Abstract
The development and progression of left ventricular hypertrophy is a consequence of multiple comorbid conditions associated with end-stage renal disease and large variations in interdialytic weight gains. The literature suggests that dietary sodium restriction alone significantly reduces interdialytic weight gains. A total of 124 hemodialysis participants in an ongoing randomized control trial participated in the validation in which psychometric properties of a self-efficacy survey were a secondary analysis. We evaluated the internal consistency, construct validity, and convergent validity of the instrument. The overall Cronbach α was 0.93. Three factors extracted explain 67.8% of the variance of the white and African American participants. The Self-Efficacy Survey has adequate internal consistency and construct and convergent validity. Future research is needed to evaluate the stability and discriminant validity of the instrument.
Keywords: consistency, construct validity, convergent validity, dietary sodium intake, fluid restriction, hemodialysis, psychometrics
In 2009, the adjusted rate of incidence for end-stage renal disease (ESRD) was 355 per million persons, with a prevalence of 1738 per million persons. Medicare is the principal payer for ESRD and hemodialysis management. The number of patients enrolled in this Medicare-funded program has increased from approximately 10 000 beneficiaries in 1973 to more than 570 000 in 2009. By 2010, projections indicated that the number of patients with ESRD would increase to more than 650 000 and total Medicare ESRD program costs to $28 billion.1 Despite the magnitude of resources committed to the treatment of ESRD and substantial improvements in the quality of dialysis therapy, patients continue to experience significant mortality and morbidity.2 Notably, survival for patients undergoing hemodialysis at 1, 2, and 5 years is 81%, 65%, and 34%, respectively.1
The primary function of hemodialysis therapy is to replace kidney function by removing waste and unwanted electrolyte and elemental concentrations (ie, sodium, potassium, and phosphorus). Intermittent hemodialysis, however, is not a perfect science and compounds the extensive cardiovascular-related risks to this patient population. In particular, left ventricular hypertrophy presents the most significant risk of morbidity and mortality to patients with ESRD.3–8
The development and progression of left ventricular hypertrophy is a consequence of multiple comorbid conditions associated with ESRD, particularly hypertension, and large variations in interdialytic weight gains (IDWG).9 Both conditions are responsive to restriction of free fluid and consequently, patients undergoing hemodialysis are counseled to restrict fluid intake to as little as 0.5 L/d.10 It may be unrealistic, however, to expect patients undergoing hemodialysis to reduce fluid intake if they do not also restrict dietary sodium intake. High dietary sodium intake elevates serum sodium content and exacerbates thirst, making it difficult for patients undergoing hemodialysis to restrict fluid intake.11,12 Thirst can become overwhelming, result in excessive fluid intake, and lead to large IDWG. The multiple medications prescribed for this patient population, the need for dialysis treatments 3 times a week, dietary requirements for calorie and protein intake, and phosphorus and potassium restrictions present complicated tasks for patients undergoing hemodialysis. Because of the complicated nature of the hemodialysis regimen, many patients undergoing hemodialysis lack confidence in their ability to adhere to the prescribed regimen.
An extensive body of evidence supports the use of perceived self-efficacy to predict subsequent performance across various behavioral domains including smoking cessation, adherence to exercise programs, and weight control programs.13–20 The basic premise underlying self-efficacy theory is that the expectations of personal mastery and success (efficacy expectation) influence the likelihood of an individual engaging in a particular behavior. In essence, an individual’s behavior is influenced by personal characteristics, beliefs about the consequences of a particular behavior, and the confidence in one’s ability to achieve that behavior.21
Several behavioral intervention studies addressing fluid volume in patients undergoing hemodialysis were found in the literature.22–35 All studies except for 229,35 focused on fluid intake, without addressing dietary sodium. However, educating patients undergoing hemodialysis about fluid control without focusing on sodium is futile,28 because the thirst instigated by high serum sodium cannot be ignored. None of these studies featured self-efficacy–based interventions or measurements of dietary self-efficacy.
Findings from our pilot work suggested that dietary sodium restriction alone resulted in a clinically significant reduction in IDWG.27 This observation led to the BalanceWise study (NIH-R01-NR010135), heretofore known as the parent study. The purpose of this randomized controlled trial was to evaluate the efficacy of a Social Cognitive Theory (SCT)-based behavioral intervention to reduce dietary sodium intake of patients undergoing hemodialysis. The BalanceWise investigators developed the Self-Efficacy Survey described later in this article. The purpose of this secondary analysis is to describe the psychometric properties of the Self-Efficacy Survey.
METHODS
Design
Data to meet the primary aim were collected as part of the parent study. The 16-week intervention paired SCT-based behavioral counseling with technology-based dietary self-monitoring. The primary aims of the parent study were to (1) assess the impact of the intervention on average daily IDWG and (2) examine the impact of the intervention on dietary sodium intake. Secondarily, the study explored the impact of the intervention on blood pressure, interdialytic and postdialytic symptoms, health-related quality of life, and the mediating effect of dietary self-efficacy.
Sample
Parent study participants were recruited from 13 dialysis centers in the Pittsburgh area, stratified by dialysis center, and randomized within center strata using permuted blocks. Participants were 18 years of age or older, with no upper age limit, and had received maintenance hemodialysis for at least 3 months. Individuals were excluded if they (1) could not read, write, or speak English; (2) planned to move out of the area or change dialysis centers within the next 4 months; (3) had a terminal illness and life expectancy of less than 12 months per clinical evaluation of dialysis center staff; (4) could not read the screen of the hand-held computer used in the intervention or use the device’s stylus to make selections from the computer screen; (5) were institutionalized (eg, in a nursing home or a personal care facility or incarcerated), which limited control over their dietary intake; or (6) resided with another participant of the study. Data collected at baseline were used for this analysis. This study received approval from the Institutional Review Board of the University of Pittsburgh and all subjects provided signed informed consent.
Measures
For this study, we evaluated the internal consistency, construct validity, and convergent validity of the measure of dietary self-efficacy (Self-Efficacy Survey). The Self-Efficacy Survey was adapted from the Cholesterol Diet Self-Efficacy Scale.13 Using an 11-point Visual Numeric Scale with responses ranging from 0 = “not confident at all” to 100 = “very confident” in 10-point increments, participants indicated how confident they were that they could adhere to the sodium restriction component of the hemodialysis diet. The items address self-efficacy to adhere to the diet, in general; limit sodium, fluid, and excess IDWG; limit common sources of excess sodium (eg, canned food, processed meats, salty snacks); follow common strategies to minimize sodium intake (eg, avoid adding table salt, read food labels, avoid fast food restaurants); adhere given their emotional state (eg, feeling blue); adhere when appetite was poor; and adhere by day of the week (eg, dialysis vs nondialysis treatment day, weekday vs weekend day). The instrument provided written instructions, with examples demonstrating sample responses.
ANALYSIS
Statistical analysis was performed utilizing SPSS version 20.0 (IBM Corp, Armonk, NY). The internal consistency of the items was calculated using the Cronbach α coefficient. The criterion standard cutoff score for adequate internal consistency of a Cronbach α of 0.70 or greater, as suggested by Nunnally, was used to determine internal consistency adequacy.36 In addition, internal consistency of the factor structure and subscales was evaluated. The factor structure was estimated using Principal Components Analysis extraction with an oblique rotation for initial factor extraction. A multifaceted approach was used in the initial factor extraction including examination of Cattell’s scree plot (a subjective means of factor extraction), percentage of variance explained and meaningfulness of factors (eigenvalues and communalities) to determine the number of factors within the structure. Convergent validity was examined by computing interitem correlations.
RESULTS
Sample
Data from the first 124 participants recruited to the study were considered for this report. Two of the participants withdrew from the study prior to completion of baseline measures and the final sample consisted of 122 participants. Respondents were primarily older, male whites, and married or living as married; the average duration of maintenance hemodialysis was greater than 4 years (Table 1). Notably, almost a third of participants reported their income to be inadequate for meeting their basic living requirements.
Table 1.
Sociodemographic Characteristics of BalanceWise Study Participants (N = 124)
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 74 | 60 |
| Female | 48 | 40 |
| Ethnicity | ||
| White/Caucasian | 64 | 52 |
| Black/African | 58 | 47 |
| American | ||
| Married or living married |
64 | 52 |
| Income inadequate to meet needs |
38 | 31 |
| Mean | SD | |
| Mean age, y | 61 | |
| Duration of ESRD treated with dialysis, mo |
51 |
Abbreviation: ESRD, end-stage renal disease.
Exploratory factor analysis
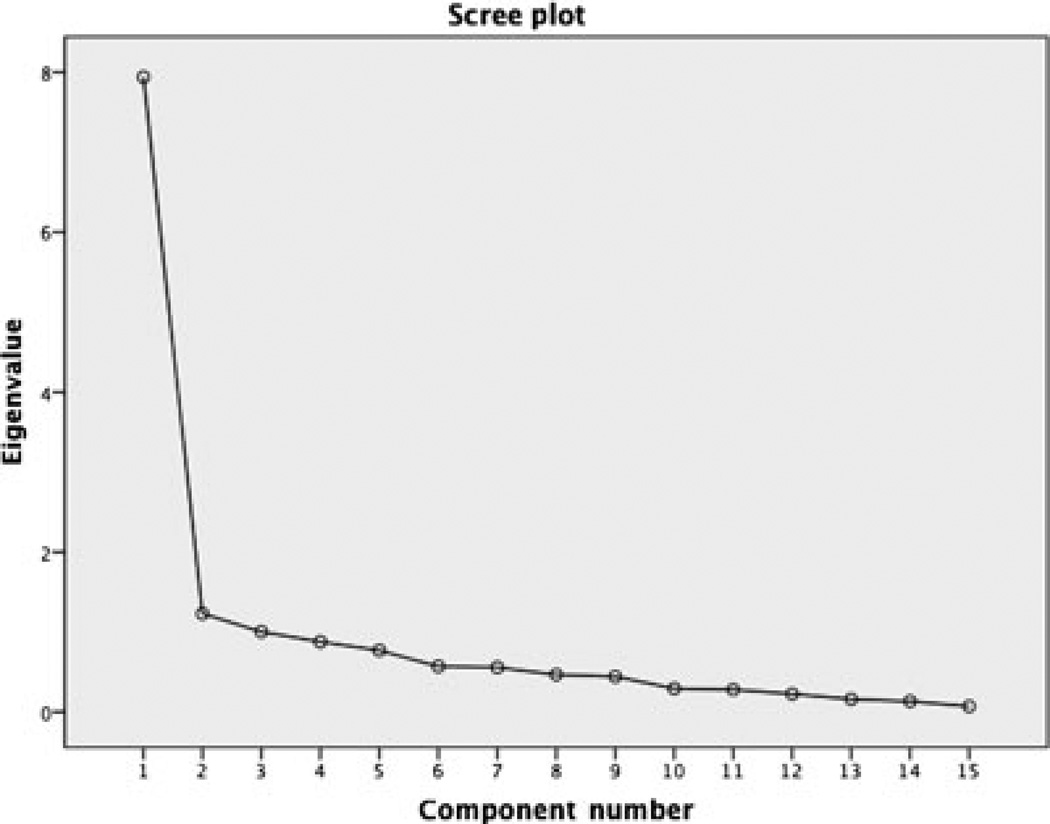
A 3-factor structure was extracted from the obliquely rotated principal components analysis, indicating that 3 distinct concepts are tested by this instrument. The scree plot (Figure) demonstrated a natural bend at either 2 or 3 factors within the structure. Total variance explained and eigenvalues verified the structure to be 3 factors. Total variance explained by factor I was 52.9%, 8.2% by factor II, and 6.7% by factor III. Eigenvalues were 7.9, 1.2, and 1.0, respectively (Table 2).
Figure.
Scree plot of factors extracted from the Self-Efficacy Restricting Dietary Salt in Hemodialysis Scale (N=124).
Table 2.
Total Variance Explained by the 3 Extracted Factors From the Self-Efficacy Restricting Dietary Salt in Hemodialysis Scale
| Initial Eigenvalues |
Extracted Sums of Squares Loadings |
|||||
|---|---|---|---|---|---|---|
| Factors | Total | % Variance | Cumulative % | Total | % Variance | Cumulative % |
| I | 7.9 | 52.9 | 52.9 | 7.9 | 52.9 | 52.9 |
| II | 1.2 | 8.2 | 61.1 | 1.2 | 8.2 | 61.1 |
| III | 1.0 | 6.7 | 67.8 | 1.0 | 6.7 | 67.8 |
Factor I focused on “sources of dietary sodium” and consisted of 5 items (Table 3) displays reliability and Table 4 displays factor loadings). Factor loadings ranged from 0.52 to 0.82 and accounted for the largest amount of variance (52.9%). The items that loaded on this factor reflect participants’ confidence in their ability to control sodium intake from particularly high sodium foods. Three items that represented confidence in their ability to employ strategies for reducing sodium intake (including ability to “avoid table salt,” “limit fast food,” and “read food labels so that [they knew] how much salt [was] is in [their] food”) cross-loaded on factor II or factor III. Forcing these items onto factor I resulted in a minimal increase of Cronbach α from 0.87 to 0.88.
Table 3.
Reliability: Internal Consistency of the Self-Efficacy Restricting Dietary Salt in Hemodialysis Scale
| Cronbachα | Cronbach α–Based on Standardized Items |
No. of Items | |
|---|---|---|---|
| Overall instrument | 0.934 | 0.936 | 15 |
| Factor I: Sources of dietary sodium | 0.872 | 0.876 | 7 |
| Factor II: Daily schedule | 0.945 | 0.945 | 3 |
| Factor III: Situational | 0.791 | 0.797 | 3 |
Table 4.
Self-Efficacy for Restricting Dietary Salt in Hemodialysis Scale Items
| Factor | Item No. | |
|---|---|---|
| How confident are you that in the next month, you will be able to … | ||
| 1 | follow the dialysis diet in general? | |
| I | 2 | control the amount of salt that you eat? |
| III | 3 | limit the amount of fluids that you drink? |
| I | 4 | avoid the amount of canned food that you eat? |
| I | 5 | avoid adding table salt to your food? |
| I | 6 | limit the amount of processed meat (such as bacon and luncheon meat) that you eat? |
| I | 7 | read food labels so that you know how much salt is in your food? |
| 8 | limit the amount of weight that you gain from fluid between dialysis treatments? |
|
| I | 9 | limit salty snacks? |
| I | 10 | limit the number of times each week you eat at fast food restaurants? |
| How confident are you that in the next month, you can limit your salt intake when you are … |
||
| III | 11 | feeling blue or depressed? |
| III | 12 | experiencing a day when your appetite is poor? |
| How confident are you that in the next month, you can limit your salt intake on.…. |
||
| II | 13 | dialysis treatment days? |
| II | 14 | weekdays when you have no dialysis treatments? |
| II | 15 | weekend days when you have no dialysis treatments? |
Factor II named “daily schedule” comprised the 3 items addressing confidence in the ability to restrict dietary sodium given the day of the week (ie, dialysis weekdays, nondialysis weekdays, and nondialysis weekend days). Factor loadings ranged from 0.89 to 0.95. Cronbach α for factor II was 0.94.
Factor III, “contextual factors,” focused on situations in which individual circumstances might impact decisions about dietary sodium intake. These included confidence in limiting sodium intake when “experiencing poor appetite.” Item 11 (How confident are you that you can limit your food intake when feeling blue?) cross-loaded on factor II. Forcing the item onto factor III, which the content most closely resembles, increased Cronbach α to 0.79 (previously 0.69). The factor loadings ranged from 0.51 to 0.79.
DISCUSSION
The primary aim of this report was to evaluate the psychometric properties of the investigator-developed instrument, Self-Efficacy for Restricting Dietary Sodium in Hemodialysis Scale (Self-Efficacy Survey), in a convenience sample of patients undergoing hemodialysis. Based on the results of this analysis, the Self-Efficacy Survey is a valid instrument for assessing self-efficacy in restricting dietary sodium in the patient population undergoing hemodialysis. In addition, the high Cronbach α coefficients for the individual Self-Efficacy Survey subscales and the instrument as a whole suggest good internal consistency.
Interventionists often turn to SCT to explicate the process of lifestyle modification. Multiple SCT and cognitive behavioral therapy–driven interventions to reduce dietary sodium intake in hypertension have demonstrated successful results.13–18,27,30–33,35,37,38 The tenets of SCT are thought to have a direct impact upon behavior and an indirect effect upon intentions. In short, optimistic self-beliefs predict actual behavioral performance, and individuals will typically perform behaviors they perceive to be within their control.21 Therefore, enhancing self-efficacy may be a useful intervention approach to assist patients undergoing hemodialysis in reducing dietary sodium intake. Because no instruments measuring confidence of patients undergoing hemodialysis in their ability to reduce dietary sodium were found in the literature, we adapted an existing instrument. At the study’s conclusion, we will use the Self-Efficacy Scale to evaluate the extent to which changes in IDWGs, resulting from our SCT-based intervention, were mediated by improved self-efficacy.
There is evidence to support that all items within the instrument were measuring the same construct. Cross-loading of items, specifically items 7 and 8, suggests the potential to remove these items with the goal of shortening the instrument and reducing participant burden. Further investigation into the content of these items and their contribution to the overall usefulness of the instrument is, therefore, warranted.
STRENGTHS AND LIMITATIONS
Sample adequacy was determined using the Kaiser-Mayer-Olkin measure of sampling adequacy. Sample size was adequate, as demonstrated by the Kaiser-Mayer-Olkin score, and participants varied in education and socioe-conomic status. Our sample included a robust African American population. However, representatives of other ethnicities or racial groups were not available in the parent study. As such, it remains unknown whether the instrument will have similar psychometrics in a more diverse sample. Because of concerns regarding respondent burden, retesting of subjects was not judged to be feasible; therefore, test-retest reliability could not be determined. Future research to examine instrument stability is warranted. Discriminant validity also could not be performed because we were unable to identify an instrument with a similar purpose that was developed for this patient population.
CONCLUSION
The Self-Efficacy Survey has adequate internal consistency and construct and convergent validity. Future research is needed to evaluate the stability and discriminant validity of the instrument.
Acknowledgments
This study was supported through grants from NIH-R01-NR010135, NIH-K24-NR012226 received by Mary Ann Sevick, and 1F31NR013410 received by Maya N. Clark, PhD, ACNP-BC, RN.
The authors acknowledge the individual contributions of Lin Hough, MPH; Ann Steenkiste, MS; Susan Stark, RD, MS; Beth Hall, RD; and Tienna Luster. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.US Renal Data System, USRDS 2011 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 2.Crews D, Charles M, Evans M, Zonderman A, Powe N. Poverty, race, and CKD in a racially and socioe-conomically diverse urban population. Am J Kidney Dis. 2010;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luft FC, Morris CD, Weinberger MH. Compliance to a low-salt diet. Am J Clin Nutr. 1997;65(suppl 2):698S–703S. doi: 10.1093/ajcn/65.2.698S. [DOI] [PubMed] [Google Scholar]
- 4.Webster JL, Dunford EK, Neal BC. A systematic survey of the sodium contents of processed foods. Am J Clin Nutr. 2010;91(2):413–420. doi: 10.3945/ajcn.2009.28688. [DOI] [PubMed] [Google Scholar]
- 5.McCausland FR, Waikar SS, Brunelli SM. Increased dietary sodium us independently associated with greater mortality among prevalent hemodialysis patients. Kidney Int. 2012;82(2):204–211. doi: 10.1038/ki.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman M, Dixit A, Donley V, et al. Factors associated with inadequate blood pressure control in hypertensive hemodialysis patients. Am J Kidney Dis. 1999;33:493–497. doi: 10.1016/s0272-6386(99)70187-3. [DOI] [PubMed] [Google Scholar]
- 7.Cook NR. Salt intake, blood pressure, and clinical outcomes. Curr Opin Nephrol Hypertens. 2008;17:310–314. doi: 10.1097/MNH.0b013e3282f4b720. [DOI] [PubMed] [Google Scholar]
- 8.Ozkahya M, Toz H, Ozerkan F, et al. Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol. 2002;15(6):655–660. [PubMed] [Google Scholar]
- 9.Saran R, Bragg-Gresham J, Rayner HC, et al. Nonad-herence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 10.Kursat S, Ozgur B, Alici T. Effect of ultrafiltration on blood pressure variability in hemodialysis patients. Clin Nephrol. 2003;59(4):289–292. doi: 10.5414/cnp59289. [DOI] [PubMed] [Google Scholar]
- 11.Denhaerynck K, Manhaeve D, Dobbels F, Garzoni D, Nolte C, De Geest S. Prevalence and consequences of non-adherence to hemodialysis regimens. Am J Crit Care. 2007;16:222–235. [PubMed] [Google Scholar]
- 12.Klag M, Whelton P, Randall B, Neaton J, Brancati F, Stamler J. End-stage renal disease in African-American and white men: 16 year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 13.Burke LE, Dunbar-Jacob J, Sereika S, Ewart CK. Development and testing of the Cholesterol-Lowering Diet Self-Efficacy Scale. Eur J Cardiovasc Nurs. 2003;2(4):265–273. doi: 10.1016/S1474-5151(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 14.Tucker M. The effects of behavioral intervention with patients, nurses, and family members on dietary non-compliance in chronic hemodialysis patients. Transplant Proc. 1989;21:3985–3988. [PubMed] [Google Scholar]
- 15.Tanner J, Craig C, Bartolucci A, et al. The effect of a self- monitoring toll on self-efficacy, health beliefs, and adherence in patients receiving hemodialysis. J Renal Nutr. 1998;8:203–211. doi: 10.1016/s1051-2276(98)90019-x. [DOI] [PubMed] [Google Scholar]
- 16.Sagawa M, Oka M, Chaboyer W. The utility of cognitive behavioral therapy on chronic haemodialysis patients’ fluid intake: a preliminary examination. Int J Nurs Stud. 2003;40:367–373. doi: 10.1016/s0020-7489(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 17.Fisher L, Cairns H, Amir-Ansari B, Scoble J, Chalder T, Treasure J. Psychological Intervention in fluid management. Palliat Support Care. 2006;4:419–424. doi: 10.1017/s1478951506060512. [DOI] [PubMed] [Google Scholar]
- 18.Christensen A, Moran P, Wiebe J, Ehler S, Lawton W. Effect of a behavioral self-regulation intervention on patient adherence in hemodialysis. Health Psychol. 2002;21:393–397. doi: 10.1037//0278-6133.21.4.393. [DOI] [PubMed] [Google Scholar]
- 19.Casey V, Johnson V, McClelland P. Impact of stepped verbal and written reinforcement of fluid balance advice within an outpatient haemodialysis unit. J Hum Nutr Diet. 2002;15:43–47. doi: 10.1046/j.1365-277x.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 20.Cummings K, Becker M, Kirtscht J, Levin N. Intervention strategies to improve compliance with medical regimens by ambulatory hemodialysis patients. J Behav Med. 1981;4:111–127. doi: 10.1007/BF00844851. [DOI] [PubMed] [Google Scholar]
- 21.van der Bijl JJ, Shortridge-Bagget LM. The theory and measurement of the self-efficacy construct. Sch Inq Nurs Pract. 2001;15(3):189–207. [PubMed] [Google Scholar]
- 22.Hart R. Utilization of token economy within a chronic dialysis unit. J Consult Clin Psychol. 1979;47:646–648. [PubMed] [Google Scholar]
- 23.Keane T, Prue D, Collins F. Behavioral contracting to improve dietary compliance in chronic renal patients. J Behav Ther Exp Psychiatry. 1981;12:63–67. doi: 10.1016/0005-7916(81)90031-8. [DOI] [PubMed] [Google Scholar]
- 24.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med. 2001;12(1):13–20. doi: 10.3909/ricm0585. [DOI] [PubMed] [Google Scholar]
- 26.Movilli E, Gaggia P, Zubani R, et al. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicenter study. Nephrol Dial Transplant. 2005;22(12):3547–3552. doi: 10.1093/ndt/gfm466. [DOI] [PubMed] [Google Scholar]
- 27.Sevick MA, Stone RA, Novak M, et al. A PDA-based dietary self-monitoring intervention to reduce sodium intake in an in-center hemodialysis patient: case report. Patient Prefer Adherence. 2008;2:177–184. [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad S. Dietary sodium restriction for hypertension in dialysis patients. Semin Dial. 2004;17(4):284–287. doi: 10.1111/j.0894-0959.2004.17328.x. [DOI] [PubMed] [Google Scholar]
- 29.Cutler J, Follman D, Allender PS. Randomized trials of sodium reduction: an overview. Am J Clin Nutr. 1997;65(suppl):643S–651S. doi: 10.1093/ajcn/65.2.643S. [DOI] [PubMed] [Google Scholar]
- 30.Robare JF, Milas NC, Bayles CM, et al. The key to life nutrition program: results from a community-based dietary sodium reduction trial. Public Health Nutr. 2009;13(5):606–614. doi: 10.1017/S1368980009991583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woollard J, Bellin L, Lord T, Puddey I, MacAdam D, Rouse I. A controlled trial of nurse counseling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22(6–7):466–468. doi: 10.1111/j.1440-1681.1995.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 32.Svetkey LP, Harsha DW, Vollmer WM, et al. Premier: a clinical trial of comprehensive lifestyle modification for blood pressure control: rationale, design, and baseline characteristics. Ann Epidemiol. 2003;13(6):462–471. doi: 10.1016/s1047-2797(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 33.Hyman DJ, Pavlik VN, Taylor WC, Goodrick K, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med. 2007;167(11):1152–1158. doi: 10.1001/archinte.167.11.1152. [DOI] [PubMed] [Google Scholar]
- 34.Appel LJ, Espeland M, Whelton PK, et al. Trial of non-pharmacologic intervention in the elderly (TONE): design and rationale of a blood pressure trial. Ann Epidemiol. 1995;5(2):119–129. doi: 10.1016/1047-2797(94)00056-y. [DOI] [PubMed] [Google Scholar]
- 35.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunnally JC. Psychometric Theory. 2nd ed. New York, NY: McGraw-Hill; 1978. [Google Scholar]
- 37.Luszcyznska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol. 2005;129(5):439–457. doi: 10.3200/JRLP.139.5.439-457. [DOI] [PubMed] [Google Scholar]
- 38.Fowles ER, Feucht J. Testing the barriers to healthy eating scale. West J Nurs Res. 2004;26(4):429–443. doi: 10.1177/0193945904263281. [DOI] [PubMed] [Google Scholar]