Abstract
Vaccination of healthcare workers (HCWs) reduces the risk of occupational infections, prevents nosocomial transmission and maintains healthcare delivery during outbreaks. Despite the European directive and national legislation on workers’ protection, immunization coverage among HCWs has often been very low. In light of Italian National Vaccination Plan 2012–2014 recommendations, the aim of this study was to assess levels of immunization and factors influencing adherence to vaccinations needed for HCWs in Puglia region, South Italy. The study was conducted using an interview-based standardized anonymous questionnaire administered to hospital employees in the period November 2009-March 2011. A total of 2198 health professionals responded in 51/69 Apulian hospitals (median age: 45 years; 65.2% nurses, 22.6% doctors and 12.2% other hospital personnel). Vaccination coverage was 24.8% for influenza, 70.1% for hepatitis B, 9.7% for MMR, 3.6% for varicella, and 15.5% for Td booster. Receiving counselling from occupational health physicians (OHPs) was associated with influenza (OR = 1.8; 95%CI = 1.5–2.2; P < 0.001), hepatitis B (OR = 4.9; 95%CI = 3.9–6.3; P < 0.001), varicella (OR = 43.7; 95%CI = 18.9–101.7; P < 0.001), MMR (OR = 8.8; 95%CI = 4.1–18.6; P < 0.001) and tetanus (OR = 50.5; 95%CI = 30.1–88.3; P < 0.001) vaccine uptake.
OHPs should be trained with standard guidelines specific for healthcare settings and HCWs’ risk groups to facilitate their crucial role in improving vaccine coverage among HCWs and increase awareness on the duty to protect both employees and patients.
Keywords: healthcare workers, vaccine, influenza, hepatitis B, measles, mumps, rubella, varicella, Td booster
Background
WHO estimates that all over the world 59 million health care workers (HCWs) are exposed every day to multiple occupational hazards,1 the most common being the risk of exposure to infected patients and/or infectious materials, including body fluids, contaminated medical supplies and equipment, environmental surfaces, or air. In addition, HCWs are at increased risk of transmitting infections to their colleagues and vulnerable patients.2,3 Vaccination of HCWs reduces the risk of occupational infections, prevents nosocomial transmission and maintains health care delivery during outbreaks. Optimal uptake of recommended vaccines safeguards HCWs from vaccine-preventable infections, thereby protects patients from becoming infected.3,4
The European directive 2000/54/EC stated the need to offer free of charge effective vaccines to workers susceptible to the biological agents to which they are exposed. The importance to keep informed workers of the benefits and drawbacks of both vaccination and non-vaccination was also recommended.5
In Italy, the Decree Law no. 81 dated 9 April 2008 recommends the protection of workers from biological hazards through vaccination and attributes the responsibility of vaccines administration to Occupational Health Physicians (OHPs). According to the law, the Italian National Vaccination Plan 2012–2014 strongly recommends to actively offer: (1) hepatitis B (HB) and influenza vaccines to all HCWs; (2) measles, mumps, rubella (MMR) and varicella vaccines to susceptible workers; and (3) diphtheria, tetanus, acellular pertussis (DTaP) booster every ten years, especially to those HCWs working in obstetric or neonatology departments.6
Since 1988, hepatitis B vaccine is offered free of charge to all new employees by the National Health Service and to those already working in high risk of infection environments.7,8 The Ministry of Health Decree dated 20 November 2000 established the need to verify the seroconversion one month after the last dose of hepatitis B vaccine to ascertain the establishment of immunological memory. Subjects who test negative are recommended for further doses.9
Finally, the National Plan of Measles and Congenital Rubella Elimination (PNEMoRC) 2010–2015, strongly recommends vaccination of susceptible adults at risk of contracting and transmitting disease, such as health professionals.10
Despite the European directive and the adoption of national legislation on HCWs’ protection, low immunization coverage and transmission of vaccine-preventable diseases among HCWs are frequently documented as well as nosocomial outbreaks.3,6,7,11-28
In France, during the 2007–2008 influenza season, vaccination coverage among workers in nursing home was 33.6%,29 in Spain a negative trend of influenza vaccination coverage from 29,2% in 2008/9 to 17,9% in 2011/12 seasons was recorded among HCWs.30 In Belgium, in 2004, 84.9% of HCWs eligible for HB immunization had received the vaccine;31 in France, in 2011, of 505 HCWs working in tertiary-care hospitals, 56.5% had been vaccinated against hepatitis B;32 in South-Western Greece HB vaccination coverage was 70.9% in 2013.33 Coverage for the other recommended vaccines is often very low and inadequate to prevent hospital epidemics.32,34 In France, a study conducted in 2011 showed vaccination coverage of 18.8% for measles and mumps, 22.2% for rubella, 1.9% for varicella and 35.7% for tetanus-diphtheria.32 Another study conducted in 2008 among French medical residents showed vaccination coverage of 65.2% for pertussis and 62.8% for measles and varicella.35
In Italy, figures on vaccination coverage among HCWs are not routinely available either at national or regional level.7 A study conducted in Sicily (South Italy) in 2013 showed a reduction of vaccination coverage for influenza from 13.2% to 3.1% during seven consecutive influenza seasons (2005–2012) among HCWs of an acute care hospital.36 During a survey performed among the medical residents of 18 Italian Universities, 11.9% reported to have been vaccinated against influenza during 2011–2012 season.37 An audit of Apulian vaccination services HCWs in 2008 revealed a susceptibility rate of 9.3% for measles, mumps or rubella and 4.6% for varicella.38
In light of a such jeopardized scenario, considering a moral imperative for HCWs to be immunized and for healthcare institutions to ensure vaccination, European public health organizations, following some experiences in the US, are pondering mandatory vaccination policies for HCWs. Overcoming concerns about individual autonomy should be ethical and acceptable if HCWs’ voluntary uptake of vaccination is not optimal, especially in settings with high-risk groups of patients.3
In February 2013, Puglia region adopted the National Vaccination Plan 2012–2014 recommendations, assigning to OHPs the responsibility for the identification of susceptible HCWs and the administration of vaccinations.39
This study aims to assess levels of immunization and factors influencing adherence to vaccinations needed for HCWs in Puglia region.
Results
A total of 2,198 HCWs returned the questionnaire, 67.7% were female, median age was 45 y (SD = 9.2; range 22–70). Of the respondents, 65.2% were nurses, 22.6% physicians and 12.2% other health care personnel. The median length of professional career was 21 y (SD = 9.8, range 0–46 y). The 59.2% reported to work in medical departments, 21.7% in intensive care units, 17.8% in surgical departments and 0.3% in other hospital services, 0.9% did not indicate any job assignment (Table 1). Of the interviewed HCWs, 63.9% referred to have contracted varicella, 69.8% measles, 48% mumps and 43.4% rubella.
Table 1. Apulian HCWs’ characteristics. Italy, 2009–2011.
| N | % | ||
| Sex | |||
| Female | 1487 | 67.7 | |
| Age (y) | |||
| ≥ median age | 1089 | 49.5 | |
| Duration of professional career (y) | |||
| ≥ median | 970 | 44.1 | |
| Job assignment | |||
| Nurse | 1433 | 65.2 | |
| Physician | 496 | 22.6 | |
| Other healthcare worker | 269 | 12.2 | |
| Department | |||
| Medicine | 1302 | 59.2 | |
| Surgery | 392 | 17.8 | |
| Intensive care | 477 | 21.7 | |
| Other services | 7 | 0.3 | |
| No reply | 20 | 0.9 | |
Overall vaccination coverage was 24.8% for influenza (computed for both seasons 2009–2010 and 2010–2011), 70.1% for hepatitis B, 9.7% for MMR, 3.6% for varicella and 15.5% for Td booster.
Determinants of seasonal influenza vaccination
In the univariate analysis, being female, having an older age and a longer length of service increased the likelihood to have been vaccinated (P < 0.05; Table 2A). Compared with nurses and the other HCWs, physicians were more likely to have been vaccinated (P < 0.01; Fig. 1). Vaccination coverage was 24.5% among workers in medical departments, 26% in surgical departments and 24.3% in intensive care units (Fig. 2). Nearly 57% of HCWs had received a proposal for vaccination against seasonal influenza. Receiving counseling from an OHP (P < 0.001) or a GP (P < 0.001) was associated with influenza vaccine uptake (Table 2A).
Table 2. Determinants of vaccination for influenza (A), hepatitis B (B), measles, mumps, rubella (C), varicella (D), and Td booster (E) in Apulian HCWs. Italy, 2009–2011.
| A | Influenza vaccination | ||||||
|---|---|---|---|---|---|---|---|
| (n = 546) | |||||||
| N | % | OR (95% CI) | chi2 | P value | |||
| Sex | |||||||
| Female | 330 | 60.4 | 1.5 (1.2–1.9) | 17.3 | 0.0000 | ||
| Age (y) | |||||||
| ≥ median age | 319 | 58.4 | 1.5 (1.2–1.8) | 14.5 | 0.0000 | ||
| Duration of professional career (y) | |||||||
| ≥ median | 357 | 65.4 | 1.4 (1.2–1.8) | 12.2 | 0.0005 | ||
| Vaccination proposal | |||||||
| OHP | 317 | 58.1 | 1.8 (1.5–2.2) | 34.2 | 0.0000 | ||
| GP | 88 | 16.1 | 2.3 (1.7–3.2) | 34.3 | 0.0000 | ||
| B | Hepatitis B vaccination | ||||||
| (n = 1,541) | |||||||
| N | % | OR (95% CI) | chi2 | P value | |||
| Sex | |||||||
| Female | 1,057 | 68.6 | 0.9 (0.8–1.1) | 2.1 | 0.1493 | ||
| Age (y) | |||||||
| ≥ median age | 696 | 45.2 | 0.4 (0.3–0.5) | 79.3 | 0.0000 | ||
| Duration of professional career (y) | |||||||
| ≥ median | 823 | 53.4 | 0.4 (0.3–0.5) | 66.9 | 0.0000 | ||
| Vaccination proposal | |||||||
| OHP | 824 | 53.5 | 4.9 (3.9–6.3) | 226.8 | 0.0000 | ||
| GP | 50 | 3.2 | 4.4 (1.7–14.1) | 11.6 | 0.0006 | ||
| Public health service | 273 | 17.7 | 7.2 (4.5–12.3) | 87.9 | 0.0000 | ||
| C | MMR vaccination | ||||||
| (n = 214) | |||||||
| N | % | OR (95% CI) | chi2 | P value | |||
| Sex | |||||||
| Female | 155 | 72.4 | 0.8 (0.6–1.1) | 2.5 | 0.1158 | ||
| Age (y) | |||||||
| ≥ median age | 51 | 23.8 | 0.3 (0.2–0.4) | 71.9 | 0.0000 | ||
| Duration of professional career (y) | |||||||
| ≥ median | 72 | 33.6 | 0.3 (0.2–0.4) | 63.0 | 0.0000 | ||
| Vaccination proposal | |||||||
| OHP | 16 | 7.5 | 8.8 (4.1–18.6) | 54.7 | 0.0000 | ||
| GP | 21 | 9.8 | 16.5 (7.7–36.4) | 106.4 | 0.0000 | ||
| Public health service | 50 | 23.4 | 54.7 (27.3–118.2) | 372.5 | 0.0000 | ||
| D | Varicella vaccination | ||||||
| (n = 80) | |||||||
| N | % | OR (95% CI) | chi2 | P value | |||
| Sex | |||||||
| Female | 44 | 55.0 | 1.7 (1.1–2.8) | 6.1 | 0.0137 | ||
| Age (y) | |||||||
| ≥ median age | 27 | 33.7 | 0.5 (0.3–0.8) | 10.3 | 0.0013 | ||
| Duration of professional career (y) | |||||||
| ≥ median | 41 | 51.2 | 0.7 (0.4–1.2) | 2.1 | 0.1506 | ||
| Vaccination proposal | |||||||
| OHP | 17 | 21.2 | 43.7 (18.9–101.7) | 243.8 | 0.0000 | ||
| GP | 7 | 8.8 | 22.5 (6.9–69.6) | 73.9 | 0.0000 | ||
| Public health service | 17 | 21.2 | 63.2 (25.3–166.1) | 286.0 | 0.0000 | ||
| E | Td booster | ||||||
| (n = 341) | |||||||
| N | % | OR (95% CI) | chi2 | P value | |||
| Sex | |||||||
| Female | 194 | 56.9 | 1.7 (1.4–2.2) | 21.4 | 0.0000 | ||
| Age (y) | |||||||
| ≥ median age | 150 | 43.9 | 0.7 (0.5–0.9) | 8.8 | 0.0030 | ||
| Duration of professional career (y) | |||||||
| ≥ median | 172 | 50.4 | 0.7 (0.5–0.8) | 12.2 | 0.0005 | ||
| Vaccination proposal | |||||||
| OHP | 117 | 34.3 | 50.5 (30.1–88.3) | 549.9 | 0.0000 | ||
| GP | 19 | 5.6 | 8.4 (3.9–18.6) | 47.7 | 0.0000 | ||
| Public health service | 49 | 14.4 | 22.1 (11.8–43.8) | 191.8 | 0.0000 | ||
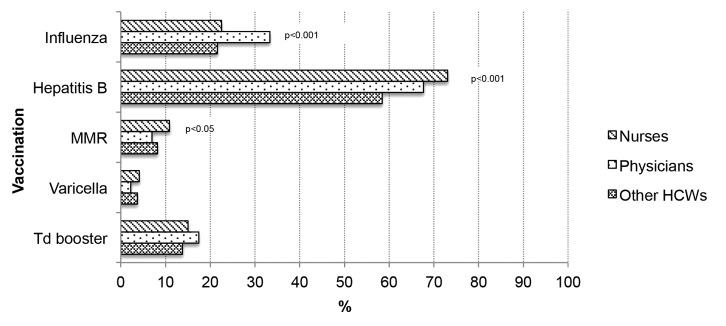
Figure 1. Vaccination coverage in Apulian HCWs, by job assignment. Italy, 2009–2011.
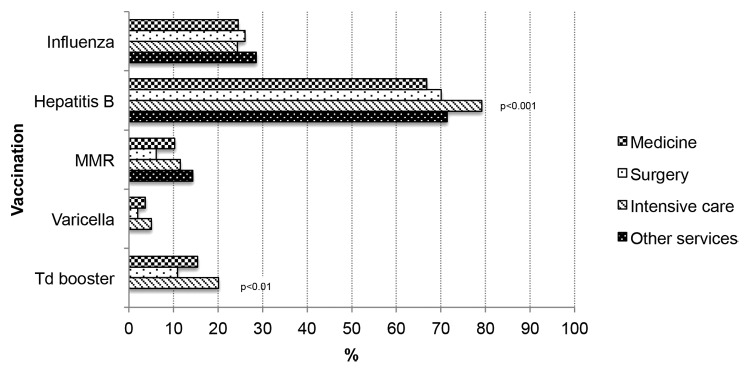
Figure 2. Vaccination coverage in Apulian HCWs, by department of service. Italy, 2009–2011.
The logistic regression confirmed the association between having been vaccinated against influenza and sex female (OR = 1.5; 95% Cl = 1.2–1.9; P < 0.01), to be a physician (OR = 1.8; 95% Cl = 1.1–3.0; P < 0.05), to have received the vaccination proposal from an OHP (OR = 2.4; 95% Cl = 1.9–3.1; P < 0.001) or from a GP (OR = 4.6; 95% Cl = 3.2–6.6; P < 0.001).
Determinants of hepatitis B vaccination
Younger age and shorter length of service were associated with the probability of having been vaccinated (P < 0.05; Table 2B). Compared with physicians and other HCWs, nurses were more likely to have been vaccinated against hepatitis B (P < 0.01; Fig. 1). Vaccination coverage was 66.8% among workers in medical departments, 70.1% in surgical departments and 79.2% in intensive care units. HCWs working in an intensive care unit were more likely to be vaccinated (P < 0.01; Fig. 2). About 60% of HCWs had received the proposal for vaccination against hepatitis B. Receiving counseling from an OHP, a GP or from the Vaccination Service (P < 0.001) was associated with hepatitis B vaccine uptake (Table 2B).
In the logistic regression, having been vaccinated against hepatitis B was associated with younger age (OR = 1.9; 95%Cl = 1.4–2.6; P < 0.001), a shorter length of service (OR = 1.5; 95%Cl = 1.1–2.0; P < 0.05), and the proposal of vaccination from the OHP (OR = 8.3; 95% Cl = 6.4–10.9; P < 0.001), the GP (OR = 9.2; 95% Cl = 3.5–23.7; P < 0.001), or the Vaccination Service (OR = 14.6; 95% Cl = 8.8–24.4; P < 0.001).
Determinants of MMR vaccination
Younger age and shorter length of service were associated with vaccination (P < 0.001; Table 2C). Nurses were more likely to be vaccinated against MMR (P < 0.05; Fig. 1). Vaccination coverage was 10.3% in those working in a department of medicine, 6.1% in a department of surgery, and 11.5% in an intensive care unit (Fig. 2). Only 5.9% of HCWs had received the proposal for vaccination against MMR. Receiving counseling from an OHP, a GP or the Vaccination Service was associated with MMR vaccine uptake (P < 0.001; Table 2C).
Overall, 17% of those susceptible to measles, 13.2% to mumps and 17.8% to rubella had received MMR vaccination.
In the logistic regression, MMR vaccination was associated with being younger (OR = 1.9; 95%Cl = 1.2-3.0; P < 0.01), a shorter length of service (OR = 1.8; 95% Cl = 1.1–2.8; P < 0.05), a vaccination proposal from the OHP (OR = 10.8; 95%Cl = 4.7–25.1; P < 0.001), the GP (OR = 13.6; 95% Cl = 5.9–31.3; P < 0.001) or the Vaccination Service (OR = 50.6; 95%Cl = 22.7–112.4; P < 0.001).
Determinants of varicella vaccination
Being female and younger was associated with the probability of having been vaccinated (P < 0.05; Table 2D). Vaccination coverage was 3.7% among workers in a department of medicine, 2.9% in a department of surgery and 5.0% in an intensive care unit (Fig. 2). Nearly 8% of HCWs received the proposal for vaccination against varicella. Receiving counseling from the OHP, the GP or the Vaccination Service (P < 0.001) was associated with vaccination (Table 2D). Overall, 7.6% of HCWs susceptible to varicella had received vaccination.
The logistic regression showed an association with sex female (OR = 2.0; 95%Cl = 1.2–3.5; P < 0.05), younger age (OR = 1.8; 95%Cl = 1.1–3.4; P < 0.05), the proposal of vaccination from the OHP (OR = 69.8; 95%Cl = 30.5–159.8; P < 0.001), the GP (OR = 42.2; 95% Cl = 14.3–123.9; P < 0.001), or the public health service (OR = 67.7; 95% Cl = 27.2–168.4; P < 0.001).
Determinants of Td booster
In the univariate analysis, being female, younger and with a shorter length of service were associated with vaccination (P < 0.001; Table 2E). Of the interviewed HCWs, 15.4% working in a department of medicine, 10.9% in a department of surgery and 20.1% in an intensive care unit had received a Td booster dose; HCWs who worked in intensive care unit were less likely to have received vaccination (P < 0.01; Fig. 2). The proposal for a booster dose was reported by 8.4% of HCWs. Receiving counseling was associated with vaccination (P < 0.001; Table 2E).
In the logistic regression, Td booster was associated with sex female (OR = 1.9; 95%Cl = 1.4–2.7; P < 0.01) and a proposal of vaccination from the OHP (OR = 67.8; 95%Cl = 38.7–118.8; P < 0.001), the GP (OR = 13.3; 95%Cl = 6.0–29.9; P < 0.001), or the public health service (OR = 40.7; 95%Cl = 20.5–80.5; P < 0.001).
Discussion
Protection against occupational biohazards needs resources and investment in terms of both setting up protective measures and training healthcare staff, but the cost of non-protection can be higher in terms of worker’s health, productivity, safety and quality of work environment. The prevention of infection diseases among HCWs is based on the adoption of primary measures in the form of the usual standard precautions together with immunization, and secondary measures in the form of post-exposure prophylaxis.40
In this study, according with the findings of a review on vaccination in Italian healthcare workers in 2010 and other national and international studies, vaccination coverage for hepatitis B is acceptable but still sub-optimal and figures for other vaccine-preventable diseases are quite low.7,11-16,30-38,41 Vaccination coverage for seasonal influenza is far from the objective of 75% to be achieved by 2015 as in the EU Council recommendation of December 2009.42 Protection against measles, mumps, rubella and varicella is totally inadequate to prevent disease transmission among susceptible HCWs and nosocomial oubreaks.7,11-18,33
Increasing awareness on the importance of vaccination in HCWs and on the duty to protect both employees and patients is the main goal of the European project HProImmune. Started in 2011, it aims to promote immunization among HCWs in different health care settings, developing communication toolkits and educational materials tailored to health professionals in both the private and the public sector.43
To address concerns on low vaccination coverage among HCWs, several international healthcare organizations are evaluating the ethics of mandatory immunization policies,14 provided that benefits outweigh harm for HCWs, patients’ welfare is enhanced, and fair rules and exemptions are defined.3
According to other studies, our findings revealed that older workers have a higher likelihood of influenza vaccine uptake, while those younger have higher vaccination coverage for hepatitis B, measles, mumps, rubella and varicella.13,15,33 Physicians get vaccinated against influenza more than other HCWs, as reported in the last CDC report on Influenza vaccination coverage among health-care personnel.44
Counseling and proposing vaccination from the OHP, the GP or the public health service appeared to be influent in the decision of HCWs to get vaccinated as reported in other studies.45-50 A survey conducted between December 2009 and April 2010 in Germany, France, United States, China, and Mexico showed as the recommendation of a GP was a leading reason for receiving the influenza pandemic vaccine.45 In another study conducted in 11 European countries in 2009, the advice from a family doctor or a nurse was deemed as the main encouraging factor for influenza vaccination.46
OHPs should be trained according to standard guidelines specific for healthcare settings and HCWs’ risk groups to facilitate their crucial role in improving vaccine coverage among HCWs. Moreover, OHPs should record reasons for vaccination refusal, since the use of a signed informed declination statement for those refusing vaccination, has recently been recommended as a possible tool to stimulate HCWs vaccination rates.51,52
Finally, medical students must be considered a priority group for campaigns promoting vaccination, not only for their training activities at the hospital wards, but especially for their future role. A new generation of adequately immunized HCWs is coming in Italy, also considering that they received most of recommended-to-HCWs vaccinations since infancy.
Methods
Between November 2009 and March 2011, the HCWs employed in 51 out of 69 Apulian hospitals were invited to fill out a standardized anonymous questionnaire.
Main investigated outcomes were: (1) demographic and job characteristics (sex, age, hospital department and length of service, job assignment); (2) susceptibility/protection to measles, mumps, rubella and varicella; (3) vaccination history against hepatitis B, tetanus, measles, mumps, rubella and varicella. In addition, the interviewees were asked if they had received a vaccination proposal from an OHP, a general practitioner (GP), or the Vaccination Service.
To assess the association between vaccination status and the other investigated variables, an univariate analysis was performed, by using double-entry contingency tables and computing chi square (Chi2) and Odds Ratios (OR) with 95% Confidence Intervals (95% CIs), considering as significant P values < 0.05. Variables significantly associated with “having been vaccinated” were included in a multivariate logistic model to calculate adjusted ORs with 95% CIs. Data were analyzed with Stata MP 11.2 for Mac OS software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank all the Apulian hospitals’ staff who took part in the survey.
Glossary
Abbreviations:
- HCW
Health care worker
- PNEMoRC
Piano Nazionale di Eliminazione del Morbillo e della Rosolia Congenita - National Plan of Measles and Congenital Rubella Elimination
- OHP
Occupational health physician
- GP
General practitioner
- DTaP
Diphtheria, Tetanus, acellular Pertussis vaccine
- Td
Tetanus, diphtheria booster
- HB
Hepatitis B
- OR
Odds Ratio
- 95%CI
95% Confidence Interval
- MMR
Measles, Mumps, Rubella vaccine
References
- 1.World Health Organization (WHO). Occupational health. Health workers. Health worker occupational heath. Available at: http://www.who.int/occupational_health/topics/hcworkers/en/. Accessed 14 May 2014.
- 2.Campins Martí M, Uriona Tuma S.. [General epidemiology of infections acquired by health-care workers: immunization of health-care workers]. Enferm Infecc Microbiol Clin 2014; 32:259 - 65; PMID: 24656968 [DOI] [PubMed] [Google Scholar]
- 3.Galanakis E, Jansen A, Lopalco PL, Giesecke J.. Ethics of mandatory vaccination for healthcare workers. Euro Surveill 2013; 18:20627; http://dx.doi.org/ 10.2807/1560-7917.ES2013.18.45.20627; PMID: 24229791 [DOI] [PubMed] [Google Scholar]
- 4.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC).. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:RR071 - 45; http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6007a1.htm Accessed15May2014 PMID: 22108587 [PubMed] [Google Scholar]
- 5.Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work. Available at: http://www.biosafety.be/PDF/2000_54.pdf. Accessed 20 May 2014.
- 6.Ministero della Salute. Italy (2012). Piano della Prevenzione Vaccinale 2012-2014. Intesa Stato-Regioni del 22 febbraio 2012. G.U. Serie Generale, n. 60 del 12 marzo 2012. Italian. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf. Accessed 20 May 2014.
- 7.Prato R, Tafuri S, Fortunato F, Martinelli D.. Vaccination in healthcare workers: an Italian perspective. Expert Rev Vaccines 2010; 9:277 - 83; http://dx.doi.org/ 10.1586/erv.10.11; PMID: 20218856 [DOI] [PubMed] [Google Scholar]
- 8.Ministero della Salute.. Italy (1988). Decreto Ministeriale del 22 dicembre 1988. “Offerta gratuita della vaccinazione contro l’epatite virale B alle categorie a rischio. Italian Official Bulletin, No. 1988; 305:30 [Google Scholar]
- 9.Ministero della Salute. (2012). Vaccinazioni per gli operatori sanitari. Available at: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua = italiano&id = 645&area = Malattie%20infettive&menu = vaccinazioni. Accessed 20 May 2014.
- 10.Ministero della Salute. Italy (2011). Piano nazionale per l'eliminazione del morbillo e della rosolia congenita 2010-2015. Intesa Stato-Regioni del 23 marzo 2011. Italian. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1519_allegato.pdf. Accessed 20 May 2014.
- 11.Blank PR, Szucs TD.. Increasing influenza vaccination coverage in recommended population groups in Europe. Expert Rev Vaccines 2009; 8:425 - 33; http://dx.doi.org/ 10.1586/erv.09.7; PMID: 19348558 [DOI] [PubMed] [Google Scholar]
- 12.Topuridze M, Butsashvili M, Kamkamidze G, Kajaia M, Morse D, McNutt LA.. Barriers to hepatitis B vaccine coverage among healthcare workers in the Republic of Georgia: An international perspective. Infect Control Hosp Epidemiol 2010; 31:158 - 64; http://dx.doi.org/ 10.1086/649795; PMID: 20038247 [DOI] [PubMed] [Google Scholar]
- 13.Maltezou HC, Katerelos P, Poufta S, Pavli A, Maragos A, Theodoridou M.. Attitudes toward mandatory occupational vaccinations and vaccination coverage against vaccine-preventable diseases of health care workers in primary health care centers. Am J Infect Control 2013; 41:66 - 70; http://dx.doi.org/ 10.1016/j.ajic.2012.01.028; PMID: 22709989 [DOI] [PubMed] [Google Scholar]
- 14.Maltezou HC, Wicker S, Borg M, Heininger U, Puro V, Theodoridou M, Poland GA.. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine 2011; 29:9557 - 62; http://dx.doi.org/ 10.1016/j.vaccine.2011.09.076; PMID: 21964058 [DOI] [PubMed] [Google Scholar]
- 15.Music T.. Protecting patients, protecting healthcare workers: a review of the role of influenza vaccination. Int Nurs Rev 2012; 59:161 - 7; http://dx.doi.org/ 10.1111/j.1466-7657.2011.00961.x; PMID: 22591085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicker S, Rose MA.. Health care workers and pertussis: an underestimated issue. Med Klin (Munich) 2010; 105:882 - 6; http://dx.doi.org/ 10.1007/s00063-010-1153-0; PMID: 21240587 [DOI] [PubMed] [Google Scholar]
- 17.Caputi G, Tafuri S, Chironna M, Martinelli D, Sallustio A, Falco A, Germinario CA, Prato R, Quarto M.. An outbreak of measles including nosocomial transmission in Apulia, south-east Italy, January-March 2008--a preliminary report. Euro Surveill 2008; 13; PMID: 18768120 [PubMed] [Google Scholar]
- 18.Tafuri S, Germinario C, Rollo M, Prato R.. Occupational risk from measles in healthcare personnel: a case report. J Occup Health 2009; 51:97 - 9; http://dx.doi.org/ 10.1539/joh.N8006; PMID: 19096198 [DOI] [PubMed] [Google Scholar]
- 19.Huttunen R, Syrjänen J.. Healthcare workers as vectors of infectious diseases. Eur J Clin Microbiol Infect Dis 2014; 33:1477 - 88; PMID: 24798250 [DOI] [PubMed] [Google Scholar]
- 20.Caseris M, Houhou N, Longuet P, Rioux C, Lepeule R, Choquet C, Yazdanpanah Y, Yeni P, Joly V.. French 2010-2011 measles outbreak in adults: report from a Parisian teaching hospital. Clin Microbiol Infect 2014; 20:O242 - 4; http://dx.doi.org/ 10.1111/1469-0691.12384; PMID: 24707854 [DOI] [PubMed] [Google Scholar]
- 21.Baxi R, Mytton OT, Abid M, Maduma-Butshe A, Iyer S, Ephraim A, Brown KE, O’Moore E.. Outbreak report: nosocomial transmission of measles through an unvaccinated healthcare worker--implications for public health. J Public Health (Oxf) 2013; In press http://dx.doi.org/ 10.1093/pubmed/fdt096; PMID: 24099734 [DOI] [PubMed] [Google Scholar]
- 22.Gilroy SA, Domachowske JB, Johnson L, Martin D, Gross S, Bode M, Costello K, Sikora R, Richey D, Watkins J, et al.. Mumps exposure of a health care provider working in a neonatal intensive care unit leads to a hospital-wide effort that prevented an outbreak. Am J Infect Control 2011; 39:697 - 700; http://dx.doi.org/ 10.1016/j.ajic.2010.12.011; PMID: 21641085 [DOI] [PubMed] [Google Scholar]
- 23.Singh MP, Chatterjee SS, Singh R, Goyal K, Ratho RK.. Rubella seronegativity among health care workers in a tertiary care north Indian hospital: implications for immunization policy. Indian J Pathol Microbiol 2013; 56:148 - 50; http://dx.doi.org/ 10.4103/0377-4929.118704; PMID: 24056653 [DOI] [PubMed] [Google Scholar]
- 24.Sood S.. Occupationally related outbreak of chickenpox in hospital staff: a learning experience. J Clin Diagn Res 2013; 7:2294 - 5; PMID: 24298507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.dos Santos AM, Ono E, Lobato RT, do Prado SI, Kopelman BI, Cavalcanti CM, Monomi MK, Weckx LY, de Moraes-Pinto MI.. Diphtheria, tetanus, and varicella immunity in health care workers in neonatal units. Am J Infect Control 2008; 36:142 - 7; http://dx.doi.org/ 10.1016/j.ajic.2007.04.283; PMID: 18313517 [DOI] [PubMed] [Google Scholar]
- 26.Botelho-Nevers E, Cassir N, Minodier P, Laporte R, Gautret P, Badiaga S, Thiberville DJ, Ninove L, Charrel R, Brouqui P.. Measles among healthcare workers: a potential for nosocomial outbreaks. Euro Surveill 2011; 16; PMID: 21251488 [PubMed] [Google Scholar]
- 27.Chironna M, Tafuri S, Santoro N, Prato R, Quarto M, Germinario CA.. A nosocomial outbreak of 2009 pandemic influenza A(H1N1) in a paediatric oncology ward in Italy, October-November 2009. Euro Surveill 2010; 15; PMID: 20067748 [DOI] [PubMed] [Google Scholar]
- 28.Walker J, Huc S, Sinka K, Tissington A, Oates K.. Ongoing outbreak of mumps infection in Oban, Scotland, November 2010 to January 2011. Euro Surveill 2011; 16; PMID: 21371413 [PubMed] [Google Scholar]
- 29.Vaux S, Noël D, Fonteneau L, Guthmann JP, Lévy-Bruhl D.. Influenza vaccination coverage of healthcare workers and residents and their determinants in nursing homes for elderly people in France: a cross-sectional survey. BMC Public Health 2010; 10:159; http://dx.doi.org/ 10.1186/1471-2458-10-159; PMID: 20338028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez-García R, Rodríguez-Rieiro C, Hernandez-Barrera V, Carrasco Garrido P, López de Andres A, Esteban-Vasallo MD, Domínguez-Berjón MF, Astray-Mochales J.. Negative trends from 2008/9 to 2011/12 seasons in influenza vaccination coverages among high risk subjects and health care workers in Spain. Vaccine 2014; 32:350 - 4; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.040; PMID: 24269621 [DOI] [PubMed] [Google Scholar]
- 31.Vranckx R, Jacques P, De Schrijver A, Moens G.. Hepatitis B vaccination coverage in Belgian health care workers. Infection 2004; 32:278 - 81; http://dx.doi.org/ 10.1007/s15010-004-2204-3; PMID: 15624891 [DOI] [PubMed] [Google Scholar]
- 32.Maltezou HC, Gargalianos P, Nikolaidis P, Katerelos P, Tedoma N, Maltezos E, Lazanas M.. Attitudes towards mandatory vaccination and vaccination coverage against vaccine-preventable diseases among health-care workers in tertiary-care hospitals. J Infect 2012; 64:319 - 24; http://dx.doi.org/ 10.1016/j.jinf.2011.12.004; PMID: 22198739 [DOI] [PubMed] [Google Scholar]
- 33.Karaivazoglou K, Triantos C, Lagadinou M, Bikas C, Michailidou M, Kalafateli M, Thomopoulos K, Assimakopoulos K, Nikolopoulou V, Jelastopulu E, et al.. Acceptance of hepatitis B vaccination among health care workers in Western Greece. Arch Environ Occup Health 2014; 69:107 - 11; http://dx.doi.org/ 10.1080/19338244.2012.750586; PMID: 24205962 [DOI] [PubMed] [Google Scholar]
- 34.Williams CJ, Liebowitz LD, Levene J, Nair P.. Low measles, mumps and rubella (MMR) vaccine uptake in hospital healthcare worker contacts following suspected mumps infection. J Hosp Infect 2010; 76:91 - 2; http://dx.doi.org/ 10.1016/j.jhin.2010.03.011; PMID: 20542592 [DOI] [PubMed] [Google Scholar]
- 35.Mir O, Adam J, Gaillard R, Gregory T, Veyrie N, Yordanov Y, Berveiller P, Chousterman B, Loulergue P.. Vaccination coverage among medical residents in Paris, France. Clin Microbiol Infect 2012; 18:E137 - 9; http://dx.doi.org/ 10.1111/j.1469-0691.2012.03788.x; PMID: 22404767 [DOI] [PubMed] [Google Scholar]
- 36.Amodio E, Restivo V, Firenze A, Mammina C, Tramuto F, Vitale F.. Can influenza vaccination coverage among healthcare workers influence the risk of nosocomial influenza-like illness in hospitalized patients?. J Hosp Infect 2014; 86:182 - 7; http://dx.doi.org/ 10.1016/j.jhin.2014.01.005; PMID: 24581755 [DOI] [PubMed] [Google Scholar]
- 37.Costantino C, Mazzucco W, Azzolini E, Baldini C, Bergomi M, Biafiore AD, Bianco M, Borsari L, Cacciari P, Cadeddu C, et al.. Influenza vaccination coverage among medical residents: An Italian multicenter survey. Hum Vaccin Immunother 2014; 10; In press http://dx.doi.org/ 10.4161/hv.28081; PMID: 24603089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tafuri S, Martinelli D, Caputi G, Arbore A, Lopalco PL, Germinario C, Prato R.. An audit of vaccination coverage among vaccination service workers in Puglia, Italy. Am J Infect Control 2009; 37:414 - 6; http://dx.doi.org/ 10.1016/j.ajic.2008.10.030; PMID: 19216005 [DOI] [PubMed] [Google Scholar]
- 39.Commissione tecnico-scientifica regionale vaccini, Apulia Region, Italy (2013). Intesa Stato-Regioni 22 febbraio 2012: “Piano Nazionale di Prevenzione Vaccinale 2012-2014”. Recepimento e adozione Calendario Regionale Vaccinale 2012 “Calendario per la vita”. Deliberazione della Giunta Regionale 18 febbraio 2013, n. 241. Bollettino ufficiale della Regione Puglia - n.41 del 19-03-2013. Available at: http://www.regione.puglia.it/index.php?page = burp&opz = getfile&file = o9.htm&anno = xliv&num = 41. Accessed 21 May 2014.
- 40.Beltrami EM, Williams IT, Shapiro CN, Chamberland ME.. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev 2000; 13:385 - 407; http://dx.doi.org/ 10.1128/CMR.13.3.385-407.2000; PMID: 10885983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbadoro P, Marigliano A, Di Tondo E, Chiatti C, Di Stanislao F, D’Errico MM, Prospero E.. Determinants of influenza vaccination uptake among Italian healthcare workers. Hum Vaccin Immunother 2013; 9:911 - 6; http://dx.doi.org/ 10.4161/hv.22997; PMID: 24064543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Commission Staff Working Document. State of play on implementation of the Council Recommendation of 22 December 2009 on seasonal influenza vaccination (2009/1019/EU). Brussels 2014. Available at: http://ec.europa.eu/health/vaccination/docs/seasonflu_staffwd2014_en.pdf. Accessed 30 May 2014.
- 43.HProImmune. Promotion of Immunization for Health Professionals in Europe. Available at: http://www.hproimmune.eu/. Accessed 30 May 2014.
- 44.Centers for Disease Control and Prevention (CDC).. Influenza vaccination coverage among health-care personnel--United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:781 - 6; PMID: 24067582 [PMC free article] [PubMed] [Google Scholar]
- 45.Blank PR, Bonnelye G, Ducastel A, Szucs TD.. Attitudes of the general public and general practitioners in five countries towards pandemic and seasonal influenza vaccines during season 2009/2010. PLoS One 2012; 7:e45450; http://dx.doi.org/ 10.1371/journal.pone.0045450; PMID: 23071519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blank PR, Schwenkglenks M, Szucs TD.. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009; 58:446 - 58; http://dx.doi.org/ 10.1016/j.jinf.2009.04.001; PMID: 19446340 [DOI] [PubMed] [Google Scholar]
- 47.Holm MV, Blank PR, Szucs TD.. Trends in influenza vaccination coverage rates in Germany over five seasons from 2001 to 2006. BMC Infect Dis 2007; 7:144; http://dx.doi.org/ 10.1186/1471-2334-7-144; PMID: 18070354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blank PR, Schwenkglenks M, Szucs TD.. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Infect Dis 2007; 7:144; PMID: 1807035418070354 [Google Scholar]
- 49.Mereckiene J, Cotter S, D’Ancona F, Giambi C, Nicoll A, Levy-Bruhl D, Lopalco PL, Weber JT, Johansen K, Dematte L, et al. , VENICE project gatekeepers group.. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008-2009. Euro Surveill 2010; 15; PMID: 21087586 [DOI] [PubMed] [Google Scholar]
- 50.Placidi D, Franco G, Bacis M, Belotti L, Biggi N, Carrer P, Cologni L, Gattini V, Lodi V, Magnavita N, et al.. [Focus on coverage and promotion of anti influenza vaccine in health workers: results and perspectives of a multicenter working group]. G Ital Med Lav Ergon 2010; 32:286 - 91; PMID: 21061711 [PubMed] [Google Scholar]
- 51.Polgreen PM, Septimus EJ, Parry MF, Beekmann SE, Cavanaugh JE, Srinivasan A, Talbot TR.. Relationship of influenza vaccination declination statements and influenza vaccination rates for healthcare workers in 22 US hospitals. Infect Control Hosp Epidemiol 2008; 29:675 - 7; http://dx.doi.org/ 10.1086/588590; PMID: 18564904 [DOI] [PubMed] [Google Scholar]
- 52.Talbot TR.. Do declination statements increase health care worker influenza vaccination rates?. Clin Infect Dis 2009; 49:773 - 9; http://dx.doi.org/ 10.1086/605554; PMID: 19622044 [DOI] [PubMed] [Google Scholar]


