Preface
Niches are local tissue microenvironments that maintain and regulate stem cells. Haematopoiesis provides a paradigm for understanding mammalian stem cells and their niches, yet the haematopoietic stem cell (HSC) niche remains incompletely defined and beset by competing models. Here we review progress in elucidating the location and cellular components of the HSC niche in the bone marrow. The niche is perivascular, created partly by mesenchymal stromal cells and endothelial cells and often, but not always, located near trabecular bone. Outstanding questions concern the cellular complexity of the niche, the role of the endosteum, and functional heterogeneity among perivascular microenvironments.
Introduction
HSC niches are present in diverse tissues throughout development beginning in the aorta-gonad-mesonephros (AGM) region and the yolk sac, followed by the placenta, fetal liver, spleen, and bone marrow1. Postnatally, the bone marrow is the primary site of HSC maintenance and haematopoiesis but in response to haematopoietic stress the niche can shift to extramedullary sites. Defining niche components and how they work in concert to regulate haematopoiesis offers the opportunity to improve regeneration following injury or HSC transplantation and to understand how disordered niche function may contribute to disease. In this review we focus on the nature of the HSC niche in bone marrow because that has been the subject of most of the recent research and controversies.
Historic context
Following Darwin, there was much emphasis on defining hierarchical evolutionary relationships among organisms. Morphologic similarities were used to construct ancestral trees that connected complex multicellular organisms to an original monocellular “stem celle”2. Lineage relationships were formulated and Ernst Haeckel proposed that cell organization in a developing organism was the recapitulation of events in the evolution of the species, with cells deriving from a “stem celle” equivalent3. Thirty years later, Artur Pappenheim proposed a less grand and more accurate formulation based on improved ability to visualize cell morphology - that cells of the blood were related to one another, with mature cell types descending from a single cell type in a “unified view of haematopoiesis”4. In so doing, he articulated the hypothesis of tissue stem cells. This concept took approximately half a century to define experimentally through the inspired work of Till and McCulloch who showed that single cells could indeed yield multilineage descendants while preserving the multipotency of the mother cell5–7. They gave substance to the idea of a stem cell and gave us methods to define the cardinal properties of those cells, self-renewal and differentiation.
Till and McCulloch based much of their work on an in vivo spleen colony-forming assay (CFU-S) now known to measure mainly multipotent progenitors rather than long-term self-renewing haematopoietic stem cells (HSCs)8,9. The imprecise nature of that assay contributed to Ray Schofield’s formulation of the niche hypothesis in 1978. Recognizing that the putative CFU-S stem cells were less robust than cells of the bone marrow at reconstituting haematopoiesis in irradiated animals, he proposed that a specialized bone marrow niche preserved the reconstituting ability of stem cells10. His colleagues at the University of Manchester concurrently sought to define what made bone marrow a nurturing context for HSCs and Michael Dexter showed that largely mesenchymal ‘stromal’ cell cultures could maintain primitive haematopoietic cells ex vivo 11. Further, Brian Lord progressively reamed long bone marrow cavities and showed that primitive cells tended to localize toward the endosteal margins, leading to the hypothesis that bone might regulate haematopoiesis (Fig. 1) 12.
Figure 1. Bone marrow anatomy.
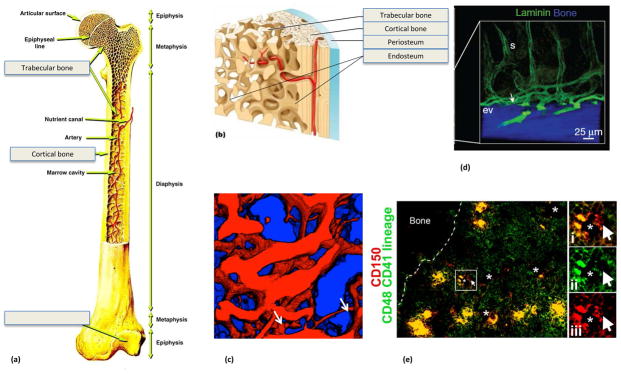
Haematopoietic stem cells (HSCs) reside primarily within bone marrow during adulthood. Bone marrow is a complex organ containing many different haematopoietic and non-haematopoietic cell types. Bone marrow is surrounded by a shell of vascularized and innervated bone. a. Minute projections of bone (trabeculae) are found throughout the metaphysis such that many cells in this region are close to bone surface. b. The interface of bone and bone marrow is known as the endosteum, which is covered by bone-lining cells that include bone-forming osteoblasts and bone-resorbing osteoclasts. Arteries carry oxygen, nutrients, and growth factors into the bone marrow, before feeding into sinusoids, which coalesce as a central sinus to form the venous circulation. Sinusoids are specialized venuoles that form a reticular network of fenestrated vessels that allow cells to pass in and out of circulation. There is a particularly rich supply of arterioles as well as sinusoids near the endosteum. c. 3-D reconstructed photomicrograph from the marrow looking toward the endosteal surface (blue) from 50 μm below the surface, revealing the rich network of vessels (red) (courtesy of Charles Lin, Joel Spencer and Juwell Wu). Smaller arteriolar vessels (white arrows) become larger sinusoidal vessels. The field of view is 350μm × 350μm. d. A cross-sectional view of blood vessels that run along the endosteal surface (ev) and that transition (white arrow) into sinusoids (s) that then course toward the central sinus (from ref31). e. The bone marrow is cellularly complex with CD150+CD48−CD41−Lineage− HSCs (arrow) residing in close contact with not only vascular and perivascular cells (*, sinusoid lumens) but also megakaryocytes (large yellow cells) and other haematopoietic cells (image from ref124).
These early studies were followed by in vitro evidence that osteoblasts differentiated in culture from human marrow stromal cells could produce haematopoietic cytokines and support primitive haematopoietic cells in culture 13. This fostered the idea that bone cells might create the HSC niche but it was essential to move to engineered mouse strains to test the hypothesis in vivo. Two studies followed, including a mouse model in which a promoter restricted in activity to osteoblastic cells was used to drive expression of a constitutively active parathyroid hormone receptor 14. Along similar lines, Linheng Li’s laboratory used a promoter since shown to be restricted in bone marrow stroma to primitive and mature osteolineage cells15 to delete the BMPr1a gene16. In both models, the number of endosteal osteoblasts and the number of primitive haematopoietic cells (scored as stem cells given the measures in use at the time) increased. These data provided the first evidence of specific heterologous cells regulating mammalian stem cells in vivo, though it remained unclear whether the regulation was direct or indirect. This demonstrated that the niche was experimentally tractable, prompting a series of studies that have since refined our understanding of the complexity of the bone marrow microenvironment.
Studies of the niche have now more precisely determined the components that regulate HSCs, and to some extent other haematopoietic progenitors, in the bone marrow. Like any interactive system there are complex regulatory relationships among cells in the bone marrow. A perturbation in one cell type that leads to an effect in another cell type does not necessarily require the interaction between the cells to be direct. The data now suggest that the early studies that observed effects on HSC frequency as a consequence of genetic manipulation in osteoblastic cells reflected indirect effects rather than the existence of an osteoblastic niche. Indeed, expression of constitutively active parathyroid hormone receptor in osteoblasts 14 likely causes widespread changes in many cell types the bone marrow, including in the vasculature. Current data suggest there are specialized niches for distinct types of haematopoietic stem and progenitor cells and that each niche may be created by multiple cell types that contribute to the niches in unique as well as redundant ways17. Indeed, there is heterogeneity among HSCs themselves18–20, raising the possibility of cellularly distinct niches for distinct subpopulations of HSCs. This review will focus on the current data and the unanswered questions.
Mapping the marrow space
A niche is defined by anatomy and function21 - a local tissue microenvironment that directly maintains and regulates a particular kind of stem cell or progenitor 22. Determining what cells neighbor HSCs and regulate HSC maintenance has been complicated by the difficulty in retaining histologic integrity when sectioning bone as well as the complexity of immunostaining methods necessary to identify HSCs.
The identification of markers that reliably identify HSCs in vivo was an important step in defining the niche 22. Despite the ability to isolate HSCs by flow cytometry for decades 23, the identification of HSCs within tissues remained a challenge because the combination of immunofluorescent markers used to isolate HSCs by flow cytometry was too complex for microscopy. Consequently, markers of poor specificity were often used. For example, “HSCs” have been localized in the bone marrow using BrdU or H2B-GFP label retention as a marker 24,25. Although there is a subset of HSCs that preferentially retains H2B-GFP and BrdU, these markers by themselves have very poor specificity - the vast majority of bone marrow cells that retain these labels are not HSCs 18,19,26.
By combining positive staining for CD150 and negative staining for CD48 and CD41, HSCs could finally be highly purified using a simple two-color stain 27. All serially transplantable HSCs in young adult mice are contained within the CD150+CD48−CD41−/low population of bone marrow cells, including the most quiescent HSCs26–29. This made it possible to localize HSCs in sections through haematopoietic tissues using markers validated to give high purity. Most CD150+CD48−CD41−Lineage− cells in the bone marrow and spleen localize adjacent to sinusoid vessels and nearly all are within 5 cell diameters of a sinusoid (Fig. 2)27,30. Indeed, HSCs are five times more likely than other haematopoietic cells to be immediately adjacent to a sinusoid 30. HSCs are distributed throughout the bone marrow, with less than 20% within 10μm of the endosteum27,30–32. Nonetheless, most HSCs are found in the trabecular region of bone marrow, suggesting that HSCs, or their niche, may be directly or indirectly regulated by factors present near bone surfaces.
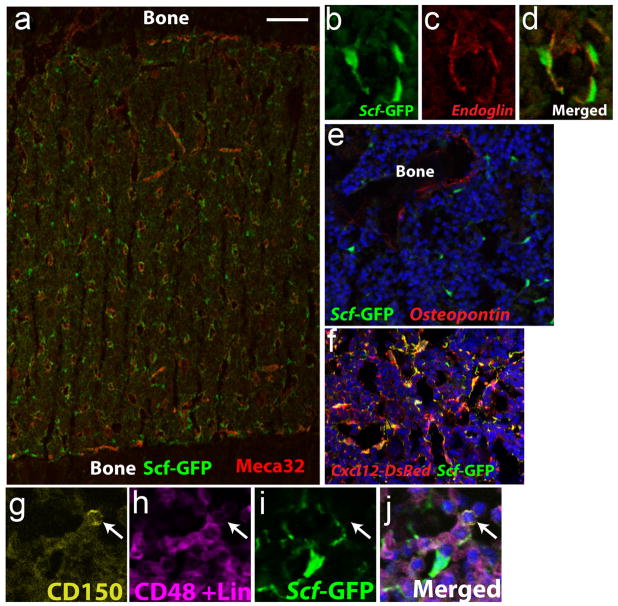
Figure 2. HSCs and their niche cells surround sinusoids throughout the bone marrow.
a. Sections through the bone marrow of Scfgfp/+ mice show that HSC niche cells (green) include mesenchymal stromal cells and endothelial cells that surround sinusoids and potentially other blood vessels throughout the bone marrow64. b–d. High magnification shows that Scf-GFP overlaps with the endothelial marker endoglin but also extends beyond the endoglin on the abluminal side of the sinusoids, indicating expression by mesenchymal stromal cells. e. Scf-GFP is not expressed by osteopontin+ bone lining cells around trabecular bone, but is expressed by some nearby perivascular cells. f. Cxcl12-DsRed exhibits a similar expression pattern, primarily by perivascular mesenchymal cells and endothelial cells around sinusoids throughout the bone marrow, in a pattern that strongly overlaps with Scf-GFP in Cxcl12DsRed/+; Scfgfp/+ mice17. g–j, Cells that are CD150+ (g) and CD48 and Lineage marker negative (h) are usually found immediately adjacent to Scf-GFP+ perivascular cells (i) in the bone marrow (see j for merge). Images are from references17,64.
The frequent localization of HSCs adjacent to blood vessels suggested that HSCs might be maintained in a perivascular niche by endothelial or perivascular cells27,33. Yet, HSC are mobile, regularly entering and exiting the circulation 34. This raised the possibility that the cells observed near vessels were in transit, perhaps delayed in entering or exiting the circulation by the process of migrating through vascular barriers. This issue could not be resolved by histologic analysis that captures a single moment in time.
The sequential imaging of mice with high resolution assessed the three dimensional position of cells in the calvarium over time 32,35. These studies indicated that primitive haematopoietic cells trafficked to specific microdomains of marrow blood vessels where CXCL12 and E-selectin were abundant, then remained in these positions for weeks, generating new cells as indicated by partitioning of a cytosolic dye. When HSCs were visualized after transplantation into irradiated mice they preferentially localized near the endosteum, consistent with that region being particularly relevant for HSC maintenance 32,36. However, it was subsequently learned that irradiation disrupts sinusoids in the bone marrow 37 raising the possibility that the only blood vessels preserved after irradiation are the arteriolar vessels near the endosteum. Therefore, the peri-endosteal localization of HSCs in these experiments may have reflected, in part, the destruction of sinusoidal niches by irradiation. Overall, the localization data emphasized the possibility of a perivascular niche. How could this be resolved with the historical data suggesting that the endosteum and osteoblasts were niche participants?
Osteoblasts: more harbinger than host
While osteoblastic cells were the first cell population shown to influence haematopoietic stem/progenitor cell frequency when perturbed in vivo 14,16, several lines of evidence raised concerns that the effect may not be direct. First, in vivo imaging studies using validated markers or labeled stem cells found few HSCs in contact with osteoblastic cells 27,33,38,31,32. Second, studies that depleted osteoblasts by biglycan deficiency30 or treatment with diphtheria toxin 39,40(Scadden unpublished) or that increased osteoblasts by strontium treatment41 had no acute effect on HSC frequency. The studies in which osteoblasts were conditionally deleted by diphtheria toxin were particularly compelling as they showed acute depletion of lymphoid progenitors but not HSCs39,40(Scadden unpublished). Third, genetic modification of primitive osteolineage cells had an effect on HSC proliferation and differentiation, but the same modification in mature osteoblasts did not42. Finally, a key adhesion molecule thought to mediate osteoblast-HSC interaction, N-cadherin, was called into question.
N-cadherin+ HSCs were proposed to adhere to N-cadherin+ osteoblasts by homophilic adhesion16,36, promoting HSC maintenance43–45; however, these studies did not test whether N-cadherin deletion affected HSC function. The levels of N-cadherin staining in HSCs were difficult to distinguish from background fluorescence and depended upon anti-N-cadherin antibodies that gave non-specific staining in some haematopoietic cells46. Other studies failed to detect N-cadherin expression by HSCs using gene expression profiling 27,47(https://gexc.stanford.edu/model/3/gene/Cdh2)48 quantitative RT-PCR, flow cytometry with multiple anti-N-cadherin antibodies, western blot, or N-cadherin:LacZ genetrap mice 18,28,30,38. Conditional deletion of N-cadherin from HSCs or from osteoblast lineage cells had no effect on HSC frequency, HSC function, or haematopoiesis 38,49,50. Collectively, these data undermined the notion of an N-cadherin+ ‘osteoblastic’ niche.
Is there any role for osteoblasts or osteolineage cells in HSC regulation? Several lines of evidence suggest that this possibility remains viable but not as initially envisioned. First, higher numbers of HSCs reside in the trabecular rich metaphysis31,51. This may simply reflect other components of bone marrow co-localizing with bony surfaces; however, conditional deletion of osterix results in chondrocytes without osteoblastic differentiation, increasing blood vessels and mesenchymal progenitors in the bone marrow but virtually eliminating haematopoiesis in the metaphysis52. These data argue that the presence of mature or maturing osteolineage cells in regions with abundant endosteum is critical for haematopoiesis. Indeed, mesenchymal progenitors capable of forming bone are sufficient to create bony ossicles that become invested by host vasculature and HSCs 53,54. This suggests that bone or bone-forming progenitors can promote the formation or maintenance of HSC niches (for example by recruiting vasculature to the bone marrow) even if they do not directly promote HSC maintenance.
Transplanted haematopoietic stem/progenitor cells preferentially localize to blood vessels in endosteal regions even without prior cytotoxic conditioning 55. Within the endosteal region, transplanted HSCs position themselves closer to the endosteal surface than progenitor cells 32. These may again reflect indirect effects of bone forming osteolineage cells as bone turnover results in high local concentrations of ionic calcium and the calcium sensing receptor promotes bone marrow engraftment by HSCs during development or after transplantation 56. Osteolineage cells also elaborate cytokines and extracellular matrix proteins that may influence a wide range of cell types, some of which may directly regulate HSC function. This is exemplified by Parathyroid Hormone Receptor (PTHr) activation, which induces expression of multiple regulatory molecules by osteoblasts (such as IL-6, RANKL, and Jagged1) that can influence other cells in the bone marrow, including the vasculature 14,57. Also, osteoblastic expression of transgenes encoding the Wnt antagonists, Dkk1 and Wif1, depletes HSCs58,59. Finally, the depletion of osteocalcin expressing cells (osteoblasts or osteocytes) in vivo resulted in an inability to mobilize at least short-term repopulating cells to the blood using G-CSF60,61 despite osteoblasts having little expression of CXCL1217,33,62, a key molecule that regulates HSC mobilization. In aggregate, these data indicate that the endosteal region is important for haematopoiesis, but the mature osteolineage cells likely have an indirect role in modulating HSCs. Rather, these cells appear to be more important in directly regulating restricted progenitors, a topic discussed below.
It is important to therefore re-focus attention on the endosteum as a regulatory region and not on the osteoblasts themselves (Fig. 3). The endosteum has a diverse group of cells and anatomic elements including a rich endowment of arteriolar and sinusoidal blood vessels (Fig. 1)31,32. The cells include endothelial cells as well as mesenchymal cells with osteolineage potential. These mesenchymal cells reside perivascularly but traffic to the endosteal surface to differentiate to osteoblasts. Undifferentiated mesenchymal cells around blood vessels may promote HSC maintenance throughout the bone marrow but the mesenchymal cells around vessels in the endosteal region may differ from those distant from endosteal surfaces.
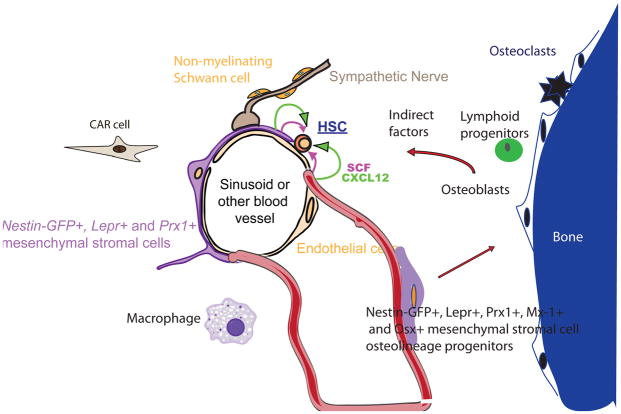
Figure 3. HSCs and restricted haematopoietic progenitors occupy distinct niches in the bone marrow.
a. HSCs are found mainly adjacent to sinusoids throughout the bone marrow27,30,31,33, where endothelial cells and mesenchymal stromal cells promote HSC maintenance by producing SCF64, CXCL1217,33,62, and likely other factors. Similar cells may also promote HSC maintenance around other types of blood vessels, such as arterioles. The mesenchymal stromal cells can be identified based on their expression of Lepr-Cre64, Prx1-Cre62, Cxcl12-GFP33, or Nestin-GFP transgene63 in mice and similar cells are likely to be identified by CD146 expression in humans54. These perivascular stromal cells, which likely include Cxcl12-abundant Reticular (CAR) cells33, are fated to form bone in vivo, express Mx-1-Cre and overlap with CD45/Ter119−PDGFRα +Sca-1+ stromal cells that are highly enriched for MSCs in culture66. b. It is likely that other cells also contribute to this niche, likely including cells near bone surfaces in trabecular rich areas. Other cell types that regulate HSC niches include sympathetic nerves91,92, non-myelinating Schwann cells (which are also Nestin+)96, macrophages95, osteoclasts97, extracellular matrix 119,120, and calcium56. Osteoblasts do not directly promote HSC maintenance but do promote the maintenance and perhaps the differentiation of certain lymphoid progenitors by secreting Cxcl12 and likely other factors13,17,39,40. Early lineage committed progenitors thus reside in an endosteal niche that is spatially and cellularly distinct from HSCs.
Perivascular regulators of HSCs
Given the localization of HSCs near blood vessels, it was critical to define the stromal cells surrounding the vessels and to test whether they promote HSC maintenance. Attention focused on the mesenchymal cells that surround blood vessels throughout the bone marrow. While mesenchymal stroma are likely to be heterogeneous, and the precise relationships between cells expressing various markers remain to be defined, perivascular mesenchymal cells that express CD146 in humans54 and Cxcl12-GFP33, Nestin-GFP63, full length Leptin receptor64, Prx-1-Cre62, Osterix-Cre62, and inducible Mx-1-Cre15 in mice all generate osteoblastic cells and all express factors that promote HSC maintenance. CXCL12-abundant ‘reticular’ (CAR) cells adjacent to sinusoids were first shown to co-localize with HSCs throughout the bone marrow 33. Ablation of Cxcl12-expressing bone marrow cells depletes HSCs as well as well as severely impairing the adipogenic and osteogenic capacity of bone marrow cells65. Human CD146+ skeletal stem cells also localize adjacent to sinusoids in the bone marrow and synthesize high levels of the HSC niche factors Stem Cell Factor (SCF) and CXCL1254.
This possibility that mesenchymal stem/stromal cells (MSCs) are part of the HSC niche was further supported by Frenette and colleagues who found that MSCs in the bone marrow express a Nestin-GFP transgene and localize around blood vessels throughout the bone marrow63. HSCs commonly localize adjacent to Nestin-GFP+ cells and the Nestin-GFP+ cells express high levels of Scf and Cxcl12. Moreover, Fibroblast Activation Protein (FAP) is expressed by bone marrow stromal cells with many characteristics of MSCs, including Cxcl12, Scf, PDGFRα and Sca-1 expression 66,67, and ablation of these FAP+ cells leads to bone marrow hypocellularity, anemia, and depletion of osteogenic cells 68,69. These studies provided strong evidence that MSCs are one component of a perivascular niche for HSCs.
Endothelial cells also contribute to the perivascular HSC niche27. The earliest functional evidence supporting this possibility was the observation that conditional deletion of the gp130 cytokine receptor in endothelial cells led to bone marrow hypocellularity and a reduction in HSC numbers70. Inhibition of VEGFR2 signaling in irradiated mice using a blocking antibody impaired the regeneration of sinusoidal endothelial cells and prevented the recovery of LSK stem/progenitor cells as well as spleen colony-forming cells (CFU-S)37. Endothelial cells can promote HSC maintenance in culture71 and bone marrow sinusoidal endothelial cells promote long-term reconstituting HSC expansion in culture72,73. E-selectin has been suggested to be exclusively expressed by endothelial cells in the bone marrow and E-selectin deficiency renders HSCs more quiescent and resistant to irradiation74. These studies suggested that endothelial cells are one component of the HSC niche, but did not address whether they directly or indirectly regulate HSC maintenance in vivo.
To formally identify the niche cells, studies examined which cell populations were the key sources of factors that promote HSC maintenance in vivo. For example, SCF is non-cell-autonomously required for HSC maintenance in vivo 75–79. Differential splicing and proteolytic cleavage yield membrane-bound and soluble forms of SCF. HSCs are depleted in Sl/Sld mutant mice80, which express soluble SCF but not the membrane-bound form, indicating that the membrane-bound form is necessary for HSC maintenance81. Importantly, mice with a mixture of wild-type and Sl/Sld stromal cells only exhibit normal haematopoiesis in the immediate vicinity of the wild-type cells, demonstrating that SCF acts locally in creating the niche82. Since HSCs need cell-cell contact with the cells that synthesize SCF, the niche could be localized by identifying the key sources of SCF for HSC maintenance.
Analysis of the SCF expression pattern in Scfgfp knock-in mice revealed that Scf is expressed perivascularly, primarily around sinusoids throughout the bone marrow64. Leptin Receptor+ (Lepr) perivascular stromal cells expressed the highest levels of Scf and endothelial cells expressed lower levels. Gene expression profiling suggested that these Lepr-expressing perivascular cells were mesenchymal. Scf-GFP expression could not be detected in osteoblasts or in haematopoietic cells. Conditional deletion of Scf from perivascular stromal cells (Lepr-Cre), or endothelial cells (Tie2-Cre) depleted HSCs64. However, deletion of Scf from haematopoietic cells (Vav1-Cre), osteoblastic cells (Col2.3-Cre), and Nestin-expressing perivascular stromal cells (Nestin-Cre and Nestin-CreER) did not affect HSC frequency64. These results proved there is a perivascular niche for HSCs in which endothelial cells and mesenchymal cells promote HSC maintenance by synthesizing SCF (Fig. 3).
It has been proposed that the endosteal region and its osteoblastic cells provide a unique zone for the maintenance of quiescent HSCs. However, when Scf was conditionally deleted from both endothelial cells and perivascular mesenchymal cells in Lepr-Cre; Tie2-Cre; Scffl/− mice, 85% of all long-term multilineage reconstituting cells, including all serially transplantable HSCs and all HSCs in the most quiescent subpopulation, were eliminated20. Therefore, even the most primitive and quiescent HSCs are maintained by a perivascular niche. Whether there are functionally distinct perivascular niches in different regions of the bone marrow, such as in the endosteal region, remains an open question.
Are other key niche factors also synthesized primarily by perivascular cells? CXCL12 is a chemokine that is required for HSC maintenance and HSC retention in the bone marrow33,83–86. Global deletion of Cxcl12, or the gene that encodes its receptor, Cxcr4, depletes HSCs from the bone marrow33,83,87. CXCL12 is primarily expressed by perivascular mesenchymal stromal cells (CAR cells, Nestin-GFP, Lepr-Cre, or Prx-1-Cre expressing cells), with 100-fold lower levels of expression in endothelial cells and 1000-fold lower levels in osteoblasts17,33,62,88,89. Conditional deletion of Cxcl12 from perivascular mesenchymal cells using Prx1-Cre and Lepr-Cre depleted and mobilized HSCs, respectively 17,62. HSCs were depleted but not mobilized when Cxcl12 was conditionally deleted from endothelial cells (Tie2-Cre)17,62. HSC frequency and bone marrow retention were not affected when Cxcl12 was conditionally deleted from osteoblasts or their progenitors (Col2.3-Cre and Osx-Cre), haematopoietic cells (Vav1-Cre), or Nestin-Cre-expressing stromal cells17,62. These data confirmed that HSCs reside in a perivascular niche in which mesenchymal stromal cells and endothelial cells each synthesize multiple factors that promote HSC maintenance and localization.
While conditional deletion of Scf and Cxcl12 with Nestin-Cre and Nestin-CreER did not have any effect on HSC frequency17,64, Nestin-GFP+ perivascular cells are almost certainly part of the HSC niche63. Each of these Nestin alleles are transgenes with different expression patterns in the bone marrow64. Nestin-Cre appears not to be expressed in the bone marrow and Nestin-CreER exhibits very limited perivascular expression that does not resemble the Scf-GFP, Cxcl12-DsRed, Nestin-GFP, or Nestin-Cherry expression patterns64. However, Nestin-GFP expression strongly overlaps with LepR-Cre expression by perivascular cells throughout the bone marrow64,67,90. Thus, it is likely that Nestin-GFP+ perivascular MSCs are a component of the HSC niche even though Nestin-Cre mediated deletion of Scf or Cxcl12 did not deplete HSCs. Going forward, it will be useful to identify other Cre alleles that are specifically expressed in Nestin-GFP+ cells to compare their function to other perivascular stromal cells.
Complexity of the perivascular HSC niche
Endothelial cells and mesenchymal stromal cells are not the only cell types that regulate the perivascular HSC niche (Fig. 3). The sympathetic nervous system regulates CXCL12 expression and HSC retention in the bone marrow 91,92. It appears that this is accomplished by sympathetic nerve fibers that synapse upon perivascular cells around a subset of blood vessels in the bone marrow, conferring circadian regulation of CXCL12 expression and HSC mobilization. Circadian oscillation in the clearance of aged neutrophils by macrophages in the bone marrow also contributes to these circadian changes in CXCL12 expression and HSC circulation93. Consistent with this, macrophages modulate CXCL12 expression by Nestin-GFP+ cells and HSC retention in the bone marrow 94,95. Non-myelinating Schwann cells appear to regulate the niche by regulating TGFβ activation and potentially by secreting other factors 96. Osteoclasts, or osteoclast activity at the endosteum may also influence HSC maintenance and bone marrow retention 56,97,98. Many different cell types are likely to directly or indirectly regulate the perivascular HSC niche.
Given the complexity of cell types implicated in the regulation of HSCs, there is no singular niche cell. Rather, the niche integrates the function of multiple participants. It is important to bear in mind that niche composition and niche function may change under different physiological conditions or in response to stress. It is also important to note that many of the Cre recombinase alleles used so far to study niche cells were active during development. While this was necessary to achieve efficient gene deletion (temporally regulated CreER alleles tend to give much lower levels of recombination) and no abnormalities in development were noted, indirect effects on surrounding cell types and compensatory changes cannot be excluded. Although endothelial cells and perivascular mesenchymal cells express SCF and CXCL12, conditional deletion of these factors from these cell types may have direct and indirect effects on HSCs.
There may also be long-range signals circulating through the blood that regulate HSC/niche function, perhaps integrating stem cell activity with overall physiology99. These may include hormones that signal reproductive or nutritional status, or even haematopoietic cytokines. For example, Thrombopoietin is required for HSC maintenance 100–103. The major sites of Thrombopoietin synthesis are in the liver and kidney, though it is also synthesized at lower levels by bone marrow stroma104,105. Conditional deletion experiments will be required to determine the physiologically important source(s) of Thrombopoietin for HSC maintenance.
There may also be functionally distinct perivascular environments in the bone marrow based on vessel type. Most studies of perivascular niches in the bone marrow have focused on sinusoids because they are the most abundant blood vessels in the bone marrow and most HSCs, MSCs, Scf-expressing cells, and Cxcl12-expressing cells are in close proximity to them 17,27,33,54,64. However, other kinds of blood vessels, such as arterioles, may play an important role in HSC maintenance. Indeed, a recent study suggests that NG2+ but Lepr negative mesenchymal cells that surround arterioles in the bone marrow are important for the maintenance of quiescent HSCs106. This conclusion was based on the observation that HSCs were depleted and driven into cycle when NG2-CreER+ cells were ablated by treatment with diphtheria toxin. However, these data would appear to conflict with the prior observation that quiescent HSCs are eliminated from the bone marrow when Scf is conditionally deleted using Tie2-Cre and Lepr-Cre, which recombine in endothelial cells and mesenchymal cells that are primarily around sinusoids throughout the bone marrow 20. It will thus be interesting to determine whether HSCs are depleted when Scf is conditionally deleted using NG2-CreER or whether NG2-CreER is expressed by cells other than periarteriolar cells in the bone marrow. Similarly, it will be important to assess whether Lepr-expressing perivascular cells contribute to arteriolar niches in the bone marrow. In the end, perivascular niches associated with both sinusoids and arterioles may regulate HSC maintenance and quiescence in the bone marrow. Dissecting the diversity in perivascular environments will require Cre alleles that are specifically expressed within distinct perivascular domains to map their functions.
Evidence has been presented that HSCs reside within relatively hypoxic domains within the bone marrow 31,107. This has been based partly on staining with pimonidazole31.. Pimonidazole stained HSCs often reside adjacent to sinusoids in the bone marrow and are found next to cells that do not stain with pimonidazole31. This suggests that pimonidazole staining does not reflect ambient oxygen or that it is cell-autonomously determined, rather than reflecting a hypoxic environment. Pimonidazole responds to reducing intermediates and may reflect more about the metabolic state of cells than ambient oxygen levels.
The dependence of HSC maintenance upon HIF-1α has also been interpreted to suggest that HSCs are maintained in a hypoxic niche108. However, a number of factors other than hypoxia regulate HIF-1α expression. A recent imaging study using a nanoprobe specifically reflective of ambient oxygen found that oxygen tension was lowest around sinusoids and highest near the endosteum (personal communication, Charles Lin). The entire marrow space was much reduced in oxygen compared with vessels entering the marrow, a feature largely lost when haematopoiesis was ablated by cytotoxic drugs. It is therefore likely that consumption of oxygen during haematopoiesis renders the marrow hypoxic but that no distinct hypoxic region exists at the endosteum.
Distinct haematopoietic progenitors have distinct niches
HSCs reside within a specialized niche that is distinct from the niches that nurture other haematopoietic progenitors. For example, while osteoblasts do not directly regulate HSC maintenance, they do regulate some B-lineage progenitors. Cultures enriched for osteoblasts support B lymphopoiesis and ablation of osteoblasts in adult mice acutely depletes common lymphoid progenitors (CLPs) 40,109(Scadden, unpublished data). Deletion of Gs-alpha in osteoblastic cells, which is necessary for PTHr signaling, markedly depleted pro- and pre-B cells in a manner that could be rescued with IL-7110. Approximately 30% of IL7R+Lineage- bone marrow cells, which are enriched for early lymphoid progenitors, localize immediately adjacent to bone lining cells at the endosteum17. Conditional deletion of CXCL12, a factor that promotes the proliferation and maintenance of B lineage progenitors84,111 and CLPs112, in osteoblastic cells depleted CLPs and certain other early lymphoid progenitors from the bone marrow without any effect on HSCs17. Therefore, some early lymphoid progenitors depend upon an osteoblastic niche that is cellularly and functionally distinct from the perivascular niche that maintains HSCs.
Other lineage-restricted niches may exist as well. For example, macrophages appear to be critical for erythroid maturation and macrophage depletion reduces normal and malignant erythropoiesis113. Other cellular components of the erythropoiesis niche will have to be identified to understand the relationship between this niche and HSC and lymphoid progenitor niches.
The approach of conditionally deleting specific niche factors from candidate niche cells and then examining the consequences for stem/progenitor cell maintenance in vivo offers the opportunity to map the niches for each stem cell and restricted progenitor in the haematopoietic system, limited only by the precision of the Cre alleles that are available.
Novel niche factors
In contrast to stem cells in a number of other tissues, HSCs cannot be sustainably expanded in culture. This has impeded the ability to safely and effectively transplant HSCs in certain clinical contexts, such as during gene therapy, in which it would be useful to expand transfected HSCs in culture and then verify the quality of the transfected HSCs prior to transplantation. One possibility is that the inability to expand HSCs in culture reflects the existence of yet-unidentified growth factors that are synthesized by the niche in vivo.
Some HSC niche factors have only recently been discovered. Addition of Pleiotrophin to culture promotes HSC maintenance 114 and Pleiotrophin deficiency is associated with HSC depletion and impaired haematopoietic regeneration after myelosuppression 115. Pleiotrophin is synthesized by sinusoidal endothelial cells and Cxcl12-expressing perivascular stromal cells and acts non-cell-autonomously to promote HSC function115. Robo4, a Slit receptor expressed by HSCs and endothelial cells, regulates HSC localization in the bone marrow 116,117. The Slit2 ligand is restricted to MSCs and possibly other osteoblast lineage cells. This suggests that Pleiotrophin and Robo4/Slit2 are important elements of the perivascular niche. Tenascin-C118, osteopontin119,120, and non-canonical Wnts25 have also been reported to positively or negatively influence HSC numbers in the bone marrow and are among a number of factors that bear further characterization in terms of cellular source or role with respect to the niche.
Perspective
Ten years of experimentation has validated the niche concept and resolved some first order questions about the molecular and cellular nature of the HSC niche in the bone marrow. The ‘parts’ list remains incomplete, but with the pace of current work it is likely that additional components will be defined and ambiguity about overlapping cell populations resolved over the next several years. This will make it possible to compare anatomically and developmentally distinct HSC niches that have different functions. HSCs expand in number daily within the fetal liver but are sustained at nearly constant levels in the bone marrow, at least in the absence of injury. How components of these niches compare may inform methods for achieving HSC expansion. Similarly, comparing homologous niches among species, such as long-lived humans versus short-lived mice, may provide insight into mechanisms for preserving the integrity of haematopoiesis under stress or in response to aging. Finally, comparing niches among tissues will assess whether the mesenchymal and endothelial populations in brain, gut, and skin share characteristics and functions with those defined in the bone marrow. Do diverse adult tissues consistently have perivascular niches for stem cell maintenance? Do regenerative tissues have niches with common mechanisms for preserving self-renewal? Are there common components that can be engineered into niches ex vivo?
With the detail now emerging in our understanding of the bone marrow niche, a number of second order questions can be addressed. Niche cells can increasingly be genetically tagged or modified, enabling both quantification and molecular manipulation. Coupled with high resolution real-time imaging and well-validated methods to measure haematopoiesis, it is becoming possible to systematically elucidate how the niche responds to stresses or physiological changes to mediate changes at the stem cell and tissue levels. When stressed by infection, myeloablation, or neoplasia, what niche components change in number or function to modify haematopoiesis? Is there a hierarchy of niche components that determine these responses? Can such information enable predictive algorithms that guide specific interventions to achieve desired outcomes?
Another set of questions concerns the manner in which the niche participates in diseases of stem cell failure, such as aplastic anemia or neoplasia. The niche may be hostile to normal progenitors in those disease states and, with neoplasia, undergo a facultative response to support altered haematopoiesis121. Can changes in the niche be a primary but non-cell-autonomous driver of neoplasia in humans as has been suggested by animal models42,122,123? The potential for unraveling how the microenvironment participates in normal and disease physiology is at hand and promises new approaches to haematologic disorders.
Acknowledgments
SJM was supported by the National Heart, Lung and Blood Institute (HL097760), the Howard Hughes Medical Institute, and the Mary McDermott Cook Chair in Pediatric Genetics. DTS was supported by the National Institutes of Health (HL044851, HL096372, EB014703) and the Gerald and Darlene Jordan Chair in Medicine. We apologize to authors whose work could not be cited due to space limitations.
References
- 1.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 2.Haeckel EHPA. Generelle Morphologie der Organismen : allgemeine Grundzüge der organischen Formen-Wissenschaft, mechanisch begründet durch die von C. Darwin reformirte Decendenz-Theorie. 1866. [Google Scholar]
- 3.Haeckel E. The Riddle of the Universe (Die Weltraetsel, 1895–1899) Prometheus Books; 1901. 1992 Reprint Edition edn. [Google Scholar]
- 4.Pappenheim A. Virchows Arch. 1896;145:587–643. [Google Scholar]
- 5.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 6.Siminovitch L, McCulloch EA, Till JE. The Distribution of Colony-Forming Cells among Spleen Colonies. J Cell Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 7.Siminovitch L, Till JE, McCulloch EA. Decline in Colony-Forming Ability of Marrow Cells Subjected to Serial Transplantation into Irradiated Mice. J Cell Physiol. 1964;64:23–31. doi: 10.1002/jcp.1030640104. [DOI] [PubMed] [Google Scholar]
- 8.Jones RJ, et al. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 9.Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 10.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. Articulation of the niche hypothesis. [PubMed] [Google Scholar]
- 11.Dexter TM, Allen TD, Lajha LG. Conditions controlling the proliferation of hemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 12.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- 13.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. Identification of heterologous cells influencing stem/progenitor cells in mammals, providing experimental evidence for the niche hypothesis. [DOI] [PubMed] [Google Scholar]
- 15.Park D, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. Identification of heterologous cells influencing stem/progenitor cells in mammals, providing experimental evidence for the nich hypothesis. [DOI] [PubMed] [Google Scholar]
- 17.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. Systematic analysis of CXCL12 expressing cells in the bone marrow demonstrating that stem cells and restricted progenitors depend upon cellularly distinct niches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Oguro H, Ding L, Morrison SJ. SLAM Family Markers Resolve Functionally Distinct Subpopulations of Hematopoietic Stem Cells and Multipotent Progenitors. Cell stem cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science (New York, N Y. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 24.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Sugimura R, et al. Noncanonical wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiel MJ, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. Definition of an immunophenotypic signature for highly enriched stem cells that permitted histologic mapping of HSC within the bone marrow and suggested the existence of a perivascular niche. [DOI] [PubMed] [Google Scholar]
- 28.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. jem.20091318 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell stem cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature cell biology. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. Identification of a perivascular stromal cell that promoted HSC maintenance. [DOI] [PubMed] [Google Scholar]
- 34.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science (New York, N Y. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 35.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. Evidence that subregions of the microvasculature express high levels of Cxcl12 where transplanted hematopoietic progenitors localize and increase in number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2008 doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 37.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell stem cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. Evidence that sinusoidal endothelial cells have specialized features and are necessary for HSC engraftment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell stem cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, et al. Osteoblasts support B lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 41.Lymperi S, et al. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 42.Raaijmakers MH, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. Demonstration that perturbing specific mesenchymal populations in the bone marrow can result in pathologic hematopoietic outcomes including neoplasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosokawa K, et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 44.Hosokawa K, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell stem cell. 2010;6:194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Wilson A, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Zon LI. Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell stem cell. 2010;6:199–202. doi: 10.1016/j.stem.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Ivanova NB, et al. A stem cell molecular signature. Science (New York, N Y. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 48.Seita J, et al. Gene Expression Commons: an open platform for absolute gene expression profiling. PLoS One. 2012;7:e40321. doi: 10.1371/journal.pone.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012 doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bromberg O, et al. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120:303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guezguez B, et al. Regional Localization within the Bone Marrow Influences the Functional Capacity of Human HSCs. Cell stem cell. 2013;13:175–189. doi: 10.1016/j.stem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. Evidence for a mesenchymal skeletal stem cell that is capable of generating bone, secreting HSC niche factors, and giving rise to bone marrow compartments that include HSC niches. [DOI] [PubMed] [Google Scholar]
- 55.Ellis SL, et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–1524. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- 56.Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 57.Dai JC, He P, Chen X, Greenfield EM. TNFalpha and PTH utilize distinct mechanisms to induce IL-6 and RANKL expression with markedly different kinetics. Bone. 2006;38:509–520. doi: 10.1016/j.bone.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaniel C, et al. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118:2420–2429. doi: 10.1182/blood-2010-09-305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferraro F, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Science translational medicine. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asada N, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell stem cell. 2013;12:737–747. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. Systematic analysis of CXCL12 expressing cells in the bone marrow demonstrating the specific role of primitive mesenchymal cells and endothelial cells in regulating HSC maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. Evidence that primitive mesenchymal cells that reside perivascularly regulate HSC maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. Systematic analysis of KitL expression in the bone marrow demonstrating the requirement for endothelial and leptin receptor-expressing perivascular cells in regulating HSC maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 66.Morikawa S, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. The Journal of experimental medicine. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinho S, et al. PDGFRalpha and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran E, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. The Journal of experimental medicine. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts EW, et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. The Journal of experimental medicine. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106:4093–4101. doi: 10.1182/blood-2005-02-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Experimental hematology. 2004;32:1226–1237. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell stem cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 75.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 76.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science (New York, NY. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogawa M, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 79.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barker JE. Sl/Sld hematopoietic progenitors are deficient in situ. Experimental hematology. 1994;22:174–177. [PubMed] [Google Scholar]
- 81.Barker JE. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Experimental hematology. 1997;25:542–547. [PubMed] [Google Scholar]
- 82.Wolf NS. Dissecting the hematopoietic microenvironment. III. Evidence for a positive short range stimulus for cellular proliferation. Cell Tissue Kinet. 1978;11:335–345. [PubMed] [Google Scholar]
- 83.Tzeng YS, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 84.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 85.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 86.Ara T, et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 87.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 88.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. The Journal of clinical investigation. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dar A, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 90.Hanoun M, Frenette PS. This niche is a maze; an amazing niche. Cell stem cell. 2013;12:391–392. doi: 10.1016/j.stem.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katayama Y, et al. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. Evidence for nervous system involvement in regulating the bone marrow HSC niche. [DOI] [PubMed] [Google Scholar]
- 92.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. Demonstration that neural circadian rhythms modulate HSC function. [DOI] [PubMed] [Google Scholar]
- 93.Casanova-Acebes M, et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 96.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 97.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 98.Mansour A, et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. The Journal of experimental medicine. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakada D, Levi BP, Morrison SJ. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 2011;70:703–718. doi: 10.1016/j.neuron.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qian H, et al. Critical roleof thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell stem cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Yoshihara H, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 102.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proceedings of the National Academy of Sciences USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaushansky K. Thrombopoietin and the hematopoietic stem cell. Blood. 1998;92:1–3. [PubMed] [Google Scholar]
- 104.Guerriero A, et al. Thrombopoietin is synthesized by bone marrow stromal cells. Blood. 1997;90:3444–3455. [PubMed] [Google Scholar]
- 105.Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89:101–107. [PubMed] [Google Scholar]
- 106.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013 doi: 10.1038/nature12612. In Press. Histologic characterization of subtypes of vascular structures and evidence that peri-arteriolar mesenchymal cells maintain HSC quiescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 109.Visnjic D, et al. Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res. 2001;16:2222–2231. doi: 10.1359/jbmr.2001.16.12.2222. [DOI] [PubMed] [Google Scholar]
- 110.Wu JY, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proceedings of the National Academy of Sciences USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. The Journal of experimental medicine. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chow A, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Himburg HA, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith-Berdan S, et al. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell stem cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith-Berdan S, Schepers K, Ly A, Passegue E, Forsberg EC. Dynamic expression of the Robo ligand Slit2 in bone marrow cell populations. Cell Cycle. 2012;11:675–682. doi: 10.4161/cc.11.4.19146. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura-Ishizu A, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119:5429–5437. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stier S, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. The Journal of experimental medicine. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nilsson SK, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 121.Schepers K, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell stem cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walkley CR, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]