Abstract
Rubella remains an important pathogen globally with approximately 100,000 cases of congenital rubella syndrome estimated to occur each year. Rubella vaccine is highly effective and safe when used across a population and, as a result, endemic rubella transmission has been interrupted in the Americas since 2009. Incomplete rubella vaccination programs result in continued disease transmission as evidenced by recent large outbreaks in Japan and elsewhere. Herein, we provide current results regarding rubella control, elimination and eradication policies, and a brief review of new laboratory diagnostics. In addition, we provide novel information regarding rubella vaccine immunogenetics and review the emerging evidence of inter-individual variability in humoral and cell-mediated innate and adaptive immune responses to rubella vaccine and their association with HLA alleles, haplotypes, and single nucleotide polymorphisms across the human genome. Finally, we conclude with a call for further research in rubella vaccine immunogenetics and its ability to inform a vaccinomics-level approach to novel vaccine candidate development and the need for a next generation vaccine that is affordable, easy to administer, and does not require a cold chain for optimal immunogenicity.
Basic Virology and Introduction
First isolated from cell culture in 1962,1 rubella virus contains a single-stranded positive sense RNA genome.2 Rubella virus belongs to the Togaviridae family and is the sole member of the Rubivirus genus. It is the causative agent of rubella disease or so-called “German measles.” Although most cases of infection lead to a mild, self-limiting measles-like disease, the real threat arises when rubella virus infects the fetus – particularly during the first trimester when infection can lead to miscarriage or congenital rubella syndrome (CRS). The link between maternal rubella infection and CRS was first suggested by the Australian ophthalmologist, Norman Gregg.3 Dr. Gregg noticed a significant increase in the number of congenital cataract cases being seen in his practice. He was able to link a history of maternal “German measles” in 78 of these cases.
In CRS, rubella virus is able to infect the placenta, spread to the fetus, and alter the function of multiple fetal systems by interfering with organ formation and causing systemic inflammation.4 There is also persistent infection associated with CRS. Rubella virus intraocular persistent infection is observed in patients diagnosed with Fuchs' uveitis syndrome (FUS).5–7 Detection of rubella virus RNA in the aqueous humor of a 28-year-old patient diagnosed with CRS and FUS verifies that infection can last for decades.8
The molecular structure of rubella virus was first observed using antigen-antibody complexes under electron microscopy in 1967 and later verified by thin section techniques.9, 10 Further studies using electron microscopy characterized assembled rubella viral particles as measuring between 50 and 85 nm in diameter.11 Rubella virus contains a pleomorphic nucleocapsid enveloped in a host-derived lipid membrane.12 Two proteinaceous spikes, E1 and E2, are anchored to the external layer of the membrane. The E1 protein is responsible for receptor-mediated endocytosis and is the immunodominant antigen.13, 14 The measurement of antibodies against the neutralizing domain of E1 can be used as a correlate of protection against rubella virus.15–19 The E2 protein is membrane bound and forms connections between rows of E1 proteins.
To date, there is no definitively known cellular receptor for rubella virus. However, the rubella E1 protein binds to myelin oligodendrocyte glycoprotein (MOG) and ectopic expression of MOG on non-permissive cells allows for in vitro infection.20 In a biological sense then, MOG is a promising cellular receptor candidate, especially for maternal infections that spread to the fetus. There is a high level of homology between rubella E2 protein and MOG, which may explain the ability of antibodies against rubella to cause demyelination of rat brain cells.21 Tissue sections from human CNS, GI tract, and placenta stain weakly to moderately for MOG, while all other normal tissues stain negative.22 The ability of rubella to infect the placenta and the neurological pathologies associated with CRS, coupled with the presence of MOG on both tissue types, supports the hypothesis that MOG is a potential receptor for rubella. The lack of MOG expression on any other tissue type (i.e., lymphocytes, respiratory, or skin), however, suggests that MOG is not the receptor involved in primary acquired rubella. Further research into the identification of the putative host receptor for rubella virus will allow useful insight into viral pathogenesis and help direct novel vaccine candidates.
Immunization with live attenuated rubella virus vaccine has the demonstrated ability to prevent infection and one of the most feared complications – CRS. While much progress has occurred, rubella remains an important pathogen and public health concern around the world. For example, the recent rubella epidemic in Japan, with more than 11,000 rubella cases occurring in the first 6 months of 2013 and at least 13 CRS cases occurring, highlights the fact that a partial vaccination strategy leads to major outbreaks.23 Seventy percent of the rubella cases in the Japanese outbreak occurred among males ages 20 to 39 years, indicating the weakness of an initial strategy that provided rubella vaccine only to adolescent girls. In 2012, Poland24 and Romania25 also experienced rubella outbreaks that predominantly affected males as a result of a vaccination strategy that initially focused on vaccination of females. For this reason, a global commitment to rubella control, elimination, and eventual eradication must be in place.
A past review of rubella is available addressing CRS and postnatally acquired rubella, immune responses to rubella, and other issues which will not be repeated here,26 as well as a comprehensive book chapter.27 The purpose of this review is to give an expert update on: worldwide rubella epidemiology, emphasizing issues with the prevention of CRS and current policies on rubella control and eradication; new techniques in laboratory diagnostics; novel information regarding the immunogenetics of rubella vaccine-induced immune responses; and future issues faced by clinicians and researchers alike.
Worldwide Epidemiology and Current Control, Elimination and Eradication Policies
In the pre-vaccine era, rubella was an acute viral disease affecting children and young adults worldwide. Thanks to the implementation of rubella vaccination strategies, the number of rubella cases has been reduced in many countries, and, since 2009, its endemic transmission in the WHO Region of the Americas has been interrupted.28
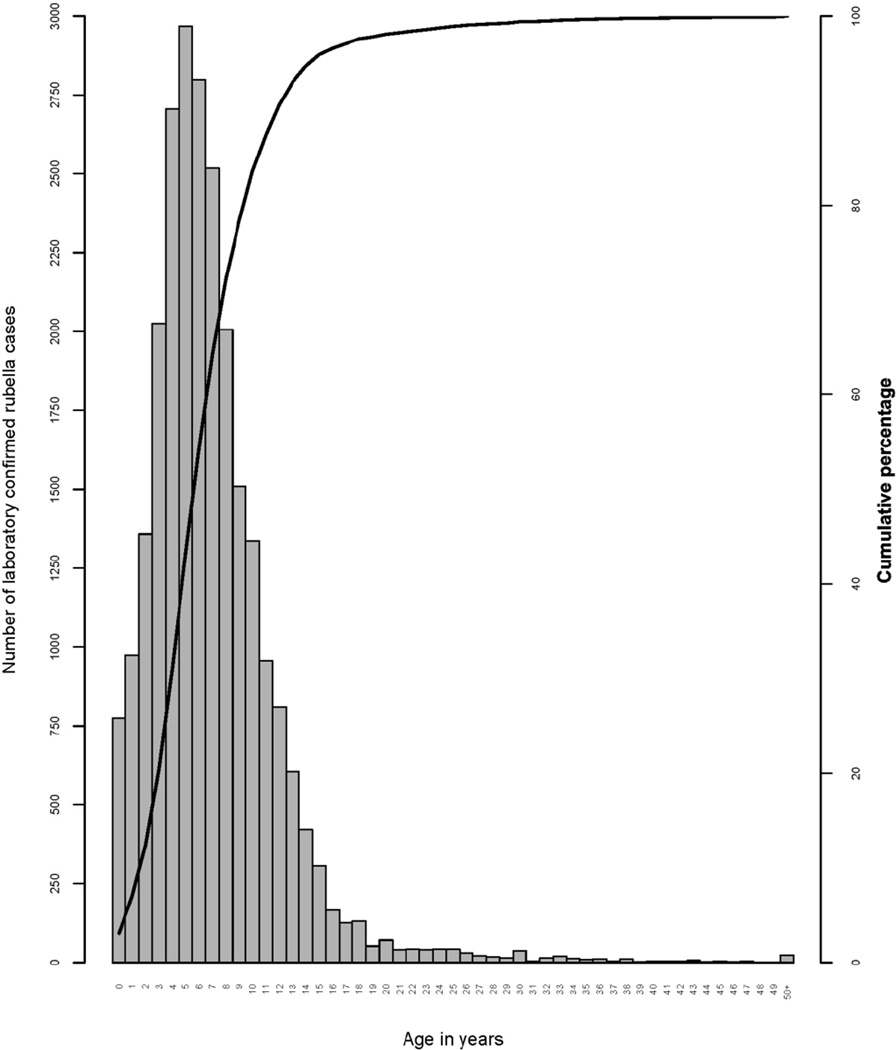
Rubella virus is spread from person to person via the respiratory route. Immunity following natural infection or vaccination is life-long. In the absence of vaccination, the mean age of rubella infection is 5–9 years of age with annual seasonal outbreaks usually occurring in the spring, and large epidemics occur every 3–8 years. The cyclical nature of rubella is related to the buildup of susceptible persons in the population and contact rates. A review of the epidemiology of rubella in Africa in the prevaccine era (2002–2009) found the median age of rubella IgM positive cases to be 7.3 years (interquartile range: 4.2–9.0 years) (Figure 1).29 The observed seasonal pattern was more marked in West Africa and Southern Africa, with Central and East Africa having a bimodal pattern with transmission throughout the year.
Figure 1. Frequency of laboratory confirmed rubella cases age in years in the African Region, 2002–2009.
Frequency of laboratory confirmed rubella cases (as reported by countries using measles case-based surveillance) by age in years with a cumulative age distribution curve, 2002–2009, World Health Organization African Region, N = 25,097. (Reprinted with permission from: Goodson JL, et al. Rubella epidemiology in Africa in the prevaccine era, 2002–2009. Journal of Infectious Diseases 2011. Suppl 1:S215–25.)
When infection with rubella occurs just before conception or during the first 8–10 weeks of gestation, it may cause multiple fetal defects in up to 90% of cases, including fetal wastage or stillbirth.30 The risk of birth defects declines with infection later in gestation, and fetal defects are rarely associated with maternal rubella after the 16th week of pregnancy, although sensorineural hearing deficit may occur with infection as late as week 20.31 The defects associated with congenital rubella syndrome (CRS) most commonly affect the eyes (e.g., cataracts, microphthalmia, glaucoma, pigmentary retinopathy, chorioretinitis), hearing (e.g., sensorineural deafness), the heart (e.g., peripheral pulmonary artery stenosis, patent ductus arteriosus or ventricular septal defects), and the brain (e.g., microcephaly). Those that survive the neonatal period may face serious developmental disabilities (e.g., visual and hearing impairments) and have an increased risk for developmental delay, including autism. In fact, rubella is and should be considered a vaccine-preventable cause of autism.32 Congenital rubella infection has also been associated with increased risk of endocrinopathies such as thyroiditis and insulin-dependent diabetes mellitus with associated long-term effects.33 Finally, a progressive encephalopathy resembling subacute sclerosing panencephalitis has also been observed in patients with CRS.34
Countries with high rates of susceptibility to rubella among women of childbearing age are at highest risk for CRS. This risk varies between and within countries based on epidemiological and socioeconomic differences. Before the introduction of rubella vaccine, the incidence of CRS varied from 0.1–0.2 per 1,000 live births during endemic periods and from 0.8–4.0 per 1,000 live births during rubella epidemics.35 Large rubella epidemics can lead to high levels of morbidity as was observed in the United States during 1964–1965. This epidemic was associated with an estimated 12.5 million cases of rubella, including >2,000 cases of encephalitis, >11,250 cases of fetal wastage, and >20,000 cases of CRS (within those 20,000 cases, there were: >8,000 cases of deafness; 3,580 deaf/blind children; and 1,800 children with mental retardation).36
The basic reproductive rate (R0) for rubella has been estimated at between 3–8 in European countries and as high as 12 in crowded developing countries.37, 38 Based on the mean age of infection in the pre-vaccine era, the herd immunity threshold for interruption of rubella transmission in the United Kingdom and the USA has been estimated at between 85%–88% and between 67%–87% in European countries based on different mixing patterns.37, 39 In an urban African setting, the herd immunity threshold has been estimated at between 85%–91%.38 Hence, if vaccination programs can achieve and maintain immunization coverage that results in sustained population immunity above this level, rubella can be eliminated.
Rubella virus is a candidate for global eradication because humans are the only known host, safe and highly effective vaccines (≥95% following a single dose) exist, accurate diagnostic and molecular assays exist, and there has been demonstration of sustained interruption of endemic transmission in the Americas since 2009.28 Rubella has a lower R0 compared with measles, indicating that rubella eradication may be easier to achieve than measles eradication. This concept is supported by experience in the United Kingdom, where measles outbreaks continue to occur among adolescents born during the period of reduced public confidence in MMR vaccine due to the now disproven association between MMR and childhood autism.40 While rubella vaccine coverage is almost the same as measles vaccination coverage, no widespread rubella outbreaks have been reported in the United Kingdom, suggesting elimination may have been achieved and maintained.41 In contrast to measles, where substantial outbreaks have been reported among populations with high coverage with one dose of measles vaccine, vaccine failure after a single dose of rubella vaccine does not appear to play a role in sustained transmission of rubella. While two doses are generally recommended, high coverage with a single dose appears to have been adequate to terminate transmission.42, 43
Three of the six WHO regions have set control or elimination targets for rubella.44 The Americas targeted rubella and CRS elimination by 2010 and achieved it in 2009. The European Region has a target of rubella elimination by 2015 and the Western Pacific Region aims to have significantly accelerated rubella control and CRS prevention (<1 CRS case per 100,000 live births) by 2015. The African, Eastern Mediterranean and South-East Asia Regions have yet to establish rubella control or elimination goals.
At the World Health Assembly (WHA) in May 2012, all 194 Member States endorsed the target of eliminating rubella in five of the six WHO Regions by 2020 as part of the Global Vaccine Action Plan (GVAP) of the Decade of Vaccines.45 Also in 2012, the core partners of the Measles and Rubella Initiative (American Red Cross, US Centers for Disease Control and Prevention, United Nations Foundation, UNICEF and WHO) launched the Global Measles and Rubella Strategic Plan, 2012–2020.46 The plan envisions a world without measles, rubella and congenital rubella syndrome with existing global control and regional elimination targets as milestones toward this end. The plan includes a five-pronged strategy to: 1) achieve and maintain high levels of population immunity by achieving ≥95% vaccination coverage with two doses of measles- and rubella-containing vaccines; 2) monitor disease using effective surveillance, and evaluate programmatic efforts; 3) develop outbreak preparedness and respond rapidly to outbreaks; 4) communicate and engage to build public confidence and demand for immunization; and 5) perform the research and development needed to support cost-effective operations and improve vaccination and diagnostic tools.
In 2011, the WHO updated its guidance on rubella vaccine use with a clear recommendation that countries that have not yet introduced rubella vaccine should take the opportunity of accelerated measles control and elimination activities to include RCV in their immunization program.47 As all countries have elimination targets, their measles vaccine delivery strategies provide a platform for advancing rubella and CRS elimination through use of combined vaccines (e.g., measles-rubella vaccine, MR, or measles-mumps-rubella vaccine, MMR). For countries newly introducing rubella vaccine, the preferred approach is to begin with a wide-age range MR campaign followed immediately with introduction of MR or MMR vaccine in the routine program – either one or two doses depending on the country schedule for measles vaccination. All subsequent follow-up campaigns should use MR vaccine or MMR vaccine. In addition, countries should make efforts to reach women of childbearing age with immunization of adolescent girls and/or women of childbearing age, either through routine services or mass campaigns.
One of the major factors limiting universal use of rubella vaccine has been concern about a “paradoxical effect” – that sustained low rubella immunization coverage in infants and young children might decrease exposure to rubella during childhood, which may lead to increased susceptibility among women of childbearing age compared to the pre-vaccine era because such women are neither vaccinated nor exposed to virus. This has the theoretical potential to result in an increased risk of CRS above the pre-vaccine era level. Country experience and mathematical modeling indicates that to avoid increasing the risk of CRS, countries should achieve and maintain immunization coverage of 80% or greater with at least one dose of RCV delivered through routine services and/or regular supplementary immunization activities* (SIAs).
Because of their high efficacy and relatively low cost, rubella vaccines are highly cost-effective. In both industrialized countries and less industrialized countries in Latin America and the Caribbean with coverage >80%, cost-benefit studies of rubella vaccination have demonstrated that the benefits outweigh the costs and that rubella vaccination is economically justified, particularly when combined with measles vaccine.48 However, no such studies have been conducted in low-income countries in Africa and Asia.49
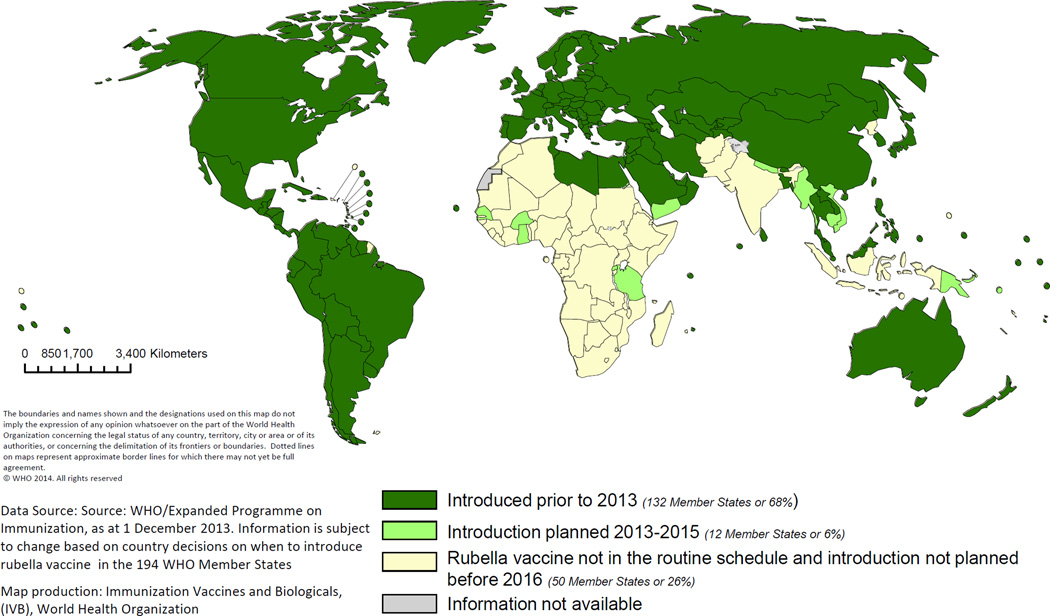
Over the past 15 years there has been a gradual increase in the number of countries using rubella vaccine in their national immunization program. By 2012, 132 (68%) countries were providing at least one dose of RCV (Figure 2), up from 99 (52%) in 2000.44 Rubella vaccination coverage through the routine immunization program is almost identical to that of measles, because all countries provide rubella vaccine combined with measles or measles and mumps vaccines. The proportion of countries having introduced rubella vaccine by 2012 ranged from 7% in the African Region to 100% of countries in the American and European Regions. During 2000–2012, the estimated global coverage with one dose of RCV** increased from 22% to 43%; by 2012, the American and European Regions of WHO had >93% estimated RCV coverage.50
Figure 2. Distribution of countries using rubella vaccine in their routine immunization schedule in 2012 and countries planning introduction during 2013–2015.
During 2000–2012, global reported rubella cases decreased 86% from 670,894 to 94,030;44however, rubella cases are substantially under-reported, particularly in countries not yet using rubella vaccine. The greatest decrease in reported rubella cases was a 95% decrease in the European Region, from 621,039 to 30,509, and a 99.9% decrease in the Americas, from 39,228 in 2000 to only 21 cases in 2012. In other regions, the number of cases increased during this period in parallel with the increase in the number of countries reporting rubella cases. Compared to reporting of acquired rubella cases, fewer countries report CRS cases, though the number increased from 75 (39%) in 2000 to 129 (66%) countries in 2012. Compared to model estimates, the number of reported CRS cases is very low, with 300 reported CRS cases in 2012 versus a model-based estimate of 110,000 CRS cases in 1996.51
Estimates suggest that the burden of CRS in regions that had not yet introduced rubella-containing vaccination by 2010 may be very high. For example, in 1996, approximately 22,000 new cases of CRS were born in Africa (uncertainty bounds: 6,127–51,472), and approximately 46,000 (uncertainty bounds: 1,016–168,910) and 12,634 (uncertainty bounds: 1,545–21,396) new cases were born in South East Asia and the Western Pacific regions, respectively. Very few countries in these regions had introduced rubella-containing vaccination by the year 2010, and therefore the current burden of CRS in these settings is likely to be similar to that estimated for 1996.
The second major factor limiting universal use of rubella vaccines is cost – the weighted average price for MR vaccine purchased by UNICEF is $0.52 compared with $0.24 for the single antigen measles vaccine. In November 2011, the GAVI Board effectively removed this barrier by setting aside $554 million to support rubella vaccine introduction in GAVI-eligible countries (i.e., for 51 of the remaining 60 countries not yet using RCV). GAVI support includes funding for an initial MR mass campaign for all children up to 15 years of age as well as a one-time grant to support introduction of RCV in the routine program. In 2012, six countries (Bangladesh, Cambodia, Ghana, Rwanda, Senegal, Vietnam) successfully applied for GAVI funding to introduce rubella vaccine and are in the process of introducing the vaccine.
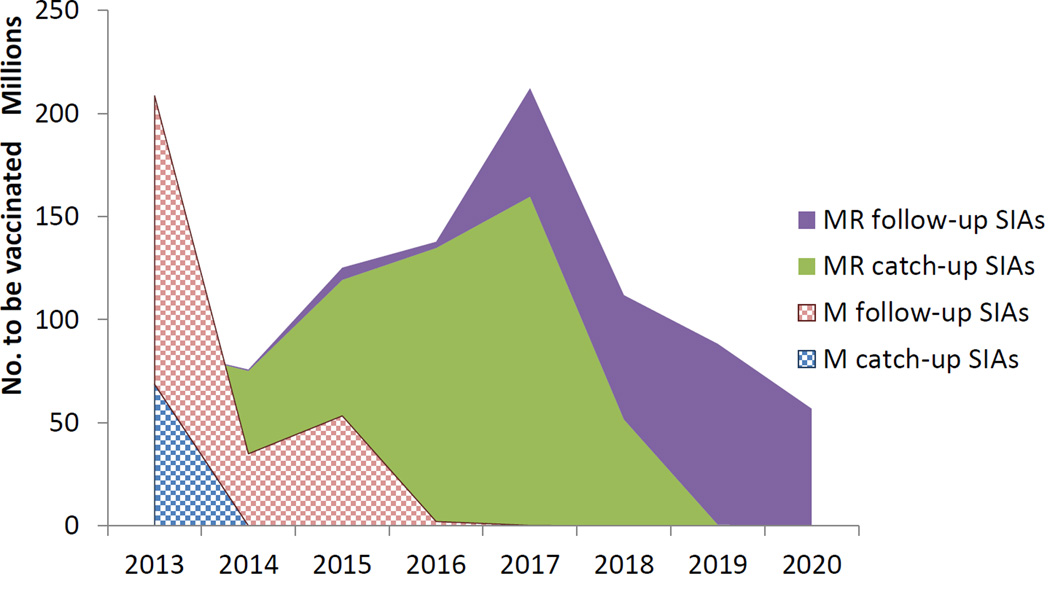
The new GAVI support for introduction of rubella vaccine offers the opportunity to accelerate progress toward both rubella and measles elimination by rapidly raising population immunity among children age 9 months up to 15 years who typically contribute most to virus transmission. To ensure sufficient MR vaccine supply for the campaigns, and to allow countries not yet using RCV time to prepare for the switch to MR vaccine as part of their routine program, the roll-out is planned over an eight-year period (2013–2020). Full implementation of the roll-out will result in nearly 1 billion children receiving MR vaccine in the campaigns and an additional 200 million infants receiving MR vaccine as a routine first dose (Figure 3). Well conducted post-campaign coverage and/or seroprevalence surveys will be required to document the actual coverage achieved in these critically important campaigns.
Figure 3. Transition from measles vaccine to combined measles-rubella vaccine Projected number to be vaccinated by year and vaccine, 2013–2u020.
“SIA” is Supplemental Immunization Activity, usually a mass vaccination campaigns targeting persons in a wide age range regardless of prior vaccination status. “M” is measles vaccine alone while “MR” is combined Measles Rubella vaccine. Catch-up SIAs are first SIAs to boost overall population immunity. In measles, for example, most catch-up SIAs covered children 9 months through 14 years of age. Follow-up SIAs usually take place several years after the catch-up SIA and usually target a narrower age range, covering children born since the last SIA. For measles, this is usually 9 months through 4 years of age.
Source: WHO/Expanded Programme on Immunization as at 1 December 2013. Information is subject to change based on country decisions on when to introduce rubella vaccine in the 194 WHO Member States.
While rubella and CRS are potentially eradicable, lack of awareness and political commitment, in part due to the difficulty in documenting the true burden of CRS, as well as competing public health priorities, remain the major barriers. At its November 2012 meeting, the Strategic Advisory Group of Experts (SAGE) concluded that, based on current trends and program performance, the current regional measles and rubella elimination targets (except for the Americas) will not be achieved on time.52 SAGE urged countries and partners to raise the visibility of measles and rubella elimination activities and to ensure that they receive adequate priority and resources as a central component of the GVAP.
New Laboratory Diagnostics
Clinical specimens for the diagnosis of rubella by virus detection usually consist of throat swabs (TS), oral fluids (OF) or nasopharyngeal secretions, and by antibody detection are usually sera or OF.53, 54 The virus has also been found in other specimens, including cataract tissue and urine. Urine and TSs or OFs are about equivalent as sources of viral RNA, but the ease of obtaining TSs or OFs make these specimens the primary ones that are collected.8, 54 Urine is often a source of infectious virus from CRS patients. Specimens for virus detection and for IgM/IgG detection can be transported by standard methods.
The timing of specimen collection is important in postnatal rubella. Rubella virus-specific IgM is present in sera in only about 50% of rubella cases on the day of rash, but, at five days after rash, most rubella cases have detectable rubella-specific IgM. Most rubella cases are virus positive on the day of rash and may be positive from seven to ten days post rash.53, 54 Since postnatal rubella is a mild disease of short duration, special effort is required to obtain samples on the day of rash or shortly thereafter. Patients with CRS and congenital rubella infection (CRI) are IgM and virus positive for months; therefore, timing is less critical for individuals suspected of having CRS or CRI.54
Alternative specimens, such as dried blood spots (DBS) and OF, have recently been shown to be adequate for surveillance of rubella using IgM detection (DBS and OF) and virus detection (OF).53, 55–57 Note that diagnostic kits are usually not approved for use with DBS, and low IgM levels in OF necessitate the use of sensitive detection assays.
Amplification of rubella virus RNA directly from a clinical specimen using RT-PCR is now common. Assays that can reliably detect 3 to 10 copies of rubella virus RNA are necessary since many specimens have small amounts of rubella RNA. Real-time and nested RT-PCR assays often have this level of sensitivity.58, 59
There is no cell type that reliably produces a cytopathic effect (CPE) in a single passage of wild-type viruses. However, virus growth can now be identified in the absence of CPE using RT-PCR, IFA, and immunocolorimetric assays (ICA) to detect viral RNA or proteins.60, 61
Sequencing of the rubella virus nucleic acid amplified directly from specimens or from infected tissue culture cells can now provide useful information on vaccine versus wild-type viruses, on the likely origin of imported cases of rubella and CRS, and for the documentation of elimination.62, 63, 64 The sensitivity of the RT-PCR system used to generate sequencing templates from infected tissue culture cells is not critical, since the amount of rubella viral RNA in rubella virus infected cells is higher than in clinical specimens.
Detection of rubella virus-specific IgM by either IgM capture ELISA or indirect IgM ELISA is the most common diagnostic test for recent postnatal infection. If acute- and convalescent-phase sera are available, a four-fold rise in rubella virus-specific IgG (usually by ELISA) is also diagnostic for postnatal rubella infection. The same ELISAs may be used to confirm CRS and CRI.54
Avidity tests have now been developed that are useful for suspect case classification in certain situations (e.g., first serum sample was collected months after clinical symptoms). Low avidity anti-rubella IgG suggests recent infection.65 Avidity tests are not widely available and vary in performance.66
Laboratory tests supporting surveillance for rubella and CRS in control and elimination programs have largely been rubella virus specific IgM tests, supported for some suspect cases by techniques that amplify rubella virus RNA.55 However, with the WHA target of eliminating rubella in five of the six WHO Regions in the next six years, control programs will move into developing countries, and laboratory testing algorithms supporting these programs are expected to change. Specifically, advanced molecular techniques and point-of-care diagnostics for rubella may be used.67, 68
Since the clinical symptoms of postnatal rubella and CRS are dramatically different, it is not surprising that there are significant differences in the immune responses of patients with these diseases. These differences can be observed on Western blots, in which antibodies in sera from CRS patients often demonstrate different reactivity to rubella proteins than those from postnatal rubella patients.14 (authors’ unpublished observations)
In many countries, much of the rubella testing is for immunity to rubella. There are slightly different criteria for rubella immunity that are recommended by various groups (most are 10 or 15 IU/ml).69 Commonly used tests (e.g., ELISA in the United States) are standardized to give positive results for 10 IU/ml.70 Other tests (e.g., immunoprecipitation) detect rubella-specific antibodies, but have not been correlated with immunity. Immunity testing is often done commercially. Monitoring of rubella vaccination programs by seroprevalence studies is used in some countries.71 The hemagglutinin inhibition (HI) test was once the standard test for antibodies to rubella virus, and many current tests were calibrated using HI assays. However, this assay is no longer a common diagnostic test. Neutralization tests for virus specific antibodies have the advantages over other tests such as ELISA because they assess the biologic function of antibodies and can be used with any virus strain. For some viruses (e.g., measles), the neutralization test has been developed as the standard assay for determinations of immunity.54
The plaque reduction neutralization test (PRN) is performed when a quantitative assessment of the neutralizing capacity of an antiserum is necessary. The assay follows a format common to many viruses. Such neutralization tests exist for laboratory-adapted rubella virus strains in a number of cell types, but an immunocolorimetric neutralization assay for rubella virus using a soluble substrate is a significant improvement over plaque development.72 Signal can be detected in three days instead of 6–11 days for plaques to develop, viewer subjectivity in plaque counting is eliminated, and wild-type viruses can be used because CPE is not required.60 Furthermore, the detection portion of the assay can be done using a microplate washer/dispenser, enhancing throughput by a factor of about three and reducing technician hands-on time by a factor of about six (authors’ unpublished observations).
In a recent study conducted by the authors,73 about 2,500 sera were titered by three technical staff in only four months using one automated machine, and a microplate washer/dispenser. More than 400 sera were titered a second time. These repeated assays suggested a good degree of reproducibility, with person-to-person differences being more than 8 times higher than the observed within-assay variability. This compares favorably to the standardized measles PRN (author’s unpublished data).74 The possibility of efficiently performing thousands of rubella neutralization titers opens the possibility of routinely using this neutralization assay for large studies, such as serosurveys.
Many postnatal rubella cases are asymptomatic and the individual defects found in CRS are not specific for CRS. Thus, laboratories bear a considerable burden in rubella and CRS diagnosis. For example, when primary rubella virus infection is suspected for a pregnant woman, false positives and false negatives may lead to incorrect clinical decisions.75
Rubella Vaccine Immunogenetics
The current live rubella virus vaccine strain licensed for use in the United States is the RA27/3 strain. It was first isolated from an infected fetus in the 1960s76 and further passaged for attenuation through either the WI-38 or MRC-5 human diploid cell lines.77 It is currently administered as a two-dose series in the U.S. as part of the measles-mumps-rubella (MMR-II) vaccine. RA27/3 elicits a robust humoral and cellular immune response. As noted above, correlate protective levels of anti-rubella antibodies are defined as titers at or above 10 IU/ml.70 However, the measurement of rubella-specific humoral immunity using serum antibodies can result in a false positives due to a previous parvovirus or Epstein-Barr virus infection, or the presence of Rh factor.78, 79, 80 Measuring the response of rubella-specific memory B cells in vaccinees may be an alternative correlate and might explain protective immunity in those individuals with low levels of serum antibodies.81 The seroconversion rate after two doses of MMR-II approaches 99% and antibodies persist for at least 21 years.82, 83 The high seroconversion rate of 99% is observed as early as 9-months-old after receiving the Wistar RA 27/3 live rubella virus vaccine strain.84 With a calculated half-life of 114 years, rubella-specific antibodies may even persist for an entire lifetime.85 Although excellent seroconversion rates are obtained with RA27/3 vaccination, there are limited occurrences of vaccine failure, and this is thought to arise when preexisting antibodies neutralize the live viral vaccine strain.86
Distinct patterns of cellular immunity to rubella virus are related to the time elapsed since vaccination. Early cellular immunity is marked by an immunosuppressive phenotype characterized by an increase in serum IL-10 and TNF-α, coupled with a decrease in IFN-γ and the proliferative properties of peripheral lymphocytes.87 However, cellular immunity measured in a cohort of 738 schoolchildren several years after vaccination was shifted toward a predominantly pro-inflammatory cytokine profile with elevated levels of IL-6, GM-CSF, and TNF-α and minimal detection of IL-10.88
There are numerous factors that influence inter-individual variations in immune responses to rubella vaccine such as: genetics; age; race; gender; antigenic exposure history (either infectious or through vaccination); interference of maternal antibodies still present during vaccination; and other confounding environmental variables.89–92 Since rubella virus is comprised of a stable and conserved genome93 with similar antigenic responses across strains,94 studying the heterogeneity of immune responses to a well characterized live rubella virus (strain RA27/3) in a cohort with documented vaccination and infection history provides the ability to understand the influence of inter-individual genetic differences.95
A successful model that allows for the control of genetic and certain environmental factors is to perform immunogenetic studies in twin subjects. Along with similar (dizygotic) or identical (monozygotic) genetic backgrounds, assumptions regarding environmental factors can be made that account for twins being raised in the same household. Twin studies also offer an exceptional model to study the heritability of immune response to live viral vaccines. With this intention, in 100 pairs of twins (45 monozygotic and 55 dizygotic), the heritability of rubella antibody levels after vaccination was calculated to be 45.7% (p = 0.003).91
Polymorphisms in genetic elements controlling the immunity to rubella virus explain a significant degree of inter-individual variations in immune response. These genetic elements include: HLA alleles; haplotypes; HLA supertypes; single-nucleotide polymorphisms in genes involved in innate and antiviral immunity; SNPs in genes not associated with classical viral response or immunity, but discovered through genome-wide association studies; and whole-genome transcription profiling, as well as other factors.
The human leukocyte antigens (HLA) play a critical role in immune response to viruses. The highly polymorphic nature of HLA genes highlights their importance in contributing to the heterogeneity of the immune response to rubella virus. HLA class I and II polymorphisms restrict the available repertoire of rubella antigens presented to T cells and therefore influence the subsequent immune response. Specific HLA alleles bind to unique motifs of rubella-derived epitopes.96,97, 98 In turn, this influences the subsequent immune response to vaccination. To investigate this relationship, extensive research has been performed in the discovery and replication of the association of HLA genes with inter-individual variations in immunity to rubella virus. Several HLA alleles (B*2705, DPA1*0201, DPB1*0401), haplotypes (A*03-C*07-B*07, DRB1*04-DQB1*03-DPB1*03, and DRB1*15/16-DQB1*06-DPB1*03) and a supertype (B27) associated with rubella-specific antibody levels have been successfully validated in an independent cohort.99 Discovery associations have been made between IL-2 secretion and HLA class II alleles (DQA1*0103, *0301, *0303; DQB1*0202, *0402, *0603), and TNF-α secretion with HLA class I alleles (B*3901, *4001, *4102, *4403; C*0303, *1601, *1703).100 Unlike other viral infection models where HLA homozygosity can have a deleterious effect, homozygosity for HLA-DPB1 was associated with increased levels of rubella-specific antibody levels.101 Adverse events have also been associated with specific HLA alleles. For example, there is a weak association between HLA-DR and transient arthritis-like joint manifestations after rubella vaccination.102 A thorough understanding of the genetic influences of HLA polymorphisms could inform the development of novel vaccine candidates and screening processes for potential adverse events, such as a construct comprised of HLA-specific epitopes that would induce immunity across a heterogenetic population. Although variations in HLA loci are estimated to account for 20% of inter-individual genetic variations in humoral immunity to rubella,99 HLA genes alone do not contribute to all of the genetically associated variations observed in immune responses to rubella.
To investigate other genetic contributions, studies next focused on candidate genes with known involvement in innate immunity and response to viral infection or vaccination. Initial discovery efforts have found: associations between polymorphisms in TNFA/TNFRSF1B and IL2B genes with levels of rubella-specific IgG and IL-6;103 associations between vitamin A (RARB), RIG-I (DDX58), TRIM5, and TRIM22 polymorphisms and the humoral immune response to rubella virus vaccine;104 additional associations of SNPs in vitamin A (RARA, RARB, RARG, and TOP2B); vitamin D signaling (RXRA) with rubella virus-specific cytokine (IFN-y, IL-2, IL-10, TNF-a, and GM-CSF) secretion;105 and OAS gene SNPs associated with rubella vaccine-specific humoral and cellular immunity.106 A greater percent of genetic impact on immune response to rubella virus can be calculated with the combined contribution of findings from HLA and candidate gene studies.
The contribution of inter-individual differences in gene expression in response to rubella virus can be assayed through whole-genome transcriptional profiling of peripheral blood mononuclear cells from subjects with extreme immunological phenotypes to rubella vaccine. For example, high antibody responders (138 IU/mL; 121, 217 IQR) express different levels of genes involved in antigen presentation (HLA-A, HLA-B and B2M) and innate immunity (EMR3 and MEFV) than low responders (10 IU/mL; 8, 11 IQR).107 These additional findings will assist in the creation of a holistic snapshot of the influence of multiple genetic elements on the immune response to rubella virus.
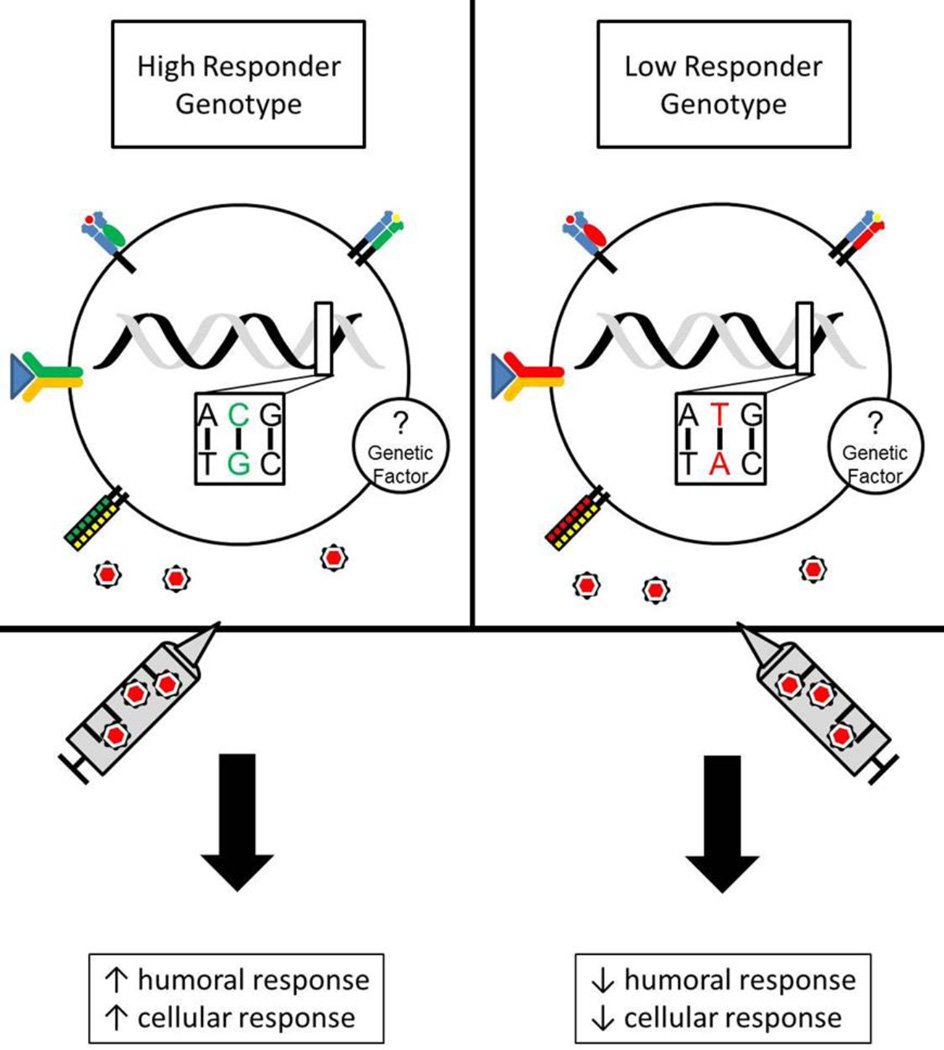
The Mayo Clinic Vaccine Research Group is currently conducting a replication study of all HLA and candidate gene SNP associations and two unique GWAS studies in independent cohorts to elucidate novel gene polymorphisms associated with inter-individual variations in immune responses to rubella vaccine. These novel and exciting data will considerably advance the understanding of rubella-specific immunogenetics. The goal of these immunogenetic studies is to explain a larger percentage of the genetic elements and inter-individual variability involved in immunity to live rubella virus vaccine (Figure 4), and in return perhaps gain additional insight into those same elements that influence rubella disease pathogenesis and severity. This insight, along with a systems-level interrogation of host and viral responses, will drive the next-generation of vaccine candidates that progress beyond traditional empirical processes, and adopt the novel and progressive approach of “Vaccinomics.”108, 109
Figure 4. Genetic influences on differences in rubella-induced immunity.
Single nucleotide polymorphisms (SNPs) in receptors known to play a role in innate and antiviral immunity (cytokine/cytokine receptors, vitamin receptors, TLRs) are associated with measurable differences in humoral (antibody titers) and cellular (secreted cytokines) immunity after rubella vaccination. A number of HLA (Class I and Class II) alleles and haplotypes are also associated with differences in rubella-specific immunity. A hypothetical subject with a High Responder Genotype (CG SNP allele) represents a holistic compilation of SNPs discovered by interrogating polymorphisms present in candidate genes exhibits increased humoral and cellular responses to rubella vaccination. Low Responder Genotype, however, displays a hypo-immune phenotype to rubella vaccination and this may be influenced by multiple SNPs (TA SNP allele) in candidate genes. There are also unknown immunogenetic factors influencing immunity to rubella vaccine. These factors will be explored through Genome-wide association studies (GWAS), next-generation sequencing, and systems biology approaches.
Future Issues
Significant health policy and public health campaigns in the Americas have demonstrated that rubella virus transmission can be interrupted, and the disease eliminated. Political will and the global desire to eliminate both rubella and measles are likely to accelerate control and eradication advances worldwide. Nonetheless, additional research needs are apparent. We do not yet understand what surrogate markers of protection, besides antibody, indicate longer term cell-mediated immunity and protection from disease. Additional advancements in high-sensitivity, low cost diagnostic assays are needed. We do not have a full understanding of potential cell-based rubella receptors, and identification of such receptors could be exploited in vaccine design, diagnostics, and the development of potential therapeutic candidates. Much more work is needed to more fully understand the immunogenetics of rubella humoral and cell-mediated immune responses, and to understand the inter-individual variability in the immune response phenotypes to rubella vaccine. Additionally, a systems-level approach to understanding the development and maintenance of acute and long-term immunity to rubella and rubella vaccine is needed. Rubella as a human pathogen, and the devastating toll of human misery and morbidity it causes, are controllable and preventable with current technologies, but having, for example, a vaccine that does not require a cold chain, nor sophisticated health care workers to administer it, would be a great advantage and research imperative. Additionally, it would be beneficial to have a non-live viral vaccine that could be used in all patients and populations. Imperative is the need for global cooperation in creating a world free of rubella.
Acknowledgements
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under award number R37AI48793 (which recently received a MERIT Award). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services. The author (P.Strebel) is a staff member of the World Health Organization. The author (P.Strebel) alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization. The authors wish to thank Caroline L. Vitse for her editorial assistance.
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational non-rubella vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on non-rubella vaccine development to Merck & Co. Inc., CSL Biotherapies, Avianax, Sanofi Pasteur, Dynavax, Novartis Vaccines and Therapeutics, PAXVAX Inc, and Emergent Biosolutions. Dr. Poland holds two patents related to vaccinia peptide research.
Footnotes
The primary purpose of SIAs is to reach children who have been missed by routine services. In general, there are two types of SIAs targeting different age groups. An initial, nationwide catch-up SIA targets all children aged 9 months to 14 years; its goal is to eliminate susceptibility to measles and/or rubella in the general population. Periodic follow-up SIAs then target all children born since the last SIA. Follow-up SIAs are conducted nationwide every 2–4 years and target children aged 9–59 months; their goal is to eliminate any measles and/or rubella susceptibility that has developed in recent birth cohorts
Coverage with rubella vaccine is estimated based on first dose measles vaccination coverage
Competing Interests
These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Weller TH, Neva FA. Propagation in Tissue Culture of Cytopathic Agents from Patients with Rubella-Like Illness. Jama. 1963;183(10):243–247. [Google Scholar]
- 2.Frey TK. Molecular biology of rubella virus. Advances in Virus Research. 1994;44:69–160. doi: 10.1016/S0065-3527(08)60328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg NM. Congenital cataract following German measles in the mother. 1941. Epidemiology and Infection. 1991;107(1):iii–xiv. doi: 10.1017/s0950268800048627. discussion xiii–xiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alford CA, Jr, Neva FA, Weller TH. Virologic and Serologic Studies on Human Products of Conception after Maternal Rubella. The New England Journal of Medicine. 1964;271:1275–1281. doi: 10.1056/NEJM196412172712501. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki J, Goto H, Komase K, Abo H, Fujii K, Otsuki N, et al. Rubella virus as a possible etiological agent of Fuchs heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1487–1491. doi: 10.1007/s00417-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 6.Cimino L, Aldigeri R, Parmeggiani M, Belloni L, Zotti CA, Fontana L, et al. Searching for viral antibodies and genome in intraocular fluids of patients with Fuchs uveitis and non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1607–1612. doi: 10.1007/s00417-013-2287-6. [DOI] [PubMed] [Google Scholar]
- 7.Prechtl HF. The organization of behavioral states and their dysfunction. Semin Perinatol. 1992;16(4):258–263. [PubMed] [Google Scholar]
- 8.Winchester SA, Varga Z, Parmar D, Brown KE. Persistent intraocular rubella infection in a patient with Fuchs' uveitis and congenital rubella syndrome. Journal of Clinical Microbiology. 2013;51(5):1622–1624. doi: 10.1128/JCM.03239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best JM, Banatvala JE, Almeida JD, Waterson AP. Morphological characteristics of rubella virus. Lancet. 1967 [Google Scholar]
- 10.Kistler GS, Best JM, Banatvala JE, Tondury G. [Electron microscopic studies on rubella infectied human tissue cultures] Schweizerische Medizinische Wochenschrift. 1967;97(42):1377–1382. [PubMed] [Google Scholar]
- 11.Oshiro LS, Schmidt NJ, Lennette EH. Electron microscopic studies of Rubella virus. The Journal of general virology. 1969;5(2):205–210. doi: 10.1099/0022-1317-5-2-205. [DOI] [PubMed] [Google Scholar]
- 12.Battisti AJ, Yoder JD, Plevka P, Winkler DC, Prasad VM, Kuhn RJ, et al. Cryo-electron tomography of rubella virus. Journal of Virology. 2012;86(20):11078–11085. doi: 10.1128/JVI.01390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petruzziello R, Orsi N, Macchia S, Rieti S, Frey TK, Mastromarino P. Pathway of rubella virus infectious entry into Vero cells. The Journal of general virology. 1996;77(Pt 2):303–308. doi: 10.1099/0022-1317-77-2-303. [DOI] [PubMed] [Google Scholar]
- 14.Katow S, Sugiura A. Antibody response to individual rubella virus proteins in congenital and other rubella virus infections. Journal of Clinical Microbiology. 1985;21(3):449–451. doi: 10.1128/jcm.21.3.449-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordoba P, Lanoel A, Grutadauria S, Zapata M. Evaluation of antibodies against a rubella virus neutralizing domain for determination of immune status. Clin Diagn Lab Immunol. 2000;7(6):964–966. doi: 10.1128/cdli.7.6.964-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell LA, Zhang T, Ho M, Decarie D, Tingle AJ, Zrein M, et al. Characterization of rubella virus-specific antibody responses by using a new synthetic peptide-based enzyme-linked immunosorbent assay. Journal of Clinical Microbiology. 1992;30(7):1841–1847. doi: 10.1128/jcm.30.7.1841-1847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell LA, Ho MKL, Rogers JE, Tingle AJ, Marusyk RG, Weber JM, et al. Rubella reimmunization: Comparative analysis of the immunoglobulin G response to rubella virus vaccine in previously seronegative and seropositive individuals. Journal of Clinical Microbiology. 1996;34:2210–2218. doi: 10.1128/jcm.34.9.2210-2218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zrein M, Joncas JH, Pedneault L, Robillard L, Dwyer RJ, LaCroix M. Comparison of a whole-virus enzyme immunoassay (EIA) with a peptide-based EIA for detecting rubella virus immunoglobulin G antibodies following rubella vaccination. Journal of Clinical Microbiology. 1993;31:1521–1524. doi: 10.1128/jcm.31.6.1521-1524.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson KM, Di Camillo C, Doughty L, Dax EM. Humoral immune response to primary rubella virus infection. ClinVaccine Immunol. 2006;13(3):380–386. doi: 10.1128/CVI.13.3.380-386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong H, Jiang Y, Tien P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. Journal of Virology. 2011;85(21):11038–11047. doi: 10.1128/JVI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besson Duvanel C, Honegger P, Matthieu JM. Antibodies directed against rubella virus induce demyelination in aggregating rat brain cell cultures. J Neurosci Res. 2001;65(5):446–454. doi: 10.1002/jnr.1173. [DOI] [PubMed] [Google Scholar]
- 22.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nature Biotechnology. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 23.Minakami H, Kubo T, Unno N. Causes of a nationwide rubella outbreak in Japan, 2012–2013. The Journal of infection. 2014;68(1):99–101. doi: 10.1016/j.jinf.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Paradowska-Stankiewicz I, Czarkowski MP, Derrough T, Stefanoff P. Ongoing outbreak of rubella among young male adults in Poland: increased risk of congenital rubella infections. Euro Surveill. 2013;18(21) [PubMed] [Google Scholar]
- 25.Janta D, Stanescu A, Lupulescu E, Molnar G, Pistol A. Ongoing rubella outbreak among adolescents in Salaj, Romania, September 2011-January 2012. Euro Surveill. 2012;17(7) [PubMed] [Google Scholar]
- 26.Banatvala JE, Brown DW. Rubella. Lancet. 2004;363(9415):1127–1137. doi: 10.1016/S0140-6736(04)15897-2. [DOI] [PubMed] [Google Scholar]
- 27.Reef SE, Plotkin SA. Rubella vaccine. In: Plotkin SA, Orenstein W, Offit PA, editors. Vaccines. 6th ed. Elsevier; 2012. p. 688. [Google Scholar]
- 28.Andrus JK, de Quadros CA, Solorzano CC, Periago MR, Henderson DA. Measles and rubella eradication in the Americas. Vaccine. 2011;29(Suppl 4):D91–D96. doi: 10.1016/j.vaccine.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 29.Goodson JL, Masresha B, Dosseh A, Byabamazima C, Nshimirimana D, Cochi S, et al. Rubella epidemiology in Africa in the prevaccine era, 2002–2009. The Journal of Infectious Diseases. 2011;204(Suppl 1):S215–S225. doi: 10.1093/infdis/jir108. [DOI] [PubMed] [Google Scholar]
- 30.Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet. 1982;2(8302):781–784. doi: 10.1016/s0140-6736(82)92677-0. [DOI] [PubMed] [Google Scholar]
- 31.Grillner L, Forsgren M, Barr B, Bottiger M, Danielsson L, De Verdier C. Outcome of rubella during pregnancy with special reference to the 17th–24th weeks of gestation. Scandinavian Journal of Infectious Diseases. 1983;15(4):321–325. doi: 10.3109/inf.1983.15.issue-4.01. [DOI] [PubMed] [Google Scholar]
- 32.Berger BE, Navar-Boggan AM, Omer SB. Congenital rubella syndrome and autism spectrum disorder prevented by rubella vaccination--United States, 2001–2010. BMC Public Health. 2011;11:340. doi: 10.1186/1471-2458-11-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginsberg-Fellner F, Witt ME, Yagihashi S, Dobersen MJ, Taub F, Fedun B, et al. Congenital rubella syndrome as a model for type 1 (insulin-dependent) diabetes mellitus: increased prevalence of islet cell surface antibodies. Diabetologia. 1984;27 Suppl:87–89. doi: 10.1007/BF00275655. [DOI] [PubMed] [Google Scholar]
- 34.Weil ML, Itabashi H, Cremer NE, Oshiro L, Lennette EH, Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. The New England Journal of Medicine. 1975;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]
- 35.Cutts FT, Robertson SE, Diaz-Ortega JL, Samuel R. Control of rubella and congenital rubella syndrome (CRS) in developing countries, part 1: burden of disease from CRS. Bulletin of the World Health Organization. 1997;75:55–68. [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkin S. Vaccines. 5 ed. Philadelphia: Saunders; 2008. Rubella vaccine; p. 471. [Google Scholar]
- 37.Edmunds WJ, Gay NJ, Kretzschmar M, Pebody RG, Wachmann H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiology and Infection. 2000;125(3):635–650. doi: 10.1017/s0950268800004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutts FT, Abebe A, Messele T, Dejene A, Enquselassie F, Nigatu W, et al. Sero-epidemiology of rubella in the urban population of Addis Ababa, Ethiopia. Epidemiology and Infection. 2000;124(3):467–479. doi: 10.1017/s0950268899003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson RM, Grenfell BT. Quantitive investigations of different vaccination policies for the control of congenital rubella syndrome (CRS) in the United Kingdom. Journal of Hygiene. 1986;96:305–333. doi: 10.1017/s0022172400066079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise J. Largest group of children affected by measles outbreak in Wales is 10–18 year olds. Bmj. 2013;346:f2545. doi: 10.1136/bmj.f2545. [DOI] [PubMed] [Google Scholar]
- 41.Manikkavasagan G, Bukasa A, Brown KE, Cohen BJ, Ramsay ME. Oral fluid testing during 10 years of rubella elimination, England and Wales. Emerging Infectious Diseases. 2010;16(10):1532–1538. doi: 10.3201/eid1610.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C, Robinson J. Cochrane in context: Vaccines for measles, mumps and rubella in children. Evidence-Based Child Health: Cochrane Review Journal. 2013;8(6):2239–2242. [Google Scholar]
- 43.Strebel P, Papania MJ, Fiebelkorn P, Halsey NA. Measles Vaccine. In: Plotkin SA, Orenstein WA, Offitt PA, editors. Vaccines. 6 ed. Elsevier; 2013. pp. 352–387. [Google Scholar]
- 44.Rubella and congenital rubella syndrome control and elimination - global progress, 2000–2012. MMWR Morbidity and mortality weekly report. 2013;62(48):983–986. [PMC free article] [PubMed] [Google Scholar]
- 45.Global Vaccine Action Plan. Decade of vaccine collaboration. Vaccine. 2013;31(Suppl 2):B5–B31. doi: 10.1016/j.vaccine.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Organization WH. Geneva: 2012. Global Measles & Rubella Strategic Plan 2012–2020. [Google Scholar]
- 47.Who P. Rubella vaccines: WHO position paper--recommendations. Vaccine. 2011;29(48):8767–8768. doi: 10.1016/j.vaccine.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 48.Hinman AR, Irons B, Lewis M, Kandola K. Economic analyses of rubella and rubella vaccines: a global review. BullWorld Health Organ. 2002;80(4):264–270. [PMC free article] [PubMed] [Google Scholar]
- 49.Babigumira JB, Morgan I, Levin A. Health economics of rubella: a systematic review to assess the value of rubella vaccination. BMC Public Health. 2013;13:406. doi: 10.1186/1471-2458-13-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Organization WH. WHO-UNICEF estimates of MCV coverage. [Accessed September 23, 2013];2013 Jul 14; http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragemcv.html.
- 51.Cutts FT, Vynnycky E. Modelling the incidence of congenital rubella syndrome in developing countries. Int J Epidemiol. 1999;28(6):1176–1184. doi: 10.1093/ije/28.6.1176. [DOI] [PubMed] [Google Scholar]
- 52.Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012 - conclusions and recommendations. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2013;88(1):1–16. [PubMed] [Google Scholar]
- 53.Abernathy E, Cabezas C, Sun H, Zheng Q, Chen MH, Castillo-Solorzano C, et al. Confirmation of rubella within 4 days of rash onset: comparison of rubella virus RNA detection in oral fluid with immunoglobulin M detection in serum or oral fluid. Journal of Clinical Microbiology. 2009;47(1):182–188. doi: 10.1128/JCM.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellini WJ, Icenogle JP. Measles and rubella viruses. In: J V, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. Washington, D.C: ASM Press; 2011. pp. 1372–1387. [Google Scholar]
- 55.Recommendations from an ad hoc Meeting of the WHO Measles and Rubella Laboratory Network (LabNet) on use of alternative diagnostic samples for measles and rubella surveillance. MMWR Morbidity and mortality weekly report. 2008;57(24):657–660. [PubMed] [Google Scholar]
- 56.Helfand RF, Cabezas C, Abernathy E, Castillo-Solorzano C, Ortiz AC, Sun H, et al. Dried blood spots versus sera for detection of rubella virus-specific immunoglobulin M (IgM) and IgG in samples collected during a rubella outbreak in Peru. Clinical and vaccine immunology : CVI. 2007;14(11):1522–1525. doi: 10.1128/CVI.00144-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin L, Vyse A, Brown DW. The role of RT-PCR assay of oral fluid for diagnosis and surveillance of measles, mumps and rubella. Bulletin of the World Health Organization. 2002;80(1):76–77. [PMC free article] [PubMed] [Google Scholar]
- 58.Hubschen JM, Kremer JR, De Landtsheer S, Muller CP. A multiplex TaqMan PCR assay for the detection of measles and rubella virus. Journal of Virological Methods. 2008;149(2):246–250. doi: 10.1016/j.jviromet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 59.Pham VH, Nguyen TV, Nguyen TT, Dang LD, Hoang NH, Abe K. Rubella epidemic in Vietnam: characteristic of rubella virus genes from pregnant women and their fetuses/newborns with congenital rubella syndrome. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;57(2):152–156. doi: 10.1016/j.jcv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Chen MH, Zhu Z, Zhang Y, Favors S, Xu WB, Featherstone DA, et al. An indirect immunocolorimetric assay to detect rubella virus infected cells. Journal of Virological Methods. 2007;146(1–2):414–418. doi: 10.1016/j.jviromet.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Z, Xu W, Abernathy ES, Chen MH, Zheng Q, Wang T, et al. Comparison of four methods using throat swabs to confirm rubella virus infection. Journal of Clinical Microbiology. 2007;45(9):2847–2852. doi: 10.1128/JCM.00289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Three cases of congenital rubella syndrome in the postelimination era--Maryland, Alabama, and Illinois, 2012. MMWR Morbidity and mortality weekly report. 2013;62(12):226–229. [PMC free article] [PubMed] [Google Scholar]
- 63.Abernathy ES, Hubschen JM, Muller CP, Jin L, Brown D, Komase K, et al. Status of global virologic surveillance for rubella viruses. The Journal of Infectious Diseases. 2011;204(Suppl 1):S524–S532. doi: 10.1093/infdis/jir099. [DOI] [PubMed] [Google Scholar]
- 64.Organization WH. Rubella virus nomenclature update: 2013. Wkly Epidemiol Rec. 2013;32(88):337–343. [PubMed] [Google Scholar]
- 65.Wandinger KP, Saschenbrecker S, Steinhagen K, Scheper T, Meyer W, Bartelt U, et al. Diagnosis of recent primary rubella virus infections: significance of glycoprotein-based IgM serology, IgG avidity and immunoblot analysis. Journal of Virological Methods. 2011;174(1–2):85–93. doi: 10.1016/j.jviromet.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Mubareka S, Richards H, Gray M, Tipples GA. Evaluation of commercial rubella immunoglobulin G avidity assays. Journal of Clinical Microbiology. 2007;45(1):231–233. doi: 10.1128/JCM.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warrener L, Slibinskas R, Chua KB, Nigatu W, Brown KE, Sasnauskas K, et al. A point-of-care test for measles diagnosis: detection of measles-specific IgM antibodies and viral nucleic acid. Bulletin of the World Health Organization. 2011;89(9):675–682. doi: 10.2471/BLT.11.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.America IDSo. President's FY 2014 Budget Supports Some ID Priorities, Shortchanges Others. [Accessed January 2, 2014];2013 Apr 11; http://www.idsociety.org/2013_POTUS_Budget_FY2014/ [Google Scholar]
- 69.Plotkin SA. Rubella Vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia, PA: W.B. Saunders; 1999. pp. 409–440. [Google Scholar]
- 70.Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol. 1996;106(2):170–174. doi: 10.1093/ajcp/106.2.170. [DOI] [PubMed] [Google Scholar]
- 71.Poethko-Muller C, Mankertz A. Seroprevalence of measles-, mumps- and rubella-specific IgG antibodies in German children and adolescents and predictors for seronegativity. PLos ONE. 2012;7(8):e42867. doi: 10.1371/journal.pone.0042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pugachev KV, Abernathy ES, Frey TK. Improvement of the specific infectivity of the rubella virus (RUB) infectious clone: determinants of cytopathogenicity induced by RUB map to the nonstructural proteins. J Virol. 1997;71(1):562–568. doi: 10.1128/jvi.71.1.562-568.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambert ND, Pankratz VS, Larrabee BR, Ogee-Nwankwo A, Chen MH, Icenogle JP, et al. High-throughput Assay Optimization and Statistical Interpolation of Rubella-Specific Neutralizing Antibody Titers. Clinical and vaccine immunology : CVI. 2014 doi: 10.1128/CVI.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26(1):59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Best JM, O'Shea S, Tipples G, Davies N, Al-Khusaiby SM, Krause A, et al. Interpretation of rubella serology in pregnancy--pitfalls and problems. British Medical Journal. 2002;325(7356):147–148. doi: 10.1136/bmj.325.7356.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plotkin SA, Cornfeld D, Ingalls TH. Studies of immunization with living rubella virus. Trials in children with a strain cultured from an aborted fetus. American Journal of Diseases of Children. 1965;110(4):381–389. doi: 10.1001/archpedi.1965.02090030401007. [DOI] [PubMed] [Google Scholar]
- 77.Plotkin SA, Farquhar JD, Ogra PL. Immunologic properties of RA27/3 rubella virus vaccine. Journal of the American Medical Association. 1973;225:585–590. doi: 10.1001/jama.225.6.585. [DOI] [PubMed] [Google Scholar]
- 78.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR-8):1–57. [PubMed] [Google Scholar]
- 79.Prevention CfDCa. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th, second printing ed. Washington, D.C: Public Health Foundation; 2012. [Google Scholar]
- 80.Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado Y. Infectious Diseases of the Fetus and Newborn. 7th edition ed. Philadelphia: Elsevier Saunders; 2010. [Google Scholar]
- 81.Kakoulidou M, Ingelman-Sundberg H, Johansson E, Cagigi A, Farouk SE, Nilsson A, et al. Kinetics of antibody and memory B cell responses after MMR immunization in children and young adults. Vaccine. 2013;31(4):711–717. doi: 10.1016/j.vaccine.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 82.Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. 2008;197(7):950–956. doi: 10.1086/528993. [DOI] [PubMed] [Google Scholar]
- 83.O'Shea S, Woodward S, Best JM, Banatvala JE, Holzel H, Dudgeon JA. Rubella vaccination: persistence of antibodies for 10–21 years. Lancet. 1988;2:909. doi: 10.1016/s0140-6736(88)92512-3. -. [DOI] [PubMed] [Google Scholar]
- 84.Forleo-Neto E, Carvalho ES, Fuentes IC, Precivale MS, Forleo LH, Farhat CK. Seroconversion of a trivalent measles, mumps, and rubella vaccine in children aged 9 and 15 months. Vaccine. 1997;15(17–18):1898–1901. doi: 10.1016/s0264-410x(97)00135-7. [DOI] [PubMed] [Google Scholar]
- 85.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 86.Vaananen P, Makela P, Vaheri A. Effect of low level immunity on response to live rubella virus vaccine. Vaccine. 1986;4(1):5–8. doi: 10.1016/0264-410x(86)90090-3. [DOI] [PubMed] [Google Scholar]
- 87.Pukhalsky AL, Shmarina GV, Bliacher MS, Fedorova IM, Toptygina AP, Fisenko JJ, et al. Cytokine profile after rubella vaccine inoculation: evidence of the immunosuppressive effect of vaccination. MediatorsInflamm. 2003;12(4):203–207. doi: 10.1080/09629350310001599639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhiman N, Haralambieva IH, Vierkant RA, Pankratz VS, Ryan E, Jacobson RM, et al. Predominant inflammatory cytokine secretion pattern in response to two doses of live rubella vaccine in health vaccinees. Cytokine. 2010;50:24–29. doi: 10.1016/j.cyto.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet InfectDis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Newport MJ, Goetghebuer T, Weiss HA, The MRCGTSG, Whittle H, Siegrist CA, et al. Genetic regulation of immune responses to vaccines in early life. Genes Immun. 2004;5(2):122–129. doi: 10.1038/sj.gene.6364051. [DOI] [PubMed] [Google Scholar]
- 91.Tan PL, Jacobson RM, Poland GA, Jacobsen SJ, Pankratz SV. Twin studies of immunogenicity - determining the genetic contribution to vaccine failure. Vaccine. 2001;19:2434–2439. doi: 10.1016/s0264-410x(00)00468-0. [DOI] [PubMed] [Google Scholar]
- 92.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin PharmacolTher. 2007;82(6):653–664. doi: 10.1038/sj.clpt.6100415. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Y, Ushijima H, Frey TK. Genomic analysis of diverse rubella virus genotypes. The Journal of general virology. 2007;88(Pt 3):932–941. doi: 10.1099/vir.0.82495-0. [DOI] [PubMed] [Google Scholar]
- 94.Best JM, Thomson A, Nores JR, O'Shea S, Banatvala JE. Rubella virus strains show no major antigenic differences. Intervirology. 1992;34(3):164–168. doi: 10.1159/000150277. [DOI] [PubMed] [Google Scholar]
- 95.Jacobson RM, Ovsyannikova IG, Poland GA. Genetic basis for variation of vaccine response: our studies with rubella vaccine. Paediatrics and Child Health. 2009;S2:S156–S159. doi: 10.1016/j.paed.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ou D, Mitchell LA, D cD, Tingle AJ, Nepom GT. Promiscuous T-cell recognition of a rubella capsid protein epitope restricted by DRB1*0403 and DRB1*0901 molecules sharing an HLA DR supertype. Human Immunology. 1998;59:149–157. doi: 10.1016/s0198-8859(98)00006-8. [DOI] [PubMed] [Google Scholar]
- 97.Ou D, Mitchell LA, Ho M, D cD, Tingle AJ, Nepom GT, et al. Analysis of overlapping T- and B-cell antigenic sites on rubella virus E1 envelope protein. Influence of HLA-DR4 polymorphism on T-cell clonal recognition. Human Immunology. 1994;39:177–187. doi: 10.1016/0198-8859(94)90258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell LA, Decarie D, Tingle AJ, Lacroix M, Zrein M. Use of synthetic peptides to map regions of rubella virus capsid protein recognized by human T lymphocytes. Vaccine. 1994;12(7):639–645. doi: 10.1016/0264-410x(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 99.Ovsyannikova IG, Jacobson RM, Vierkant RA, O'Byrne MM, Poland GA. Replication of rubella vaccine population genetic studies: validation of HLA genotype and humoral response associations. Vaccine. 2009;27(49):6926–6931. doi: 10.1016/j.vaccine.2009.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ovsyannikova IG, Ryan JE, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine. 2009;27:3359–3366. doi: 10.1016/j.vaccine.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kennedy RB, Ovsyannikova IG, Vierkant RA, Jacobson RM, Poland GA. Effect of human leukocyte antigen homozygosity on rubella vaccine-induced humoral and cell-mediated immune responses. Human Immunology. 2010;71(2):128–135. doi: 10.1016/j.humimm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitchell LA, Tingle AJ, MacWilliam L, Horne C, Keown P, Gaur LK, et al. HLA-DR class II associations with rubella vaccine-induced joint manifestations. Journal of Infectious Diseases. 1998;177:5–12. doi: 10.1086/513807. [DOI] [PubMed] [Google Scholar]
- 103.Dhiman N, Haralambieva IH, Kennedy RB, Vierkant RA, O'Byrne MM, Ovsyannikova IG, et al. SNP/haplotype associations in cytokine and cytokine receptor genes and immunity to rubella vaccine. Immunogenetics. 2010;62:197–210. doi: 10.1007/s00251-010-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ovsyannikova IG, Haralambieva IH, Dhiman N, O'Byrne MM, Pankratz VS, Jacobson RM, et al. Polymorphisms in the vitamin A receptor and innate immunity genes influence the antibody response to rubella vaccination. Journal of Infectious Diseases. 2010;201(2):207–213. doi: 10.1086/649588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O'Byrne MM, Jacobson RM, et al. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Human Genetics. 2010;127:207–221. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haralambieva IH, Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, et al. 2'–5'-Oligoadenylate synthetase single-nucleotide polymorphisms and haplotypes are associated with variations in immune responses to rubella vaccine. HumImmunol. 2010;71(4):383–391. doi: 10.1016/j.humimm.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haralambieva IH, Oberg AL, Ovsyannikova IG, Kennedy RB, Grill DE, Middha S, et al. Genome-wide characterization of transcriptional patterns in high and low antibody responders to rubella vaccination. PLos ONE. 2013;8(5):e62149. doi: 10.1371/journal.pone.0062149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poland GA, Kennedy RB, McKinney BA, Ovsyannikova IG, Lambert ND, Jacobson RM, et al. Vaccinomics, adversomics, and the immune response network theory: Individualized vaccinology in the 21st century. Seminars in Immunology. 2013;25(2):89–103. doi: 10.1016/j.smim.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: Is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathogens. 2011;7(12):e1002344. doi: 10.1371/journal.ppat.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]