Abstract
Hematogenous dissemination is thought to be a late event in cancer progression. We showed recently that pancreas cells can be detected in the bloodstream before tumor formation, in a genetic model of pancreatic ductal adenocarcinoma (PDAC). To confirm these findings in humans, we used microfluidic geometrically enhanced immunocapture to detect circulating pancreas epithelial cells (CECs) in patient blood samples. We captured >3 CECs/ml in 7 of 21 (33%) of patients with cystic lesions and no clinical diagnosis of cancer (Sendai criteria negative), 8 of 11 (73%) with PDAC, and in 0 of 19 patients without cysts or cancer (controls). These findings indicate that cancer cells are present in the circulation of patients before tumors develop, which might be used in risk assessment.
Keywords: early detection, IPMN, circulating tumor cells, pancreatic cancer
A widely-accepted paradigm in cancer biology is that epithelial cancers progress in a linear manner whereby cancer-defining properties are acquired sequentially1. In this model, cancer cells acquire metastatic potential after large primary tumors are established. However, in pancreatic ductal adenocarcinoma (PDAC), the linear progression model cannot be reconciled with clinical observations. A number of patients undergoing pancreatectomy for chronic pancreatitis will develop disseminated PDAC although only precancerous pancreatic intraepithelial neoplasias (PanINs) and no tumors are found on histologic analysis2. Additionally, in patients with small primary tumors (<2cm) who have no clinical evidence of metastatic disease, five year survival after pancreatectomy is <18% due to recurrent metastatic disease3. These data suggest that metastatic seeding may occur before the formation of large primary tumors. Moreover, we recently showed that hematogenous dissemination occurs prior to tumor formation, in a lineage-labeled genetic model of PDAC4 at which time the pancreas contained only PanIN. Based on the clinical characteristics of PDAC and our findings within a recapitulative mouse model, we hypothesized that bloodstream seeding of pancreas-derived epithelial cells can occur in patients with clinical evidence of only precancerous lesions of the pancreas and no detectable invasive carcinoma.
To test our hypothesis, we performed a blinded prospective pilot study of three cohorts: 1) patients with no history of cancer presenting for average-risk, age-appropriate colonoscopy screening and no adenomas detected; 2) patients with precancerous cystic lesions (intraductal papillary mucinous neoplasm (IPMN) or mucinous cystic neoplasms (MCN)) of the pancreas with no evidence of tumor or metastasis on CT or MRI, who did not qualify for surgery under Sendai criteria5 (including no evidence of dysplasia or cancer on FNA, if done); and 3) patients with cytology-confirmed PDAC. Peripheral blood was obtained from consented patients prior to procedure. We analyzed blood samples using geometrically enhanced differential immunocapture (GEDI), a microfluidic platform that has been shown to detect circulating tumor cells from patients with prostate cancers with high sensitivity6, 7. Here, we functionalized the GEDI device using antibodies to epithelial cell adhesion molecule (EpCAM) to capture circulating epithelial cells (CECs). Captured cells were then stained with 4',6-diamidino-2-phenylindole (DAPI) to visualize nuclei and fluorescently conjugated antibodies to CD45, a universal marker of leukocytes, and cytokeratin 19 (CK19), a marker of epithelial-derived cells or pancreas and duodenal homeobox protein-1 (Pdx-1), a pancreas-specific transcription factor. A blinded observer (BJK) enumerated CECs using two definitions, A) CD45-, DAPI+ and B) CK+,CD45-,DAPI+ using a fluorescence microscope. Definition A was confirmed retrospectively with automated cell enumeration and four-color immunofluorescence for epithelial and pancreas-specific markers (Figure S1 and S2, Supplemental Methods).
We prospectively enrolled 48 patients (Table 1). Cyst- and cancer-free patients tended to be younger compared with cystic lesion and PDAC cohorts (p=0.003). However, there were no differences in other demographics. Most (85%) cystic lesions were classified as side-branch IPMNs. The size of cystic lesions varied from 5 to 28mm. Patients with PDAC had a wide range of primary tumor diameter (15–91mm) and tumor stage (I to IV).
Table 1.
Patient Characteristics
| Age | Race | Sex | FHx | BMI | Smoking | EtOH (Avg/wk) |
CA19-9 (Serum) |
CEA (Serum) |
CECs | Size of Cyst/Tumor (mm) |
Cyst type/ Cancer Stage |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer-free controls n=19 | 53 | Cauc | M | 35.7 | Never | 0 | 0 | |||||
| 40 | Cauc | M | 26.3 | Never | 1 | 0 | ||||||
| 64 | Cauc | F | 28.3 | Never | 0 | 0 | ||||||
| 61 | Cauc | M | 31.0 | Never | 1 | 0 | ||||||
| 48 | Cauc | F | 21.0 | Never | 0 | 0 | ||||||
| 62 | AAM | F | 35.4 | Never | 0 | 0 | ||||||
| 74 | Cauc | F | 19.8 | Never | 0 | 0 | ||||||
| 56 | AAM | F | 24.7 | Never | 0.5 | 0 | ||||||
| 70 | Cauc | M | 26.4 | Previous | 0 | 0 (CK) | ||||||
| 51 | AAM | F | 56.6 | Never | 0 | 0 (CK) | ||||||
| 60 | Cauc | M | 29.5 | Never | 0 | 0 (CK) | ||||||
| 66 | Cauc | M | 21.9 | Never | 1.5 | 0 (CK) | ||||||
| 84 | AAM | F | 29.3 | Never | 0 | 0 (CK) | ||||||
| 53 | AAM | F | 46.1 | Never | 0 | 0 (CK) | ||||||
| 59 | AAM | F | 26.6 | Never | 0 | 1 (CK) | ||||||
| 50 | AAM | F | 36.6 | Never | 1.5 | 2 (CK) | ||||||
| 58 | AAM | F | 30.2 | Never | 0 | 3 (CK) | ||||||
| 73 | AAM | F | 26.6 | Never | 0 | 0 (CK) | ||||||
| 50 | Cauc | F | 24.0 | Never | 0 | 0 (CK) | ||||||
| MEAN | 59.6 | 30.3 | 0.3 ± 0.8 | |||||||||
| Cystic Lesion n=21 | 67 | Cauc | F | 31.6 | Never | 0 | 0 | 9 | Side-branch IPMN, multiple | |||
| 62 | Cauc | F | Y | 20.2 | Never | 3 | 0 | 0 | 8 | Side-branch IPMN | ||
| 64 | Cauc | M | 34.9 | Never | 5 | 86.7 | 0 | 16 | MCN | |||
| 75 | Cauc | M | 24.3 | Never | 0 | 0 | 15 | Side-branch IPMN | ||||
| 65 | Cauc | F | Y | 22.4 | Never | 0 | 6 | 9.5 | Side-branch IPMN | |||
| 60 | Cauc | M | 28.3 | Never | 0 | 1.9 | 22 | 16 | 14 | Side-branch IPMN | ||
| 72 | Cauc | M | 21.3 | Current | 3 | <1 | 22 | 10 | MCN | |||
| 81 | Cauc | M | 27.7 | Never | 6 | 0 | 15 | Side-branch IPMN | ||||
| 58 | Cauc | M | 20.7 | Current | 20 | 0 | 5 | Side-branch IPMN | ||||
| 64 | Cauc | F | Y | 18.0 | Never | 5 | 46 | 3.8 | 0 | 20 | Side-branch IPMN | |
| 73 | Cauc | F | 24.6 | Never | 0 | 4 | 14 | Side-branch IPMN | ||||
| 69 | Cauc | F | 27.8 | Previous | 4 | 0 (CK) | 11 | Side-branch IPMN | ||||
| 68 | Cauc | M | 26.3 | Previous | 7 | 0 (CK) | 3 | Side-branch IPMN |
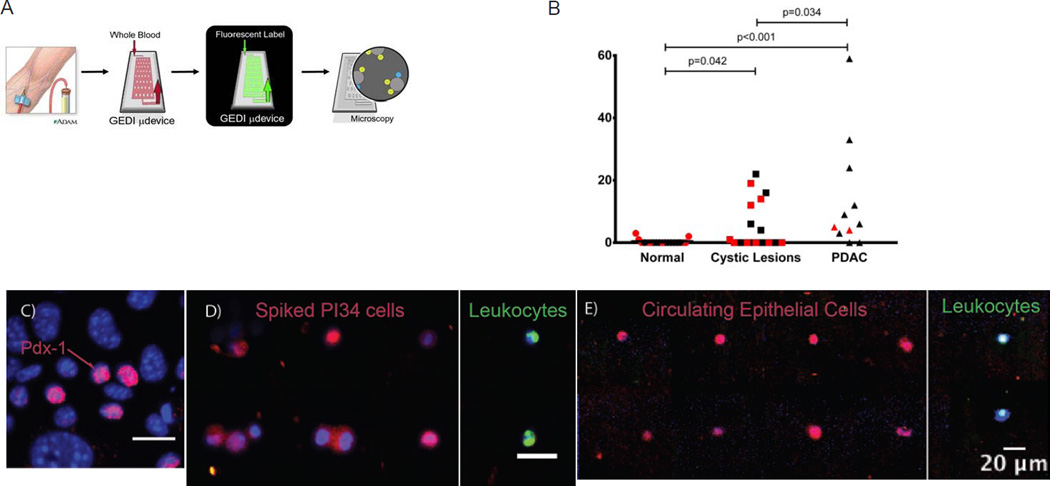
Sixteen of nineteen cancer-free controls had no CECs by either definition (Fig. 1B). When CECs were detected, there were no more than 3/ml. Seven of 9 (78%) patients with PDAC had detectable CECs, with an average of 16.2 ± 19.5 CEC/ml blood (p<0.0001 compared to cancer-free patients by Mann-Whitney test). 8 of 20 (40%) patients with cystic lesions of the pancreas had detectable CECs, averaging 4.5 ± 7.3 CECs/ml blood (p=0.022 compared to cancer-free patients), and there was a significant difference in CECs across the three groups by one-way ANOVA (p=0.015). Interestingly, there was no significant difference in the number of CECs detected among cyst lesion patients based on the immunofluorescence definition used (Fig. 1B; black denotes definition A, red denotes CEC analysis from different patients using definition B); that is, a similar percentage of cyst lesion patients contained CECs by either definition, and, when CECs were detected in these patients, a similar concentration was found. We found no correlation with CEC count and tumor or cyst size, cancer stage or serum CA19-9 and CEA.
Figure 1. Detection of CECs in patients using GEDI.
(A) Depiction of the GEDI device. (B) Vertical scatterplots of CEC concentrations (per ml blood) for Cancer-Free patients (Control), patients with cystic lesions of the pancreas without dysplasia or tumor (Cystic Lesion) and patients with PDAC. Lines indicate means. Bars indicate statistically significant differences by Mann-Whitney test. Representative images of individual GEDI-captured nucleated cells from (C) control human blood spiked with PI34 cells and (D) blood from a patient with PDAC. Cells were stained for CD45 (green), Pdx-1 (red) and DNA (DAPI, blue). Scale bar, 20µm.
To confirm the pancreas origin of CECs, we stained cells for Pdx-1, a pancreas-specific transcription factor, expressed in up to 60% of all CECs in mouse models of PDAC (Fig. 1B). Adherent and GEDI-captured primary PDAC cells also expressed nuclear Pdx-1 (21% of PI34 and 10.7% of Panc-01; Fig. 1C). However, no nuclear Pdx-1 was detected within human breast (MCF-7) or prostate (LNCaP, CWR22Rv1) cancer cells or CD45+ leukocytes (data not shown). These data suggest that Pdx-1 is a specific marker of pancreas-derived cells. In our analyses, 29% of all CECs exhibited nuclear Pdx-1 staining (Fig. 1D). These data confirm that at least a portion of all GEDI-captured epithelial cells derive from the pancreas.
In conclusion, we report for the first time that pancreas epithelial cells can enter the bloodstream in patients with cystic lesions of the pancreas prior to the clinical diagnosis of cancer. Using state-of-the-art microfluidic technology7 and immunofluorescence staining, we confirmed the pancreas origin of captured CECs. Thus, these findings suggest that the ability to seed the bloodstream may precede the formation of detectable tumors, supporting our findings in genetic mouse models of PDAC4. These data are supported by the recent finding that 24.6% of resected side-branch IPMNs that do not satisfy Sendai criteria contain regions of high-grade dysplasia or invasive carcinoma8. Data from our mouse model predict that these cells represent early, occult cancer cells4, although we do not yet have evidence to support this in humans. Studies are underway to interrogate the genomic signature of CECs from cystic lesion patients—if these cells represent the earliest forms of cancer, we predict that they would contain a complement of somatic mutations associated with PDAC. Genomic analyses of CECs represent a technical challenge which recently has been elegantly addressed using massively parallel sequencing of RNA from captured tumor cells from patients with PDAC9; however, cyst lesion patients contain many fewer CECs, complicating genomic analysis. Further, it is still unknown if patients with CECs are destined to form tumors. If associated with subsequent tumor formation, CEC detection could be utilized as a biomarker for cancer risk stratification in patients at-risk for PDAC. Studies underway in this regard will prospectively follow GEDI-analyzed cystic lesion patients to determine if CEC number or genomic analysis are predictive of an eventual diagnosis of PDAC.
Supplementary Material
Acknowledgements
Supported by NIH/NIDDK (K08DK088945 (ADR) and pilot grant funding and Core facilities from P30DK050306 (ADR)), Pancreatic Cancer Action Network (Career Development Award to ADR), Basser Research Center (ADR), National Pancreas Foundation (ADR), Cornell Center for Microenvironment and Metastasis (BLK and ADR; U54CA143876), NSF (TBL), Friends of Scandinavia (FIT), and Sloan Foundation (SMS). We thank Anil K. Rustgi for critical reading of the manuscript and Emily Jacobson for aiding in patient recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study concept: ADR, BJK, Study design: ADR, MLK, BJK, Enrollment of patients: ADR, MLK, GGG, JGL, VC, JAD, NA, TNS, ST, LRM, Acquisition of data: ADR, FIT, SMS, TBL, MLK, BJK, TNS, ST, Analysis and interpretation of data: ADR, BJK, Statistical analysis: ADR, YXY, Drafting of manuscript: ADR, Critical Revision of manuscript: ADR, FIT, MLK, GGG, VC, JAD, YXY, BJK, BZS
Conflicts of interest: none
References
- 1.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis. 2003;35:482–485. doi: 10.1016/s1590-8658(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–e20. doi: 10.1097/mpa.0b013e31814de421. [DOI] [PubMed] [Google Scholar]
- 4.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 6.Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–29. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirby BJ, Jodari M, Loftus MS, Gakhar G, Pratt ED, Chanel-Vos C, Gleghorn JP, Santana SM, Liu H, Smith JP, Navarro VN, Tagawa ST, Bander NH, Nanus DM, Giannakakou P. Functional characterization of circulating tumor cells with a prostatecancer- specific microfluidic device. PLoS One. 2012;7:e35976. doi: 10.1371/journal.pone.0035976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Bundy BD, Buchler MW, Werner J. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256:313–320. doi: 10.1097/SLA.0b013e31825d355f. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, Ulman MJ, Xega K, Contino G, Alagesan B, Brannigan BW, Milos PM, Ryan DP, Sequist LV, Bardeesy N, Ramaswamy S, Toner M, Maheswaran S, Haber DA. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.