Abstract
Background
Pharmacy refill adherence assesses medication-filling behaviors whereas self-report adherence assesses medication-taking behaviors. We contrasted the association of pharmacy refill and self-reported antihypertensive medication adherence with cardiovascular disease (CVD) incidence.
Methods and Results
Adults (n=2075) from the prospective Cohort Study of Medication Adherence among Older Adults (CoSMO) recruited between August 2006 and September 2007 were included. Antihypertensive medication adherence was determined using a pharmacy refill measure, Medication Possession Ratio-MPR (low, medium, high MPR: <0.5, 0.5 to <0.8, ≥0.8, respectively) and a self-reported measure, 8-item Morisky Medication Adherence Scale-MMAS-8 (low, medium, high MMAS-8: <6, 6 to <8, and 8, respectively). Incident CVD events (stroke, myocardial infarction, congestive heart failure, or CVD death) through February 2011 were identified and adjudicated. The prevalence of low, medium and high adherence was 4.5 %, 23.7%, and 71.8% for MPR and 14.0%, 34.3%, and 51.8% for MMAS-8. During a median 3.8 years follow-up, 240 (11.5%) people had a CVD event. After multivariable adjustment and compared to those with high MPR, the hazard ratios (HR) for CVD associated with medium and low MPR were 1.17 (95% confidence interval [CI] 0.87, 1.56) and 1.87 (95% CI: 1.06, 3.30), respectively. Compared to those with high MMAS-8, the HRs (95% CI) for MMAS-8 for medium and low MMAS-8 were 1.04 (0.79–1.38) and 0.89 (0.58–1.35), respectively.
Conclusions
Pharmacy refill but not self-report antihypertensive medication adherence was associated with incident CVD. The differences in these associations may be due to distinctions in what each adherence measure assesses.
Keywords: hypertension, cardiovascular disease, medication adherence, Medication Possession Ratio, MMAS-8
INTRODUCTION
Hypertension is a common risk factor for cardiovascular disease (CVD) morbidity and mortality 1. Effective medical therapies exist to lower BP and reduce the risk for CVD; yet, suboptimal adherence persists as a major public health challenge with approximately 50% of patients not taking chronic medications as prescribed 2, 3. Poor adherence to antihypertensive therapy has been associated with worse BP control, increased hospitalization rates, higher health care costs and lower survival 3–9.
To address this public health challenge, it is important to consider that adherence to prescribed medications includes 2 key patient behaviors: 1) filling medications and 2) taking medications as prescribed after they are filled. Although these behaviors are related, they may reflect distinct activities which may differ among individual patients11–13. Pharmacy refill adherence assesses whether patients fill their medications over specified time intervals whereas self-reported adherence assesses whether patients take their medications after they are filled. Prior studies have demonstrated poor adherence measured by pharmacy refill in hypertensive patients to be associated with an increased risk for CVD4–8, 14. Few studies have reported on the association of low adherence, assessed by a self-reported antihypertensive medication adherence scale, on increased risk for CVD events10. To our knowledge no large study has examined differences in the association between antihypertensive medication adherence and CVD events using both validated objective and multi-item self-reported adherence measures.
In this study, we sought to determine the prospective association of pharmacy refill and, separately, self-reported antihypertensive medication adherence, with CVD events in older adults. We used a well-established objective measure of pharmacy refill adherence, the Medication Possession Ratio (MPR) and a validated multi-item self-report measure widely used in national and international research settings3, 15, 16, the Morisky Medication Adherence Scale 8-item (MMAS-8).
METHODS
Study Population and Study Design
The Cohort Study of Medication Adherence in Older Adults (CoSMO) is a prospective cohort study of factors associated with antihypertensive medication adherence and CVD outcomes in elderly adults with established hypertension. The study design, response rates, and baseline characteristics have been published previously 3. In brief, women and men aged 65 years or older with essential hypertension were randomly selected from the roster of a large managed care organization in southeastern Louisiana. From August 21, 2006 to September 30, 2007, 2,194 participants were recruited and completed the baseline survey 3. The participants were followed through February 2011 to identify CVD events and mortality. The current analyses were limited to participants who had no hospitalizations for stroke, myocardial infarction (MI), or congestive heart failure (CHF) in the year prior to the administration of the baseline survey (N=2,075). CoSMO was approved by the Ochsner Clinic Foundation’s Institutional Review Board and the privacy board of the managed care organization 3.
Data Collection Overview
Using an established conceptual framework of risk factors associated with antihypertensive medication adherence and clinical outcomes, we obtained data through participant surveys, medical records, administrative databases of the managed care organization, and vital records17 (details below). Surveys were administered via telephone by trained interviewers.
Primary Exposures
The primary exposure was antihypertensive medication adherence 3, 17. Medication adherence was measured objectively using pharmacy refill data to calculate the Medication Possession Ratio (MPR). For the main analyses, data were extracted for the year prior to the baseline survey from pharmacy utilization databases and included all antihypertensive prescriptions filled, date filled, drug class, and number of pills dispensed used to calculate days’ supply. The MPR is the sum of the days' supply obtained between the first pharmacy fill and the last refill, with the supply obtained in the last refill excluded, divided by the total number of days in this time period 18. In this population of patients with treated established hypertension, two pharmacy refills in a drug class in the one year time period were required. MPR was calculated for each antihypertensive medication class and averaged across all classes to assign a single MPR to each participant for the year19; MPR values > 1 were truncated at 1.0. Low, medium and high MPR adherence were defined as <0.5, 0.5 to <0.8 and ≥0.8, respectively 20, 21.
Self-reported medication adherence was assessed using the eight-item Morisky Medication Adherence Scale (MMAS-8) 19, 22; MMAS-8 was captured at baseline and during annual follow up surveys. For the main analyses, MMAS-8 collected during the baseline survey was used. This adherence measure was designed to facilitate the identification of barriers to, and behaviors associated with, adherence to chronic medications. In prior studies, the scale has been determined to be reliable 22, significantly associated with blood pressure control 3, 22, and modestly associated with pharmacy refill 19. Overall, 99.4% of participants completed all 8 items of the scale in the baseline survey. The remaining 0.6% (n=12) of participants completed 6 or 7 of the 8 items on the MMAS-8 and the missing items were generated using the CoSMO sample median score for the item. Using previously published cut points, low, medium, and high MMAS-8 adherence were defined as scores of <6, 6 to <8, and 8, respectively 22.
Outcome variables
Composite CVD Outcome
The primary outcome was the composite CVD endpoint of stroke, MI, CHF or CVD death. All outcomes were ascertained through February 28, 2011. Data were collected on hospitalizations for CVD events and mortality. Hospitalizations for CVD events were determined by a combination of review of administrative records, medical record review and physician adjudication. A comprehensive search of primary and secondary International Classification of Diseases, Ninth Revision (ICD9) codes from administrative claims databases was used to identify hospitalizations for MI: codes 410.xx (except 410.x2); CHF: codes 402.x1, 428.xx, and stroke: codes 430.xx, 431.xx, 432.xx, 433.xx, 434.xx that occurred over the follow-up period. Deaths occurring over the follow-up period were first identified via searches of the Social Security Death Index and cross-checked against administrative claims databases and obituaries. For each identified death, death certificates were obtained from the respective health departments and cause of death (CVD or non-CVD) was recorded. When possible, supplemental data were abstracted from medical records to confirm cause of death. Then, trained research nurses abstracted information from medical records and death certificates for each CVD event onto standardized forms23. For hospitalizations occurring outside of the system, a patient release was obtained prior to requesting and reviewing the medical record. Medical information for each abstracted event was reviewed independently by two physician adjudicators (EDF, RNR) who were blinded to participant adherence status. If both adjudicators agreed on the outcome classification, it was binding. If there was a disagreement, they conferred, reconsidered their classification and could request consultation from a third independent and blinded adjudicator. In all cases, conflicting opinions among the adjudicators were resolved after conferment.
Socio-demographics, Clinical and Behavioral Variables
Based on an established conceptual model17, information was collected from participants’ surveys, the medical record, and claims databases of the managed care organization. Sociodemographic and clinical characteristics obtained from the participant survey included marital status, age, sex, race, educational attainment, height and weight, depressive symptoms, and duration of hypertension. Body mass index was calculated as weight (kg)/height (m)2. Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression Scale 24. Self-reported healthy lifestyle behaviors included nonsmoking status, less than 2 alcoholic drinks per week, and use of lifestyle modifications (exercise, salt reduction, fruit and vegetable consumption)25 to lower blood pressure. Claims data were used to calculate the Charlson Comorbidity Index 26, 27 and to determine the number of classes of antihypertensive medication filled by each participant.
Blood Pressure (BP)
Using standardized forms, trained research staff (blinded to participant adherence category) recorded seated systolic and diastolic BP measurements from medical records for clinic visits occurring during the year before the baseline survey and after the baseline survey through February 2011 or a CVD event (follow-up), whichever came first. BP levels were averaged for visits when more than one measurement was taken. Then, the average BP level across all visits during baseline and, separately, during follow up was calculated 3. Uncontrolled BP was defined as mean systolic BP ≥140 mm Hg or diastolic BP ≥ 90 mm Hg28.
Statistical Analysis
Participant characteristics were calculated overall and by level of adherence on each measure (MPR and MMAS-8, separately). The statistical significance of trends across adherence levels was determined using Cochran-Armitage trend tests. The association of adherence (MPR and MMAS-8, separately) with uncontrolled BP during follow-up was determined using logistic regression models. Cumulative incidence of CVD events was calculated by level of adherence using the Kaplan-Meier method. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of CVD associated with low and medium versus high adherence. Separate models were used for MPR and MMAS-8. Initial models were adjusted for age, sex, race, marital status, and education, and subsequent models were further adjusted using the Charlson Comorbidity Index, number of classes of antihypertensive medication, body mass index, depressive symptoms, and healthy lifestyle behaviors. Differences in the association between antihypertensive medication adherence and the composite CVD outcome across subgroups defined by race and sex were evaluated by including an interaction term in final multivariable regression models (e.g. MPR * race). Analyses were performed using SAS version 9.2 (SAS Institute, Cary NC).
Sensitivity Analyses
In sensitivity analyses, the HRs for CVD associated with MMAS-8 and MPR each modeled using a single cut-point (MMAS-8 <8 versus 8 and MPR <0.8 versus ≥ 8) were calculated. Also, the HRs for CVD associated with MMAS-8 and MPR modeled as continuous variable (log transformed) were calculated. To investigate whether differences in CVD risk by adherence measure could be explained by differing definitions of low MMAS-8 adherence, we used alternate cut-points to define low, medium, and high MMAS-8 (<4.5, 4.5 to <7, and ≥7 to 8, respectively). This strategy resulted in a similar percentage of participants with low adherence by MMAS-8 and MPR. We also assessed the association with CVD incidence of two measures of MMAS-8 averaged over 1 year. For this analysis, we restricted the sample to participants who completed baseline and first follow up MMAS-8, had pharmacy refill data in the year prior to the first follow up survey, and did not have a CVD event in the time between the two surveys(n=1,690).
Lastly, prescription-based proportion of days covered (PDC) was calculated as an alternate measure of pharmacy refill29. For each antihypertensive medication class, we calculated PDC as the number of days with medication available divided by the number of days between the first and last pharmacy refill in the one year time period. An overall PDC for each participant was calculated as the average of the class-specific PDC.
Results
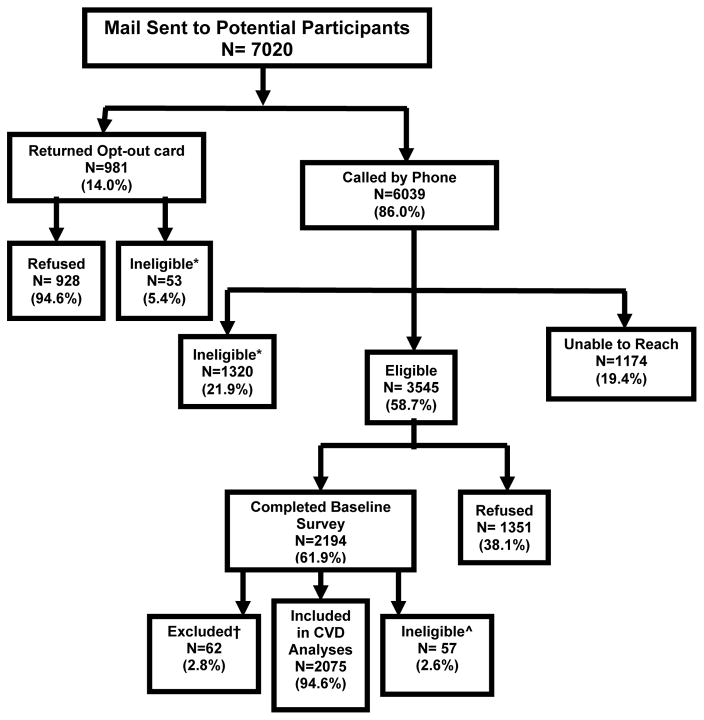
Of the 2,194 participants in the CoSMO cohort, 57 were hospitalized for a CVD event in the year prior to baseline and were not eligible for these analyses. There were 62 participants excluded from analyses because of missing pharmacy refill data (Figure 1). There were no differences in age, sex, race, education or duration of hypertension (p>0.05 for all comparisons) between those missing versus not missing pharmacy refill data. The mean age of the 2,075 participants included in the analyses was 75 years (standard deviation, 5.6), 30.4% were black, 59.8% were women, and 57.0% were married. Also, 62.9% had been diagnosed with hypertension for 10 or more years, 48.3% had 2 or more comorbid conditions, and 34.1% had uncontrolled hypertension (Table 1). Over a median follow-up of 3.8 years (maximum 4.8 years), 240 (11.6%) individuals had a CVD event (i.e., MI, stroke, CHF, or CVD death). Overall, 2.3% of the participants had a stroke event, 4.1% had a MI event, 6.2% had a CHF event, and 3.5% had a CVD death.
Figure 1. Recruitment and Follow up Flowchart for the Cohort Study of Medication Adherence among Older Adults (CoSMO).
Adapted from Krousel-Wood et al. Med Clin N Am 93:753–769, 2009
*Ineligible during the recruitment phase due to no confirmed diagnosis of hypertension (22.9%), hard of hearing (16.4%), too ill to complete survey (12.6%), deceased (11.5%), cognitive screen failure (11.1%), not currently prescribed antihypertensive medication (8.4%), no longer enrolled in managed care organization (6.9%), non-English speaker (5.8%), confined to a nursing home (1.9%), moved out of state (1.1%), current treatment for cancer (1%), or miscellaneous reason (<1%).
^ Ineligible in the follow up phase due to hospitalization for CVD outcome in the year prior to the baseline survey
†Reason for exclusion: missing Medication Possession Ratio (MPR) data in the year prior to the baseline survey
CVD-cardiovascular disease
Table 1.
Baseline Characteristics of the Study Participants according to Antihypertensive Medication Adherence Category
| Pharmacy Refill Adherence | Self-Reported Adherence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall | MPR <0.5 | MPR 0.5-<0.8 | MPR ≥0.8 | P-value* | MMAS-8 <6 | MMAS-8 6 to <8 | MMAS-8 =8 | P-value† | |
| Total Number | 2,075 | 93 | 492 | 1490 | 290 | 711 | 1074 | ||
|
| |||||||||
| Age ≥75 years, n(%) | 1012 (48.8) | 46(49.5) | 238(48.4) | 728(48.9) | 0.958 | 118(40.7) | 361(50.8) | 533(49.6) | 0.047 |
| Female, n(%) | 1241 (59.8) | 60(64.5) | 298(60.6) | 883(59.3) | 0.314 | 188(64.8) | 408(57.4) | 645(60.1) | 0.458 |
| Black race, n(%) | 631 (30.4) | 47(50.5) | 200(40.7) | 384(25.8) | <0.001 | 114(39.3) | 231(32.5) | 286(26.3) | <0.001 |
| Married, n(%) | 1182 (57.0) | 48(51.6) | 261(53.1) | 873(58.6) | 0.020 | 163(56.2) | 412(58.0) | 607(56.5) | 0.873 |
| High school education or greater, n(%) | 1652 (79.6) | 67(72.0) | 369(75.0) | 1216(81.7) | <0.001 | 232(80.0) | 559(78.6) | 861(80.2) | 0.686 |
| Hypertension duration ≥ 10 Years, n(%) | 1301 (62.9) | 48(52.2) | 302(61.8) | 951(64.0) | 0.036 | 171(59.2) | 451(63.6) | 679(63.5) | 0.279 |
| Charlson Comorbidity Index score ≥ 2 n (%)‡ | 1003 (48.3) | 43(46.2) | 278(56.5) | 682(45.8) | 0.004 | 145(50.0) | 357(50.2) | 501(46.7) | 0.160 |
| Body mass index: ≥ 30 kg/m2, n(%) | 1592 (76.8) | 75(80.7) | 387(78.8) | 1130(75.9) | 0.110 | 244(84.4) | 552(77.6) | 796(74.2) | <0.001 |
| 3+ classes of antihypertensive medication, n(%)‡ | 901 (43.4) | 14(15.1) | 225(45.7) | 662(44.4) | 0.001 | 124(42.8) | 316(44.4) | 461(42.9) | 0.833 |
| Depressive symptoms, n(%) | 267 (12.9) | 18(19.4) | 88(17.9) | 161(10.8) | <0.001 | 62(21.4) | 99(13.9) | 106(9.9) | <0.001 |
| Never a smoker, n(%) | 1024 (49.7) | 51(55.4) | 242(49.8) | 731(49.4) | 0.392 | 136(47.1) | 337(48.0) | 551(51.6) | 0.091 |
| <2 alcoholic drinks per week, n(%) | 1627 (78.7) | 81(88.0) | 393(80.4) | 1153(77.6) | 0.015 | 223(77.7) | 563(79.3) | 841(78.6) | 0.910 |
| Increasing fruits and vegetables, n(%) | 1412 (68.1) | 65(69.9) | 344(69.9) | 1003(67.3) | 0.285 | 189(65.2) | 487(68.5) | 736(68.5) | 0.376 |
| Exercising more, n(%) | 912 (44.0) | 38(40.9) | 212(43.1) | 662(44.4) | 0.427 | 115(39.7) | 317(44.6) | 480(44.7) | 0.208 |
| Reducing salt, n(%) | 1660 (80.0) | 72(77.4) | 396(80.5) | 1192(80.0) | 0.813 | 220(75.9) | 565(79.5) | 875(81.5) | 0.033 |
| Uncontrolled blood pressure, n(%) | 662 (34.1) | 36(41.9) | 181(38.7) | 445(32.0) | 0.003 | 115(42.4) | 241(36.4) | 306(30.3) | <0.001 |
P-value for comparison of characteristics among low, medium, and high MPR groups
P-value for comparison of characteristics among low, medium, and high MMAS-8 groups
- in the prior year
- High school education or greater, n=1(0.05%) missing
- Hypertension duration ≥ 10 Years, n=8 (0.39%) missing
- Charlson Comorbidity Index score ≥ 2, n=2 (0.10%) missing
- Body mass index: ≥ 30 kg/m2, n=2 (0.10%) missing
- Never smoker, n=16 (0.77%) missing
- <2 alcoholic drinks per week, n=8 (0.39%) missing
- Uncontrolled blood pressure, n=131 (6.31%) missing
Pharmacy Refill Adherence
The prevalence of low, medium and high antihypertensive medication adherence was 4.5%, 23.7%, and 71.8%, respectively, for MPR. Participant characteristics by MPR are shown in the left panel of Table 1. Participants with worse medication adherence by MPR were more likely to be black and have fewer than 2 alcoholic drinks per week, depressive symptoms, and uncontrolled blood pressure at baseline. They were less likely to be married, have at least a high school education, be taking 3 or more classes of antihypertensive medications, have Charlson comorbidity ≥ 2, and have hypertension ≥ 10 years. Lower adherence by MPR at baseline was associated with higher odds ratio of uncontrolled blood pressure during follow-up: 1.55 (95% CI 1.23, 1.96) for medium versus high MPR and 2.06 (95% CI 1.26, 3.36) for low versus high MPR.
The proportion of the sample with a CVD event was 16.1%, 13.4% and 10.7% among those with low, medium and high MPR (Figure 2a and Table 2). After multivariable adjustment and compared to those with high MPR, the HR (95% CI) for the composite CVD outcome associated with medium and low MPR were 1.17 (0.87, 1.56) and 1.87 (1.06, 3.30), respectively (Table 2). The associations between adherence by MPR and the composite CVD outcome for MPR were consistent across race and sex groups (Supplemental Table 1; P-interaction for race = 0.801, for sex = 0.339).
Figure 2. Cumulative incidence of Cardiovascular Disease Outcome by Level of Antihypertensive Medication Adherence at Baseline.
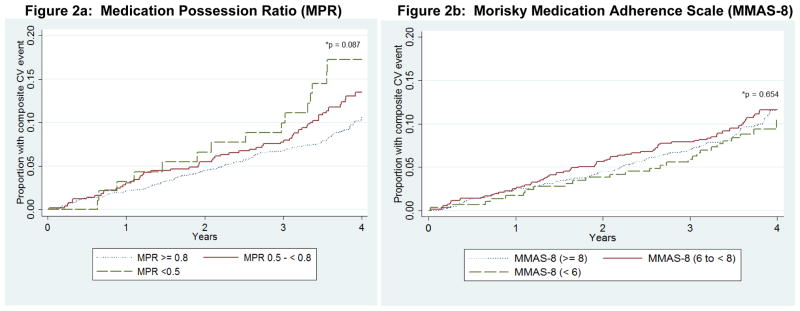
Figure 2a: Medication Possession Ratio (MPR)
Figure 2b: Morisky Medication Adherence Scale (MMAS-8)
*P-value for Log-Rank test
CVD-Cardiovascular Disease
MPR—Medication Possession Ratio
MMAS-8—Morisky Medication Adherence Scale 8-item
Composite CVD outcome-myocardial infarction, congestive heart failure, stroke, or cardiovascular death
Table 2.
Unadjusted and Adjusted Hazard Ratios for the Cardiovascular Disease Outcome associated with Antihypertensive Medication Adherence
| n(%) with CVD outcome | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | |
|---|---|---|---|---|---|
| Overall | 240(11.6) | ||||
|
| |||||
| Pharmacy Refill | |||||
| Low (MPR <0.5) | 15 (16.1) | 1.58 (0.93,2.68 ) | 1.70 (0.99,2.90) | 2.06 (1.19,3.56)† | 1.87 (1.06,3.30)* |
| Medium (MPR 0.5-<0.8) | 66 (13.4) | 1.29 (0.96,1.72) | 1.31 (0.98,1.75) | 1.16 (0.87,1.56) | 1.17 (0.87,1.56) |
| High (MPR ≥0.8) | 159 (10.7) | 1(reference) | 1(reference) | 1(reference) | 1(reference) |
|
| |||||
| Self-Reported Adherence | |||||
| Low (MMAS-8 <6) | 30 (10.3) | 0.90 (0.60, 1.36) | 0.99 (0.66,1.48) | 0.91 (0.61,1.37) | 0.89 (0.58,1.35) |
| Medium (MMAS-8 6 to <8) | 88 (12.4) | 1.09 (0.83,1.43) | 1.08 (0.82,1.42) | 1.03 (0.78,1.36) | 1.04 (0.79,1.38) |
| High (MMAS-8 =8) | 122 (11.4) | 1(reference) | 1(reference) | 1(reference) | 1(reference) |
p<0.05,
p<0.01
HR-Hazards Ratio; 95% CI - 95% confidence interval
Composite CVD outcome-myocardial infarction, congestive heart failure, stroke, or cardiovascular death
CoSMO-Cohort Study of Medication Adherence among Older Adults
MPR-medication possession ratio
MMAS-8 – Morisky Medication Adherence Scale-8 item;
CVD-cardiovascular disease;
Model 1 adjusted for age, sex, race, marital status, and education.
Model 2 adjusted for age, sex, race, marital status, education, Charlson Comorbidity
Index, number of classes of antihypertensive medications, and depressive symptoms.
Model 3 adjusted for age, sex, race, marital status, education, Charlson Comorbidity Index, number of classes of antihypertensive medications, depressive symptoms, smoking status, body mass index, alcohol intake, and healthy lifestyles for blood pressure control (fruit and vegetable intake, exercise, and sodium reduction
Self-Reported Adherence-MMAS-8
The prevalence of low, medium and high antihypertensive medication adherence was 14.0%, 34.3%, and 51.8% for MMAS-8. Participant characteristics by category of MMAS-8 are shown in the right panel of Table 1. Participants with worse medication adherence by MMAS-8 were more likely to be black, and have a BMI ≥ 30 kg/m2, depressive symptoms, and uncontrolled blood pressure at baseline; they were less likely to be ≥ 75 years old and report reducing salt to control their blood pressure. Lower adherence by MMAS-8 at baseline was associated with uncontrolled blood pressure during follow-up with odds ratios (95% CI) of 1.15 (0.92, 1.45) for medium versus high MMAS-8, and 1.58 (1.17, 2.12) for low versus high MMAS-8.
In unadjusted analyses, there was no association between MMAS categories and CVD events (Figure 2b and Table 2). After multivariable adjustment and compared to those with high MMAS-8, the HRs (95% CI) for the composite CVD outcome associated with medium and low MMAS were 1.04 (0.79, 1.38) and 0.89 (0.58, 1.35), respectively. These results were consistent across race and sex subgroups (Supplemental Table 1; P-interaction for race = 0.093, for sex = 0.663).
Sensitivity Analyses
When adherence by MPR and MMAS-8 were separately evaluated for association with CVD events using a single cut-point, the results were qualitatively similar (adjusted HRs (95% CI) of 1.00 (0.78, 1.30) for MMAS-8 < 8 versus 8 and 1.32 (0.99, 1.75) for MPR <0.8 versus ≥0.8. When MPR was modeled as a log-transformed continuous variable, a 25% decrease in MPR was associated with a HR (95% CI) for CVD events of 1.04 (1.00–1.09; p value = 0.073). When MMAS-8 was modeled as a log-transformed continuous variable, a 25% decrease in MMAS-8 was associated with a HR (95% CI) of 1.21 (0.57-2.58; p value = 0.623) for CVD events.
When alternate cut-points were used to define low, medium and high adherence on the MMAS-8 (<4.5, 4.5 to <7, and ≥7 to 8, respectively), no association was present between MMAS-8 and CVD events (adjusted HRs (95% CI) 1.04 (0.78, 1.38) for 4.5 to ≥7 versus >7 and 1.57 (0.64, 3.86) for <4.5 versus >7). Averaging the two measures of MMAS-8 collected at baseline and first follow-up, no association was present between MMAS-8 and subsequent CVD. The adjusted HRs (95% CI) for those with medium and low compared to high MMAS-8 were 0.90 (0.65, 1.37) and 0.98 (0.56, 1.73), respectively.
When PDC was used as the measure of pharmacy refill adherence to assess the association with CVD outcome, the results were qualitatively similar to MPR (Supplemental Table 2).
Discussion
In the current study conducted in a real world setting of older insured patients with established hypertension, low adherence to antihypertensive medications identified using a pharmacy refill measure and self-report was significantly associated with uncontrolled blood pressure at baseline and during follow-up for both measures. Compared to their counterparts with high adherence, those with low adherence to antihypertensive medication, defined as an MPR <0.5, had a nearly 2-fold higher risk of a CVD event over a median follow up of 3.8 years. In contrast, there was no association between adherence measured by MMAS-8 and CVD. The results were consistent in sensitivity analyses. For pharmacy refill adherence, these findings are similar to prior studies revealing that pharmacy refill adherence is associated with lower risk of long-term adverse clinical outcomes in patients treated for hypertension 4–8, 14. However, fewer studies have contrasted pharmacy refill versus self-reported antihypertensive medication adherence on CVD outcomes.
Prior investigations exploring associations between self-reported adherence and CVD outcomes in older adults have yielded inconsistent results 30, 31. In outpatients treated for stable coronary artery disease, a single self-reported medication adherence question was associated with a greater than 2-fold increase in CVD events 30. In the Second Australian National Blood Pressure Study trial, participants reporting good adherence using a 4-item scale were marginally less likely to experience some types of cardiovascular events10. In contrast, Wu and colleagues found that self-report adherence using a similar single item was not associated with outcomes in patients treated for heart failure31, and discussed issues regarding reliability32, 33 and the potential for recall and social desirability biases34 with self-reported measures. Previous research has demonstrated reliability and validity of the MMAS-8 in its association with blood pressure 22, 35 and modest association between pharmacy refill and MMAS-8 19. An explanation for the differences in association between adherence and long-term outcomes using the 2 measures may reflect that MPR and MMAS-8 measure different aspects of adherence behavior which may vary within the same patient and differentially impact associations with long-term outcomes. MPR assesses medication filling behavior whereas MMAS-8 assesses medication-taking behavior (presumably after medications are obtained via pharmacy refill or via office samples) and provides information on reasons why patients may not be taking their medications. Thus, MMAS-8 reflects medication-taking determinants further down the chain of linked adherence behaviors (linked behaviors such as going to the doctor’s office for diagnosis and prescription, then filling prescriptions, and then taking the medications) 12, 36. In addition, although blacks and those with depressive symptoms were identified as low adherers by both measures, low adherers by MPR in this study were less likely to be married, to have higher education and to be taking three or more medications. These differences may be markers of poor health outcomes and contribute to CVD events. For example, marital status has been shown to confer health advantages in the general population and in those undergoing CVD procedures37. Differences in what each measure assesses and the characteristics of those identified as low adherers may have important implications when determining associations with long-term outcomes. Finally, our prior work revealed low incidence of declining adherence using MMAS-8 in older patients with established hypertension over 2 years of follow up (4.3% annual rate of decline in adherence)35. Pharmacy refill is calculated using monthly data points over twelve months and may be more reflective of adherence behavior over time.
Healthcare providers may benefit from having information on both self-report and pharmacy refill adherence behavior. During medical encounters, MMAS-8 is a quick way to identify which patients are adhering to prescribed therapies, to obtain important information on barriers to adherence that can be targeted for interventions to improve medication adherence and blood pressure control in the short-term, and to monitor change in adherence35. MPR can be used to identify those not filling prescriptions over time and assess risk of adverse outcomes in the long-term. Unlike MMAS-8, MPR does not provide information on barriers to adherence. Together, the objective and self-reported adherence measures provide complementary information that can guide appropriate engagement of patients and providers in the management of high blood pressure and other chronic conditions. Pharmacy refill measures may be particularly important for research studies and population management projects given the association between this measure and CVD events.
Study Limitations and Strengths
The current study was limited to English-speaking adults 65 years of age and older with health insurance in one region of the US and relatively high adherence rates, and thus, may not be generalizable to all persons with hypertension. Higher adherence in older versus younger adults have been reported15, 38–41. We report a similar prevalence of high adherence rates as a recent meta-analysis including 11 studies of patients taking antihypertensive medications (good adherence rates were 59% (95% CI 42% – 77%)14. Neither pharmacy refill nor self- reported adherence measures provide evidence that medications are actually taken correctly by patients, and primary nonadherence was not assessed. There is no gold standard for measuring medication adherence, and each method has strengths and limitations 42, 43. Although pharmacy refill data are becoming increasingly available, a low proportion of adults receive care in settings where pharmacy refill data are readily available 44. All pharmacy claims captured through the managed care organization were included in the analyses; however, it is possible that some participants filled prescriptions outside of the system and these claims were not included. Furthermore, the diverse nature of the MMAS-8 questions – while an important feature for identification of the varied barriers to adherence –may impede the scale’s ability to predict future events. Self-report tools are subject to recall and social desirability biases resulting in misclassification of participants and overestimation of adherence behavior. Although one prior study found no social desirability in responses provided on the MMAS-8, future investigation is warranted22. Although we confirmed CVD events against medical records to increase reporting accuracy, use of ICD-9 codes to identify CVD events may be subject to misclassification bias. The potential misclassification, however, is unlikely to be differential among the adherence groups.
This study has many strengths, including its prospective design, relatively large sample size, broad range of data collected (survey, administrative, and clinical data), availability of both pharmacy refill and self-reported adherence data, diversity of the sample with respect to sociodemographic characteristics and the presence of risk factors, and ability to perform sensitivity analyses. Because the CoSMO study is limited to community-dwelling older adults in a managed care organization, confounding by access to care and health insurance is reduced. Finally, because hypertension is a prevalent disease in older adults, the results of this study may be useful in the evaluation and management of a substantial segment of the population.
Conclusion
Poor antihypertensive medication adherence assessed using pharmacy refill data was associated with uncontrolled BP and increased risk for CVD events in community-dwelling patients with established hypertension. In contrast, while associated with BP control, self-report antihypertensive medication adherence using MMAS-8 was not associated with CVD incidence. The differences in the association of pharmacy refill versus self-report with CVD may be due to traits in the behaviors each adherence measure assesses. Self-report tools may provide important information to clinicians regarding barriers to adherence that can be targeted for intervention; pharmacy refill measures may provide medication filling patterns and enable assessment of barriers to refilling medications and of adverse event risk in clinical, population-based, and research settings. Identification of patients with poor medication adherence in outpatient settings may be important in facilitating patients achieving controlled blood pressure and ultimately improved cardiovascular health.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by the National Institute on Aging (Grant Number R01 AG022536, M. Krousel-Wood principal investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Financial disclosure: Drs. Muntner and Morisky have consulted for Amgen. Dr. Morisky is developer and owner of the MMAS-8 and receives royalties for its use.
Findings presented in part (abstract ID 817) at the American Heart Association Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism 2013 Scientific Sessions, 3-22-2013
Disclosure: Drs. Morisky has consulted for Amgen. Dr. Muntner has received research grants, consulting fees and served on advisory boards for Amgen. Dr. Morisky is developer and owner of the MMAS-8 and receives royalties for its use.
Permission for use of MMAS-8 is required. Licensure agreement is available from Dr. Donald E. Morisky, Department of Community Health Sciences, University of California, Los Angeles, Fielding School of Public Health.
Author Contributions:
All authors listed on the manuscript meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. Contributions are as follows: Marie Krousel-Wood: study design and oversight, acquisition of study subjects and data, interpretation of data and preparation of article; Elizabeth Holt: analysis and interpretation of data and preparation of article; Cara Joyce, Adriana Dornelles, Rachael Ruiz: analysis and interpretation of data and review of article; Larry Webber: study design, data interpretation, article review; Edward D Frohlich and Richard N Re: adjudication of cardiovascular outcomes, interpretation of data, and article review; Donald Morisky, Jiang He and Paul K Whelton: interpretation of data and article review; Paul Muntner: study design, data interpretation, article preparation and review. Dr. Krousel-Wood had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Reference List
- 1.Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002 Nov 2;360(9343):1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002 Dec 11;288(22):2880–3. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 3.Krousel-Wood MA, Muntner P, Islam T, Morisky DE, Webber LS. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009 May;93(3):753–69. doi: 10.1016/j.mcna.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JE, Wan JY, Tang J, Ghani MA, Cushman WC. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010 Jun;25(6):495–503. doi: 10.1007/s11606-009-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011 Mar;29(3):610–8. doi: 10.1097/HJH.0b013e328342ca97. [DOI] [PubMed] [Google Scholar]
- 6.Esposti LD, Saragoni S, Benemei S, Batacchi P, Geppetti P, Di BM, Marchionni N, Sturani A, Buda S, Esposti ED. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47–54. doi: 10.2147/CEOR.S15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kettani FZ, Dragomir A, Cote R, Roy L, Berard A, Blais L, Lalonde L, Moreau P, Perreault S. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009 Jan;40(1):213–20. doi: 10.1161/STROKEAHA.108.522193. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009 Oct 20;120(16):1598–605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 9.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005 Jun;43(6):521–30. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 10.Nelson MR, Reid CM, Ryan P, Willson K, Yelland L. Self-reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2) Med J Aust. 2006 Nov 6;185(9):487–9. doi: 10.5694/j.1326-5377.2006.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 11.Steiner JF. Self-reported adherence measures: what do they assess and how should we use them? Med Care. 2012 Dec;50(12):1011–2. doi: 10.1097/MLR.0b013e318270abaf. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JF. Rethinking adherence. Ann Intern Med. 2012 Oct 16;157(8):580–5. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 13.Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication acquisition and self-reported adherence in veterans with hypertension. Med Care. 2009 Apr;47(4):474–81. doi: 10.1097/mlr.0b013e31818e7d4d. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013 Oct;34(38):2940–8. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 15.Korb-Savoldelli V, Gillaizeau F, Pouchot J, Lenain E, Postel-Vinay N, Plouin PF, Durieux P, Sabatier B. Validation of a French version of the 8-item Morisky medication adherence scale in hypertensive adults. J Clin Hypertens (Greenwich ) 2012 Jul;14(7):429–34. doi: 10.1111/j.1751-7176.2012.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira-Filho AD, Barreto-Filho JA, Neves SJ, Lyra DP., Junior Association between the 8-item Morisky Medication Adherence Scale (MMAS-8) and blood pressure control. Arq Bras Cardiol. 2012 Jul;99(1):649–58. doi: 10.1590/s0066-782x2012005000053. [DOI] [PubMed] [Google Scholar]
- 17.Krousel-Wood M, Thomas S, Muntner P, Morisky D. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004 Jul;19(4):357–62. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 18.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005 Jul;11(7):449–57. [PubMed] [Google Scholar]
- 19.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009 Jan;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006 Apr;12(3):239–45. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P, Levitan EB, Joyce C, Holt E, Mann D, Oparil S, Krousel-Wood M. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich ) 2013 Feb;15(2):112–7. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisky DE, Ang A, Krousel-Wood MA, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav Res Ther. 1997 Apr;35(4):373–80. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- 25.National Health and Nutrition Examination Study. [2-1-2011];2011 http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/sp_bpq_c.pdf.
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009 Jul;15(7):457–64. [PMC free article] [PubMed] [Google Scholar]
- 30.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007 Sep 10;167(16):1798–803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008 Apr;14(3):203–10. doi: 10.1016/j.cardfail.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evangelista L, Doering LV, Dracup K, Westlake C, Hamilton M, Fonarow GC. Compliance behaviors of elderly patients with advanced heart failure. J Cardiovasc Nurs. 2003 Jul;18(3):197–206. doi: 10.1097/00005082-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung. 2001 Jul;30(4):294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 34.Wutoh AK, Elekwachi O, Clarke-Tasker V, Daftary M, Powell NJ, Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. J Acquir Immune Defic Syndr. 2003 Jun 1;33(Suppl 2):S106–S114. doi: 10.1097/00126334-200306012-00007. [DOI] [PubMed] [Google Scholar]
- 35.Krousel-Wood M, Joyce C, Holt E, Muntner P, Webber LS, Morisky DE, Frohlich ED, Re RN. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011 Nov;58(5):804–10. doi: 10.1161/HYPERTENSIONAHA.111.176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green LW, Mullen P, Friedman RB. Epidemiological and community approaches to patient compliance. In: Cramer JA, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991. pp. 373–86. [Google Scholar]
- 37.Barbash IM, Gaglia MA, Jr, Torguson R, Minha S, Satler LF, Pichard AD, Waksman R. Effect of marital status on the outcome of patients undergoing elective or urgent coronary revascularization. Am Heart J. 2013 Oct;166(4):729–36. doi: 10.1016/j.ahj.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Lee GK, Wang HH, Liu KQ, Cheung Y, Morisky DE, Wong MC. Determinants of medication adherence to antihypertensive medications among a Chinese population using Morisky Medication Adherence Scale. PLoS One. 2013;8(4):e62775. doi: 10.1371/journal.pone.0062775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002 Jun;18(6):649–56. [PubMed] [Google Scholar]
- 40.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population-based study in the elderly. Am J Hypertens. 1997 Jul;10(7 Pt 1):697–704. doi: 10.1016/s0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 41.Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013 Jun;11(2):54–65. doi: 10.3121/cmr.2013.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawkshead JK-WM. Techniques of measuring medication adherence in hypertensive patients in outpatient settings: advantages and limitations. Dis Manag Health Outcomes. 2007;15:109–18. [Google Scholar]
- 43.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006 Apr;26(4):483–92. doi: 10.1592/phco.26.4.483. [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics. Health, United States, 2007 Chartbook on trends in the health of Americans. Hyattsville, MD: 2007. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.