Abstract
The aim of this study was to evaluate acyclic ligands which can be applied for labeling proteins such as monoclonal antibodies and their fragments with scandium radionuclides. Recently, scandium isotopes (47Sc, 44Sc) are more available and their properties are convenient for radiotherapy or PET imaging. They can be used together as “matched pair” in theranostic approach. Because proteins denaturize at temperature above 42 °C, ligands which efficiently form complexes at room temperature, are necessary for labelling such biomolecules. For complexation of scandium radionuclides open chain ligands DTPA, HBED, BAPTA, EGTA, TTHA and deferoxamine have been chosen. We found that the ligands studied (except HBED) form strong complexes within 10 min and that the radiolabelling yield varies between 96 and 99 %. The complexes were stable in isotonic NaCl, but stability of 46Sc-TTHA, 46Sc-BAPTA and 46Sc-HBED in PBS buffer was low, due to formation by Sc3+stronger complexes with phosphates than with the studied ligands. From the radiolabelling studies with n.c.a. 47Sc we can conclude that the most stable complexes are formed by the 8-dentate DTPA and EGTA ligands.
Keywords: Scandium radionuclides, Targeted therapy, Acyclic multidentate ligands
Introduction
Radionuclides emitting low and medium energy beta-particles and having several days half-life are attractive candidates for radioimmunotherapy. The most promising radioisotope in this category is 177Lu, which has favourable decay characteristics, as e.g. half-life of 6.71 days.
This radionuclide can be produced in nuclear reactors by n,γ reaction and is commercially available at high levels of specific activity and chemical purity.
Having large cross-section of 2090 b [1], 177Lu can be directly produced with a relatively high specific activity by neutron activation of 176Lu (2.6 % of natural abundance). Nevertheless, some amount of stable 176Lu cannot be avoided, which may cause some problems concerning receptor saturation with biomolecules labelled with stable isotope, especially when the number of receptors is limited. For this purpose an alternative production route via neutron capture, starting with enriched 176Yb targets, has been demonstrated [2–4]. In this method, the isotopically enriched 176Yb target undergoes the (n,γ) reaction to produce 177Yb, which subsequently decays by β−-emission (T 1/2 = 1.9 h) to 177Lu. However, this indirect production route requires difficult radiochemical separation of 177Lu from the irradiated Yb2O3 target. Taking into account that ytterbium and lutetium are neighbouring trivalent lanthanides and that the Yb/Lu mass ratio in the irradiated target can be as high as several thousand, separation is a very difficult task [2, 5, 6].
The usefulness of other radionuclides, which may enhance the therapeutic effect of radiopharmaceuticals, should be also investigated. As a good alternative to application of carrier-free 177Lu Lehenberger et al. [7] proposed application of reactor produced 161Tb. The properties of this radionuclide are similar to those of 177Lu, but separation from the target is considerably easier.
Application of 47Sc, as an alternative radionuclide to 177Lu, was proposed in earlier works [8–11]. 47Sc is a low energy β−-emitter with a 3.35 days half-life, and shows a primary γ-ray at 159 keV, which is suitable for imaging. It is important to mention that other scandium radionuclides, 43Sc and 44Sc, are also promising β+-emitters, suitable for PET technique. Therefore, therapeutic 47Sc together with diagnostic 44Sc or 43Sc can be used as “matched pair” in teranostic approach. Essential decay characteristics and production parameters for n.c.a. 47Sc, 177Lu and 161Tb are presented in Table 1.
Table 1.
Production parameters of n.c.a. 177Lu, 161Tb and 47Sc
| Radionuclide | T1/2 (days) | Nuclear reactions | Eβmax (MeV) | Main photons, keV (%) | Cross-section (barn) (Vertes 2011) | Cost of enriched target ($/mg) | Separation from the target |
|---|---|---|---|---|---|---|---|
| 177Lu | 6.647 |
176Yb(n,γ)177Yb |
0.14 | 54 (33.5) | 3 | 13 | Very difficult |
| 208.4 (60.9) | |||||||
| 229 (37.2) | |||||||
| 161Tb | 6.906 |
160Gd(n,γ)161Gd |
0.15 | 25.7 (21) | 1.5 | 5 | Difficult |
| 48.9 (16) | |||||||
| 74.6 (9.8) | |||||||
| 47Sc | 3.341 | 47Ti(n,p)47Sc | 0.16 | 159.4 (68) | 0.15 | 10 | Easy |
The production method of highly active 47Sc in a nuclear reactor was described by Mausner and coworkers [8, 12]. Enriched 47TiO2 target was irradiated with high energy neutrons (En > 1 MeV) to produce 47Sc in the 47Ti(n,p)47Sc reaction. Various methods of 47Sc separation from metallic Ti and TiO2 targets, based on TBP extraction or cation and anion exchange processes, have been reported [12, 13]. Recently, to avoid the slow dissolution of the target in hot concentrated H2SO4, a new, simple and fast method based on irradiation of the Li472TiF6 or 47TiO2 target, dissolution in HF solution and ion exchange isolation of 47Sc has been investigated [11].
As shown in Table 1, the advantage of 47Sc production contrary to that of 177Lu and 161Tb, is relatively easy isolation of the radionuclide from the target, but the disadvantage is smaller cross-section of the nuclear reaction.
To date, only few reports concerning labelling studies with Sc radionuclides were published [14–17] Authors of these papers concluded, that for labelling peptides, such as octreotide, the macrocyclic ligand 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), is the most suitable. The stability of the DOTA complexes results from their kinetical inertness, therefore efficient labelling of DOTA-conjugates requires elevated (90 °C) temperature. Due to its rather long half-life, 47Sc can be also considered for preparation of alternative therapeutic agents with longer pharmacokinetics, such as 47Sc-labelled proteins, as e.g. monoclonal antibodies, its fragments and nanobodies. Unfortunately, because proteins denaturize at temperature above 42 °C, ligands other than DOTA, which form complexes at room temperature, are necessary for labelling such biomolecules. The acyclic chelators are not as kinetically stable as the macrocyclic chelators (DOTA, NOTA, TETA etc.), but formation of their complexes at room temperature is much faster. In this paper we report formation and stability studies on 47Sc complexes with various acyclic polydentate ligands, which exhibit faster than DOTA kinetics of complex formation.
Experimental
Materials
The analytical grade reagents were used for radiochemical investigations. Water was obtained from a Millipore water purification system. Ammonium acetate and hydrofluoric acid (40 %) were purchased from Sigma-Aldrich. All resins (Chelex® 100, Dowex® 1X8, Dowex® 50WX4) were purchased in 100–200 mesh size from Dow Chemical.
The analytical grade TiO2 was obtained from Sigma-Aldrich. Enriched 47TiO2 was supplied by Isoflex, San Francisco, USA. The isotopic composition was: 46Ti-4.0, 47Ti-65.8, 48Ti-26.8, 49Ti-1.8, 50Ti-1.6 %.
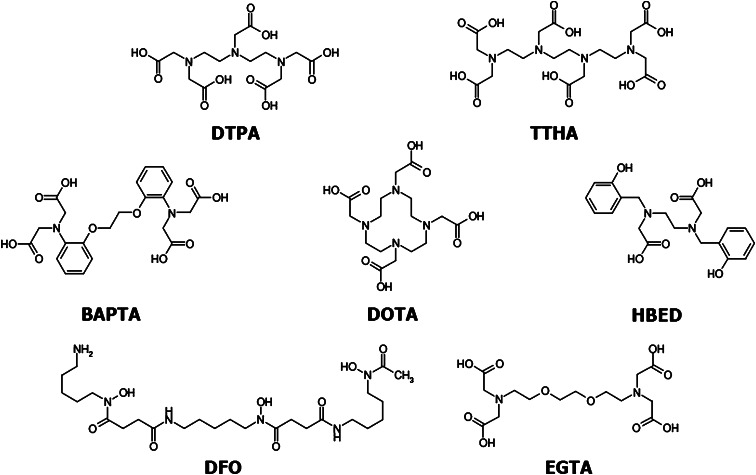
We have chosen the following acyclic ligands for the studies: 8-dentate diethylenetriaminepentaacetic acid (DTPA), 6-dentate N,N-bis(2-hydroxybenzyl)ethylenediamine-N,N-diacetic acid (HBED), 6-dentate 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 8-dentate ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 10-dentate triethylenetetraamine-N,N′,N′′,N′′′-hexaacetic acid (TTHA) and deferoxamine (DFO). For comparison, cyclic 8-dentate ligand, the 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) has been chosen.
Radionuclides
For reasons of availability we used in preliminary experiments instead of 47Sc, the longer lived 46Sc radionuclide. The former is produced by fast neutron irradiation of the 47Ti target, while 46Sc can be produced in a simple way by direct thermal neutron irradiation of natural scandium. The 46Sc radionuclide was obtained by neutron irradiation of the Sc2O3 target at a neutron flux of 1.5 × 1014 n cm−2 s−1 for 200 h in the Maria research reactor in Świerk (Poland). The specific activity of the radionuclide was 3.8 GBq/mg. The target was then dissolved in 0.1 M HCl.
47Sc was produced by fast neutrons in the 47Ti(n,p)47Sc reaction on enriched (66 %) 47Ti target. The 2 mg samples of 47TiO2 target were irradiated in the fast neutron channel of nuclear reactor in Świerk (Poland) for 143 h at fast neutron flux (>1 MeV) of 3 × 1013 n cm−2 s−1, and thermal neutron flux of 2.5 1014 n cm−2 s−1. The irradiated 47TiO2 was then dissolved in 1 ml of concentrated hydrofluoric acid (2–4 h with gentle heating at 80 °C) and the solution was diluted to 20 ml with 1 M HF. The solution was next passed through anion exchange bed (Dowex® 1X8). 47Sc was quantitatively eluted using the 0.04 M HNO3 + 0.06 M HF solution. For additional purification the eluent was evaporated to dryness and the residue was dissolved in 0.1 M HNO3. Afterwards 47Sc was adsorbed on cation exchange column Dowex® 50WX4 and finally eluted in two 1 ml fractions using 0.25 M ammonium acetate.
Syntheses of radiolabelled complexes
The experimental conditions for labelling, such as metal-to-ligand molar ratio and time of reaction were optimized to achieve high complexation yield. The 46Sc complexes were synthesized by mixing 164 nmol of carrier added 46Sc in chloride form with aqueous solutions of chelators in various molar ligand-to-metal ratios (1/1, 2/1, 5/1, 10/1). In the case of n.c.a. 47Sc 1 MBq was used and the ligand amount varied from 2 to 15 nmol. The complexes were prepared at room temperature at pH = 6.0 (20 mM acetate buffer). The radiolabelling yield was determined by thin layer chromatography (TLC) using silica gel plates (Polygram, Macherey–Nagel). The NH3/H2O (1/25) mixture was used as the mobile phase. The distribution of activity on paper strips was measured by cutting the paper into 1-cm pieces and counting in the NaI (Tl) well counter. The 46Sc-complexes moved with the solvent front (R f = 1), while free 46Sc remained at the starting point.
Stability of complexes
Stability of the 46,47Sc acyclic complexes in isotonic NaCl solution and PBS buffer was assessed by adding 20 μl of radiocomplex solution to 500 μl of 0.9 % NaCl and 0.01 M PBS buffer. The solutions were incubated at 37 °C and kinetics of the Sc complex dissociation was measured by taking aliquots of the NaCl and PBS solution at different time points and measuring the liberated 46Sc by ITLC analysis.
Results and discussion
Radiolabelling and kinetics
As already mentioned, the cyclic polyamino-polycarboxylate ligands like DOTA, due to formation of kinetically inert complexes, are excellent ligands for binding M3+ to biomolecules. DOTA labelled with 90Y, 177Lu, 213Bi and 68Ga is widely used in peptide radiopharmaceuticals such as octreotide, bombesin, substance P etc. However, complex formation with macrocyclic DOTA derivatives generally requires, in contrast to open-chain analogues, heating at elevated temperatures (>90 °C). Because the aim of our studies was elaboration of a method suitable for labelling proteins with 47Sc, we studied at first the kinetics of formation of Sc-DOTA complexes at room temperature. Figure 1 presents kinetics of scandium complexation by DOTA at 25 and 70 °C.
Fig. 1.
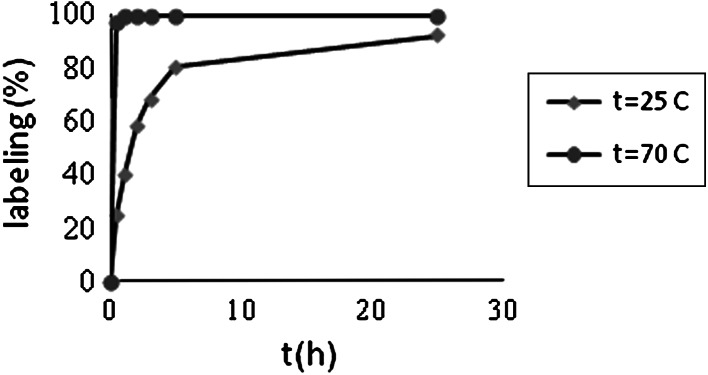
Labelling yield of the DOTA ligand with 46Sc at room and elevated temperature
As shown in Fig. 1 the kinetics of complexation at room temperature is slow, while increase of temperature to 70 °C drastically increases kinetics of labelling. Therefore, macrocyclic ligands, like DOTA, are not suitable for labelling of thermal non-resistant molecules, like proteins. In the present work, we examined selected acyclic polyamino-polycarboxylate ligands, which form complexes with scandium cations more rapidly than does DOTA. The ligands demonstrating high affinity for 3+ metal cations such as Fe3+, Ga3+ and lanthanides were selected for our studies. Structures of the ligands are presented in Fig. 2.
Fig. 2.
Structures of the ligands
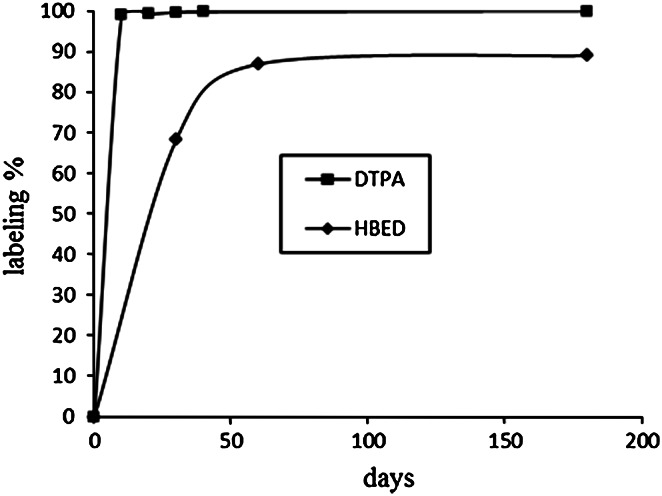
Kinetics of 46Sc complexation by open chain ligands was studied, in contrary to the DOTA, only at room temperature (Fig. 3). Comparison of Fig. 3. with the Fig. 1 shows much faster labelling at room temperature, of the open chain ligands than of the cyclic DOTA. In the case of DTPA the labelling yield after 10 min reached more than 99 % of the equilibrium value, while in the case of DOTA achievements of about 90 % value required more than 20 h. However, as shows in Fig. 3, the open chain ligand HBED seems, not to attain within 30 h equilibrium value.
Fig. 3.
Labelling yield of DTPA and HBED ligands with 46Sc at room temperature
The labelling yield of acyclic ligands with 46Sc was studied by us at different metal-to-ligand molar ratios, see Table 2. Our studies showed that at pH = 6.0 more than 96 % of 46Sc was complexed with just 1:1 ligand-to-metal molar ratio.
Table 2.
Labelling of the ligands with carrier added 46Sc
| Sc:ligand molar ratio (Sc = 1.6×10−7 M) | Labelling yield (%) | ||||
|---|---|---|---|---|---|
| EGTA | TTHA | DTPA | DFO | BAPTA | |
| 1:1 | 99.3 | 98.0 | 99.1 | 96.2 | 98.2 |
| 1:2 | 100 | 100 | 100 | 100 | 99.0 |
| 1:10 | 100 | 100 | 100 | 100 | 100 |
In vitro stability studies
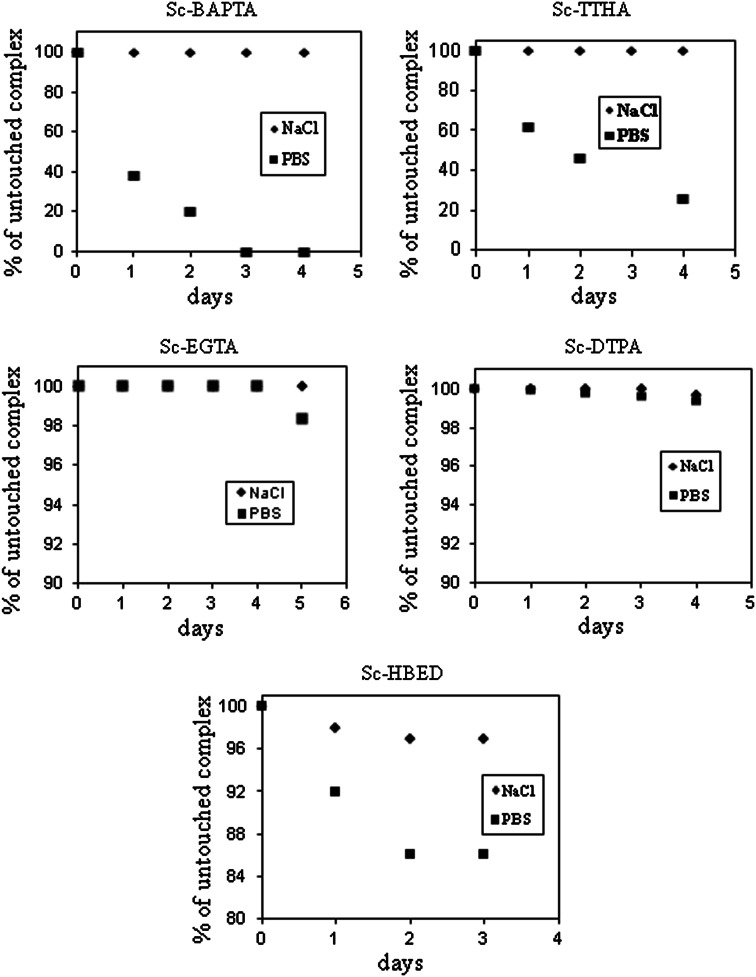
For radionuclide therapy applications, radionuclides must remain associated with the targeting chelate-protein to avoid the toxicity released in their dissociation. As shown in Fig. 4, all synthesized 46Sc-complexes exhibited high stability in isotonic NaCl solution. After 120 h incubation in the NaCl solution more than 96 % of 46Sc remained in complexed form. In 0.1 M PBS buffer 46Sc-DTPA and 46Sc-EGTA complexes were stable over the whole course of the experiment. Stability of 46Sc-TTHA and 46Sc-BABTA in PBS solution was very low due to replacement of ligands by phosphates in the first coordination sphere. In the case of 46Sc-HBED slow decomposition of the complex was observed.
Fig. 4.
Stability of 46Sc-BABTA, 46Sc-TTHA, 46Sc-EGTA, 46Sc-DTPA and 46Sc-HBED complexes in 0.9 % NaCl and 0.1 M PBS buffer
Labelling of the ligands with n.c.a. 47Sc
The obtained results on complex formation and stability of 46Sc complexes in PBS buffer shows that only Sc-DTPA and Sc-EGTA can be used as precursors for scandium radiopharmaceuticals. Therefore, we have chosen the two ligands for further studies with n.c.a. 47Sc. Various concentrations of the ligands were investigated in order to evaluate their usability for binding 47Sc to biomolecules. Radiolabelling was performed with quantities of the ligands from 2 to 15 nmol (Table 3). For comparison, results of labelling the commonly used macrocyclic ligand DOTA [8] is presented in Table 4.
Table 3.
Labelling of the open chain ligands with n.c.a. 47Sc
| Amount of ligand (nmol) | Labelling yield (%) | |
|---|---|---|
| DTPA | EGTA | |
| 2 | 38.81 | 97.20 |
| 5 | 94.89 | 98.80 |
| 10 | 95.40 | 99.40 |
| 15 | 100.0 | 100.0 |
Table 4.
Labelling of the macrocyclic ligand with n.c.a. 47Sc
| Amount of DOTA (nmol) | Labelling efficiency (%) |
|---|---|
| 22.5 | 79.4 |
| 36.0 | 96.2 |
| 58.5 | 97.3 |
From comparison of the Table 3 with the Table 4 one can conclude that both ligands, DTPA and EGTA, form complexes with n.c.a. 47Sc, in lower amount of ligand than those formed by DOTA. Particularly in the case of EGTA, only 2 nmol of the ligand is sufficient to achieve labelling higher than 97 %. The reason is that EGTA and DTPA as a 8-dentate ligand, forms strong complexes by binding via four carboxylic oxygen atoms, two ether oxygen atoms and two nitrogen atoms (EGTA) [18] or five carboxylic and tree nitrogen (DTPA). As was reported by Thakur et al. [19], contrary to our results Am3+, Cm3+ and Eu3+ form stronger complexes with DTPA than EGTA. Formation of stronger complexes by Sc3+ with EGTA than DTPA is probably related with stronger interaction of smaller and harder Sc3+ with ether than with carboxylic oxygen atoms.
Conclusion
In this paper formation and in vitro stability of a series of polyamino-polycarboxylate ligands labelled with 46Sc and 47Sc has been studied. We found that acyclic ligands (except HBED) form complexes much faster than the cyclic DOTA. The radiolabelling yield of acyclic ligands was very high and for the Sc:L molar ratio of 1:1 varied after 10 min from 96 to 99 %. The obtained complexes were stable in isotonic NaCl solution. However, stability of 46Sc-TTHA, 46Sc-BABTA and 46Sc-HBED in PBS buffer was low, due to formation by Sc3+stronger complexes with phosphates than with TTHA, BABTA and HBED. We have shown that when using n.c.a. 47Sc only 2 nmol of the EGTA is sufficient to obtain labelling yield greater than 97 %. Therefore, Sc-EGTA is a promising moiety for coupling 47Sc to proteins. However, biological studies on animal models are needed for evaluation the in vivo stability of radiometal-labelled chelates.
Acknowledgments
The authors thank Prof. Sławomir Siekierski for helpful discussions. This work was carried out as a part of the projects of the Ministry of Science and Higher Education of Poland Nr. NR 050057 06.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Firestone RB, Shirley VS, Baglin CM, Chu SYF, Zipkin J. Table of isotopes. 8. New York: Wiley; 1996. [Google Scholar]
- 2.Lebedev NA, Novgorodov AF, Misiak R, Brockmann J, Rösch F. Radiochemical separation of no-carrier-added 177Lu as produced via the 176Yb(n,γ)177Yb → 177Lu process. Appl Radiat Isot. 2000;53:421–425. doi: 10.1016/S0969-8043(99)00284-5. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto K, Matsuoka H, Uchida S. Production of no-carrier added 177Lu via the 176Yb(n,γ)177Yb → 177Lu process. J Radioanal Nucl Chem. 2003;255:575–579. doi: 10.1023/A:1022557121351. [DOI] [Google Scholar]
- 4.Balasubramanian PS. Separation of carrier-free lutetium-177 from neutron irradiated natural ytterbium target. J Radioanal Nucl Chem. 1994;185:305. doi: 10.1007/BF02041303. [DOI] [Google Scholar]
- 5.Horwitz EP, McAlister DR, Bond AH, Barrans RE, Williamson JM. A process for the separation of 177Lu from neutron irradiated 176Yb targets. Appl Radiat Isot. 2005;63:23–36. doi: 10.1016/j.apradiso.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Bilewicz A, Żuchowska K, Bartoś B. Separation of Yb as YbSO4 from the 176Yb target for production of 177Lu via the 176Yb(n,γ)177Yb → 177Lu process. J Radioanal Nucl Chem. 2009;280:167–169. doi: 10.1007/s10967-008-7437-7. [DOI] [Google Scholar]
- 7.Lehenberger S, Barkhausen Ch, Cohrs S, Fischer E, Grünberg J, Hohn A, Köster U, Schibli R, Türler A, Zhernosekov K. The low-energy β− and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl Med Biol. 2011;38:917–924. doi: 10.1016/j.nucmedbio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Mausner LF, Kolsky KL, Joshi V, Srivastava SC. Radionuclide development at BNL for nuclear medicine therapy. Appl Radiat Isot. 1998;49:285–294. doi: 10.1016/S0969-8043(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 9.Kopecky P, Szelecsenyi F, Molnàir T, Mikecz P, Tfirkànyi F. Excitation functions of (p,xn) reactions on natTi: monitoring of bombarding proton beams. Appl Radiat Isot. 1993;44:687–692. doi: 10.1016/0969-8043(93)90133-U. [DOI] [Google Scholar]
- 10.Bokhari TH, Mushtaq A, Khan IU. Separation of no-carrier-added radioactive scandium from neutron irradiated titanium. J Radioanal Nucl Chem. 2009;283:389–393. doi: 10.1007/s10967-009-0370-6. [DOI] [Google Scholar]
- 11.Bartoś B, Majkowska A, Krajewski S, Bilewicz A. New separation method of no-carrier-added 47Sc from titanium targets. Radiochim Acta. 2012;100:457–463. doi: 10.1524/ract.2012.1938. [DOI] [Google Scholar]
- 12.Pietrelli L, Mausner LF, Kolsky KL. Separation of carrier free 47Sc from titanium targets. J Radioanal Nucl Chem. 1992;157:335–345. doi: 10.1007/BF02047448. [DOI] [Google Scholar]
- 13.Kolsky KL, Joshi V, Mausner LF, Srivastava SC. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl Radiat Isot. 1998;49:1541–1549. doi: 10.1016/S0969-8043(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 14.Mausner LF, Joshi V, Kolsky KL, Meinken GE, Mease RC, Sweet MP, Srivastava SC. Evaluation of chelating agents for radioimmunotherapy with scandium-47. J Nucl Med. 1995;36:104P. [Google Scholar]
- 15.Majkowska-Pilip A, Bilewicz A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem. 2011;105:313–320. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Pruszynski M, Majkowska A, Loktionova NS, Roesch F. Radiolabeling of DOTATOC with the longer-lived, generator derived positron emitter 44Sc. Appl Radiat Isot. 2012;70:974–979. doi: 10.1016/j.apradiso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Huclier-Markai S, Sabatie A, Ribet S, Kubıcek V, Paris M, Vidaud C, Hermann P, Cutler CS. Chemical and biological evaluation of scandium(III)–polyaminopolycarboxylate complexes as potential PET agents and radiopharmaceuticals. Radiochim Acta. 2011;99:653–662. doi: 10.1524/ract.2011.1869. [DOI] [Google Scholar]
- 18.Inomata Y, Okamura D, Morita K, Yukawa Y, Howell FS. The comparison of crystal structures of lanthanide complexes with 3,12-bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioic acid (H4egta) J Mol Struct. 2003;659:23–34. doi: 10.1016/S0022-2860(03)00420-4. [DOI] [Google Scholar]
- 19.Thakur P, Conca JL, Choppin GR. Complexation studies of Cm(III), Am(III), and Eu(III) with linear and cyclic carboxylates and polyaminocarboxylates. J Coord Chem. 2011;64:3214–3236. doi: 10.1080/00958972.2011.616927. [DOI] [Google Scholar]