural killer cells99 and antiviral nitric oxide.100 The effects induced by DE1E3 Ad5 result in a multi-dimensional defense barrier against infection by IFV, and these protective effects persist for at least 3 weeks and up to 47 d when administered in a single-dose regimen.95 Therefore, rAd, as a prophylactic drug, may provide protection against IFV infection prior to inducing specific immunity by a rAd encoding IFV Ag. M2 ion channel blockers and neuraminidase inhibitors may contribute mutational pressure for further selection of resistant isolates of IFV.101 Conversely, Ad5-DVD induces an anti-influenza effect by changing the biological state of the respiratory tract and activating a specific innate immunity to prevent IFV growth without directly attacking the IFV95. Therefore, it is conceivable that Ad5-DVD may confers no mutational pressure that could induce drug resistance. Moreover, administration of the neuraminidase inhibitor, oseltamivir (OSV), suppresses respiratory mucosal secretory IgA responses and increases the risk of re-infection, whereas mucosal vaccination with rAd5 enhances mucosal innate immunity against IFV.102 Unlike LAIV, Ad5-DVD cooperates with contemporary influenza drugs because of its lack of antiviral drug targets.30,95
Influenza-Specific, Broadly Neutralizing Antibodies
HA is currently a major target of influenza vaccine research. The HA protein is a trimer of approximately 13.5 nm (135 Å) and is found on the surface of the virus. The trimeric HA ectodomains consist of the HA1 and HA2 domains, which are assembled into a head domain and a stem domain.103 HA head domains are the major protective antibody-binding site (Fig. 1), and neutralizing antibodies can induce a serum hemagglutination inhibition (HI) effect.104 The HA head domain evolves with a high mutation rate to avoid initial antibody suppression;103 therefore, the classical influenza vaccine must be continuously updated to defend against the challenge of new mutational viral strains.
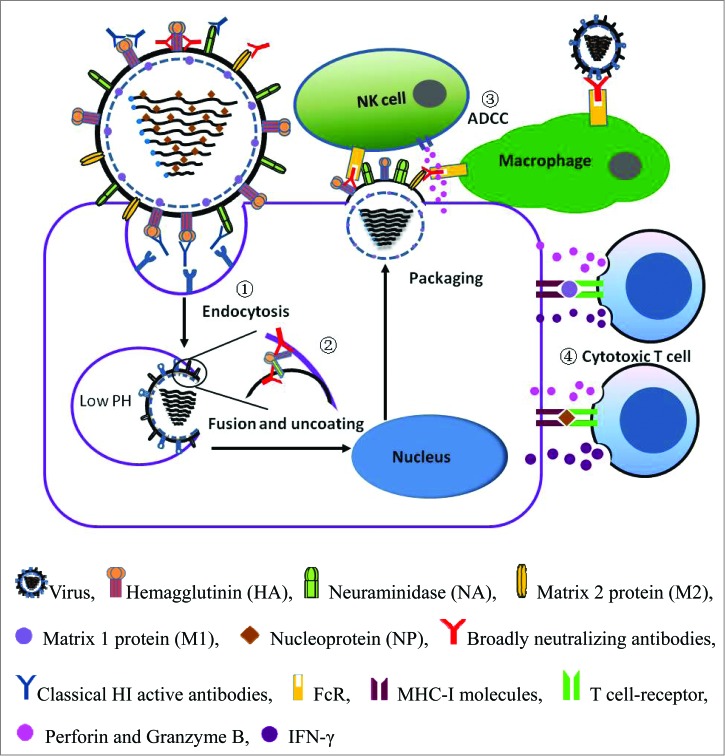
Figure 1.
Schematic diagram of influenza virus infection and the adaptive immune responses involved in host defense. (1) Classical HI antibodies prevent receptor-mediated endocytosis of the virus by binding to the HA head domains, which are typically variable. (2) Broadly neutralizing antibodies prevent membrane fusion by binding to the highly conserved HA stem. (3) Broadly neutralizing antibodies specific for HA stem and viral internal proteins mediate ADCC of infected cells, which is dependent on binding to FcR. (4) The influenza A virus internal proteins, M1 and NA, induce cytotoxic T lymphocyte-specific responses, which are dependent on MHC-I molecules.
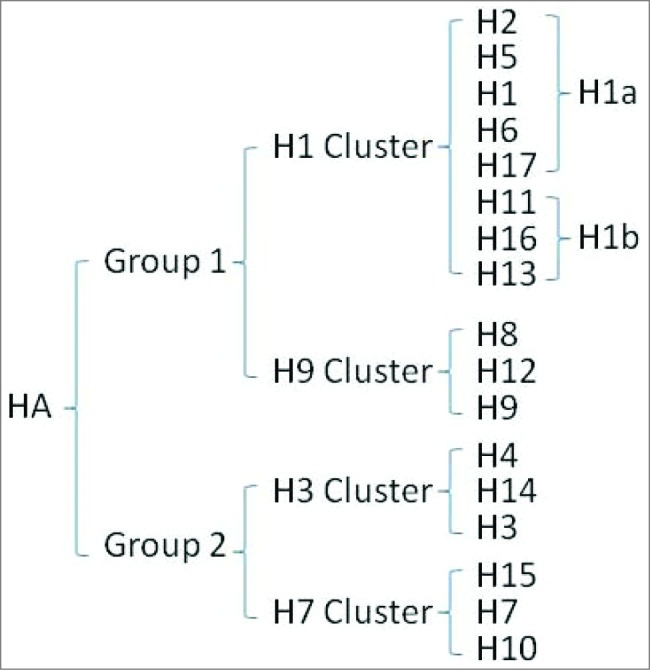
Influenza virus A viruses are divided into 17 subtypes based on HA and are further segregated into 2 phylogenetic groups (Fig. 2).4 In 1993, the Japanese researcher Y. Okuno first found a monoclonal antibody, designated C179, that neutralized all H1 and H2 strains of influenza A virus.120 Further research showed that C179 also neutralized the H5 strain.121 The monoclonal antibody C179 not only protected a mouse model from challenge with H1 and H2 influenza virus infection but also treated H1, H2 and H5-induced bronchopneumonia in the mouse model.105,106 Then, new antibodies, CR6261 and F10, were found to neutralize additional influenza viruses in group 1, including H1, H2, H5, H6, H8 and H9.107-109 In 2010, Wang et al. identified the monoclonal antibody 12D1, which neutralized H3 virus in group 2 strains and protected from challenge by the H3 subtype strain.110 However, researchers did not identify an antibody that neutralized all group 1 and group 2 viral strains until FI6v3 was found in 2011.111 Furthermore, CR9114 neutralizes both influenza A and B viruses and protects against lethal challenge with H1N1, H3N2 and influenza B viruses112 (Table 1).
Figure 2.
HA Subtypes.
Table 1.
Broadly neutralizing antibodies against influenza A virus
| MAb | Group | Subtype | Challenge | Model | Reference |
|---|---|---|---|---|---|
| C179 | 1 | H1, H2, H5 | H1N1/H5N2 | Mice | 105,106 |
| CR6261 | 1 | H1, H2, H5, H6, H8, H9 | H1N1/H5N1 | Mice | 107,108 |
| F10 | 1 | H1, H2, H5, H6, H11, H13, H16, H9 | H1N1/H5N1 | Mice | 109 |
| 12D1 | 2 | H3 | H3N2 | Mice | 110 |
| CR8020 | 2 | H3, H7, H10 | H3N2/H7N7 | Mice | 190 |
| FI6v3 | 1 and 2 | All | H1N1 | Mice, ferret | 111 |
| CR911* | 1 and 2 | All | H1N1/H3N2 | Mice | 112 |
Also neutralizes influenza B virus and protects a model from challenge with lethal influenza B virus.
In contrast to the most abundant influenza antibodies that interfere with receptor binding by binding to the head of HA, the above-mentioned, broadly neutralizing antibodies recognize a highly conserved and hydrophobic helical region in the membrane-proximal stem of HA, the ‘fusion peptide’,108 which plays a decisive role in the membrane fusion process. When HA undergoes a conformational change at low pH (5∼6) in the endosome, the fusion peptide is exposed and inserted into the endosomal membrane, causing the endosome and virus to fuse, followed by the release of viral RNA and successful infection.113 Universal monoclonal antibodies (mAbs) block infection by inserting their heavy chains into the conserved fusion peptide in the stem region, thereby preventing membrane fusion109 (Fig. 1).
Both x-ray crystallography and electron microscopy models suggest that universal monoclonal antibodies bind to the stem region of HA trimers and block the pH-induced conformational changes in HA.112 HA is active as a trimer on the viral surface, and the trimeric stem domain is the key to the induction of universal neutralizing antibodies; therefore, mutational escape is not possible due to the critical function and conserved helical structure of the stem. However, it is difficult to simulate the trimeric HA stem domain.
Several research groups have been developing a broadly protective influenza vaccine based on the stem domain.114–116 However, none of the vaccines have produced a properly folded stem trimer. Wei et al. constructed a rAd vector encoding a stem mutant trimer that was recognized by the mAb C179.69 Lin et al. used baculovirus-insect cell expression to obtain trimeric HA proteins that resulted in high levels of neutralizing antibodies when coupled with a PELC/CpG adjuvant.114 Lin et al. also constructed a glycan-masked HA mutant that overlapped with broadly neutralizing epitopes of the mAb CR6261. The trimeric HA mutant induced HA-inhibition and virus-neutralizing antibodies.115 Because the head covers conserved Ag epitopes on the stem region, the neutralizing antibodies that were induced by the complete trimeric HA recognized the head of HA but only blocked a few subtypes in the same group of influenza A viruses.103
In addition to recognizing conformational epitopes, the monoclonal antibody 12D1 also binds to a linear epitope present between amino acids 76–106 in the stem of HA.110 Hu et al. found three neutralizing mAbs (1F2,1F4, and 1E1) that could neutralize different influenza virus strains between group 1 and group 2, including subtypes of H1(H1N1), H3 (H3N2), H5 (PR8-H5), H7 (PR8-H7), and H9 (H9N2). The three mAbs could specifically recognize a conserved linear epitope that is part of the fusion peptide on HA2.117 Nevertheless, a number of linear and conformational neutralizing epitopes within the HA stem shows that this region is complicated. Therefore, further research is needed to understand the wide diversity of interaction between neutralizing antibodies and the HA stem region.
Universal Antibody-Dependent, Cell-Mediated Cytotoxicity
Typically, stem-specific Abs prevent membrane fusion between the endosome and virion membranes but do not induce a HI effect via the receptor-binding site, as do classical head-specific Abs. Further research revealed that only anti-stem mAbs were capable of mediating antibody-dependent cell-mediated cytotoxicity (ADCC) of infected cells, which is dependent on the binding of Fc receptors (FcRs) to IgG116 (Fig. 1).
Influenza A virus internal antigens are also involved in potent ADCC effects. Cells infected with influenza virus express nucleoprotein (NP) on their surface,118 and a natural anti-NP antibody was detected in human serum119 that specifically promoted heterosubtype influenza virus clearance in mice via ADCC involving FcRs.120 The high conservation of NP Ag and the ADCC effect may provide a critically necessary component of a universal influenza vaccine. Jegerlehner et al. found that mice immunized with M2 coupled to hepatitis B core (M2-HBc) produce M2-specific protective Abs that failed to neutralize the virus in vitro. NK cells are important for protection induced by M2-HBc. They also found that the dominant M2-specific Ab isotype after infection of vaccinated mice is IgG2b, followed by IgG2a.121 These 2 isotypes have been shown to be the most important mediators of ADCC in mice.122 The M2-specific mAb Z3G1 recognizes a broad spectrum of M2 variants from natural viral isolates. Passive immunotherapy with Z3G1 significantly protected mice from influenza A infection via ADCC.123,124 These results indicate that M2 may also induce protection through an ADCC-dependent mechanism.
Influenza Virus-Specific T-Cell-Mediated Immunity
To effectively prevent influenza virus infection, an ideal influenza vaccine should induce a cell-mediated immune response to limit disease severity when mucosal and humoral immunities are inadequate or are circumvented by a reassortant virus. In cell-mediated immunity, the T-cell response effectively clears the virus and promotes the recovery from influenza virus infection.125 Mice lacking CD8+ T cells have significantly delayed pulmonary viral clearance and a significantly higher mortality rate than control mice.126 However, mice devoid of Abs and mature B cells can survive primary influenza infection. These mice cleared virus from the lungs in a process dependent upon CD8+ T cells, and these Ab knockout mice can produce antigen-specific immune protection against challenge infection.127,128 Adoptive transfer of cytotoxic T lymphocytes (CTLs) to mice challenged with a lethal dose of influenza virus has been shown to cause a significant reduction of the infectious virus levels in the lungs and prevented death.129 Further studies found that the adoptive cross-reactive CTL clone A7 protects mice from a simultaneously lethal challenge with H1N1 and H2N2 subtypes and promotes complete recovery.130 In a nonhuman primate model of influenza, IFN-γ+ CD8+ T cells mediated the early clearance of an antigenically novel influenza virus.131 Furthermore, memory CTLs (mCTLs) reduced the titers of heterologous type A viruses 2–3 d earlier than in naïve controls.132 The frequencies of pre-existing T cells specific for conserved CD8 epitopes have a strong inverse correlation with illness severity and the total symptom score of influenza,133 demonstrating that cross-reactive T cell responses play an important role in the early clearance of newly emerging pandemic influenza viruses.
Activation of T cells is initiated by major histocompatibility complex class I (MHC-I)-displaying viral epitopes from within the infected cell to T cells. In humans, MHC is also called human leukocyte antigen (HLA). Polymorphic HLA molecules occur at significantly different frequencies in different ethnicities; therefore, a single CD8+ T cell epitope derived from a conserved influenza viral protein may be insufficient to induce strong cellular immunity in different populations.128,129
Although hundreds of HLA alleles are present in the human population, a large fraction of HLA Class I molecules have overlapping repertoires of binding specificity. Therefore, HLA Class I molecules can be grouped into 9 supertype families based on overlapping peptide-binding repertories and consensus B- and F-pocket structures.134–136 It is possible to account for the predominance of all known HLA class I molecules with only 9 main functional binding specificities. Assarsson et al. identified 54 non-redundant conserved epitopes (38 class I and 16 class II) that bind to the common HLA alleles and belong to the corresponding 6 class I (A1, A2, A3, A24, B7, B44) and 1 class II (DR) supertypes that provide high coverage among different ethnicities. The theoretical population coverage for the class I and class II epitopes was high throughout the major different populations, with an average of 98.5%. On average, each individual was calculated to bind 6.5 epitopes.137
Influenza virus epitope information can be accessed from the Immune Epitope Database and Analysis Resource (IEDB, http://www.immuneepitope.org/). The IEDB contains data related to both B cell and T cell epitopes from infectious pathogens.138 Available online since January 2005, the IEDB data are derived from over 4000 literature references and imported from previously developed databases.139 The IEDB provides various online tools that cover a broad range of research areas relating to epitope discovery and analysis to assist in vaccine discovery and development.138 Particularly, tools to visualize data are hosted, such as tools for viewing 3D structural data that provides antibody and Ag interaction information.140 Researchers can easily access relevant epitope information from the IEDB to assist in the development of prophylactic or therapeutic approaches against infectious diseases.
The published data for influenza-derived epitopes indicate that the major highly conserved epitopes broad binding to class I HLA supertype molecules are located within NP, M1 and PB1137,141-143 (Fig. 1). These influenza T cell epitope data were obtained using mice and other mammalian models; however, it is difficult to provide proof-of-concept support for the protective capacity of T cells against influenza illness in humans.144 Sridhar et al. followed 342 healthy adults through the pandemic waves of influenza and correlated the responses of pre-existing T cells with clinical outcomes. They found that individuals who developed less severe illness had higher frequencies of pre-existing T cells specific for the conserved CD8+ epitopes. The total symptom score had the strongest inverse correlation with the frequency of IFN-γ+IL-2−CD8+T cells. In the absence of cross-reactive neutralizing antibodies, CD8+T cells specific to conserved viral epitopes play a key role in the reduction of influenza symptoms and cross-protection against influenza. This protective immune response correlation may guide universal influenza vaccine development.133
Cooperation Between CD8+T-Cell- and Virus-Specific Non-Neutralizing Antibodies
Laidlaw et al. found that virus-specific CD8+ T cells or virus-specific non-neutralizing antibodies are relatively ineffective at conferring heterosubtypic protective immunity alone. However, both cooperatively elicit robust cross-protective immunity against H1N1 and H3N2,145 and this synergistic effect is dependent on alveolar macrophages. Therefore, the basis for a potential ‘universal’ vaccine is the capacity to elicit both CD8+ T cells and antibodies specific for highly conserved influenza proteins. Ad is a respiratory virus and is therefore an ideal vector to activate alveolar macrophages.
rAd-Vectored Universal Influenza Vaccine
Many studies have evaluated the protective effect of Ad-vectored influenza vaccine against various subtypes,146–148 and some have completed phase I clinical trials.148 The HA protein plays critical roles in the early stages of virus infection by binding to viral receptors and mediating membranes fusion between viruses and cells.149 Therefore, the HA protein is an attractive target of influenza vaccine research. The Ad-vector-based, full-length H5N1 HA (A/Vietnam/1203/04) has been shown to induce homologous and heterotypic (A/Hong Kong/156/197) HI responses146. Furthermore, another study assessed the protective efficacy of rAd-HA against challenge with variant H5N1 strains. Immunization of mice with rAd-HA/H5N1/Hong Kong/156/97 provided effective protection from heterologous H5N1 (A/Hong Kong/483/97, A/Vietnam/1203/04, and A/Hong Kong/156/197) disease, death, and primary viral replication, even without a strong humoral neutralizing response against A/Vietnam/1203/04 virus.147 Two studies of Ad-vectored HA (H3N2) vaccines have revealed that cross-protection from heterotypic challenge can also occur in the absence of neutralizing humoral immunity in swine and mice.70,150 H1N1 HA has a similar protection efficacy. Vaccination with plasmid DNA encoding H1N1 HA and boosting with a rAd vector encoding HA stimulated broadly neutralizing antibodies that recognized diverse H1N1 strains dating from 1934 to 2007 and conferred protection against divergent H1N1 viruses in mice and ferrets.69 These studies indicate that cellular immunity likely plays a major role in heterotypic immunity. In addition, CD4+ and CD8+ T cell-mediated immunity may play important roles in protecting against this virus and promoting recovery after influenza infection.151 However these studies also show that the HA protein provides limited heterotypic protection for the same subtype and is unable to induce cross-subtype and cross-group protection. The HA2 subunit, which comprises most of the HA stem region, shows high sequence conservation among the different HA subtypes. Therefore, the HA2 region would be a very attractive target to induce broader neutralizing Abs then full-length HA.108,152-154 Results of recent studies that reevaluated the HA stalk subunit are likely to contribute to the development of more effective rAd vectors encoding HA stems. In one study, Ad-vectored HA2 failed to prevent homologous virus infection but partially enhanced viral clearance and recovery from influenza infection.146 Recent research has shown that glycan-masked H5HA elicits stem-specific antibodies that overlap with broadly neutralizing epitopes of the CR6261 mAb, which neutralizes most group 1 subtypes.115 In another study, a conserved HA stalk domain (H1N1) expressed in transiently transfected cells induced stem-specific antibodies that were cross-reactive among group 1 HA subtypes (H2N2 and H5N1).152 Further studies assessed the protective efficacy of HA stem with different heads. The chimeric HA antigens induced high titers of stalk-reactive Abs in mice and ferrets,155,156 and such humoral immunity broadly protected from lethal challenge by divergent group 1 and group 2 viruses, including H5N1 and H7N9 viruses156-158. However, it is important to note that, to date, no cross-group protection has been observed from vaccination with only HA or the HA stem.155
These previous reports suggest that the conserved HA stem may provide much weaker protective Ag compared with the whole HA protein and may induce only mild immunity and protection. This mild immunity may be partly caused by the lack of CAR on DCs, which result in resistance of DCs to Ad infection.159 Moreover, Ag presentation by transduced non-professional APCs may lead to suboptimal T cell activation or even tolerance induction.160 An alternative method to strengthen the immunity efficacy of Ag is by retargeting Ag or rAd vector to CD40 on APCs such as DCs.161-163 CD40 and its ligand (CD40L) not only play a crucial role in the expansion and survival of T cells and B cells to initiate and sustain immune responses but also promote DC maturation into fully competent APCs.164,165 Fan et al. generated a recombinant rAd encoding a secreted and codon-optimized HA2 fusion with murine CD40L. Mice immunized with this recombinant viral vaccine were completely protected against lethal challenge with cross-group influenza A virus subtypes, including H1N1, H3N2, and H9N2.166 The results also show that codon-optimization of HA2 as well as the use of CD40L as a targeting ligand/molecular adjuvant were indispensable for enhancing HA2-specific mucosal IgA and serum IgG levels.
In addition to the HA stem, conserved internal viral proteins, such as NP and matrix protein 1/2 (M1/2), induce cross-immunity between different subtypes in the same group. Epstein et al. predicted DNA prime-rAd boost vaccination to conserved NP and M2 in ferrets and mice, and this vaccine strategy protected against virulent H1N1 and H5N1 challenges.86,167 However, antibodies induced by conserved internal viral epitopes failed to replace HA stem-specific neutralizing Abs that play a key role in the prevention of infection and merely reduced the disease symptoms.121 NP has also been shown to provide limited protection against high challenge doses of H5N1 in ferrets.168 To enhance the immune-inducing efficacy of NP, Hashem et al. constructed rAd vectors encoding a secreted NP-CD40L fusion protein (SNP40L). SNP40L expressed in rAd-infected cells could be secreted and target CD40 on APCs. CD40L, as an adjuvant and targeting molecule, can enhance the breadth, potency, and durability of NP-specific immune responses involving both CD8+ T cells and anti-NP Abs and provide complete cross-group protection against H1N1 and H3N2 strains in mice.169 Therefore, secreted Ag fusion with CD40L may be a potential platform to improve the immunogenicity and protective efficacy of other HA stem and conserved internal viral proteins. The present vaccine development strategies include expressing HA Ag combined with other conserved internal viral proteins, such as NP and M1/2, to induce highly effective and lasting CD8+ T cell responses and ADCC effects.
An adenoviral vector-based vaccine that contains HA and conserved NP (H5N1) elicited cell-mediated CD8+ T cell immune responses as well as neutralizing antibodies against clade 1 and clade 2 strains within the same subtype viruses (H5N1),170 and similar observations were recorded in another study. An Ad-based HA vaccine protected mice from challenge with different clade strains of the same subtype.171 Furthermore, lung virus titers were significantly reduced in mice that were challenged with the cross-subtype strain after vaccination with rAd-HA and rAd-NP.171 However, another study showed that co-administration of Ad-based HA and NP did not confer better protection than HA alone.172 Future research will assess the relative risks and benefits of different combinations of vaccines.
Kim et al. reported that intranasal vaccination with rAd encoding H5 and M2e induced significant HA- and M2e-specific Ab responses and protected vaccinated mice against heterosubtypic (H1N1) challenge. This cross-subtype protection is based on stalk-specific Abs that prevent the release of viral genetic material into the cells and on M2e-specific Abs that mediate the lysis of virus-infected cells by ADCC.173
Holman et al. developed a multi-antigen Ad vector, cAdVax-FluAv, containing the HA, NA and M1 genes. Mice vaccinated with cAdVax-FluAv survived after challenge with lethal clade 1 and clade 2 H5N1 viruses.174 A single Ad vector encoding a multi-subunit of the influenza virus has many advantages, such as a reduction of the vaccine dose, avoidance of the complexity of production, and induction of an optimized immune response.
Codon optimization was used to elicit immune responses to viral antigens by improving the expression of the protein in host cells.175 Steitz et al. demonstrated that a single-dose of codon-optimized, Ad-based H1N1 vaccine encoding HA Ag induced more robust cellular and humoral responses than wild type HA Ag in mice.176 Codon-optimization of HA2 also seems to induce significantly higher levels of local and systemic anti-HA2 Abs than wild-type HA2 in mice.166
In addition to adenovirus type 5, Ad type 4 (Ad4) has been evaluated as a candidate vector to circumvent pre-existing adenoviral immunity. A pre-clinical evaluation showed that rAd serotype 4 vaccine expressing HA was safe and induced HA-specific humoral and cellular immunity.177 Currently, the Ad4-vectored vaccine has been investigated in multiple phase I clinical trials, including for H5N1 influenza, HIV infections, and anthrax infection.178 Other nonhuman adenovirus vectors include chimpanzee adenovirus AdC7,45 bovine adenovirus subtype 3,47 canine adenovirus type 2,55 and porcine adenovirus.56
Vaccine-Associated Immunity Escape
Current study results present a challenge to HA-specific universal antibodies. To et al. found that nonneutralizing Ab titers were significantly higher for patients with severe disease than for those with mild disease during the 2009 H1N1 influenza pandemic. Early IgG response within 2 to 4 d after symptom onset indicated that the nonneutralizing antibody present in patients was likely to be preexisting or was the result of a secondary heterotypic antibody response against conserved epitopes.179 This study concluded that higher levels of nonneutralizing antibodies in the early stage of infection may be associated with worse clinical severity and poorer outcomes. Khurana et al. evaluated the mismatched influenza vaccine-associated enhanced respiratory disease (VAERD) after pandemic H1N1 (pH1N1) infection in a swine model. Cross-reactive HA2-specific Abs induced by inactivated H1N2 promoted H1N1 virus fusion and enhanced influenza virus respiratory disease.180 Gauger et al. have also confirmed that high levels of IgG serum Abs targeting the mismatched pH1N1 HA2 stalk domain were exclusively detected in IIVV-vaccinated swine and associated with increased pH1N1 virus infectivity in MDCK cells. IIVV-vaccinated swine challenged with mismatched pH1N1 were not protected from infection and demonstrated severe respiratory disease consistent with VAERD.181 Conversely, infection-enhancing HA2 Abs were detected at minimal levels in the serum of intranasal LAIV vaccinates, and VAERD was not observed, though both IIVV and LAIV vaccinates induced low and similar mean levels of Abs against mismatched pH1N1 HA1.181 Therefore, when challenged with mismatched virus, pigs lacking protective Abs in the presence of high titer anti-HA2 Abs may have an increased risk of VAERD. Although the mechanism of the differences in the type of Abs elicited by WIV and intranasal LAIV is still unknown, the results of this study suggest that the immune balance among globular-specific protective Abs, stalk-targeting Abs and local IgA may play an important role in the infection outcome. However, VAERD did not interfere with the adaptive immune response following challenge with H1N1.178
There have been no reports of HA2-specific Abs related VAERD in other animal models. However, Dougan et al. found another mechanism of VAERD in a mouse model. Influenza virus infects HA-specific B cells via its receptor, disrupting antibody secretion and causing HA-specific B cell death in mice. Infection and killing of antigen-specific B cells impair the kinetics of the memory response that is established by infection or vaccination.182 Therefore, it is necessary to further investigate the possibility of establishing a balanced immune response induced by a combined vaccine.
Furthermore, a vaccine based on conserved internal viral proteins induces T cell immunity, which may lead to selective immune pressure on the influenza virus, similar to Ab-mediated antigenic drift.183 Under this selective pressure, virus escape mutants arise at the residues that anchor the epitope peptide to MHC.184 Gras et al. demonstrated that influenza virus escapes CD8+ T-cell immunity through mutations at highly conserved NP418-426peptides.185 Therefore, this type of theoretical vaccine-associated T-cell immune escape must be further estimated.
Prospect of a Universal Influenza Vaccine
There is no doubt that great progress has been made in the development of a rAd-vectored influenza vaccine. However, it is too early for the use of a rAd-vectored vaccine as an alternative to the classical IIVV and LAIV because many problems are still unresolved. There are many difficulties in the development of a universal vaccine that induces a cross-reactive CD8+ T-cell immune response, including time-related attrition of immune competence,125,186 the protective capacity in different HLA populations, and vaccine-associated immune escape and immunopathology. Early studies have shown that cytotoxic T-cell memory has a half-life of approximately 2–3 y.125 Therefore, a booster immunization is needed every 2–3 y to maintain an adequate level of memory T-cells. Furthermore, the future design of a universal vaccine should consider major HLA allele populations, as well as rare-allele ethnicities.
The ideal influenza vaccine should induce universal and efficient cross-reactive antibodies to conserved antigens, such as the HA stem, and this strategy has been assessed in mouse and ferret models. Furthermore, the ideal influenza vaccine should also induce a strong T-cell immune response and maintain long memory potential. Significant progress has been made to identify a universal antibody and produce cross-reactive T-cell immunity, but generating an effective universal vaccine remains difficult.
Moreover, cross immunity-associated immune evasion and its effect on virus evolution must be further assessed. Another major problem is that the relationship between the vaccine dosage and immune effect remains unclear.187 Serum HI antibody titers of more than or equal to 1:40 reduce the risk of influenza infection by at least a 50%.188 However, no such correlation of protection exists for a rAd-vectored vaccine encoding the HA,174 HA1/HA2,146 NP or M286 genes. ADCC activity is impaired in neutrophils from aged subjects;189 therefore, an ADCC-dependent universal vaccine may be ineffective in the elderly.
Future directions for the universal influenza vaccine should focus on multi-faceted based on cross-reactive antibodies, T-cell immune responses and long memory potential. Standardized virus strains and animal models are necessary to develop standard methods for evaluating different candidate vaccines. Additional studies will probe the relationship between the dosage of the vaccine and the immune effect. Overall, the development of a universal influenza vaccine based on the Ad vector is early in its development, but the approach holds great potential in the fight against influenza.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant (2012AE001) from the Department of Science and Technology of Yunnan Province. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1.WHO Influenza (Seasonal). Disponibile al link: http://wwwwhoint/mediacentre/factsheets/fs211/en/, 2014 [Google Scholar]
- 2.WHO Influenza update. http://wwwwhoint/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/, 2014 [Google Scholar]
- 3.Lagace-Wiens PR, Rubinstein E, Gumel A. Influenza epidemiology–past, present, and future. Crit Care Med 2010; 38:e1-9; PMID:20029350; http://dx.doi.org/ 10.1097/CCM.0b013e3181cbaf34 [DOI] [PubMed] [Google Scholar]
- 4.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al.. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269-74; PMID:22371588; http://dx.doi.org/ 10.1073/pnas.1116200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emanuel EJ, Wertheimer A. Who should get influenza vaccine when not all can? Public Health Ethik 2010; 1:191. [Google Scholar]
- 6.Tutykhina IL, Logunov DY, Shcherbinin DN, Shmarov MM, Tukhvatulin AI, Naroditsky BS, Gintsburg AL. Development of adenoviral vector-based mucosal vaccine against influenza. J Mol Med (Berl) 2011; 89:331-41; PMID:21104066; http://dx.doi.org/ 10.1007/s00109-010-0696-0 [DOI] [PubMed] [Google Scholar]
- 7.Lambe T. Novel viral vectored vaccines for the prevention of influenza. Mol Med 2012; 18:1153-60; PMID:22735755; http://dx.doi.org/ 10.2119/molmed.2012.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toro H, Tang DC, Suarez DL, Sylte MJ, Pfeiffer J, Van Kampen KR. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine 2007; 25:2886-91; PMID:17055126; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang ZQ, Yang Y, Wilson JM, Ertl HC. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 1996; 219:220-7; PMID:8623532; http://dx.doi.org/ 10.1006/viro.1996.0239 [DOI] [PubMed] [Google Scholar]
- 10.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, et al.. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 2012; 4:115ra2; PMID:22218691; http://dx.doi.org/ 10.1126/scitranslmed.3002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al.. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473:523-7; PMID:21562493; http://dx.doi.org/ 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY): Royal Society of Medicine, 1953:570-3 [DOI] [PubMed] [Google Scholar]
- 13.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 2006; 24:849-62; PMID:16297508; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Topics Microbiol Immunol 2010; 343:195-224; PMID:20376613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 Å resolution. Science 2010; 329:1071-5; PMID:20798318; http://dx.doi.org/ 10.1126/science.1187292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell WC. Adenoviruses: update on structure and function. J Gen Virol 2009; 90:1-20; PMID:19088268; http://dx.doi.org/ 10.1099/vir.0.003087-0 [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HS. The life and times of adenoviruses. Adv Virus Res 1999; 54:1-13; PMID:10547672; http://dx.doi.org/ 10.1016/S0065-3527(08)60363-2 [DOI] [PubMed] [Google Scholar]
- 18.Young CS. The structure and function of the adenovirus major late promoter. Curr Topics Microbiol Immunol 2003; 272:213-49; PMID:12747552 [DOI] [PubMed] [Google Scholar]
- 19.Kovesdi I, Hedley SJ. Adenoviral producer cells. Viruses 2010; 2:1681-703; PMID:21994701; http://dx.doi.org/ 10.3390/v2081681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croyle M, Cheng X, Wilson J. Development of formulations that enhance physical stability of viral vector for gene therapy. Gene Ther 2001; 8:1281-90; PMID:11571564; http://dx.doi.org/ 10.1038/sj.gt.3301527 [DOI] [PubMed] [Google Scholar]
- 21.Alcock R, Cottingham MG, Rollier CS, Furze J, De Costa SD, Hanlon M, Spencer AJ, Honeycutt JD, Wyllie DH, Gilbert SC, et al.. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Transl Med 2010; 2:19ra2-ra2; PMID:20371486; http://dx.doi.org/ 10.1126/scitranslmed.3000490 [DOI] [PubMed] [Google Scholar]
- 22.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, et al.. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PloS One 2009; 4:e6999; PMID:19756141; http://dx.doi.org/ 10.1371/journal.pone.0006999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molinier-Frenkel V, Lengagne R, Gaden F, Hong S-S, Choppin J, Gahery-Ségard H, Boulanger P, Guillet JG. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol 2002; 76:127-35; PMID:11739678; http://dx.doi.org/ 10.1128/JVI.76.1.127-135.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy CJ, Ault A, Sivasubramani SK, Gorres JP, Wei CJ, Andersen H, Gall J, Roederer M, Rao SS. Aerosolized adenovirus-vectored vaccine as an alternative vaccine delivery method. Respir Res 2011; 12:153; PMID:22103776; http://dx.doi.org/ 10.1186/1465-9921-12-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambe T, Carey JB, Li Y, Spencer AJ, van Laarhoven A, Mullarkey CE, Vrdoljak A, Moore AC, Gilbert SC. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci Rep 2013; 3:1443; PMID:23485942; http://dx.doi.org/ 10.1038/srep01443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appaiahgari MB, Vrati S . Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert opinion on biological therapy 2015; 15:337-51; PMID:25529044 [DOI] [PubMed] [Google Scholar]
- 27.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977; 36:59-74; PMID:886304; http://dx.doi.org/ 10.1099/0022-1317-36-1-59 [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Grace M, Casale J, Chang AT, Musco ML, Bordens R, Greenberg R, Schaefer E, Indelicato SR. Characterization of replication-competent adenovirus isolates from large-scale production of a recombinant adenoviral vector. Hu Gene Ther 1999; 10:113-21; PMID:10022536; http://dx.doi.org/ 10.1089/10430349950019246 [DOI] [PubMed] [Google Scholar]
- 29.Hermens WT, Verhaagen J. Adenoviral vector-mediated gene expression in the nervous system of immunocompetent Wistar and T cell-deficient nude rats: preferential survival of transduced astroglial cells in nude rats. Hum Gene Ther 1997; 8:1049-63; PMID:9189763; http://dx.doi.org/ 10.1089/hum.1997.8.9-1049 [DOI] [PubMed] [Google Scholar]
- 30.Tang DC, Zhang J, Toro H, Shi Z, Van Kampen KR. Adenovirus as a carrier for the development of influenza virus-free avian influenza vaccines. Expert Rev Vaccines 2009; 8:469-81; PMID:19348562; http://dx.doi.org/ 10.1586/erv.09.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian S, Kim JJ, Harding F, Altaras GM, Aunins JG, Zhou W. Scaleable production of adenoviral vectors by transfection of adherent PER.C6 cells. Biotechnol Prog 2007; 23:1210-7; PMID:17715941 [DOI] [PubMed] [Google Scholar]
- 32.Gorziglia MI, Kadan MJ, Yei S, Lim J, Lee GM, Luthra R, Trapnell BC. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol 1996; 70:4173-8; PMID:8648763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorziglia MI, Lapcevich C, Roy S, Kang Q, Kadan M, Wu V, Pechan P, Kaleko M. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J Virol 1999; 73:6048-55; PMID:10364357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitani K, Graham FL, Caskey CT, Kochanek S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc Natl Acad Sci U S A 1995; 92:3854-8; PMID:7731995; http://dx.doi.org/ 10.1073/pnas.92.9.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, Morse MA, Lyerly HK, Amalfitano A, Clay TM. Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther 2009; 16:673-82; PMID:19229288; http://dx.doi.org/ 10.1038/cgt.2009.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28:950-7; PMID:19925902; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.145 [DOI] [PubMed] [Google Scholar]
- 37.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng'ang'a D, Brandariz KL, Abbink P, et al.. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011; 29:5203-9; PMID:21619905; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichla-Gollon SL, Lin SW, Hensley SE, Lasaro MO, Herkenhoff-Haut L, Drinker M, Tatsis N, Gao GP, Wilson JM, Ertl HC, et al.. Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol 2009; 83:5567-73; PMID:19279092; http://dx.doi.org/ 10.1128/JVI.00405-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol 2003; 77:799-803; PMID:12477888; http://dx.doi.org/ 10.1128/JVI.77.1.799-803.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al.. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263-71; PMID:12857895; http://dx.doi.org/ 10.1128/JVI.77.15.8263-8271.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holterman L, Vogels R, van der Vlugt R, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der Helm E, Lemckert A, Gillissen G, et al.. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol 2004; 78:13207-15; PMID:15542673; http://dx.doi.org/ 10.1128/JVI.78.23.13207-13215.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemckert AA, Grimbergen J, Smits S, Hartkoorn E, Holterman L, Berkhout B, Barouch DH, Vogels R, Quax P, Goudsmit J, et al.. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J Gen Virol 2006; 87:2891-9; PMID:16963747; http://dx.doi.org/ 10.1099/vir.0.82079-0 [DOI] [PubMed] [Google Scholar]
- 43.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, et al.. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 2007; 81:4654-63; PMID:17329340; http://dx.doi.org/ 10.1128/JVI.02696-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahl CA, Bonnell J, Hiriyanna S, Fultz M, Nyberg-Hoffman C, Chen P, King CR, Gall JG. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine 2010; 28:5691-702; PMID:20600496; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy S, Kobinger GP, Lin J, Figueredo J, Calcedo R, Kobasa D, Wilson JM. Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine 2007; 25:6845-51; PMID:17728024; http://dx.doi.org/ 10.1016/j.vaccine.2007.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatsis N, Tesema L, Robinson E, Giles-Davis W, McCoy K, Gao G, Wilson JM. Chimpanzee-origin adenovirus vectors as vaccine carriers. Gene Ther 2005; 13:421-9; http://dx.doi.org/ 10.1038/sj.gt.3302675 [DOI] [PubMed] [Google Scholar]
- 47.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther 2008; 16:965-71; PMID:18301400; http://dx.doi.org/ 10.1038/mt.2008.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao YW, Xia XZ, Wang LG, Liu D, Huang G. [Construction and experimental immunity of recombinant replication-competent canine adenovirus type 2 expressing hemagglutinin gene of H5N1 subtype tiger influenza virus]. Wei Sheng Wu Xue Bao 2006; 46:297-300; PMID:16736595 [PubMed] [Google Scholar]
- 49.Patel A, Tikoo S, Kobinger G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PloS One 2010; 5:e15301; PMID:21179494; http://dx.doi.org/ 10.1371/journal.pone.0015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews QL. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol Pharm 2011; 8:3-11; PMID:21047139; http://dx.doi.org/ 10.1021/mp100214b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McConnell MJ, Danthinne X, Imperiale MJ. Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol 2006; 80:5361-70; PMID:16699016; http://dx.doi.org/ 10.1128/JVI.00256-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Cheng C, Ko SY, Kong WP, Kanekiyo M, Einfeld D, Schwartz RM, King CR, Gall JG, Nabel GJ. Delivery of human immunodeficiency virus vaccine vectors to the intestine induces enhanced mucosal cellular immunity. J Virol 2009; 83:7166-75; PMID:19420074; http://dx.doi.org/ 10.1128/JVI.00374-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, et al.. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol 2010; 84:10522-32; PMID:20686035; http://dx.doi.org/ 10.1128/JVI.00450-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, Casimiro DR, Duerr A, Robertson MN, Buchbinder SP, et al.. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest 2012; 122:359-67; PMID:22201684; http://dx.doi.org/ 10.1172/JCI60202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 2010; 28:1932-41; PMID:20188249; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang J, Olive M, Pulmanausahakul R, Schnell M, Flomenberg N, Eisenlohr L, Flomenberg P. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 2006; 350:312-22; PMID:16499941; http://dx.doi.org/ 10.1016/j.virol.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 57.Tang J, Olive M, Champagne K, Flomenberg N, Eisenlohr L, Hsu S, Flomenberg P. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Ther 2004; 11:1408-15; PMID:15269714; http://dx.doi.org/ 10.1038/sj.gt.3302316 [DOI] [PubMed] [Google Scholar]
- 58.Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther 2009; 9:1521-31; PMID:19780714; http://dx.doi.org/ 10.1517/14712590903307388 [DOI] [PubMed] [Google Scholar]
- 59.Dharmapuri S, Peruzzi D, Aurisicchio L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin Biol Ther 2009; 9:1279-87; PMID:19645630; http://dx.doi.org/ 10.1517/14712590903187053 [DOI] [PubMed] [Google Scholar]
- 60.Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y, Hayakawa T, Mizuguchi H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J Immunol 2007; 178:1767-73; PMID:17237426; http://dx.doi.org/ 10.4049/jimmunol.178.3.1767 [DOI] [PubMed] [Google Scholar]
- 61.Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, Cabrini G. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol 2006; 80:11241-54; PMID:16956941; http://dx.doi.org/ 10.1128/JVI.00721-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol 2004; 78:5368-81; PMID:15113916; http://dx.doi.org/ 10.1128/JVI.78.10.5368-5381.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoggins JW, Nociari M, Philpott N, Falck-Pedersen E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J Virol 2005; 79:11627-37; PMID:16140740; http://dx.doi.org/ 10.1128/JVI.79.18.11627-11637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Geest B, Snoeys J, Van Linthout S, Lievens J, Collen D. Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum Gene Ther 2005; 16:1439-51; PMID:16390275; http://dx.doi.org/ 10.1089/hum.2005.16.1439 [DOI] [PubMed] [Google Scholar]
- 65.Farrow AL, Rachakonda G, Gu L, Krendelchtchikova V, Nde PN, Pratap S, Lima MF, Villalta F, Matthews QL. Immunization with Hexon modified adenoviral vectors integrated with gp83 epitope provides protection against Trypanosoma cruzi infection. PLoS Negl Trop Dis 2014; 8:e3089; PMID:25144771; http://dx.doi.org/ 10.1371/journal.pntd.0003089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu L, Krendelchtchikova V, Krendelchtchikov A, Oster RA, Fujihashi K, Matthews QL. A recombinant adenovirus-based vector elicits a specific humoral immune response against the V3 loop of HIV-1 gp120 in mice through the “Antigen Capsid-Incorporation” strategy. Virol J 2014; 11:112; PMID:24935650; http://dx.doi.org/ 10.1186/1743-422X-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian X, Su X, Li X, Li H, Li T, Zhou Z, Zhong T, Zhou R. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PloS One 2012; 7:e41381; PMID:22848478; http://dx.doi.org/ 10.1371/journal.pone.0041381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther 2007; 15:2146-53; PMID:17895860; http://dx.doi.org/ 10.1038/sj.mt.6300319 [DOI] [PubMed] [Google Scholar]
- 69.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, et al.. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 2010; 329:1060-4; PMID:20647428; http://dx.doi.org/ 10.1126/science.1192517 [DOI] [PubMed] [Google Scholar]
- 70.Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, Marks D, Elmets CA, Tang DC. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 2005; 23:1029-36; PMID:15620476; http://dx.doi.org/ 10.1016/j.vaccine.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 71.Hashem A, Jaentschke B, Gravel C, Tocchi M, Doyle T, Rosu-Myles M, He R, Li X. Subcutaneous immunization with recombinant adenovirus expressing influenza A nucleoprotein protects mice against lethal viral challenge. Hum Vaccines Immunother 2012; 8:425-30; PMID:22370512; http://dx.doi.org/ 10.4161/hv.19109 [DOI] [PubMed] [Google Scholar]
- 72.Song K, Bolton DL, Wei CJ, Wilson RL, Camp JV, Bao S, Mattapallil JJ, Herzenberg LA, Herzenberg LA, Andrews CA, et al.. Genetic immunization in the lung induces potent local and systemic immune responses. Proc Natl Acad Sci U S A 2010; 107:22213-8; PMID:21135247; http://dx.doi.org/ 10.1073/pnas.1015536108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holst PJ, Ørskov C, Thomsen AR, Christensen JP. Quality of the transgene-specific CD8+ T cell response induced by adenoviral vector immunization is critically influenced by virus dose and route of vaccination. J Immunol 2010; 184:4431-9; PMID:20212099; http://dx.doi.org/ 10.4049/jimmunol.0900537 [DOI] [PubMed] [Google Scholar]
- 74.Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J Virol 2010; 84:5986-96; PMID:20357087; http://dx.doi.org/ 10.1128/JVI.02563-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suda T, Kawano M, Nogi Y, Ohno N, Akatsuka T, Matsui M. The route of immunization with adenoviral vaccine influences the recruitment of cytotoxic T lymphocytes in the lung that provide potent protection from influenza A virus. Antiviral Res 2011; 91:252-8; PMID:21722671; http://dx.doi.org/ 10.1016/j.antiviral.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 76.Steitz J, Wagner RA, Bristol T, Gao W, Donis RO, Gambotto A. Assessment of route of administration and dose escalation for an adenovirus-based influenza A Virus (H5N1) vaccine in chickens. Clin Vaccine Immunol 2010; 17:1467-72; PMID:20660133; http://dx.doi.org/ 10.1128/CVI.00180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoelscher MA, Jayashankar L, Garg S, Veguilla V, Lu X, Singh N, Katz JM, Mittal SK, Sambhara S. New pre-pandemic influenza vaccines: an egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin Pharmacol Ther 2007; 82:665-71; PMID:17957181; http://dx.doi.org/ 10.1038/sj.clpt.6100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al.. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881-93; PMID:19012954; http://dx.doi.org/ 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Z, Zeng M, Yang G, Siegel F, Cain LJ, van Kampen KR, Elmets CA, Tang DC. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J Virol 2001; 75:11474-82; PMID:11689629; http://dx.doi.org/ 10.1128/JVI.75.23.11474-11482.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol 2008; 82:2350-7; PMID:18094185; http://dx.doi.org/ 10.1128/JVI.02372-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PloS One 2008; 3:e3548; PMID:18958172; http://dx.doi.org/ 10.1371/journal.pone.0003548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: an update. Transl Respir Med 2014; 2:3; PMID:25505695; http://dx.doi.org/ 10.1186/2213-0802-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis 2005; 58:195-207; PMID:16116250 [PubMed] [Google Scholar]
- 84.Shmarov MM, Sedova ES, Verkhovskaya LV, Rudneva IA, Bogacheva EA, Barykova YA, Shcherbinin DN, Lysenko AA, Tutykhina IL, Logunov DY, et al.. Induction of a protective heterosubtypic immune response against the influenza virus by using recombinant adenoviral vectors expressing hemagglutinin of the influenza H5 virus. Acta Naturae 2010; 2:111-8; PMID:22649637 [PMC free article] [PubMed] [Google Scholar]
- 85.Park KS, Lee J, Ahn SS, Byun YH, Seong BL, Baek YH, Song MS, Choi YK, Na YJ, Hwang I, et al.. Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology 2009; 395:182-9; PMID:19836045; http://dx.doi.org/ 10.1016/j.virol.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 86.Price GE, Soboleski MR, Lo CY, Misplon JA, Pappas C, Houser KV, Tumpey TM, Epstein SL. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine 2009; 27:6512-21; PMID:19729082; http://dx.doi.org/ 10.1016/j.vaccine.2009.08.053 [DOI] [PubMed] [Google Scholar]
- 87.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, Pushko P, Tumpey TM. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol 2009; 83:5726-34; PMID:19321609; http://dx.doi.org/ 10.1128/JVI.00207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lau YF, Wright AR, Subbarao K. The contribution of systemic and pulmonary immune effectors to vaccine-induced protection from H5N1 influenza virus infection. J Virol 2012; 86:5089-98; PMID:22379093; http://dx.doi.org/ 10.1128/JVI.07205-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JG, et al.. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J Infect Dis 2011; 204:1491-9; PMID:21957153; http://dx.doi.org/ 10.1093/infdis/jir596 [DOI] [PubMed] [Google Scholar]
- 90.Draghia R, Caillaud C, Manicom R, Pavirani A, Kahn A, Poenaru L. Gene delivery into the central nervous system by nasal instillation in rats. Gene Ther 1995; 2:418; PMID:7584117 [PubMed] [Google Scholar]
- 91.Damjanovic D, Zhang X, Mu J, Medina MF, Xing Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet Vaccines Ther 2008; 6:5; PMID:18261231; http://dx.doi.org/ 10.1186/1479-0556-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang D, Pereboev A, Korokhov N, He R, Larocque L, Gravel C, Jaentschke B, Tocchi M, Casley WL, Lemieux M, et al.. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40 (+) cells. Gene Ther 2007; 15:298-308; PMID:18046426; http://dx.doi.org/ 10.1038/sj.gt.3303085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith JH, Brooks P, Johnson S, Tompkins SM, Custer KM, Haas DL, Mair R, Papania M, Tripp RA. Aerosol vaccination induces robust protective immunity to homologous and heterologous influenza infection in mice. Vaccine 2011; 29:2568-75; PMID:21300100; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health A Curr Issues 1985; 15:197-214; http://dx.doi.org/ 10.1080/15287398509530647 [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Tarbet EB, Feng T, Shi Z, Van Kampen KR, De-chu CT. Adenovirus-vectored drug-vaccine duo as a rapid-response tool for conferring seamless protection against influenza. PloS One 2011; 6:e22605; PMID:21818346; http://dx.doi.org/ 10.1371/journal.pone.0022605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res 2008; 132:1-14; PMID:18036698; http://dx.doi.org/ 10.1016/j.virusres.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaguchi T, Kawabata K, Kouyama E, Ishii KJ, Katayama K, Suzuki T, Kurachi S, Sakurai F, Akira S, Mizuguchi H. Induction of type I interferon by adenovirus-encoded small RNAs. Proc Natl Acad Sci U S A 2010; 107:17286-91; PMID:20855616; http://dx.doi.org/ 10.1073/pnas.1009823107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thiele AT, Sumpter TL, Walker JA, Xu Q, Chang CH, Bacallao RL, Kher R, Wilkes DS. Pulmonary immunity to viral infection: adenovirus infection of lung dendritic cells renders T cells nonresponsive to interleukin-2. J Virol 2006; 80:1826-36; PMID:16439539; http://dx.doi.org/ 10.1128/JVI.80.4.1826-1836.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu J, Huang X, Yang Y. A Critical Role for Type I IFN-dependent NK Cell Activation in Innate Immune Elimination of Adenoviral Vectors In Vivo. Mol Ther 2008; 16:1300-7; PMID:18443600; http://dx.doi.org/ 10.1038/mt.2008.88 [DOI] [PubMed] [Google Scholar]
- 100.Higashimoto Y, Yamagata Y, Itoh H. Complex effect of adenovirus early region proteins on innate immune system. Inflamm Allergy Drug Targets 2006; 5:229-37; PMID:17168793; http://dx.doi.org/ 10.2174/187152806779010927 [DOI] [PubMed] [Google Scholar]
- 101.Poland GA, Jacobson RM, Ovsyannikova IG. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis 2009; 48:1254-6; PMID:19323631; http://dx.doi.org/ 10.1086/598989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takahashi E, Kataoka K, Fujii K, Chida J, Mizuno D, Fukui M, Hiro-O Ito, Fujihashi K, Kido H. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect 2010; 12:778-83; PMID:20452454; http://dx.doi.org/ 10.1016/j.micinf.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 103.Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U S A 2014; 111:125-30; PMID:24344259; http://dx.doi.org/ 10.1073/pnas.1308701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767-77; PMID:4509641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smirnov YA, Lipatov AS, Gitelman AK, Claas EC, Osterhaus AD. Prevention and treatment of bronchopneumonia in mice caused by mouse-adapted variant of avian H5N2 influenza A virus using monoclonal antibody against conserved epitope in the HA stem region. Arch Virol 2000; 145:1733-41; PMID:11003481; http://dx.doi.org/ 10.1007/s007050070088 [DOI] [PubMed] [Google Scholar]
- 106.Okuno Y, Matsumoto K, Isegawa Y, Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol 1994; 68:517-20; PMID:8254764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, et al.. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS One 2008; 3:e3942; PMID:19079604; http://dx.doi.org/ 10.1371/journal.pone.0003942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science 2009; 324:246-51; PMID:19251591; http://dx.doi.org/ 10.1126/science.1171491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Bakker A, Cox F, van Deventer E, Guan Y, et al.. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009; 16:265-73; PMID:19234466; http://dx.doi.org/ 10.1038/nsmb.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 2010; 6:e1000796; PMID:20195520; http://dx.doi.org/ 10.1371/journal.ppat.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al.. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011; 333:850-6; PMID:21798894; http://dx.doi.org/ 10.1126/science.1205669 [DOI] [PubMed] [Google Scholar]
- 112.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al.. Highly conserved protective epitopes on influenza B viruses. Science 2012; 337:1343-8; PMID:22878502; http://dx.doi.org/ 10.1126/science.1222908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaudin Y, Ruigrok RW, Brunner J. Low-pH induced conformational changes in viral fusion proteins: implications for the fusion mechanism. J Gen Virol 1995; 76 (Pt 7):1541-56; PMID:9049361; http://dx.doi.org/ 10.1099/0022-1317-76-7-1541 [DOI] [PubMed] [Google Scholar]
- 114.Lin SC, Huang MH, Tsou PC, Huang LM, Chong P, Wu SC. Recombinant trimeric HA protein immunogenicity of H5N1 avian influenza viruses and their combined use with inactivated or adenovirus vaccines. PloS One 2011; 6:e20052; PMID:21655326; http://dx.doi.org/ 10.1371/journal.pone.0020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin SC, Liu WC, Jan JT, Wu SC. Glycan masking of hemagglutinin for adenovirus vector and recombinant protein immunizations elicits broadly neutralizing antibodies against H5N1 avian influenza viruses. PloS One 2014; 9:e92822; PMID:24671139; http://dx.doi.org/ 10.1371/journal.pone.0092822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014; 20:143-51; PMID:24412922; http://dx.doi.org/ 10.1038/nm.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu W, Chen A, Miao Y, Xia S, Ling Z, Xu K, Wang T, Xu Y, Cui J, Wu H, et al.. Fully human broadly neutralizing monoclonal antibodies against influenza A viruses generated from the memory B cells of a 2009 pandemic H1N1 influenza vaccine recipient. Virology 2013; 435:320-8; PMID:23084424; http://dx.doi.org/ 10.1016/j.virol.2012.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol 1981; 126:1814-9. [PubMed] [Google Scholar]
- 119.Sukeno N, Otsuki Y, Konno J, Yamane N, Odagiri T, Arikawa J, Ishida N. Anti-nucleoprotein antibody response in influenza A infection. Tohoku J Exp Med 1979; 128:241-9; PMID:494246; http://dx.doi.org/ 10.1620/tjem.128.241 [DOI] [PubMed] [Google Scholar]
- 120.LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, Kaminski DA. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol 2011; 186:4331-9; http://dx.doi.org/ 10.4049/jimmunol.1003057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol 2004; 172:5598-605; http://dx.doi.org/ 10.4049/jimmunol.172.9.5598 [DOI] [PubMed] [Google Scholar]
- 122.Denkers EY, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol 1985; 135:2183-6. [PubMed] [Google Scholar]
- 123.Wang R, Song A, Levin J, Dennis D, Zhang NJ, Yoshida H, Koriazova L, Madura L, Shapiro L, Matsumoto A, et al.. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res 2008; 80:168-77; PMID:18598723; http://dx.doi.org/ 10.1016/j.antiviral.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 124.Song A, Myojo K, Laudenslager J, Harada D, Miura T, Suzuki K, Kuni-Kamochi R, Soloff R, Ohgami K, Kanda Y. Evaluation of a fully human monoclonal antibody against multiple influenza A viral strains in mice and a pandemic H1N1 strain in nonhuman primates. Antiviral Res 2014; 111:60-8; PMID:25218949; http://dx.doi.org/ 10.1016/j.antiviral.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 125.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med 1983; 309:13-7; PMID:6602294; http://dx.doi.org/ 10.1056/NEJM198307073090103 [DOI] [PubMed] [Google Scholar]
- 126.Bender BS, Croghan T, Zhang L, Small PA Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med 1992; 175:1143-5; PMID:1552285; http://dx.doi.org/ 10.1084/jem.175.4.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol 1998; 160:322-7. [PubMed] [Google Scholar]
- 128.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med 1997; 186:2063-8; PMID:9396777; http://dx.doi.org/ 10.1084/jem.186.12.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 1978; 273:238-9; PMID:306072; http://dx.doi.org/ 10.1038/273238a0 [DOI] [PubMed] [Google Scholar]
- 130.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med 1984; 160:814-26; PMID:6206190; http://dx.doi.org/ 10.1084/jem.160.3.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinfurter JT, Brunner K, Capuano SV 3rd, Li C, Broman KW, Kawaoka Y, Friedrich TC. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog 2011; 7:e1002381; PMID:22102819; http://dx.doi.org/ 10.1371/journal.ppat.1002381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 1998; 8:683-91; PMID:9655482; http://dx.doi.org/ 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- 133.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305-12; PMID:24056771; http://dx.doi.org/ 10.1038/nm.3350 [DOI] [PubMed] [Google Scholar]
- 134.Sette A, Sidney J. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Immunol 1998; 10:478-82; PMID:9722926; http://dx.doi.org/ 10.1016/S0952-7915(98)80124-6 [DOI] [PubMed] [Google Scholar]
- 135.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 1999; 50:201-12; PMID:10602880; http://dx.doi.org/ 10.1007/s002510050594 [DOI] [PubMed] [Google Scholar]
- 136.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol 2008; 9:1; PMID:18211710; http://dx.doi.org/ 10.1186/1471-2172-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Assarsson E, Bui HH, Sidney J, Zhang Q, Glenn J, Oseroff C, Mbawuike IN, Alexander J, Newman MJ, Grey H, et al.. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol 2008; 82:12241-51; PMID:18842709; http://dx.doi.org/ 10.1128/JVI.01563-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peters B, Sidney J, Bourne P, Bui HH, Buus S, Doh G, Fleri W, Kronenberg M, Kubo R, Lund O, et al.. The immune epitope database and analysis resource: from vision to blueprint. PLoS Biol 2005; 3:e91; PMID:15760272; http://dx.doi.org/ 10.1371/journal.pbio.0030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vita R, Peters B, Sette A. The curation guidelines of the immune epitope database and analysis resource. Cytometry A 2008; 73:1066-70; PMID:18688821; http://dx.doi.org/ 10.1002/cyto.a.20585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ponomarenko J, Papangelopoulos N, Zajonc DM, Peters B, Sette A, Bourne PE. IEDB-3D: structural data within the immune epitope database. Nucleic Acids Res 2011; 39:D1164-70; PMID:21030437; http://dx.doi.org/ 10.1093/nar/gkq888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu C, Zanker D, Valkenburg S, Tan B, Kedzierska K, Zou QM, Doherty PC, Chen W. Systematic identification of immunodominant CD8+ T-cell responses to influenza A virus in HLA-A2 individuals. Proc Natl Acad Sci U S A 2011; 108:9178-83; PMID:21562214; http://dx.doi.org/ 10.1073/pnas.1105624108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alexander J, Bilsel P, del Guercio M-F, Marinkovic-Petrovic A, Southwood S, Stewart S, Ishioka G, Kotturi MF, Botten J, Sidney J, et al.. Identification of broad binding class I HLA supertype epitopes to provide universal coverage of influenza A virus. Hum Immunol 2010; 71:468-74; PMID:20156506; http://dx.doi.org/ 10.1016/j.humimm.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu J, Wu B, Zhang S, Tan S, Sun Y, Chen Z, Qin Y, Sun M, Shi G, Wu Y, et al.. Conserved epitopes dominate cross-CD8+ T-cell responses against influenza A H1N1 virus among Asian populations. Eur J Immunol 2013; 43:2055-69; PMID:23681926; http://dx.doi.org/ 10.1002/eji.201343417 [DOI] [PubMed] [Google Scholar]
- 144.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al.. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274-80; PMID:22286307; http://dx.doi.org/ 10.1038/nm.2612 [DOI] [PubMed] [Google Scholar]
- 145.Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, et al.. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog 2013; 9:e1003207; PMID:23516357; http://dx.doi.org/ 10.1371/journal.ppat.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, Robbins PD, Swayne DE, Donis RO, Katz JM, et al.. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol 2006; 80:1959-64; PMID:16439551; http://dx.doi.org/ 10.1128/JVI.80.4.1959-1964.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, Bright RA, Katz JM, Mittal SK, Sambhara S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 2006; 367:475-81; PMID:16473124; http://dx.doi.org/ 10.1016/S0140-6736(06)68076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Mayall T, Ervin JE, Greenberg RN, Strout C, et al.. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis 2013; 13:238-50; PMID:23369412; http://dx.doi.org/ 10.1016/S1473-3099(12)70345-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual Rev Biochem 2000; 69:531-69; PMID:10966468; http://dx.doi.org/ 10.1146/annurev.biochem.69.1.531 [DOI] [PubMed] [Google Scholar]
- 150.Wesley RD, Tang M, Lager KM. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine 2004; 22:3427-34; PMID:15308368; http://dx.doi.org/ 10.1016/j.vaccine.2004.02.040 [DOI] [PubMed] [Google Scholar]
- 151.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis 2006; 12:48-54; PMID:16494717; http://dx.doi.org/ 10.3201/eid1201.051237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, García-Sastre A, Palese P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 2010; 1:pii: e00018-10; PMID:20689752; http://dx.doi.org/ 10.1128/mBio.00018-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, et al.. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci 2010; 107:13701-6; PMID:20615991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, García-Sastre A, Moran TM, et al.. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A 2010; 107:18979-84; PMID:20956293; http://dx.doi.org/ 10.1073/pnas.1013387107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 2013; 87:6542-50; PMID:23576508; http://dx.doi.org/ 10.1128/JVI.00641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 2014; 88:2340-3; PMID:24307585; http://dx.doi.org/ 10.1128/JVI.03183-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, García-Sastre A, et al.. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 2013; 87:10435-46; PMID:23903831; http://dx.doi.org/ 10.1128/JVI.01715-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Krammer F, Palese P. Universal influenza virus vaccines: need for clinical trials. Nat Immunol 2014; 15:3-5; PMID:24352315; http://dx.doi.org/ 10.1038/ni.2761 [DOI] [PubMed] [Google Scholar]
- 159.Noureddini SC, Curiel DT. Genetic targeting strategies for adenovirus. Mol Pharm 2005; 2:341-7; PMID:16196486; http://dx.doi.org/ 10.1021/mp050045c [DOI] [PubMed] [Google Scholar]
- 160.Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. T cell priming versus T cell tolerance induced by synthetic peptides. J Expe Med 1995; 182:261-6; PMID:7540654; http://dx.doi.org/ 10.1084/jem.182.1.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Franco D, Liu W, Gardiner DF, Hahn BH, Ho DD. CD40L-containing virus-like particle as a candidate HIV-1 vaccine targeting dendritic cells. J Acquir Immune Defic Syndr 2011; 56:393-400; PMID:21239998; http://dx.doi.org/ 10.1097/QAI.0b013e31820b844e [DOI] [PubMed] [Google Scholar]
- 162.Kim YS, Kim YJ, Lee JM, Han SH, Ko HJ, Park HJ, Pereboev A, Nguyen HH, Kang CY. CD40-targeted recombinant adenovirus significantly enhances the efficacy of antitumor vaccines based on dendritic cells and B cells. Hum Gene Ther 2010; 21:1697-706; PMID:20604681; http://dx.doi.org/ 10.1089/hum.2009.202 [DOI] [PubMed] [Google Scholar]
- 163.Cao J, Wang X, Du Y, Li Y, Wang X, Jiang P. CD40 ligand expressed in adenovirus can improve the immunogenicity of the GP3 and GP5 of porcine reproductive and respiratory syndrome virus in swine. Vaccine 2010; 28:7514-22; PMID:20851084; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 164.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev 2003; 14:297-309; PMID:12787567; http://dx.doi.org/ 10.1016/S1359-6101(03)00024-8 [DOI] [PubMed] [Google Scholar]
- 165.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009; 21:265-72; PMID:19524453; http://dx.doi.org/ 10.1016/j.smim.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fan X, Hashem AM, Chen Z, Li C, Doyle T, Zhang Y, Yi Y, Farnsworth A, Xu K, Li Z, et al.. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol 2015; 8:211-20; PMID:25052763; http://dx.doi.org/ 10.1038/mi.2014.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lo CY, Wu Z, Misplon JA, Price GE, Pappas C, Kong WP, Tumpey TM, Epstein SL. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine 2008; 26:2062-72; PMID:18378366 [DOI] [PubMed] [Google Scholar]
- 168.Rao SS, Kong WP, Wei CJ, Van Hoeven N, Gorres JP, Nason M, Andersen H, Tumpey TM, Nabel GJ. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PloS One 2010; 5:e9812; PMID:20352112; http://dx.doi.org/ 10.1371/journal.pone.0009812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hashem AM, Gravel C, Chen Z, Yi Y, Tocchi M, Jaentschke B, Fan X, Li C, Rosu-Myles M, Pereboev A, et al.. CD40 ligand preferentially modulates immune response and enhances protection against influenza virus. J Immunol 2014; 193:722-34; PMID:24928989; http://dx.doi.org/ 10.4049/jimmunol.1300093 [DOI] [PubMed] [Google Scholar]
- 170.Hoelscher MA, Singh N, Garg S, Jayashankar L, Veguilla V, Pandey A, Matsuoka Y, Katz JM, Donis R, Mittal SK, et al.. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis 2008; 197:1185-8; PMID:18462165; http://dx.doi.org/ 10.1086/529522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vemula SV, Ahi YS, Swaim AM, Katz JM, Donis R, Sambhara S, Mittal SK. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PloS One 2013; 8:e62496; PMID:23638099; http://dx.doi.org/ 10.1371/journal.pone.0062496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Patel A, Gray M, Li Y, Kobasa D, Yao X, Kobinger GP. Co-administration of certain DNA vaccine combinations expressing different H5N1 influenza virus antigens can be beneficial or detrimental to immune protection. Vaccine 2012; 30:626-36; PMID:22119588; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 173.Kim EH, Park HJ, Han GY, Song MK, Pereboev A, Hong JS, Chang J, Byun YH, Seong BL, Nguyen HH. Intranasal adenovirus vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice. J Virol 2014; 88(17):9693-703; PMID:24920793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, Mytle N, Dong JY. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine 2008; 26:2627-39; PMID:18395306; http://dx.doi.org/ 10.1016/j.vaccine.2008.02.053 [DOI] [PubMed] [Google Scholar]
- 175.Li KB, Zhang XG, Ma J, Jia XJ, Wang M, Dong J, Zhang XM, Xu H, Shu YL. [Codon optimization of the H5N1 influenza virus HA gene gets high expression in mammalian cells]. Bing Du Xue Bao 2008; 24:101-5; PMID:18533341 [PubMed] [Google Scholar]
- 176.Steitz J, Barlow PG, Hossain J, Kim E, Okada K, Kenniston T, Rea S, Donis RO, Gambotto A. A candidate H1N1 pandemic influenza vaccine elicits protective immunity in mice. PloS One 2010; 5:e10492; PMID:20463955; http://dx.doi.org/ 10.1371/journal.pone.0010492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsu KH, Lubeck MD, Bhat BM, Bhat RA, Kostek B, Selling BH, Mizutani S, Davis AR, Hung PP. Efficacy of adenovirus-vectored respiratory syncytial virus vaccines in a new ferret model. Vaccine 1994; 12:607-12; PMID:8085377; http://dx.doi.org/ 10.1016/0264-410X(94)90264-X [DOI] [PubMed] [Google Scholar]
- 178.Gauger PC, Loving CL, Lager KM, Janke BH, Kehrli ME Jr., Roth JA, Vincent AL. Vaccine-associated enhanced respiratory disease does not interfere with the adaptive immune response following challenge with pandemic A/H1N1 2009. Viral Immunol 2013; 26:314-21; PMID:24033080; http://dx.doi.org/ 10.1089/vim.2013.0018 [DOI] [PubMed] [Google Scholar]
- 179.To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, Ng JC, Chan JF, Chan KH, Yuen KY. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin Vaccine Immunol 2012; 19:1012-8; PMID:22573737; http://dx.doi.org/ 10.1128/CVI.00081-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 2013; 5:200ra114; PMID:23986398; http://dx.doi.org/ 10.1126/scitranslmed.3006366 [DOI] [PubMed] [Google Scholar]
- 181.Gauger PC, Loving CL, Khurana S, Lorusso A, Perez DR, Kehrli ME Jr., Roth JA, Golding H, Vincent AL. Live attenuated influenza A virus vaccine protects against A(H1N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology 2014; 471-473C:93-104; PMID:25461535; http://dx.doi.org/ 10.1016/j.virol.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 182.Dougan SK, Ashour J, Karssemeijer RA, Popp MW, Avalos AM, Barisa M, Altenburg AF, Ingram JR, Cragnolini JJ, Guo C, et al.. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature 2013; 503:406-9; PMID:24141948; http://dx.doi.org/ 10.1038/nature12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Quinones-Parra S, Loh L, Brown LE, Kedzierska K, Valkenburg SA. Universal immunity to influenza must outwit immune evasion. Front Microbiol 2014; 5:285; PMID:24971078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Valkenburg SA, Quinones-Parra S, Gras S, Komadina N, McVernon J, Wang Z, Halim H, Iannello P, Cole C, Laurie K, et al.. Acute emergence and reversion of influenza A virus quasispecies within CD8+ T cell antigenic peptides. Nat Commun 2013; 4:2663; PMID:24173108; http://dx.doi.org/ 10.1038/ncomms3663 [DOI] [PubMed] [Google Scholar]
- 185.Gras S, Kedzierski L, Valkenburg SA, Laurie K, Liu YC, Denholm JT, Richards MJ, Rimmelzwaan GF, Kelso A, Doherty PC, et al.. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci U S A 2010; 107:12599-604; PMID:20616031; http://dx.doi.org/ 10.1073/pnas.1007270107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Valkenburg SA, Venturi V, Dang TH, Bird NL, Doherty PC, Turner SJ, Davenport MP, Kedzierska K. Early priming minimizes the age-related immune compromise of CD8(+) T cell diversity and function. PLoS Pathog 2012; 8:e1002544; PMID:22383879; http://dx.doi.org/ 10.1371/journal.ppat.1002544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Madore DV, Meade BD, Rubin F, Deal C, Lynn F, Meeting C. Utilization of serologic assays to support efficacy of vaccines in nonclinical and clinical trials: meeting at the crossroads. Vaccine 2010; 28:4539-47; PMID:20470795; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69-75; PMID:367490 [DOI] [PubMed] [Google Scholar]
- 189.Fulop T Jr., Foris G, Worum I, Leovey A. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol 1985; 61:425-32; PMID:2994926 [PMC free article] [PubMed] [Google Scholar]
- 190.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al.. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011; 333:843-50; PMID:21737702; http://dx.doi.org/ 10.1126/science.1204839 [DOI] [PMC free article] [PubMed] [Google Scholar]