Abstract
Filifactor alocis, a Gram-positive anaerobic rod, is now considered one of the marker organisms associated with periodontal disease. Although there was heterogeneity in its virulence potential, this bacterium was shown to have virulence properties that may enhance its ability to survive and persist in the periodontal pocket. To gain further insight into a possible mechanism(s) of pathogenesis, the proteome of F. alocis strains was evaluated. Proteins including several proteases, neutrophil-activating protein A and calcium-binding acid repeat protein, were identified in F. alocis. During the invasion of HeLa cells, there was increased expression of several of the genes encoding these proteins in the potentially more virulent F. alocis D-62D compared to F. alocis ATCC 35896, the type strain. A comparative protein in silico analysis of the proteome revealed more cell wall anchoring proteins in the F. alocis D-62D compared to F. alocis ATCC 35896. Their expression was enhanced by coinfection with Porphyromonas gingivalis. Taken together, the variation in the pathogenic potential of the F. alocis strains may be related to the differential expression of several putative virulence factors.
Keywords: Filifactor alocis, Microbiology, Proteases, Proteome, Virulence
1 Introduction
Periodontal disease is associated with a complex microbial milieu harboring several pathogens that can initiate or directly contribute to host tissue destruction. Bacteria such as Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter (Actinobacillus) actinomycetemcomitans, Tannerella forsythia, and Treponema denticola have previously been demonstrated to be major pathogens associated with periodontal diseases [1–3]. A paradigm shift for infection-induced periodontal diseases based on data emerging from the oral microbiome project now suggest the involvement of as-yet-culturable and fastidious organisms [4–7]. Collectively, these studies have demonstrated that there are changes in the periodontal status associated with shifts in the composition of the bacterial community in the periodontal pocket [8, 9].
Filifactor alocis (F. alocis), a Gram-positive, assacharolytic, obligate anaerobic rod, is one of the marker organisms that is now identified to be significant to the pathogenetic structure of biofilms associated with periodontal inflammation and is suggested to be an important organism for periodontal disease [10–12]. Further, in comparison to the other traditional periodontal pathogens, F. alocis is present in the periodontal pocket in higher numbers and is least detected in healthy or periodontitis-resistant patients [13–15]. This organism first isolated in 1985 from the gingival sulcus in gingivitis and periodontis patients was classified as Fusobacterium alocis [16]. However, based on phylogenetic analysis using 16s rRNA sequences, it was reclassified in 1999 into the genus Filifactor [17].
In a previous report, we have demonstrated that F. alocis has virulence properties that may enhance its ability to survive and persist in the periodontal pocket [18]. F. alocis was relatively resistant to oxidative stress and its growth was stimulated under those conditions [18]. In addition, as reported elsewhere, there is evidence that the secretion of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α from gingival epithelial cells and apoptosis of these cells can be induced by F. alocis [19]. A comparative analysis of several F. alocis isolates showed heterogeneity in their virulence potential [18]. Further, in coculture with P. gingivalis, these F. alocis strains showed variations in their invasive capacity of epithelial cells [18]. While synergistic interactions during polymicrobial infections have resulted in enhanced pathogenesis of periodontopathogens such as P. gingivalis [20], a mechanism(s) for F. alocis is unclear. It is likely that surface and secretory proteins from F. alocis may play a role in this process.
Proteome analyses have contributed significantly toward a deeper understanding of the molecular mechanisms of invasion, adaptation, survival, and pathogenesis in several oral pathogens such as Streptococcus mutants [21] Streptococcus oralis [22], Fusobacterium nucleatum [23], and P. gingivalis [24]. In this report, we have evaluated the proteome of F. alocis ATCC 35896 and a potentially more virulent strain designated F. alocis D-62D. Our comparative analysis revealed variation in several hypothetical proteins and those known to be important virulence factors in other bacteria. Several proteases identified in the proteome of F. alocis D-62D were missing in F. alocis ATCC 35896. There was differential expression of the genes encoding these proteins during the infection of HeLa cells.
2 Materials and methods
2.1 Bioinformatics analysis
The DNA and amino acid sequences were aligned using Bioedit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The phylogenetic relationship of these sequences between the oral pathogens was analyzed using MEGA version 4.0 [25]. The signal peptide and potential cleavage sites were predicted using both Neural network and Hidden Markov Model [26]. Metabolic pathway analysis was carried out using the Kyoto Encyclopedia of Genes and Genomes (www.genome.jp/kegg/) [27]. Signal peptide prediction and cleavage site prediction were performed using Signal P 3.0 [28]. Transmembrane helices were predicted using the TMHMM server [29]. The presence of LysM domains, peptidoglycan-binding domains, and choline-binding domains were determined by screening against the Pfam database [30] (E value cutoff of <1 × 10−5). Lipoprotein predictions were performed as previously described [31]. The sorting motifs and WxL motifs were searched using string search and the results were screened manually determining the motif sequences. Sortase substrates were identified by manual screening and a Hidden Markov model [32].
2.2 Bacterial strains and growth conditions
F. alocis ATCC 35896 was purchased form the American Type Culture Collection (Rockville, MD, USA). F. alocis D-62D was a gift from Dr. Floyd Dewhirst, the custodian of the Moores' anaerobic microbial collection (The Forsyth Institute, Boston, MA, USA). The identity of the F. alocis D-62D was confirmed by 16s rRNA gene sequencing (D-62D, accession # GU968904). F. alocis strains were grown initially in Robertson's bullock heart medium followed by adaptation to Brain heart infusion broth supplemented with hemin (5 μg/mL), vitamin K (0.5 μg/mL), cysteine (1 μg/mL), and arginine (17.42 μg/mL). Porphyromonas gingivalis strains were grown in Brain heart infusion (BHI) broth (Difco) supplemented with hemin (5 μg/mL), vitamin K (0.5 μg/mL), and cysteine (0.1%). Blood agar medium was prepared by the addition of sheep blood (5%) and agar (2%). The bacterial cultures were incubated at 37°C in an anaerobic chamber (Coy Manufacturing) in 10% H2,10% CO2, and 80%N2. Growth rates were determined spectrophotometrically (optical density at 600 nm [OD600]).
2.3 Epithelial cell culture
HeLa cells were grown and maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin (100 IU/mL), streptomycin (100 IU/mL), and amphotericin B (2.5 mg/mL) (Invitrogen, Carlsbad, CA, USA). Confluent stock cultures were trypsinized, adjusted to approximately 5 × 103 cells/mL, seeded (1 mL per well) into 12-well plates (Nunc, Rochester, NY, USA), and further incubated for 48 h to reach semiconfluency (105 cells per well).
2.4 Adherence and standard antibiotic protection assay
Invasion of epithelial cells was quantified using the standard antibiotic protection assay [33]. Briefly, an isolated bacterial colony harvested from solid agar plate was grown to exponential phase in BHI broth. The bacterial cells were then centrifuged, washed three times in PBS, and adjusted to 107 CFU/mL of bacteria in DMEM. The epithelial cell monolayer was washed three times with PBS, infected with bacteria at an multiplicity of infection (MOI) of 1:100 (105 epithelial cells) and then incubated at 37°C for 30 and 45 min under 5% CO2. Nonadherent bacteria were removed by washing with PBS while cell surface bound bacteria were killed with metronidazole (200 μg/mL, 60 min). F. alocis was sensitive to 100 μg/mL of metronidazole. After removal of the antibiotic, the internalized bacteria were released by osmotic lysis of the epithelial cells in sterile distilled water. Lysates were serially diluted, plated (in duplicate) on BHI agar, and incubated for 6–10 days. The number of bacterial cells recovered was expressed as a percentage of the original inoculum. The number of adherent bacteria was obtained by subtracting the number of intracellular bacteria from the total bacteria obtained in the absence of metronidazole [34]. Coinfection was performed as described previously [2]. F. alocis and P. gingivalis inocula were prepared by mixing equal volumes (1 × 107 cells/well) of bacterial suspension, which was then incubated for 5 min in the anaerobic chamber prior to infection. The serially diluted lysate was plated on BHI blood agar and incubated for 6–10 days. The bacterial colonies were phenotypically identified.
2.5 Real-time PCR analysis
Total RNA (1 μg) was reverse transcribed to cDNA using SV total RNA isolation system (Promega, CA) in the presence of random primers (50 ng) according to the manufacturer's recommendations. Real-time PCR was carried out using the Smart cycler II, Cephid. The primers for the real-time analysis (Supporting Information Table 1) were designed using Primer3 software (http://primer3.sourceforge.net/). The amplification efficiency of each primer set was determined empirically by using cDNA template dilutions over four orders of magnitude. The amplification efficiency for each primer set varied between 95.1 and 102.5%, showing that the amplicons were generated with comparable efficiency. The realtime PCR reaction contained 12.5 μL of QuantiTect SYBR Green qPCR master mix (Qiagen), 0.2 μM of each gene-specific primer, and 1 μL of cDNA template. The cycling conditions were 50°C for 2 min, 95°C for 2 min, then 40 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Distilled water was included as a negative control in each run. All reactions were carried out in triplicate and melting curve analysis indicated that in each reaction a single product was amplified. For all reactions, 16s ribosomal RNA gene (HMPREF 0389_03102) was selected as normalizer. The critical threshold cycle (Ct) for each gene and the relative expression ratio of the selected genes were calculated and analyzed using the relative expression software tool (REST) http://www.gene-quantification.info [35].
2.6 Cell fractionation and SDS-PAGE analysis
Bacterial cells were fractionated as described previously [36]. Briefly, an overnight liquid culture of F. alocis was harvested by centrifugation (5000 × g, 10 min, 4°C). The supernatant and the pellet were collected separately. To further remove cell debris, the supernatant was again centrifuged at 4000 × g for 15 min (4°C), and then concentrated using the Amicon ultrafiltration system (EMD Millipore). The concentrated supernatant was further subjected to ultracentrifugation at 100 000 × g for 1 h at 4°C to produce the extracellular fraction. The pellet containing the cells was resuspended in ice-cold PBS. Cells were lysed in a French press (American Instrument company, MD, USA) at 20 000 lb/inch2. The lysate was cleared of unlysed cells by centrifugation (5000 × g, 10 min, 4°C). To separate the membrane fractions from the cytosolic fractions, the lysate was further ultracentrifuged (100 000 × g, 1 h, 4°C). The supernatant containing cytosolic fractions was decanted from the pellet that contained the membrane fraction.
SDS-PAGE was used at 4–12% Bis-Tris separating gel in MOPS-SDS running buffer according to the manufacturer's instructions (NuPAGE Novex gels; Invitrogen). Samples were prepared (65% sample, 25% 4× NuPAGE LDS sample buffer, 10% NuPAGE reducing agent), heated at 72°C for 10 min, and then electrophoresed at 200 V for 65 min in the XCell SureLock Mini-Cell System (Invitrogen). The protein bands were visualized with Simply Blue Safe Stain (Invitrogen).
2.7 2D-PAGE analysis
2DE was carried out using the 2D gel strips (7 cm) of pI 3–10 in a Protean IEF cell (Bio-Rad, USA) following the method of Poznanovic [37]. Briefly, the protein concentration of the sample was measured using the Bio-Rad spectrophotometer. The protein samples were diluted to a final concentration of 30–50 μg protein and 20 μL of the sample was added to solubilization buffer (7 M urea, 2 M thiourea, 2% w/v CHAPS, 65 mM DTT, bromophenol blue 0.002%, Zwittergent (3–10) 1% w/v). The first-dimension IPG strip was run by adding 125 μL of the diluted sample in the rehydration buffer (730 mg DTT, 70 mg Iodoacetemide) for 8 h at voltage gradients of 3 h at 300 V, 5 h at linear gradients of 300–3500 V, 18 h at 3500 V. After equilibration, the IPG strips were loaded on the gel and electrophoresed at 200 V, 0.3 A for 4–5 h, and then stained with Coomassie Simply Blue strain. 2D gel analysis was performed twice using biological replicates.
2.8 MS and data analysis
An LCQ Deca XP Plus system (www.thermo.com) was used to analyze the extracted peptides from each gel piece [38]. The four part protocol used for the MS and MS/MS analyses, included one full MS analysis (from 450 to 1750 m/z) followed by three MS/MS events using data-dependent acquisition, where the most intense ion from a given full MS scan was subjected to collision-induced dissociation, followed by the second and third most intense ions. The nanoflow buffer gradient was extended over 45 min in conjunction with the cycle repeating itself every 2 s, using a 0–60% ACN gradient from buffer B (95% ACN with 0.1% formic acid) developed against buffer A (2% ACN with 0.1% formic acid) at a flow rate of 250–300 nL/min, with a final 5-min 80% bump of buffer B before equilibration. In order to move the 20 μL sample from the autosampler to the nanospray unit, flow stream splitting (1:1000) and an automated valve together with a nanotrap column was used. The spray voltage and current were set at 2.2 kV and 5.0 μA, with a capillary voltage of 25 V in positive ion mode. For peptides, 160°C was used as the spray temperature. Data collection was achieved using the Xcalibur software (Thermo Electron), then screened with Bioworks 3.1. The MASCOT software (www.matrixscience.com) was used for each analysis to produce unfiltered data and out files. Statistical validation of peptide and protein findings was achieved using X TANDEM (www.thegmp.org) and SCAFFOLD meta analysis software (www.proteomesoftware.com). The presence of two different peptides at a probability of at least 95% was required for consideration as being positively identified. General protein database search was conducted using UniprotKb–Protein knowledgebase database (http://www.uniprot.org/uniprot/Filifactoralocis). F. alocis ORF database is based on the latest release of the F. alocis genome at the NCBI genome project (http://www.ncbi.nlm.nih.gov/nuccore/CP002390.1).
3. Results and discussion
3.1 Several putative virulence factors are modulated in F. alocis during invasion of HeLa cells
In a previous report, F. alocis D-62D showed an increased invasive capacity of HeLa cells compared to the type strain (F. alocis ATCC 35896) [18]. Several putative virulence factors including neutrophil-activating protein A (NAPA) (HMPREF0389_01654) and calcium-binding acid repeat protein (CBARP) (HMPREF0389_01532) were identified from the proteome of F. alocis ATCC 35896 [18]. In addition, an interrogation of the genome of F. alocis ATCC 35896 revealed several proteases such as metal-dependent proteases, CaaX proteases, sialoglycoproteases, and calcium-dependent proteases (http://www.ncbi.nlm.nih.gov/genomeprj/46625). Proteases are important virulence factors in oral pathogens including P. gingivalis [39, 40]. To determine if the variation in the invasive capacity of epithelial cells by F. alocis strains may be correlated with the expression of several of these putative virulence factors, we evaluated their expression during infection of HeLa cells. Infection with a coculture of P. gingivalis and the F. alocis strains at 30 and 45 min post infection showed an upregulation of several of the putative virulence factors only in F. alocis D-62D. Increased expression of the Caax protease (HMPREF0389_00677) at 30 min and ATP-dependent protease La (HMPREF0389_00279) at 45 min were observed for F. alocis ATCC 35896. As shown in Fig. 1A, there was an upregulation of the genes encoding the Caax protease (HMPREF0389_00590), Caax protease (HMPREF0389_00677), Xaa-pro-dipeptidase (HMPREF0389_01538), Protease (HMPREF0389_00122), NAPA (HMPREF0389_01654), and CBARP (HMPREF0389_01532) at 30 min post infection of HeLa cells with F. alocis D-62D (Fig. 1A). Expressions of these genes, except the Xaa-pro-dipeptidase (HMPREF0389_1538) protease, were not upregulated in HeLa cells infected with F. alocis ATCC 35896. At 45-min post infection, the expression level of the Xaa-pro-dipeptidase gene was increased more than threefold while the Protease–00122 (HMPREF0389_00122) gene was upregulated more than 4.5-fold. The NAPA and CBARP genes were found to be upregulated 5- and 7.8-folds, respectively (Fig. 1B). Taken together, these results suggest that there is differential expression of several putative virulence genes in F. alocis D-62D compared to the type strain F. alocis ATCC 35896. The relative significance of these genes in the virulence potential of F. alocis is unclear and is under study in the laboratory.
Figure 1.
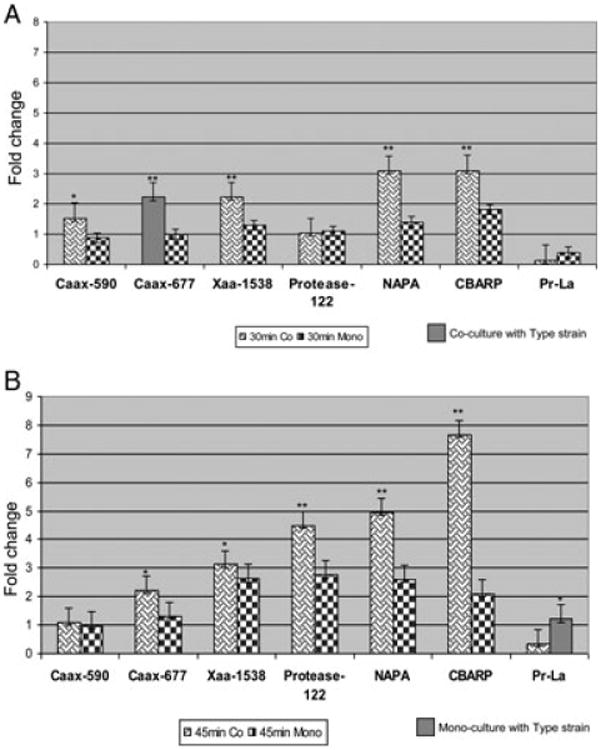
Quantitative PCR analysis of several putative virulence genes in F alocis. (A) 30-min mono- and coculture. (B) 45-min mono- and coculture. Hela cells were infected with F. alocis ATCC 35896 and D-62D strains [(MOI of 1:100(105 epithelial cells)] in mono or coculture with Porphyromonas gingivalis W83 as previously reported [18]. RNA was isolated at 30- and 45-min post infection using the SV total RNA isolation system (Promega). cDNA was made using the Transcriptor High Fidelity cDNA synthesis kit (Roche). Real-time PCR was performed using the Smart cycler (Cepheid) with gene-specific oligonucleotides. Caax-590 (HMPREF0389_00590); Caax-677 (HMPREF0389_00677); Xaa-1538, Xaa pro dipeptidase (HMPREF0389_01538); NAPA (neutrophil-acitivating factor protein A) (HMPREF0389_01654); Protease-00122 (HMPREF0389_00122); CBARP (calcium-binding acid repeat proteins) (HMPREF0389_01532); Protease La (Pr-La) (HMPREF0389_00279). Fold change was calculated using the formula. Fold change = 2 – ΔΔCt, where ΔΔCt = ΔCt of the sample –ΔCt of reference.
(**p < 0.01; *p < 0.05).
3.2 Proteome variation in F. alocis strains
A comparative SDS-PAGE analysis of cell fractions from F. alocis ATCC 35896 strain and the D-62D strain showed variation in their protein profile [18]. A random pick of 22 intense protein bands among the various fractions were subjected to MS/MS analysis. As shown in Fig. 2, the identity of these proteins includes several proteins that are known virulence factors in other bacteria [18, 19, 21, 23, 41–51]. To further evaluate other variations that may contribute to the relative pathogenic potential of F. alocis, the proteome of F. alocis D-62D was compared to the type strain F. alocis ATCC 35896. Extracellular and membrane fractions from both these strains were subjected to 2D PAGE analysis (Figs. 3–6). The pattern of spots were reproducible both in technical and biological replicates. The spots were manually excised from the respective gels, in-gel digested with trypsin, and subjected to MS/MS analysis. A total of approximately 1568 peptides were identified that were above the threshold (p < 0.05) and had a Mascot score of ≥ 15 with individual ion score of more than 20. A total of 986 nonredundant peptides corresponding to 219 proteins were identified with at least two peptides above the threshold (p < 0.05) for each protein. A complete proteome profiling of various fractions of F. alocis strains are summarized in Tables 1–4.
Figure 2.
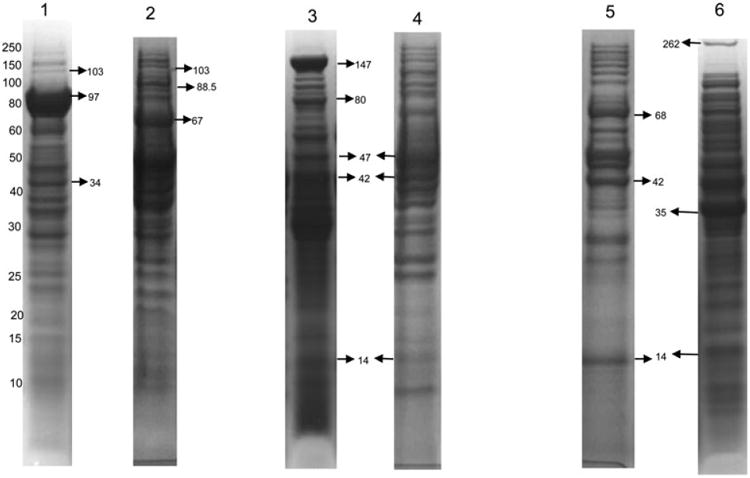
PAGE of the F. alocis protein fractions from the type strain and D-62D. All the lanes show protein bands and their corresponding molecular masses (kDa). Each lane was loaded with 35 μg of protein. The prominent protein bands (shown by arrow) were excised and analyzed by MS. Lane 1: F. alocis ATCC 35896—extra cellular fraction. 103 kDa: calcium-binding acid repeat protein (HMPREF0389_01448); 97 kDa: conserved hypothetical protein (HMPREF0389_01431); 34 kDa: cobalt import ATP-binding protein (HMPREF0389_00901). Lane 2: F alocis D-62D-extra cellular fraction. 103 kDa: calcium-binding acid repeat protein (HMPREF0389_01448); 88.5 kDa: protease (HMPREF0389_00122); 67 kDa: S layer Y domain containing protein (HMPREF0389_00223). Lane 3: F alocis ATCC 35896—membrane fraction. 147 kDa: S layer Y domain containing protein (HMPREF0389_01139); 80 kDa: membrane protein (HMPREF0389_000638); 47 kDa: Caax protease (HMPREF0389_00590); 42 kDa: Xaa pro-dipeptidase (HMPREF0389_01538); 14 kDa: neutrophil-activating protein A (HMPREF0389_01654). Lane 4: Falocis D-62D— membrane fraction; 47 kDa: Caax protease (HMPREF0389_00590); 42 kDa: Xaa pro-dipeptidase (HMPREF0389_01538); 14 kDa: neutrophil-activating protein A (HMPREF0389_01654). Lane 5: F alocis ATCC 35896—cytosolic fraction. 68 kDa: fibronectin-binding protein (HMPREF 0389_00575); 42 kDa: Xaa pro-dipeptidase (HMPREF0389_01538); 14 kDa: neutrophil-activating protein A (HMPREF0389_01654). Lane 6: F. alocis D-62D—cytosolic fraction. 262 kDa: cell wall associated serine proteinase (HMPREF0389_01110); 35 kDa: electron transfer flavo-protein alpha (HMPREF0389_00742); 14 kDa: neutrophil-activating protein A (HMPREF0389_01654).
Figure 3.
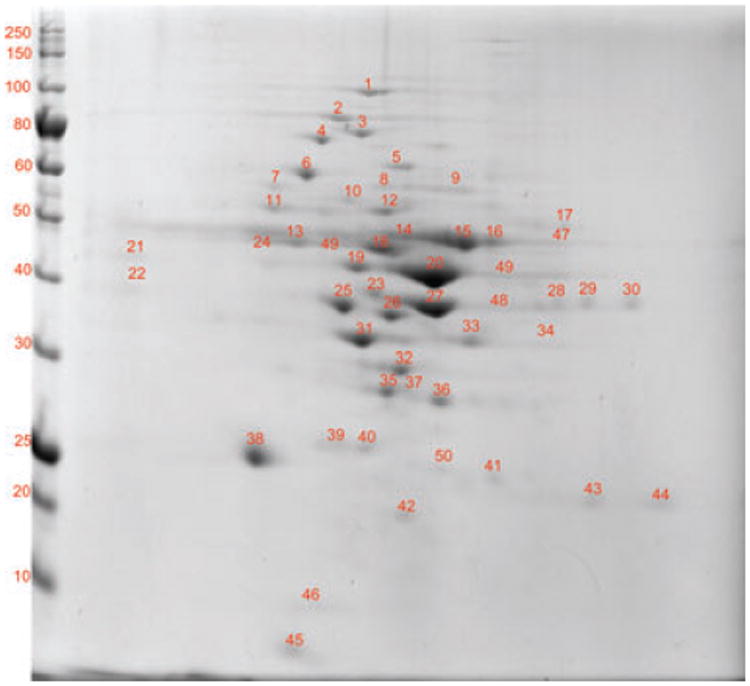
2D-PAGE of the membrane fraction of F. alocis ATCC 35896 strain. 2D page was performed using 7-cm IPG strips of pI 3–10 in Protean IEF cell and 30–50 μg of protein and electrophoresed at 200 V, 0.3 A for 4–5 h, and stained with Coomassie simply blue strain. A total of 50 distinct spots were identified and processed for MS/MS analysis.
Figure 6.
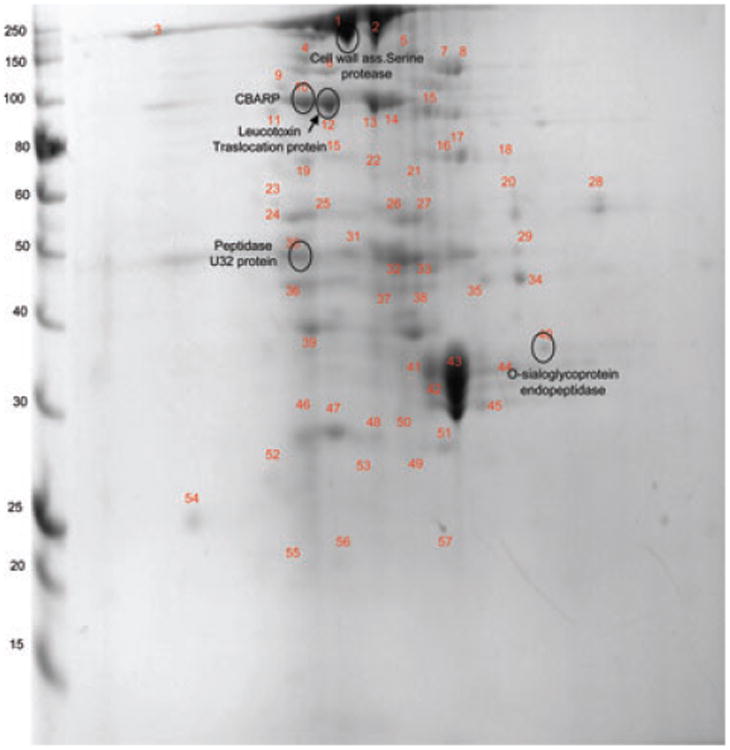
2D-PAGE of the extra-cellular fraction of F. alocis D-62D strain. 2D page was performed using 7-cm IPG strips of pI 3–10 in Protean IEF cell and 30–50 μg of protein and electrophoresed at 200 V, 0.3 A for 4–5 h, and stained with Coomassie simply blue strain. A total of 60 distinct spots were identified and processed for MS/MS analysis.
Table 1. F. alocis AJCC 35896 — membrane fraction.
| Spot | Accessiona) | Protein descriptiona) | Mol. wt/Cal- culated mol. wt (kDa) |
PMF Scoreb)/ (em PAI) |
Total peptides matchedc) |
PSORT prediction score and categoryd) |
Domainse) | HI scoref) |
Nature of proteing) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | HMPREF0389_01740 | Hypothetical protein | 104.4/104.3 | 35/0.09 | 5 | 0.243 CM | SecA ATPase domain | −0.56 | Nonsecretory protein |
| 2. | HMPREF0389_00724 | ATP-dependent chaperone protein (CIpB) | 97.5/97.6 | 14.98/1.85 | 82 | 0.380 CM | AAA domain (ATP associated with wide cellular activity) | −0.85 | Nonsecretory protein |
| 3. | HMPREF0389_01580 | Leucotoxin translocation ATP-binding protein LktB | 81/81.15 | 56/0.98 | 5 | 0.455 M | Four TMMH Domain P-loop NTPase domain | 0.78 | Cleavage site with no N terminal signal sequence |
| 4. | HMPREF0389_00784 | Copper amine oxidase N-domain protein | 80/94.2 | 27/0.05 | 6 | 0.227C 0.112M |
No conserved domain | −0.19 | Nonsecretory protein |
| 5. | HMPREF0389_00575 | Fibronectin-binding protein | 68.3/68.3 | 22/0.11 | 12 | 0.421 CM | N terminal fibronectin-binding domain with prokaryotes | −0.56 | Nonsecretory protein |
| 6. | HMPREF0389_00021 | Hypothetical protein | 70/69.2 | 76/0.63 | 8 | 2.356 C 0.114M |
DUF 2156 uncharacterized conserved domain | −0.56 | Nonsecretory protein |
| 7. | HMPREF0389_00233 | TraG family protein | 66.7/66.8 | 23/0.32 | 4 | 0.238 M | Two TMMH domain | 3.23 | N terminal signal sequence Secretory protein |
| 8. | HMPREF0389_00426 | Type IV pilus assembly protein (PilB) | 61/61.51 | 25 | 7 | 0.274 C 0.111 CM |
Bacterial type II secretion system protein E signature domain | −0.12 | Nonsecretory protein |
| 9. | HMPREF0389_00804 | Periplasmic Oligo peptide binding protein | 60/60.3 | 106/0.31 | 14 | 0.640 CM | ABC type transporter signal domain | 2.26 | N terminal cleavage site. Possible lipoprotein transporter |
| 10. | HMPREF0389_01305 | Chaperonin GroL | 57.7/60 | 2327/4.90 | 130 | 4.213 C 0.065 M |
Type-I chaperonin doman | −0.19 | Nonsecretory protein |
| 11. | HMPREF0389_00773 | Amino acyl histidine dipeptidase | 55/54.8 | 41/0.29 | 4 | 3.927 C 0.964 M |
M20 peptidase D domain | 0.41 | Nonsecretory protein |
| 12. | HMPREF0389_01130 | Fe hydrogenase large subunit family protein | 55.5/54.8 | 71/0.08 | 3 | 0.251C 0.284 M |
Iron hydrogenase domain | 0.89 | Nonsecretory protein |
| 13. | HMPREF0389_01643 | Hypothetical protein | 53/53.37 | 25/0.08 | 4 | 0.120 CM | No conserved domains identified | 1.85 | N terminal signal peptide Secretory protein |
| 14. | HMPREF0389_01385 | Mg chelate like protein-magnesium transporter | 52.8/52.4 | 113/0.09 | 8 | 0.489 M | Five TMMH domains Divalent cation transporter domain | −0.19 | Weak cleavage site with no N terminal signal peptide |
| 15. | HMPREF0389_00816 | Signal recognition particle protein | 49.7/49.8 | 22/0.07 | 27 | 0.483 CM | SRP-Signal peptide binding domain | −0.45 | Nonsecretory protein |
| 16. | HMPREF0389_01719 | Hypothetical protein | 48.9/48.5 | 41/0.07 | 5 | 0.168 CM | Bacterial trigger factor C terminus domain | −0.11 | Nonsecretory protein |
| 17. | HMPREF0389_01646 | Trigger factor | 48.5/48.6 | 41/0.09 | 5 | 0.168 CM | Ribosome-associated trigger factor domain | 0.23 | Nonsecretory protein |
| 18. | HMPREF0389_00590 | Caax amino protease family | 47/47.15 | 75/0.16 | 6 | 0.579 CM | Eight TMMH domains Caax protease—self-immunity domain | −0.75 | Cleavage site with no N terminal signal sequence |
| 19. | HMPREF0389_01716 | Hypothetical protein | 47.2 | 25/0.09 | 5 | 0.215 M | Glutamate DH multi domain | −0.78 | Nonsecretory protein |
| 20. | HMPREF0389_01584 | Arginine deaminase | 46.6/46.5 | 247/0.54 | 27 | 2.31 C 0.193 M |
Amidotransferase domain | 1.85 | Nonsecretory protein |
| 21. | HMPREF0389_01173 | Dehydrogenase/methenyl tetrahydrofolate cyclohydrolase | 49/49.5 | 41/0.11 | 8 | 2.135C 0.129M |
Tetrahydrofolate catalytic domain | 0.89 | Nonsecretory protein |
| 22. | HMPREF0389_00745 | Acetyl coA acetyl transferase | 41/41.3 | 819/1.74 | 54 | 4.235 C 0.110M |
Thiolase domain | −0.89 | Nonsecretory protein |
| 23. | HMPREF0389_01570 | Acetyl ornithine transaminase | 43.9/44 | 550/1.22 | 52 | 0.203 C 0.432 CM |
AAT superfamily domain | 0.89 | Nonsecretory protein |
| 24. | HMPREF0389_00225 | Transcriptional regulatory protein | 48.3/47.9 | 64/0.09 | 5 | 0.128 CM | No conserved domains | −0.68 | Nonsecretory protein |
| 25. | HMPREF0389_00021 | Hypothetical protein | 37.3/37.2 | 41/0.11 | 5 | 0.425C 0.056CM |
DUF 2156 domain | −0.23 | Nonsecretory protein |
| 26. | HMPREF0389_00599 | Hypothetical protein | 39.5/39.2 | 24/0.24 | 4 | 3.872 M | Five TMMH domains | 1.74 | N terminal signal sequence Secretory protein |
| 27. | HMPREF0389_01692 | Hypothetical protein | 44/44.2 | 908/1.75 | 53 | 0.298 C 0.123 CM |
Translation elongation factor domain | −0.56 | Nonsecretory |
| 28. | HMPREF0389_01658 | Acetate kinase | 43.7/43 | 80/0.16 | 6 | 0.106 C 0.056 M |
Acetate kinase domain | −0.35 | No signal sequence |
| 29. | HMPREF0389_01707 | Hypothetical protein | 42.5/42.3 | 80/0.16 | 6 | 1.12 C 0.89 M |
Acetate kinase domain | 0.12 | Nonsecretory protein |
| 30. | HMPREF0389_01733 | Hypothetical protein | 44/43.98 | 201/0.24 | 16 | 3.21 C 0.11 M |
Translation elongation factor domain | −0.19 | Nonsecretory protein |
| 31. | HMPREF0389_01077 | Dihydrodipicolinate reductase | 37/38.01 | 21/0.11 | 3 | 2.31 C 0.239 M |
Dehyrogenase domain | 0.78 | Nonsecretory protein |
| 32. | HMPREF0389_00704 | TRAP transporter solute receptor—TAXI family | 36/35.9 | 47/0.31 | 9 | 0.119M | One TMMH domain | 0.36 | N terminal signal peptide Secretory protein |
| 33. | HMPREF0389_00145 | TIM —barrel protein | 36.2/36.15 | 38/0.09 | 5 | 3.564 C 0.117 M |
Phosphate-binding domain | −0.56 | Nonsecretory protein |
| 34. | HMPREF0389_00742 | Electron transfer flavoprotein alpha subunit protein | 35/35.1 | 246/0.43 | 15 | 0.160 M | ETF electron acceptor domain | −0.56 | Nonsecretory protein |
| 35. | HMPREF0389_01141 | Hypothetical protein | 30/30 | 24/0.11 | 9 | 4.65 C 0.362 M |
No conserved domain | − | Nonsecretory protein |
| 36. | HMPREF0389_01354 | NG, NG dimethyl arginine, dimethyl amino hydrolase | 29/29.2 | 48/0.11 | 5 | 2.568 C 0.116 M |
Amidotransferase domain | −0.36 | Nonsecretory protein |
| 37. | HMPREF0389_00553 | Septum site determining protein MinD | 29/29.5 | 55/0.11 | 3 | 0.126 M | Membrane-associated ATPase domain | −0.56 | Nonsecretory protein |
| 38. | HMPREF0389_01240 | 3-Oxy acyl carrier protein Amino acid carrier protein | 27.5/28 | 341/1.23 | 19 | 0.747 M | Eight TMMH domains | 0.45 | Uncleavable N terminal signal sequence |
| 39. | HMPREF0389_00128 | GTP sensing transcriptional pleotropic repressor CodY | 28/28.4 | 72/0.40 | 7 | 0.374 C 0.089 M |
GAF-like domain (found to repress the dipeptide transport operon) | 0.36 | Nonsecretory protein |
| 40. | HMPREF0389_01469 | Glutaconate coA transferase | 29.5/30 | 1385/7.46 | 191 | 0.110C 0.056 M |
CoA tranferase domain | −0.51 | Nonsecretory protein |
| 41. | HMPREF0389_01209 | Ruberythrin | 21.4/21.6 | 48/0.34 | 10 | 4.84 C 0.213 M |
Ferritin-like di iron binding domain | −0.65 | Nonsecretory protein |
| 42. | HMPREF0389_01167 | CRISPR-associated protein | 18./20.1 | 22/3.9 | 16 | 3.56 C 0.251 M |
CRISPR domain (clustered regularly interspaced short palindromic repeats) | −0.36 | Nonsecretory protein |
| 43. | HMPREF0389_01230 | Hypothetical protein | 22/22.02 | 123/1.06 | 13 | 0.447 CM | No conserved domain | 0.11 | Nonsecretory protein |
| 44. | HMPREF0389_00682 | CBS domain protein | 23.5/23.7 | 38/0.40 | 6 | 3.61 C 0.197 M |
CBS-Bateman domain | 0.12 | Nonsecretory protein |
| 45. | HMPREF0389_00796 | Superoxide reductase | 12.5 | 53/0.56 | 3 | 4.235 C 1.89 M |
SOR-like domain | −0.45 | Nonsecretory protein |
| 46. | HMPREF0389_00708 | Alkaline shock protein | 14/14.02 | 80/0.24 | 3 | 0.175 C 0.052 M |
DUF322 domain | 0.36 | Nonsecretory protein |
| 47. | HMPREF0389_00532 | D-Alanine–D-alanine ligase | 40/40.3 | 45/0.330 | 10 | 3.21 C 0.112 M |
Substrate binding LTTR domain | 0.89 | Nonsecretory protein |
| 48. | HMPREF0389_00019 | Membrane protein | 37.8/36.2 | 32/0.31 | 7 | 0.703 CM | TMMH domain | −0.78 | Nonsecretory protein |
| 49. | HMPREF0389_00429 | Serine-glucine-hydroxymethyl transferase | 47/46.9 | 24/0.09 | 7 | 2.123 C 0.075 M |
SHMT domain | −0.48 | Nonsecretory protein |
| 50. | HMPREF0389_00639 | ATP/GTP-binding protein | 28/32.2 | 120/0.09 | 10 | 0.348 CM | Protein transfer/ATP-GTP binding domain | 1.85 | Nonsecretory protein |
Accession numbers and protein descriptions are from the NCBI- F alocis genome project (http://www.ncbi.nlm.nih.gov/genomeprj/46625).
Peptide mass fingerprinting score from Mascot.
Number of matched peptides derived from Mascot.
Psort prediction score classifying protein as C, cytoplasmic; CM, cytoplasmic membrane; M, membrane; CW, cell wall; PP, periplasm; Ex, extra cellular.
Conserved domain prediction using the NCBI-Conserved domain database search.
Hydropathy index (HI) score from the iPsort predictions on the nature of protein, high positive score indicate presence of a signal sequence negative scores predict proteins as nonsecretory.
iPsort prediction showing protein to contain signal sequence, cleavage site and classification of protein to be secretory or nonsecretory.
Table 4. F. alocis D-62D— extracellular fraction.
| Spot | Accessiona) | Protein descriptiona) | Mol. wt/Cal- culated mol. wt (kDa) |
PMF scoreb)/ (emPAl) |
Total peptides matchedc) |
PSORT prediction score and categoryd) |
Domainse) | HI scoref) |
Nature of proteing) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | HMPREF0389_01110 | Cell wall associated serine proteinase | 262 | 29/0.01 | 32 | CM-9.39 EC-0.61 |
Membrane lipid attachment domain | 1.61 | N terminal signal peptide |
| 2. | HMPREF0389_01728 | Conserved hypothetical protein | 246.5 | 25/0.04 | 4 | EC-4.32 CW-5.69 |
Peptidoglycan anchor domain | 1.37 | N terminal signal peptide |
| 3. | HMPREF0389_01692 | Hypothetical protein | 223 | 98/0.16 | 4 | C-7.50 EC-0.73 |
Hemolysin III type calcium-binding signature domain Inorganic pyrophosphate domain | – | Nonsecretory |
| 4. | HMPREF0389_01419 | Conserved hypothetical protein | 145.2 | 43/0.11 | 7 | C-7.5 EC-0.73 |
DNA-binding domain | – | Nonsecretory |
| 5. | HMPREF0389_01687 | Pyruvate-flavodoxin oxidoreductase | 130 | 156/0.29 | 45 | C-7.50 EC-0.73 |
4 Fe-4S ferredoxin-type iron–sulfur binding domain | 1.86 | N terminal signal peptide |
| 6. | HMPREF0389_01448 | Calcium-binding acid repeat protein | 103 | 25/020 | 16 | Unknown | SLH domains-3 numbers | 2.0 | N terminal signal peptide |
| 7. | HMPREF0389_00724 | ATP-dependent chaperone protein | 97.5 | 902/0.68 | 45 | C-9.97 EC-0.02 |
Chaperonin cIpA/B signature domain | – | Nonsecretory |
| 8. | HMPREF0389_01431 | Conserved hypothetical protein | 97.5 | 21/0.07 | 4 | EC-2.92 | Precursor signal domain | 1.02 | N terminal signal sequence |
| 9. | HMPREF0389_00122 | Protease | 88.5 | 32/0.10 | 7 | EC-0.73 | Peptidase collagenase family domain | – | Nonsecretory |
| 10. | HMPREF0389_00279 | ATP-dependent protease La | 87.9 | 26/0.07 | 5 | C-9.97 EC-0.02 |
Serine protease La binding domain | – | Nonsecretory |
| 11. | HMPREF0389_000638 | Membrane protein | 80.45 | 18/0.11 | 6 | M | Membrane protein precursor signal | – | |
| 12. | HMPREF0389_01580 | Leucotoxin translocation ATP-binding protein | 81.1 | 59/0.09 | 8 | CM-10 | Peptidase C-39, ABC transporter 2 domains | – | Nonsecretory |
| 13. | HMPREF0389_01452 | Conserved hypothetical protein | 76.8 | 12/0.05 | 3 | CM-9.87 EC-0.03 |
S-layer precursor signal domain | 2.32 | N terminal signal peptide |
| 14. | HMPREF0389_01452 | Conserved hypothetical protein | 76.8 | 16/0.07 | 3 | CM-9.8 EC-0.12 |
S-layer precursor signal domain | 2.32 | N terminal signal peptide |
| 15. | HMPREF0389_01750 | Hypothetical protein | 71/69.8 | 22/0.05 | 8 | 3.10C 1.05 M |
Collagen-binding protein B domain | 0.12 | Nonsecretory protein |
| 16. | HMPREF0389_00315 | Conserved hypothetical protein | 70.7 | 138/0.08 | 6 | C-7.5 EC-0.75 |
Secretory system-2 pilus domain | – | Nonsecretory |
| 17. | HMPREF0389_00315 | Conserved hypothetical protein | 70.7 | 35/0.11 | 4 | C-7.50 EC-0.73 |
Secretory system-2 pilus domain | – | Nonsecretory |
| 18. | HMPREF0389_00575 | Fibronectin-binding protein | 68.1 | 102/0.25 | 12 | C-7.50 EC-0.73 |
Protein A binding adherence fibronectin/fibrinogen domain | – | Nonsecretory |
| 19. | HMPREF0389_00223 | S layer Y containing domain | 66.7 | 651/0.69 | 22 | CW-9.2 EC-0.78 |
S layer homology domain | 1.88 | N terminal single peptide |
| 20. | HMPREF0389_01573 | V-type ATP synthetase alpha chain | 66.4 | 21/0.09 | 3 | C-9.97 | ATP-binding V type domain | – | Nonsecretory |
| 21. | HMPREF0389_00803 | Conserved hypothetical protein | 63.8 | 30/0.05 | 4 | Unknown | Histidine kinase domain | – | Nonsecretory |
| 22. | HMPREF0389_00261 | Conserved hypothetical protein | 62.9 | 17/0.07 | 4 | Unknown | EF-hand calcium-binding domain | – | Nonsecretory |
| 23. | HMPREF0389_00804 | Oligopeptide-binding protein | 60.25 | 27/0.07 | 7 | EC-0.91 | Lipid attachment domain at two positions | 2.26 | N terminal signal peptide |
| 24. | HMPREF0389_01605 | Formate tetra hydrofolate ligase | 60.17 | 16/0.07 | 3 | C-7.0 EC-0.73 |
FTH dignature domain | – | Nonsecretory |
| 25. | HMPREF0389_00868 | DAK 2 domain protein | 58.8 | 11/0.06 | 2 | C-7.50 EC-0.73 |
DhaL profile domain | – | Nonsecretory |
| 26. | HMPREF0389_00596 | UDP-N-muramyl tripeptide synthetase | 57.06 | 145/0.13 | 16 | C-7.50 EC-0.73 |
Cell wall tripeptide synthetase domain | – | Nonsecretory |
| 27. | HMPREF0389_00480 | Amido transferase family protein | 54.7 | 345/0.54 | 34 | C-7.5 EC-0.73 |
GATB domain | – | Nonsecretory |
| 28. | HMPREF0389_01130 | Ferrous hydrogenase | 54.3 | 32/0.07 | 3 | C-7.5 EC-0.73 |
Ferrodoxin type Fe-S binding domain | 1.88 | N terminal signal peptide |
| 29. | HMPREF0389_01374 | Conserved hypothetical protein | 48.5 | 52/0.07 | 6 | C-9.5 EC-0.2 |
YNIH -BH1805-YDJI domain | – | Nonsecretory |
| 30. | HMPREF0389_00504 | Peptidase U32 family protein | 47.7 | 22/0.09 | 7 | C-7.50 EC-0.73 |
Peptidase U 32 domain | – | Nonsecretory |
| 31. | HMPREF0389_01584 | Arginine deiminase | 46.6 | 23/0.07 | 12 | C-7.5 EC-0.73 |
Arginine deiminase domain | Nonsecretory | |
| 32. | HMPREF0389_01344 | NLP/P60 domain protein | 45.4 | 26/0.07 | 8 | EC-9.60 C-0.15 |
G5 domain | – | Nonsecretory |
| 33. | HMPREF0389_01570 | Acetyl ornithine transaminase | 43.5 | 195/0.44 | 16 | C-9.97 EC0.02 |
AA transfer class 3 domain | – | Nonsecretory protein |
| 34. | HMPREF0389_00538 | Processive diacylglycerol glucosyl transferase | 42.2 | 11/0.05 | 2 | Unknown | MGDG domain | – | Nonsecretory |
| 35. | HMPREF0389_01465 | Conserved hypothetical protein | 41.5 | 29/0.13 | 4 | C-7.5 EC-0.73 |
Hydrolase domain | – | Nonsecretory |
| 36. | HMPREF0389_00744 | Butryl coA dehydrogenase | 41.25 | 11/0.07 | 2 | C-7.5 EC-0.73 |
Acetyl coA DH1 and DH2 domain | – | Nonsecretory |
| 37. | HMPREF0389_00745 | Acetyl coA acetyl transfserase | 40.9 | 51/0.07 | 9 | C-7.2 EC-0.93 |
Thiolase 1,2,3 domain | – | Nonsecretory |
| 38. | HMPREF0389_01008 | Membrane lipoprotein | 39.54 | 39/0.06 | 5 | Unknown | N terminal lipoprotein lipid attachment domain | 2.31 | N terminal signal peptide |
| 39. | HMPREF0389_01567 | N acetyl gamma glutamyl phosphate reductase | 38.7 | 79/0.34 | 13 | Unknown | NAC gama glutamyl phosphate domain | – | |
| 40. | HMPREF0389_01445 | O-Sialoglycoprotein endo peptidase. | 36.6/36.2 | 22/0.55 | 5 | 1.806 C 1.17M 1.17M |
Metal-dependent protease-molecular chaperone domain | –0.45 | Potent cleavage site without N terminal signal sequence |
| 41. | HMPREF0389_00940 | Phosphate acetyl transferase | 36.0 | 26/0.11 | 2 | C-7.5 EC-0.73 |
Acetyl transferase metal binding domain | – | Nonsecretory |
| 42. | HMPREF0389_00704 | TRAP transporter solute receptor protein | 35.9 | 344/0.30 | 16 | Unknown | Membrane lipoprotein lipid attachment domain | 1.12 | N terminal signal peptide |
| 43. | HMPR45EF0389_01401 | Conserved hypothetical protein | 34.9 | 30/0.09 | 6 | EC-0.73 | Precursor signal YQFA trans membrane domain | 3.67 | Strong N terminal signal peptide |
| 44. | HMPREF0389_00901 | Cobalt import ATP-binding protein | 33.92 | 22/0.08 | 8 | CM-8.79 EC-0.09 |
CBIO and ATP transporter domain | – | Nonsecretory |
| 45. | HMPREF0389_01198 | L-aminopeptidase | 33.3 | 12/0.09 | 4 | C-7.50 EC-0.73 |
Peptidase DMPA hydrolase domain | – | Nonsecretory |
| 46. | HMPREF0389_01569 | Acetyl glutamate kinase | 31.03 | 741/2.34 | 59 | EC-0.73 | Arginine amino acid kinase | – | |
| 47. | HMPREF0389_01545 | Copper amine oxidase N-domain protein | 30.5 | 22/0.09 | 6 | Unknown | Precursor signal domain | 1.96 | N terminal signal sequence |
| 48. | HMPREF0389_01619 | Iron-sulfur cluster-binding protein | 29.1 | 24/0.51 | 2 | C-7.5 EC-0.73 |
4Fe-4S-ferredoxin type iron-sulfur binding domain | – | Nonsecretory |
| 49. | HMPREF0389J582 | Phosphoglycerate mutase | 29.1 | 45/0.56 | 7 | C-6.23 | PGAM domain | – | Nonsecretory |
| 50. | HMPREF0389_00100 | Glutamate racemase | 29.4 | 47/0.06 | 10 | EC-0.75 C-3.45 | Aspartate glutamate racemase signature | – | |
| 51. | HMPREF0389_01471 | Glutaconyl coA decarboxylase | 29.4 | 383/0.22 | 18 | CM | Acetyl CoA CT- N and C terminal domains | – | |
| 52. | HMPREF0389_00119 | Pyrroline-5-carboxylate reductase | 28.8 | 10/0.07 | 2 | C-9.95 | P5CR domain | – | Nonsecretory |
| 53. | HMPREF0389_00743 | Electron transfer flavoprotein beta | 28.2 | 26/0.12 | 3 | EC-0.73 C-6.32 |
ET-Flavoprotein beta domain | – | |
| 54. | HMPREF0389_01259 | Histidinol phosophatase | 25.2 | 36/0.10 | 3 | C-7.50 EC-0.73 |
PHP-C terminal domain | – | Nonsecretory |
| 55. | HMPREF0389_00321 | Conserved hypothetical protein | 23.9 | 21/0.10 | 4 | Unknown | No conserved domain | 2.01 | N terminal signal peptide |
| 56. | HMPREF0389_00975 | TetR family transcriptional regulator | 22.8 | 65/0.12 | 6 | C-7.50 EC-0.73 |
HTH-TETR-2 domain | – | Nonsecretory |
| 57. | HMPREF0389_01744 | Conserved hypothetical protein | 22.7 | 11/0.09 | 2 | C-7.5 EC-0.73 |
Uncharacterized domain | – | Nonsecretory |
| 58.h) | HMPREF0389_01071 | Tetracyclin resistant protein | 20.8 | 34/0.11 | 4 | C-7.50 EC-0.73 |
No conserved domain | – | Nonsecretory |
| 59.h) | HMPREF0389_01503 | Conserved hypothetical protein | 15.7 | 11/0.07 | 2 | Unknown | No conserved domain | – | Nonsecretory |
| 60.h) | HMPREF0389_01654 | Neutrophil-activating factor protein A | 14/16.2 | 12/0.07 | 2 | 2.310 C 0.099 M |
DPS domain | 0.32 | Nonsecretory |
| 61.h) | HMPREF0389_01741 | Conserved hypothetical protein | 8.7 | 32/0.07 | 2 | Unknown | No conserved domain | – | Nonsecretory |
Accession numbers and protein descriptions are from the NCBI F alocis genome project (http://www.ncbi.nlm.nih.gov/genomeprj/46625).
Peptide mass fingerprinting score from Mascot.
Number of matched peptides derived from Mascot.
Psortb prediction score classifying protein as: C, cytoplasmic; CM, cytoplasmic membrane; M, membrane; CW, cell wall; PP, periplasm; Ex, extra cellular. Non classical secretory protein in silico prediction through “Secretome.”
Conserved domain prediction using the NCBI-Conserved domain database search.
Hydropathy index (HI) score from the iPsort predictions on the nature of protein, high positive score indicate presence of a signal sequence negative scores predict proteins as non secretory.
iPsort prediction showing protein to contain signal sequence, cleavage site and classification of protein to be secretory or nonsecretory.
Low molecular weight protein spots not clearly seen in Fig. 6 but confirmed by MS analysis.
In the membrane fraction, there were 50 distinct reproducible spots identified in the F. alocis ATCC strain (Fig. 3) as compared to 56 spots in F. alocis D-62D strain (Fig. 4). The proteins identified in the membrane fraction are summarized in Tables 1 and 2. Several groups of very intense spots in the range of 30 –60 kDa were observed in F. alocis ATCC 35896. While some of these intense spots were noted at the same range in the D-62D strain, there were higher molecular weight protein spots between 260 and 70 kDa that were missing in the ATCC strain (Table 1). Intense protein spots corresponding to copper amine oxidase (HMPREF0389_00784), leukotoxin ATP-binding translocation protein (HMPREF0389_1580) were present in both the strains, however, protein spots corresponding to CBARP (HMPREF0389_1532), layer Y-domain protein (HMPREF0389_1139), peptidoglycan biosynthesis transpeptidase (HMPREF0389_00555), and fibronectin-binding protein (HMPREF0389_00575) were present only in the F. alocis D-62D strain (Table 2). All these spots corresponded to high molecular weight proteins ranging from 205 to 68 kDa. Several hypothetical proteins, common to both F. alocis strains were identified in the membrane fraction. Based on the domain prediction, proteins corresponding to protein secretory pathways such as hypothetical protein (HMPREF0389_1740)–(Sec pathway) and Type IV pilus assembly protein (HMPREF0389_00426)–(Type II secretion system) were noted in the ATCC strain. The protein export membrane protein (HMPREF0389_1478) predicted to be involved in the Sec pathway was identified in the F. alocis D-62D strain. While the membrane fraction of F. alocis ATCC strain showed only the Caax amino protease (HMPREF0389_00590), the more virulent F. alocis D-62D strain had nine proteins belonging to the protease/peptidase family: peptidoglycan biosynthesis transpeptidase, peptidase M23/M37, oligo endopeptidase F, amino acyl histidine dipeptidase, Caax amino protease, Xaa pro-dipeptidase (HMPREF0389_1538), signal peptidase, ATP-dependent RIP metalloprotease, O-sialoglycoprotein endopeptidase. It is noteworthy that the Xaa pro dipeptidase was shown to be highly upregulated during coculture of F. alocis with P. gingivalis [18]. Other high molecular weight proteins, identified in F. alocis D-62D include acetyl ornithine transaminase (HMPREF0389_1570), membrane protein (HMPREF0389_00019), pyridoxine biosynthesis protein (HMPREF0389_00858), NG, NG dimethyl arginine, dimethyl amino hydrolase (HMPREF0389_1354), toxin antitoxin component ribbon helix–helix fold protein (HMPREF0389_00243), and hypothetical protein (HMPREF0389_01030). Homologs of several proteins involved in cell wall biosynthesis in S. mutans [21] (peptidoglycan biosynthesis transpeptidase (HMPREF0389_00555)), antibiotic resistance in F. nucleatum [23] (tetracycline-resistant protein (HMPREF0389_01071)), and virulence in P. gingivalis [52] (trigger factor (HMPREF0389_1646)), and DNA-binding response regulator protein (HMPREF0389_01693 with respective domain) were also observed in the membrane fraction of both strains.
Figure 4.
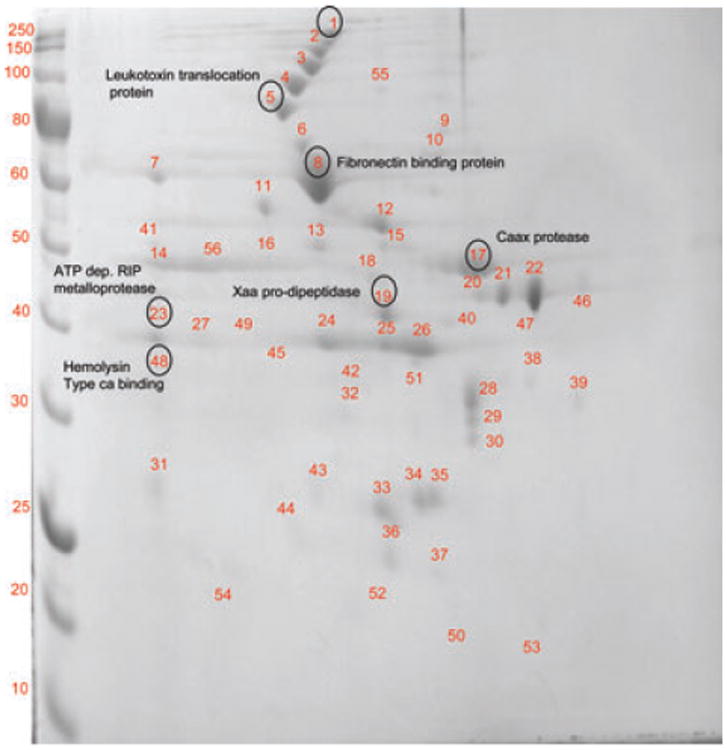
2D-PAGE of the membrane fraction of F alocis D-62D strain. 2D page was performed using 7-cm IPG strips of pI 3–10 in Protean IEF cell and 30–50 μg of protein and electrophoresed at 200 V, 0.3 A for 4–5 h and stained with Coomassie simply blue strain. A total of 54 distinct spots were identified and processed for MS/MS analysis.
Table 2. F. alocis D-62 D—membrane fraction.
| Spot | Accessiona) | Protein descriptiona) | Mol. wt/Cal- culated mol. wt (kDa) |
PMF scoreb)/ (emPAl) |
Total peptides matchedc) |
PSORT prediction score and categoryd) |
Domainse) | HI scoref) |
Nature of proteing) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | HMPREF0389_01532 | Calcium-binding acid repeat proteins | 205/209 | 21/19.8 | 2 | 4.23 C 0.112 M |
Lipase domain | 1.785 | N terminal signal sequence |
| 2. | HMPREF0389_01139 | S-layery domain containing protein | 140/147 | 89/89.2 | 17 | 0.300 M | SLH domain | 1.167 | Signal peptide Cleavage protein |
| 3. | HMPREF0389_00555 | Peptidoglycan biosynthesis transpeptidase | 107/107.7 | 41/0.39 | 5 | 2.104 M | Pencillin-binding protein transpeptidase domain | 3.25 | Uncleavable N terminal signal peptide sequence |
| 4. | HMPREF0389_01225 | Copper amine oxidase N domain protein | 80.2/80.3 | 25/0.06 | 6 | 3.00 CW/M | N terminal copper amine oxidase domain | 2.75 | Cleavable N terminal signal sequence Secretory protein |
| 5. | HMPREF0389_01580 | Leucotoxin translocation ATP-binding protein LktB | 81/81.15 | 71/0.91 | 5 | 0.455 M | Four TMMH domain P loop NTPase domain | 0.78 | Cleavage site with no N terminal signal sequence |
| 6. | HMPREF0389_01693 | Hypothetical protein | 75/75.1 | 25/0.12 | 4 | 0.232C | Two TMMH domains | 2.35 | Uncleavable N terminal signal sequence Secretory protein |
| 7. | HMPREF0389_01750 | Hypothetical protein | 71/69.8 | 30/0.05 | 8 | 3.10 C | Collagen binding protein B domain | 0.12 | Nonsecretory protein |
| 8. | HMPREF0389_00575 | Fibronectin-binding protein | 68.3/68.3 | 20/0.11 | 10 | 0.421 CM | N terminal fibronectin-binding domain with prokaryotes | −0.56 | Nonsecretory protein |
| 9. | HMPREF0389_00239 | Peptidase M23/M37 family | 69.8/69.9 | 23/0.33 | 5 | 2.37 M | NlpC/P60 family Lipoprotein domain | −0.63 | Non classical secretory proteinh) |
| 10. | HMPREF0389_00416 | Type IV pilus assembly protein | 65/64.8 | 24/0.07 | 2 | 3.65 C 1.230 M |
Tfp pilus assembly protein domain | Nonsecretory protein | |
| 11. | HMPREF0389_00926 | Oligo endo peptidase F | 65.7/65.9 | 22/0.56 | 2 | 4.37 C 0.128 M |
Peptidase family M3B domain | 0.23 | Nonsecretory protein |
| 12. | HMPREF0389_00804 | Periplasmic oligo peptide binding protein | 60.3/60.1 | 106/0.31 | 14 | 0.640 CM | ABC type transporter signal domain | 2.26 | N terminal cleavage site. Possible lipoprotein transporter |
| 13. | HMPREF0389_00773 | Amino acyl histidine dipeptidase | 55/54.8 | 41/0.29 | 4 | 3.927 C 0.964 M |
M20 peptidase D domain | 0.41 | Nonsecretory protein |
| 14. | HMPREF0389_01064 | Amino acid carrier protein | 50.8/50.2 | 125/0.09 | 18 | 4.789 M | Nine TMMH domainsSodium: alanine symporter family domain | −0.98 | Nonsecretory protein |
| 15. | HMPREF0389_01173 | Dehydrogenase/methenyl tetrahydrofolate cyclohydrolase Bifunctional protein GlmU | 49/49.5 | 42/0.10 | 9 | 2.135 C 0.129M |
Tetrahydrofolate catalytic domain | −0.75 | Nonsecretory protein |
| 16. | HMPREF0389_01385 | Mg chelate like protein-magnesium transporter | 52.8/53 | 124/0.12 | 9 | 0.489 M | Five TMMH domains Divalent cation transporter domain | −0.19 | Weak cleavage site with no IM terminal signal peptide |
| 17. | HMPREF0389_00590 | Caax amino protease family | 47/47.15 | 127/0.07 | 15 | 0.579 CM | Eight TMMH domains Caax protease-self-immunity domain | −0.75 | Cleavage site with no N terminal signal sequence |
| 18. | HMPREF0389_01276 | Iron permease FTR1 family | 47.4/47 | 52/7.4 | 6 | 4.286 M | Six TMMH domainsFTR1 domain | 2.29 | Cleavable N terminal signal sequence Secretory protein |
| 19. | HMPREF0389_01538 | Xaa pro dipeptidase | 42/39.8 | 75/0.16 | 6 | 4.491 C 0.477 M |
Metallopeptidase family M24 (play roles in regulation of biological processes rather than general protein degradation) | 0.12 | Nonsecretory protein |
| 20. | HMPREF0389_00799 | Signal peptidase (amino peptidase l family) | 42.6/42.2 | 39/0.32 | 6 | 4.673 M | Eight TMMH domains | −0.84 | Nonsecretory protein |
| 21. | HMPREF0389_01222 | Hypothetical protein | 44/43.5 | 27/0.23 | 17 | 4.302 C 0.470 M |
Metal-dependent hydrolase domain | −0.12 | Nonsecretory protein |
| 22. | HMPREF0389_01570 | Acetyl ornithine transaminase | 43.9/44 | 208/0.59 | 15 | 0.203C 0.432CM |
AAT superfamily domain | 0.89 | Nonsecretory protein |
| 23. | HMPREF0389_00112 | ATP-dependent RIP metalloprotease | 38.3/38.1 | 85/7.3 | 8 | 4.501 M | Three TMMH domains PDZ metalloprotease domain | 2.96 | Cleavable N terminal signal sequence Secretory protein |
| 24. | HMPREF0389_00090 | Heat inducible transcr858iptional repressor protein | 39.7/39.76 | 21/0.11 | 3 | 3.59 C 1.35 M |
HrcA protein C domain (negatively regulate the transcription of heat shock qenes) | −0.56 | Nonsecretory protein |
| 25. | HMPREF0389_00672 | Hypothetical protein | 38.7/38.5 | 21/0.84 | 4 | 4.375 M | Five TMMH domain | 0.74 | Nonsecretory protein |
| 26. | HMPREF0389_00599 | Hypothetical protein | 39.5/39.2 | 24/0.24 | 4 | 3.872 M | Five TMMH domains | 1.74 | N terminal signal sequence Secretory protein |
| 27. | HMPREF0389_01104 | Hypothetical protein | 38.3/38.4 | 21/0.32 | 6 | 2.39 C 1.36 M |
DHH family domain | −0.36 | Nonsecretory |
| 28. | HMPREF0389_00019 | Membrane protein | 37.8/36.2 | 28/0.25 | 5 | 1.102 M 2.838 Ex |
TMMH domain | −0.78 | Nonsecretory protein |
| 29. | HMPREF0389_00858 | Pyridoxine biosynthesis protein | 30/30.27 | 22/0.14 | 4 | 4.836 C 0.148 M |
No conserved domain | −0.63 | Nonsecretory protein |
| 30. | HMPREF0389_01354 | NG, NG dimethyl arginine, dimethyl amino hydrolase | 29/29.2 | 48/0.11 | 5 | 2.568 C 0.116 M |
Amidotransferase domain | −0.36 | Nonsecretory protein |
| 31. | HMPREF0389_01172 | Hypothetical protein | 27.52/27.3 | 38/0.24 | 4 | 4.251 M | Six TMMH domains membrane transporter domain protein | −0.74 | Nonsecretory protein |
| 32. | HMPREF0389_00639 | ATP/GTP-binding protein | 28/32.2 | 26/0.62 | 3 | 0.348 CM | Protein transfer/ATP-GTP binding domain | −1.09 | Nonsecretory |
| 33. | HMPREF0389_01587 | Aspartate racimase | 26.3/26.4 | 25/0.13 | 4 | 3.018 C 1.706 M |
Aspartate racemase multidomain | −0.12 | Nonsecretory protein |
| 34. | HMPREF0389_00107 | UMP kinase | 25.6/25.1 | 24/0.13 | 6 | 4.580 C 0.365 M |
UMPK domain | −0.56 | Nonsecretory protein |
| 35. | HMPREF0389_01594 | GTP pyrophospokinase | 27.1/27.3 | 4.535 C 0.428 M |
Nucleotidyl transferase (NT) domain | − | Nonsecretory protein | ||
| 36. | HMPREF0389_00415 | Fimbrial assembly protein PilN | 25.9/26.1 | 32/0.71 | 5 | 3.37 C 1.15M |
Fimbrial assembly protein domain | 0.12 | Nonsecretory protein |
| 37. | HMPREF0389_01157 | Hypothetical protein | 19/19.4 | 27/0.23 | 3 | 4.667 C 0.115M |
DUF 1877 domain | −0.56 | Nonsecretory protein |
| 38. | HMPREF0389_01657 | Membrane protein | 37.5/36.8 | 22 | 2 | 0.442 CM | Six TMMH domains and precursor signal inner protein domain | 1.366 | N terminal cleavage signal |
| 39. | HMPREF0389_00742 | Electron transfer flavoprotein alpha subunit protein | 35/35.1 | 246/0.43 | 15 | 0.160 M | ETF electron acceptor domain | −0.56 | Nonsecretory protein |
| 40. | HMPREF0389_00637 | NLP/P60 family protein | 27/36.1 | 22/0.45 | 4 | 3.860 CM | Lipoprotein domain | 2.35 | N terminal signal sequence Secretory protein |
| 41. | HMPREF0389_00816 | Signal recognition particle protein | 49.7/49.8 | 21/0.25 | 25 | 0.483 CM | SRP signal peptide binding domain | −0.45 | Nonsecretory protein |
| 42. | HMPREF0389_01566 | Oligo peptide/dipeptide ABC transporter substrate/ATP binding protein | 31.3/31 | 78/7.42 | 16 | 2.175 M | Copper amine oxidase N terminal domain | 2.87 | A gram positive N terminal signal peptide sequence Secretory protein |
| 43. | HMPREF0389_00768 | Peroxiredoxin | 20/19.9 | 26/0.36 | 6 | 4.129 C 0.490 M |
Thiol specific antioxidant domain | −0.65 | Nonsecretory protein |
| 44. | HMPREF0389_01476 | Hypothetical protein | 20.1/20 | 48/0.42 | 4 | 1.973 M | DUF 1836 uncharacterized domain | −0.78 | Nonsecretory |
| 45. | HMPREF0389_00295 | Ribose ABC transporter, periplasmic ribose binding protein | 32/32.4 | 65/0.56 | 6 | 0.300 M | Periplasmic binding fold domain | 2.84 | N terminal signal peptide sequence |
| 46. | HMPREF0389_01217 | Hypothetical protein | 37.3/37 | 21/0.09 | 4 | 3.837 C 1.038 M |
ParB-like nuclease domain | −0.68 | Nonsecretory protein |
| 47. | HMPREF0389_01478 | Protein export membrane protein | 35/35.3 | 21/0.09 | 4 | 4.754 M | Nine TMMH domains SecD and SecF domain | 3.23 | N terminal signal peptide sequence |
| 48. | HMPREF0389_01477 | Hemolysin III type calcium-binding protein. | 31/31.1 | 32/1.01 | 6 | 4.807 M | Six TMMH Domains-Hemolysin –III domain | −0.23 | Nonsecretory |
| 49. | HMPREF0389_00894 | Zinc ABC transporter periplasmic zinc binding protein | 34.1/34.3 | 54/5.2 | 7 | 4.811 C 0.105 M |
Periplasmic solute binding protein domain | 2.84 | Cleavable N terminal signal sequence Secretory protein |
| 50. | HMPREF0389_00243 | Toxin antitoxin component, ribbon —helix-helix fold protein | 10.6/10.5 | 45/1.03 | 5 | 3.913 C 0.509 PP |
RelB antitoxin domain | 0.11 | Nonsecretory |
| 51. | HMPREF0389_01445 | O-Sialoglycoprotein endopeptidase. | 36.6/36.2 | 22/0.55 | 5 | 1.806 C 1.17 M |
Metal-dependent protease–molecular chaperone domain | −0.45 | Potent cleavage site without IM terminal signal sequence |
| 52. | HMPREF0389_01654 | Neutrophil-activating factor protein A | 14/16.2 | 29/0.56 | 2 | 2.310 C 0.099 M |
DPS domain | 0.32 | Nonsecretory |
| 53. | HMPREF0389_01030 | Hypothetical protein | 15.6/15.9 | 24/0.22 | 4 | 4.662 C 0.103 M |
No conserved domain | −0.41 | Nonsecretory |
| 54. | HMPREF0389_00964 | Ferric uptake regulatory protein | 17/17.2 | 21/0.32 | 4 | 3.74 C 0.820 M |
Iron-dependent DNA binding reporessor/activator domain | −0.88 | Nonsecretory protein |
| 55. | HMPREF0389_ 01266 | Cation transporting ATPase | 99.7/99.76 | 49/0.72 | 6 | 2.781 M | C terminus cation transporting ATPase domain | −0.41 | Nonsecretory protein |
| 56. | HMPREF0389_01748 | Hypothetical protein | 52/52.1 | 26/0.32 | 4 | 2.489 M | Anion permease transmembrane domain | 2.67 | Potent cleavage site without IM terminal signal sequence |
Accession numbers and protein descriptions are from the NCBI F alocis genome project (http://www.ncbi.nlm.nih.gov/genomeprj/46625).
Peptide mass fingerprinting score from Mascot.
Number of matched peptides derived from Mascot.
Psort prediction score classifying protein as: C, cytoplasmic; CM, cytoplasmic membrane; M, membrane; CW, cell wall; PP, periplasm; Ex, extra cellular.
Conserved domain prediction using the NCBI-Conserved domain database search.
Hydropathy index (HI) score from the iPsort predictions on the nature of protein, high positive score indicate presence of a signal sequence negative scores predict proteins as nonsecretory.
iPsort prediction showing protein to contain signal sequence, cleavage site and classification of protein to be secretory or non-secretory,
Nonclassical secretory protein in silico prediction through “Secretome.”
In the extracellular fraction, a total of 54 and 57 nonredundant reproducible proteins were identified in F. alocis ATCC 35896 and D-62D, respectively (Figs. 5 and 6, Tables 3 and 4). Intense protein spots were found between 30 and 75 kDa in the ATCC 35896 strain. Though similar proteins were identified in the two strains, proteins such as cell wall serine protease (HMPREF0389_01110), conserved hypothetical protein (HMPREF0389_01728), hypothetical protein (HMPREF0389_01692), protease (HMPREF0389_00122), ATP-dependent protease La (HMPREF0389_00279), and leucotoxin translocation ATP-binding protein (HMPREF0389_01580) were identified only in the extracellular fraction of F. alocis D-62D, the more virulent strain. Leucotox in is known as a membrane-active toxin that specially targets human polymorphonuclear leucocytes and monocytes [53]. While it can remain associated with the bacterial cell surface, its secretion is mediated by a Type I secretion system in Gram-negative bacteria [54]. It is unknown if there is a defect in the secretion system of F. alocis ATCC 35896 or other factors may alter the secretion of the leucotoxin. In A. actinomycetemcomitans, lipopolysaccharide can mediate leukotoxin secretion [55]. Proteins such as fibronectin-binding protein [56] and S layer protein [57] that are secreted by Type-1 secretion system in other bacteria were found in the extracellular fraction of both the F. alocis strains likely, suggesting the presence of a Type 1 secretory system. A secretion mechanism and the impact of leucotoxin secretion in F. alocis pathogenesis are under study in the laboratory. An intense protein spot of molecular weight 34.9 kDa corresponding to the conserved hypothetical protein (HMPREF0389_01401) was noted exclusively in F. alocis D-62D. (Table 4). Even though the lower molecular weight proteins between 10 and 25 kDa were noted in F. alocis ATCC 35896, conserved hypothetical proteins (HMPREF0389_00741), (HMPREF0389_00321), and (HMPREF0389_01744) were found only in F. alcois D-62D. These three conserved hypothetical proteins showed no specific conserved domains. It was also interesting to note that six hypothetical proteins including HMPREF0389_01693, HMPREF0389_01750, HMPREF0389_00607, HMPREF0389_01489, HMPREF0389_01177, and HMPREF0389_01239 were unique to the F. alocis ATCC 35896. Among them, three were found to be involved with the regulation of cell function.
Figure 5.
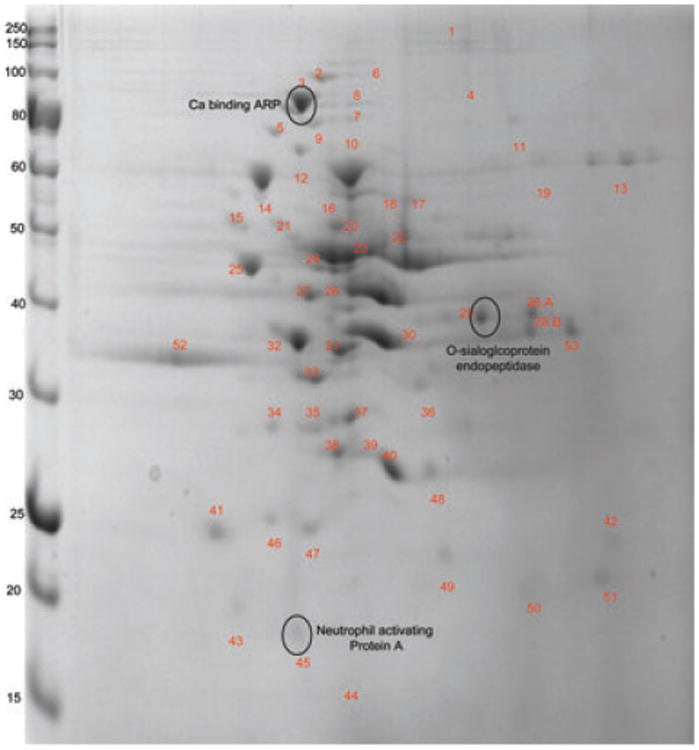
2D-PAGE of the extra-cellular fraction of F. alocis–ATCC-35896 strain. 2D page was performed using 7 cm IPG strips of pI 3–10 in Protean IEFcell and 30–50 μg of protein and electrophoresed at 200 V, 0.3 A for 4–5 h, and stained with Coomassie simply blue strain. A total of 55 distinct spots were identified and processed for MS/MS analysis.
Table 3. F. alocis AJCC 35896 — extracellular fraction.
| Spot | Accessiona) | Protein descriptiona) | Mol. wt/Cal- culated mol. wt (kDa) |
PMF scoreb)/ (emPAl) |
Total peptides matchedc) |
PSORT prediction score and categoryd) |
Domainse) | HI scoref) |
Nature of proteing,h) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | HMPREF0389_01419 | Conserved hypothetical protein | 145.4 | 32/0.39 | 4 | C-7.50 EC-0.73 |
DNA—ATP-binding domain | – | Nonsecretory |
| 2. | HMPREF0389_01687 | Pyruvate-flavodoxin oxidoreductase | 130 | 24/0.07 | 2 | C-7.50 EC-0.73 |
4 Fe-4S ferredoxin-type iron-sulfur binding domain | 1.86 | N terminal signal peptide |
| 3. | HMPREF0389_01448 | Calcium-binding acid repeat protein | 103 | 34/0.42 | 6 | Unknown | SLH domains —3 numbers | 2.0 | N terminal signal peptide |
| 4. | HMPREF0389_01431 | Conserved hypothetical protein | 97.5 | 71/0.92 | 5 | EC -2.92 | Precursor signal domain | 1.02 | N terminal signal sequence |
| 5. | HMPREF0389_00279 | ATP-dependent protease La | 87.9 | 22/0.12 | 4 | C-9.97 EC-0.02 |
Serine protease La binding domain | – | Nonsecretory |
| 6. | HMPREF0389_000638 | Membrane protein | 80.45 | 23/0.33 | 7 | M | Membrane protein precursor signal | – | Nonsecretory |
| 7. | HMPREF0389_01452 | Conserved hypothetical protein | 76.8 | 20/0.11 | 10 | CM-9.8 EC-0.12 |
S-layer precursor signal domain | 2.32 | N terminal signal peptide |
| 8. | HMPREF0389_01693 | Conserved hypothetical protein | 75.1 | 25/0.12 | 12 | Unknown | GGDEF response regulatory domain | 1.2 | N terminal signal peptide |
| 9. | HMPREF0389_01750 | Hypothetical protein | 71/69.8 | 30/0.05 | 8 | 3.10 C 1.05 M |
Collagen binding protein B domain | – | Nonsecretory protein |
| 10. | HMPREF0389_00315 | Conserved hypothetical protein | 70.7 | 28/0.07 | 6 | C-7.50 EC-0.73 |
Secretory system-II pilus domain | Nonsecretory | |
| 11. | HMPREF0389_00575 | Fibronectin-binding protein | 68.1 | 34/0.32 | 7 | C-7.50 EC-0.73 |
Protein A binding adherence fibronectin/fibrinogen domain | – | Nonsecretory |
| 12. | HMPREF0389_00223 | S layer Y containing domain | 66.7 | 22/0.33 | 3 | CW-9.2 EC-0.78 |
S layer homology domain | 1.88 | N terminal single peptide |
| 13. | HMPREF0389_00803 | Conserved hypothetical protein | 63.8 | 24/0.07 | 3 | Unknown | Histidine kinase domain | – | Nonsecretory |
| 14. | HMPREF0389_01605 | Formate tetra hydrofolate ligase | 60.17 | 28/0.07 | 2 | C-7.00 EC-0.73 |
FTH dignature domain | – | Nonsecretory |
| 15. | HMPREF0389_00804 | Oligopeptide-binding protein | 60.25 | 32/0.11 | 4 | EC-0.91 | Lipid attachment domain at two positions | 2.26 | N terminal signal peptide |
| 16. | HMPREF0389_00868 | DAK 2 domain protein | 58.8 | 22/0.07 | 2 | C-7.50 EC-0.73 |
DhaL profile domain | – | Nonsecretory |
| 17. | HMPREF038_00596 | UDP-N –muramyl tripeptide synthetase | 57.06 | 102/0.24 | 16 | C-7.50 | Cell wall tripeptide synthetase domain | – | Nonsecretory |
| 18. | H22MPREF0389_00480 | Amido transferase family protein | 54.7 | 22/0.08 | 4 | C-7.50 EC-0.73 |
GATB domain | – | Nonsecretory |
| 19. | HMPREF0389_01130 | Ferrous hydrogenase | 54.3 | 51/0.16 | 12 | C-7.5 EC-0.73 |
Ferrodoxin type Fe-S binding domain | 1.88 | N terminal signal peptide |
| 20. | HMPREF0389_00225 | Trancriptional regulatory protein | 48 | 84/0.18 | 17 | C-7.5 EC-0.73 |
No conserved domains | – | Nonsecretory |
| 21. | HMPREF0389_01374 | Conserved hypothetical protein | 48.5 | 289/0.30 | 20 | C-9.5 EC-0.2 |
YNIH-BH1805-YDJI domain | Nonsecretory | |
| 22. | HMPREF0389_00504 | Peptidase U32 family protein | 47.7 | 22/0.07 | 4 | C-7.50 EC-0.73 |
Peptidase U 32 domain | – | Nonsecretory |
| 23. | HMPREF0389_01584 | Arginine deiminase | 46.6 | 56/0.18 | 18 | C-7.5 EC-0.73 |
Arginine deiminase domain | – | Nonsecretory |
| 24. | HMPREF0389_01570 | Acetyl ornithine transaminase | 43.5 | 182/0.44 | 19 | C-9.97 EC-0.02 |
AA transfer class 3 domain | – | Nonsecretory |
| 25. | HMPREF0389_01465 | Conserved hypothetical protein | 41.5 | 55/0.16 | 8 | C-7.5 EC-0.73 |
Hydrolase domain | Nonsecretory | |
| 26. | HMPREF0389_000744 | Butryl coA dehydrogenase | 41.25 | 64/0.16 | 12 | C-7.50 EC-0.73 |
Acetyl coA DH1 and DH2 domain | – | Nonsecretory |
| 27. | HMPREF0389_00745 | Acetyl coA acetyl transfserase | 40.9 | 78/0.15 | 32 | C-7.2 EC-0.93 |
Thiolase 1,2,3 domain | – | Nonsecretory |
| 28A. | HMPREF0389_01567 | N acetyl gamma glutamyl phosphate reductase | 38.7 | 212/0.56 | 20 | Unknown | NAC gama glutamyl phosphate domain | – | Nonsecretory |
| 28B. | HMPREF0389_00567 | Glyceraldehyde 3 phosphate dehydrogenase | 37.9/39.5 | 39/0.06 | 5 | 0.351 C | Nil | – | Nonsecretory |
| 29. | HMPREF0389_01445 | O-Sialoglycoprotein endopeptidase | 36.6/36.2 | 248/0.65 | 24 | 1.806 C 1.17M |
Metal-dependent protease—molecular chaperone domain | –0.45 | Potent cleavage site without N terminal signal sequence |
| 30. | HMPREF0389_00607 | Conserved hypothetical protein | 36.2 | 22/0.09 | 5 | Unknown | Precursor signal domain | 1.71 | N terminal signal peptide |
| 31. | HMPREF0389_00704 | TRAP transporter solute receptor protein | 35.9 | 35/0.12 | 6 | Unknown | Lipoportein lipid attachment domain | 1.12 | N terminal signal peptide |
| 32. | HMPREF0389_01401 | Conserved hypothetical protein | 34.9 | 32/0.09 | 7 | EC-0.73 | Precursor signal YQFA trans membrane domain | 3.67 | Strong N terminal signal peptide |
| 33. | HMPREF0389_00901 | Cobalt import ATP-binding protein | 33.9-5.2 | 9 | CM-8.73 EC-0.09 |
CBIO-ATP binding domain | – | Nonsecretory | |
| 34. | HMPREF0389_01489 | Conserved hypothetical protein | 32.3 | 67/0.25 | 7 | Unknown | No conserved domains | 2.21 | Signal peptide at the N terminal |
| 35. | HMPREF0389_01569 | Acetyl glutamate kinase | 31.03 | 42/0.08 | 7 | EC-0.73 | Arginine amino acid kinase | Nonsecretory | |
| 36. | HMPREF0389_01545 | Copper amine oxidase N-domain protein | 30.5 | 43/0.13 | 6 | Unknown | Precursor signal domain | 1.96 | N terminal signal sequence |
| 37. | HMPREF0389_01471 | Glutaconyl coA decarboxylase | 29.4 | 313/0.18 | 16 | CM | Acetyl CoA CT- N and C terminal domains | – | Nonsecretory |
| 38. | HMPREF0389_ | Glutamate racemase | 29.4 | 42/0.12 | 6 | EC-0.75 C-3.45 |
Aspartate glutamate racemase signature | – | Nonsecretory |
| 39. | HMPREF0389_00100 | Septum site determining protein | 28.9 | 306/0.84 | 17 | CM | Septum site determining cell division inhibitor domain | 28.9 | N terminal signal peptide |
| 40. | HMPREF0389_01597 | Electron transfer flavoprotein beta | 28.2 | 14/0.07 | 2 | EC-0.73 C-6.32 |
ET-Flavoprotein beta domain | – | Nonsecretory |
| 41. | HMPREF0389_01177 | Conserved hypothetical protein | 27.1 | 45/0.14 | 6 | Unknown | No conserved domains | – | |
| 42. | HMPREF0389_01259 | Histidinol phosphatase | 25.2-6.36 | 32/0.11 | 4 | C-7.50 EC-0.73 |
PHP-C terminal domain | – | Nonsecretory |
| 43. | HMPREF0389_01071 | Tetracyclin resistant protein | 20.8-6.35 | 59/0.13 | 11 | C-7.50 EC-0.73 |
No conserved domain | – | Nonsecretory |
| 44. | HMPREF0389_01503 | Conserved hypothetical protein | 15.7-9.1 | 43/0.11 | 10 | Unknown | No conserved domain | – | Nonsecretory |
| 45. | HMPREF0389_01654 | Neutrophil-activating factor protein A | 14/16.2 | 33/0.12 | 8 | 2.310 C 0.099 M |
DPS domain | 0.32 | Nonsecretory |
| 46. | HMPREF0389_01741 | Conserved hypothetical protein | 8.7-9.4 | 12/0.04 | 2 | Unknown | No conserved domain | – | Nonsecretory |
| 47. | HMPREF0389_01594 | GTP pyrophosphokinase | 25.1/26.8 | 28/0.07 | 2 | 0.244 C | GTP kinase domain | –0.84 | Nonsecretory |
| 48. | HMPREF0389_ 01239 | Hypothetical protein | 23/27.3 | 69/0.10 | 2 | 0.094 C | None | 1.833 | Signal peptide Lipoprotein cleavage signal |
| 49. | HMPREF0389_01176 | N-acetylmuramoyl-L-alanine amidase | 27 | 16/0.09 | 4 | Multiple localization | Autolysin precursor signal domain | Nonsecretory | |
| 50. | HMPREF0389_00927 | Hypothetical protein | 23.9 | 11/0.03 | 2 | C-7.5 EC-0.73 |
Unknown | – | Nonsecretory |
| 51. | HMPREF0389_01268 | 3H domain protein | 20.5 | 22/0.07 | 2 | C-7.5 EC-0.73 |
Biotin 3H domain | – | Nonsecretory |
| 52. | HMPREF0389_00921 | Thioredoxin family protein | 33.2 | 12/0.07 | 2 | Multiple localization | Reductase peptide methionine | Nonsecretory | |
| 53. | HMPREF0389_00877 | Radical SAM domain containing protein | 36 | 11/0.06 | 4 | C-7.5 EC-0.73 |
Oxidoreductase SAM domain | – | Nonsecretory |
Accession numbers and protein descriptions are from the NCBI F alocis genome project (http://www.ncbi.nlm.nih.gov/genomeprj/46625).
Peptide mass fingerprinting score from Mascot.
Number of matched peptides derived from Mascot.
Psortb prediction score classifying protein as: C, cytoplasmic; CM, cytoplasmic membrane; M, membrane; CW, cell wall; PP, periplasm; Ex, extra cellular.
Conserved domain prediction using the NCBI-Conserved domain database search.
Hydropathy index (HI) score from the iPsort predictions on the nature of protein, high positive score indicate presence of a signal sequence negative scores predict proteins as non secretory.
iPsort prediction showing protein to contain signal sequence, cleavage site and classification of protein to be secretory or non-secretory,
Nonclassical secretory protein in silico prediction through “Secretome.”
3.3 Amino acid metabolism
Consistent with its assacharolytic properties, several proteins that play an important role in this process were identified in both membrane and extracellular fractions of F. alcois. Certain oral bacteria such as F. nucleatum lack essential amino acid synthetic pathways and rely on the ability to import and degrade di- and oligopeptides [58]. With the occurrence of a wide range of such dipeptidases, metalloproteases and O-sialoglycoproteases (refer Table 5), F. alocis, could also lack some inherent amino acid synthesis pathways but could alternate through degradation of proteins with the help of such proteases and peptidase. It is important to note that protein spots corresponding to ornithine transaminase (HMPREF0389_01570), acetyl glutamate kinase (HMPREF0389_01569), glutamate racemase (HMPREF0389_00100), and amidotransferase (HMPREF0389_00478) involved in ornithine biosynthesis were identified. These proteins were found both in the membrane and the extracellular fraction of the D-62D strain. Proteins involved in ornithine catabolism and urea breakdown, namely arginine deiminase (HMPREF0389_01584), were also noted. Taken together, it is likely that F. alocis could have a well-developed nitrogen assimilatory pathway that is needed for alternative mode of amino acid synthesis [59].
Table 5. Proteases in the genome of F. alocis.
| Name | Annotation |
|---|---|
| RIP metalloprotease | HMPREF0389_00112 |
| Protease | HMPREF0389_00122 |
| ATP-dependent protease La | HMPREF0389_00279 |
| Zinc protease | HMPREF0389_00298 |
| ATP-dependent zinc metalloprotease FtsH | HMPREF0389_01001 |
| Caax amino protease family protein | HMPREF0389_00677 |
| Caax amino protease | HMPREF0389_00590 |
| Metalloprotease | HMPREF0389_00692 |
| Glycoprotease family protein | HMPREF0389_01443 |
| Xaa pro dipeptidase | HMPREF0389_01538 |
| O-sialoglycoprotein endopeptidase | HMPREF0389_01445 |
| Serine protease HtrA | HMPREF0389_01460 |
| ATP-dependent Clp protease | HMPREF0389_01648 |
| Carboxy-processing protease | HMPREF0389_00522 |
| Oligoendopeptidase F | HMPREF0389_00926 HMPREF0389_00527 |
Certain proteins such as oxy acyl carrier protein (HMPREF0389_ 01112) involved in fatty acid metabolism and not usually identified among the oral biofilm forming pathogens, was identified in F. alocis [60]. Strain-specific proteins such as fibronectin-binding protein (HMPREF0389_00575) and dipicolinate reductase (HMPREF0389_01077) involved in amino acid metabolism and virulence [61] were noted in the D-62D strain of F. alocis.
3.4 Proteases
In bacteria, proteolysis plays an important role in many biological processes such as posttranslational regulation of gene expression, processing, and maturation of various surface-associated proteins in Gram-positive bacteria [62, 63]. Proteases have been one of the virulence attributes among many oral pathogens [7,39,64]. Expression of various surface proteins depends on proteolysis that could strongly influence both the level of activity of proteases and their cellular localization [44]. In our study, strain variations were noted among the proteases in both membrane and the extracellular fraction of F. alocis strains. Among the membrane-bound proteases of F. alocis, Caax protease (HMPREF0389_00590) has been identified in both the strains of F. alocis. The Caax amino proteases could be involved in the protein and/or peptide modification and secretion [50]. Caax amino-terminal proteases of S. gordonii have been demonstrated to play a role in transport of proteins and protect the bacteria against bacteriocins, other than their metalloprotease activity [65]. The Xaa-pro-dipeptidase (HMPREF0389_01538), O-sialoendopeptidase (HMPREF0389_01445), peptidase M23/37 (HMPREF0389_00239), and oligo endopeptidase F (HMPREF0389_00926) were also identified only in the membrane fraction of the F. alocis D-62D strain. However, protease (HMPREF0389_00122) was identified only in the extracellular fraction of D-62D strain. Based on motif search and domain prediction studies, this protease is predicted to possess a collagen peptidase function. The role of such protease could be important in F. alocis pathogenesis as it has the potential to damage the connective tissue of the gingiva. Several oral pathogens are known to produce or induce host-derived collagenases that are implicated in tissue destruction in periodontal diseases [66–68]. The role of several of the proteases in F. alocis pathogenesis is under further investigation.
3.5 Secretory system
In general, certain oral pathogens such as Fusobacteria are known to secrete few proteins and lack the genes encoding the components of the major secretory systems [58]. However, in F. alocis a conserved hypothetical protein (HMPREF0389_00315)—a secretory system-II pilus domain containing protein was identified in the ATCC strain. Proteins involved in type-II secretory pathway, namely, Type IV pilus assembly protein (HMPREF0389_00426) and trigger factor (HMPREF0389_01646), were also identified in the membrane fraction of the F. alocis ATCC 35896. The membrane fraction of the F. alcois D-62D showed proteins involved in the Sec pathway such as, fimbrial assembly protein (HMPREF0389_00415) and protein export membrane protein (HMPREF0389_01478) (containing SecD and SecF domains). A total of seven proteins predicted to be involved in protein transport were noted in the D-62D strain. The relative abundance of these proteins was less in the type strain compared to the F. alocis D-62D (Table 6). Hence, it is likely that F. alocis has a well-developed type-II and Sec-dependent protein transport pathway that could vary based on the relative virulence of the pathogen and/or its interaction with other organisms.
Table 6. Proteome profiling and categorization of various fractions of F. alocis strains.
| Strains and fractions | Protein classification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Protease/ peptidases |
Lipo proteins |
Protein metabolism |
Hypothetical protein |
Energy metabolism |
Protein secretory pathways |
Transport and binding proteins |
Antitoxin and antigens |
DNA metabolism |
Regulatory protein |
|
| ATCC–MEMBRANE FRACTION | 2 | 1 | 11 | 11 | 5 | 2 | 4 | 1 | 3 | 4 |
| D-62D–MEMBRANE FRACTION | 8 | 1 | 8 | 10 | 5 | 1 | 7 | 2 | 3 | 5 |
| ATCC–EXTRA CELLULAR FRACTION | 4 | 1 | 8 | 14 | 6 | 1 | 4 | 0 | 3 | 8 |
| D-62D–EXTRA CELLULAR FRACTION | 6 | 1 | 16 | 15 | 7 | 2 | 8 | 3 | 5 | 8 |
3.6 Virulence
Several proteins known to be involved in virulence in other bacteria were observed in both the membrane and extracellular fractions of F. alocis D-62D. CBARP (HMPREF0389_01532), leucotoxin translocation ATP-binding protein (HMPREF0389_01580), fibronectin-binding protein (HMPREF0389_00575), Type IV pilus assembly protein (HMPREF0389_00416), fimbrial assembly protein (HM-PREF0389_00415), Hemolysin III type calcium-binding protein (HMPREF0389_01477), toxin–antitoxin component protein (HMPREF0389_00243), and NAPA (HM-PREF0389_01654) were observed in the membrane fraction. There was also expression of CBARP, leucotoxin translocation ATP-binding protein, fibronectin-binding proteins, and NAPA in the extracellular fraction, in addition to the TetR family transcription regulator (HMPREF0389_00975) and tetracycline-resistant protein (HMPREF0389_01071). Similar to T. denticola, Clostridium botulinum, and P. gingivalis, there was expression of S-layer protein (HMPREF0389_01139) that was only observed in the membrane fraction of F. alocis D-62D [58]. Several glycolytic enzymes such as phosphoglycerate mutase (HMPREF0389_1582) and glyceraldehyde-3-phosphate dehydrogenase (HMPREF0389_00567) that are basically involved in energy metabolism were both identified in the extracellular fraction of F. alocis. Such proteins could have a moonlighting function as plasminogen-binding protein and adhesins for fibronectin and plasminnogens, respectively [69]. Protein moonlighting contributes to bacterial virulence in a number of important human pathogens [70].
3.7 In silico analysis of the membrane proteins
Surface proteins are important factors in the interaction of pathogenic bacteria with their environment and host [71]. A comparative bioinformatic analysis of the membrane proteins between the D-62D and the type strains of F. alocis revealed more cell wall anchor domain containing proteins (Table 7; Supporting Information Table 2). One subgroup of these proteins is the sortase-dependent proteins that have a C terminal LP×TG motif [72]. There are various groups of sortase enzymes with specific motifs, namely, a sortase B motif of NP(Q/K)(T/S)(N/G/S)(D/A), Sortase D motif of LP×TA, and other atypical sortase anchor motifs resembling the LP×TG motifs with minor variations [72]. Our study found more proteins with these motifs in the F. alocis D-62D strain in contrast to the type strain. Using the annotated genome sequence of F. alocis, we observed that more than 25% of the membrane proteins of the D-62D strain showed N-terminal signal sequences. The majority of these proteins also carried a signal peptidase I cleavage site that could have secretory/membrane targeting functions. Approximately 25% of the total membrane proteins were found to possess transmembrane helix domain and hence may be involved in protein transport [73]. Several proteins with a common motif known to play a role in membrane anchorage were observed in both F. alocis strains. More of these proteins were unique to F. alocis D-62D. Proteins can also be anchored to the envelope by LysM domains that bind to the peptidoglycan in the bacterial cell wall [74]. There are four LysM domain proteins as compared to two in the type strain. Kleerebezem and co-workers [75] identified a novel C terminal W×L domain that they proposed could be a binding domain for the cell envelope. In this study, six proteins containing this domain were identified in the D-62D strain of F. alocis. It is noteworthy that of the 56 membrane proteins identified in F. alocis D-62D, 20 contain the peptidoglycan-binding domain that amounts to 36% of the total membrane proteins identified. Upon sequence alignment of the membrane proteins of the D-62D strain, it was evident that 25 proteins contain a C-terminal glycine rich domain with a concensus GxxGxGxGx motif (Supporting Information Fig. 1). Such C-terminal glycine rich domain was not identified in the type strain membrane proteins. Such glycine rich motifs are crucial for RNA binding [76]. Hence, from this in silico study of membrane proteins it could be inferred that F. aloics might have a well-developed protein secretory and transport system that could play a role in its pathogenic potential. The metabolic pathway analysis revealed no significant difference between the two strains.
Table 7. Genome-wide survey of F. alocis cell wall anchored proteins.
| Features | F. alocis ATCC strain (n = 50) | F. alocis D-62D strain (n = 56) |
|---|---|---|
| Export features | ||
| Signal sequence a) | 5 | 14 |
| Signal peptidase I cleavageb) | 4 | 9 |
| Transmembrane helix domain c) | 7 | 15 |
| Cell wall anchored proteins | ||
| N or C-terminally anchored proteins d) | 8 | 10 |
| Sortase A. LP×TG anchors e) | – | 2 |
| Atypical sortase anchors e) | 1 | 3 |
| Sortase B motif e) | 1 | – |
| NP(Q/K)(T/S)(N/G/S)(D/A) | ||
| Sortase D motif e) | – | 1 |
| LP×TA | ||
| Peptidoglycan-binding domains f) | 10 | 20 |
| LysM domains g) | 2 | 4 |
| Lipoprotein anchors h) | – | 2 |
| GW repeats i) | 5 | 10 |
| WxL domains j) | 3 | 6 |
| Glycin-rich C terminal motif k) | – | 25 |
Signal sequence prediction was done with the hidden Markov model in SignalP3.0 [28], with p values of > 0.95 as the cutoff.
Cleavage site prediction was done with the neural network model in SignalP3.0, with Cmax values of > 0.52 and Ymax values of > 0.32 as cutoffs.
TMMH prediction was made using SignalP3.0 [28].
The N terminal and C terminal domains were identified using the HMM.
LPXTG anchors, atypical sortase anchors, sortase D motifs were predicted by the hidden Markov model for sortase substrates [32].
From the Pfam database, with a cutoff E value of ≤ 10−5.
Lipoprotein prediction was done as described by Sutcliffe and Harrington [31].
Manual screening for the presence of GW residues in repetitive regions was performed.
Prediction based on the presence of the [LI]TW[TS]L motif in the C-terminal sequence.
Sequence alignment and C terminal motif identifications were made using MEGA version 4.0 [25].
4 Concluding remarks
This study identified several membrane-associated and extracellular proteins from F. alocis. Although the number of proteins identified was lower than the expected, bacteria are known to modulate specific genes in response to their environment [77]. In practice, many low abundance proteins, particularly intrinsic cytoplasmic membrane proteins although representing 15–30% of the proteome of a bacterium, are either not detected or represent less than 1% of the proteins displayed on the 2D gel [77]. Proteins including several proteases, NAPA, and CBARP were identified in F. alocis. The genes encoding these proteins were upregulated during infection of epithelial cells. A comparative in silico analysis among the D-62D and type strains of F. alocis revealed more cell wall anchoring proteins in the D-62D strain, suggesting the role of such proteins in protein secretion and virulence. Their expression was enhanced by coinfection with P. gingivalis. The variation in the pathogenic potential of the F. alocis strains may be related to the differential expression of several putative virulence factors. Future work is needed to understand the regulation of these genes in addition to their relative significance in F. alocis. A genome-wide association study of F. alocis strains is in progress to evaluate variations at the genome level.
Acknowledgments
This work was supported by Loma Linda University and Public Health Grants DE022724, DE13664, and DE019730 from NIDCR (to H.M.F.). We thank Floyd E. Dewhirst for the gift of the F. alocis D-62D isolate.
Abbreviations
- BHI
Brain heart infusion
- NAPA
neutrophil-activating protein A
Footnotes
References
- 1.Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito A, Inagaki S, Kimizuka R, Okuda K, et al. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2008;54:349–355. doi: 10.1111/j.1574-695X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 3.Saito A, Inagaki S, Ishihara K. Differential ability of periodontopathic bacteria to modulate invasion of human gingival epithelial cells by Porphyromonas gingivalis. Microb Pathog. 2009;47:329–333. doi: 10.1016/j.micpath.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front Biosci. 2007;12:3965–3974. doi: 10.2741/2363. [DOI] [PubMed] [Google Scholar]
- 5.Dewhirst FE, Chen T, Izard J, Paster BJ, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross EL, Leys EJ, Gasparovich SR, Firestone ND, et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. 2010;48:4121–4128. doi: 10.1128/JCM.01232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paster BJ, Boches SK, Galvin JL, Ericson RE, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J, Yang L, Paster BJ, Ganly I, et al. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS One. 2011;6:e22788. doi: 10.1371/journal.pone.0022788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffen AL, Beall CJ, Campbell JH, Firestone ND, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Elios MM, Amedei A, Cappon A, Del PG, de BM. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol Med Microbiol. 2007;50:157–164. doi: 10.1111/j.1574-695X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 11.Feuille F, Ebersole JL, Kesavalu L, Stepfen MJ, Holt SC. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infect Immun. 1996;64:2094–2100. doi: 10.1128/iai.64.6.2094-2100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontology 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar PS, Griffen AL, Barton JA, Paster BJ, et al. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 14.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, et al. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38:7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- 16.Cato EP, Moore LVH, Moore WEC. Fusobacterium alocis sp. nov. and Fusobacterium sulci sp. nov. from the human gingival sulcus. Int J Syst Bacteriol. 1985:475–477. [Google Scholar]
- 17.Jalava J, Eerola E. Phylogenetic analysis of Fusobacterium alocis and Fusobacterium sulci based on 16S rRNA gene sequences: proposal of Filifactor alocis (Cato, Moore and Moore) comb. nov. and Eubacterium sulci (Cato, Moore and Moore) comb. nov. Int J Syst Bacteriol. 1999;49:1375–1379. doi: 10.1099/00207713-49-4-1375. [DOI] [PubMed] [Google Scholar]
- 18.Aruni AW, Roy F, Fletcher HM. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by Porphyromonas gingivalis. Infect Immun. 2011;79:3872–3886. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mofatt CE, Whitmore SE, Griffen AL, Leys EJ, Lamont RJ. Filifactor alocis interactions with gingival epithelial cells. Mol Oral Microbiol. 2011;26:365–373. doi: 10.1111/j.2041-1014.2011.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey MM, Rumbaugh KP, Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011;7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo LH, Wang HL, Liu XD, Duan J. Identification of protein differences between two clinical isolates of Streptococcus mutans by proteomic analysis. Oral Microbiol Immunol. 2008;23:105–111. doi: 10.1111/j.1399-302X.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilkins JC, Homer KA, Beighton D. Altered protein expression of Streptococcus oralis cultured at low pH revealed by two-dimensional gel electrophoresis. Appl Environ Microbiol. 2001;67:3396–3405. doi: 10.1128/AEM.67.8.3396-3405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Haroni M, Skaug N, Bakken V, Cash P. Proteomic analysis of ampicillin-resistant oral Fusobacterium nucleatum. Oral Microbiol Immunol. 2008;23:36–42. doi: 10.1111/j.1399-302X.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 24.Lamont RJ, Meila M, Xia Q, Hackett M. Mass spectrometry-based proteomics and its application to studies of Porphyromonas gingivalis invasion and pathogenicity. Infect Disord Drug Targets. 2006;6:311–325. doi: 10.2174/187152606778249935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 26.Johnson LS, Eddy SR, Portugaly E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 2010;11:431. doi: 10.1186/1471-2105-11-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–D360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendtsen JD, Nielsen H, von HG, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Krogh A, Larsson B, von HG, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 30.Punta M, Coggill PC, Eberhardt RY, Mistry J, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe IC, Harrington DJ. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology. 2002;148:2065–2077. doi: 10.1099/00221287-148-7-2065. [DOI] [PubMed] [Google Scholar]
- 32.Boekhorst J, de Been MW, Kleerebezem M, Siezen RJ. Genome-wide detection and analysis of cell wall-bound proteins with LP×TG-like sorting motifs. J Bacteriol. 2005;187:4928–4934. doi: 10.1128/JB.187.14.4928-4934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz O, Young PA, Lamont RJ, Kenny GE. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology. 2003;149:2417–2426. doi: 10.1099/mic.0.26483-0. [DOI] [PubMed] [Google Scholar]
- 34.Castaneda-Roldan EI, velino-Flores F, Dall'Agnol M, Freer E, et al. Adherence of Brucella to human epithelial cells and macrophages is mediated by sialic acid residues. Cell Microbiol. 2004;6:435–445. doi: 10.1111/j.1462-5822.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 35.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulkerson JF, Jr, Mobley HL. Membrane topology of the NixA nickel transporter of Helicobacter pylori: two nickel transport-specific motifs within transmembrane helices II and III. J Bacteriol. 2000;182:1722–1730. doi: 10.1128/jb.182.6.1722-1730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poznanovic S, Schwall G, Zengerling H, Cahill MA. Isoelectric focusing in serial immobilized pH gradient gels to improve protein separation in proteomic analysis. Electrophoresis. 2005;26:3185–3190. doi: 10.1002/elps.200500224. [DOI] [PubMed] [Google Scholar]
- 38.Henry LG, Sandberg L, Zhang K, Fletcher HM. DNA repair of 8-oxo-7,8-dihydroguanine lesions in Porphyromonas gingivalis. J Bacteriol. 2008;190:7985–7993. doi: 10.1128/JB.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, et al. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 41.Abdullah KM, Lo RY, Mellors A. Cloning, nucleotide sequence, and expression of the Pasteurella haemolytica A1 glycoprotease gene. J Bacteriol. 1991;173:5597–5603. doi: 10.1128/jb.173.18.5597-5603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aruni W, Vanterpool E, Osbourne D, Roy F, et al. Sialidase and sialoglycoproteases can modulate virulence in Porphyromonas gingivalis. Infect Immun. 2011;79:2779–2791. doi: 10.1128/IAI.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bethe G, Nau R, Wellmer A, Hakenbeck R, et al. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol Lett. 2001;205:99–104. doi: 10.1111/j.1574-6968.2001.tb10931.x. [DOI] [PubMed] [Google Scholar]
- 44.Biswas S, Biswas I. Role of HtrA in surface protein expression and biofilm formation by Streptococcus mutans. Infect Immun. 2005;73:6923–6934. doi: 10.1128/IAI.73.10.6923-6934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dramsi S, Magnet S, Davison S, Arthur M. Covalent attachment of proteins to peptidoglycan. FEMS Microbiol Rev. 2008;32:307–320. doi: 10.1111/j.1574-6976.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 46.Gazi MI, Cox SW, Clark DT, Eley BM. Comparison of host tissue and bacterial dipeptidyl peptidases in human gingival crevicular fluid by analytical isoelectric focusing. Arch Oral Biol. 1995;40:731–736. doi: 10.1016/0003-9969(95)00032-k. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein JM, Kordula T, Moon JL, Mayo JA, Travis J. Characterization of an extracellular dipeptidase from Streptococcus gordonii FSS2. Infect Immun. 2005;73:1256–1259. doi: 10.1128/IAI.73.2.1256-1259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leiting B, Pryor KD, Wu JK, Marsilio F, et al. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J. 2003;371:525–532. doi: 10.1042/BJ20021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nepomuceno RS, Tavares MB, Lemos JA, Griswold AR, et al. The oligopeptide (opp) gene cluster of Streptococcus mutans: identification, prevalence, and characterization. Oral Microbiol Immunol. 2007;22:277–284. doi: 10.1111/j.1399-302X.2007.00368.x. [DOI] [PubMed] [Google Scholar]
- 50.Pei J, Grishin NV. Type II CAAX prenyl endopeptidases belong to a novel superfamily of putative membrane-bound metalloproteases. Trends Biochem Sci. 2001;26:275–277. doi: 10.1016/s0968-0004(01)01813-8. [DOI] [PubMed] [Google Scholar]
- 51.Reynaud af GA, Culak R, Molenaar L, Chattaway M, et al. Comparative analysis of virulence determinants and mass spectral profiles of Finnish and Lithuanian endodontic Enterococcus faecalis isolates. Oral Microbiol Immunol. 2007;22:87–94. doi: 10.1111/j.1399-302X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura M, Ohara N, Kondo Y, Shoji M, et al. Proteome analysis of Porphyromonas gingivalis cells placed in a subcutaneous chamber of mice. Oral Microbiol Immunol. 2008;23:413–418. doi: 10.1111/j.1399-302X.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 53.Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins (Basel) 2011;3:242–259. doi: 10.3390/toxins3030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res. 2010;89:561–570. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang G, Kawai T, Komatsuzawa H, Mintz KP. Lipopolysaccharides mediate leukotoxin secretion in Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol. 2012;27:70–82. doi: 10.1111/j.2041-1014.2011.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lannergard J, Flock M, Johansson S, Flock JI, Guss B. Studies of fibronectin-binding proteins of Streptococcus equi. Infect Immun. 2005;73:7243–7251. doi: 10.1128/IAI.73.11.7243-7251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toporowski MC, Nomellini JF, Awram P, Smit J. Two outer membrane proteins are required for maximal type I secretion of the Caulobacter crescentus S-layer protein. J Bacteriol. 2004;186:8000–8009. doi: 10.1128/JB.186.23.8000-8009.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapatral V, Anderson I, Ivanova N, Reznik G, et al. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol. 2002;184:2005–2018. doi: 10.1128/JB.184.7.2005-2018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doroshchuk NA, Gel'fand MS, Rodionov DA. Regulation of nitrogen metabolism in gram-positive bacteria. Mol Biol (Mosk) 2006;40:919–926. [PubMed] [Google Scholar]
- 60.Rathsam C, Eaton RE, Simpson CL, Browne GV, et al. Two-dimensional fluorescence difference gel electrophoretic analysis of Streptococcus mutans biofilms. J Proteome Res. 2005;4:2161–2173. doi: 10.1021/pr0502471. [DOI] [PubMed] [Google Scholar]
- 61.Berges DA, DeWolf WE, Jr, Dunn GL, Newman DJ, et al. Studies on the active site of succinyl-CoA:tetrahydrodipicolinate N-succinyltransferase. Characterization using analogs of tetrahydrodipicolinate. J Biol Chem. 1986;261:6160–6167. [PubMed] [Google Scholar]
- 62.Gottesman S, Wickner S, Maurizi MR. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 63.Laskowska E, Kuczynska-Wisnik D, Skorko-Glonek J, Taylor A. Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol Microbiol. 1996;22:555–571. doi: 10.1046/j.1365-2958.1996.1231493.x. [DOI] [PubMed] [Google Scholar]
- 64.Curtis MA, duse-Opoku J, Rangarajan M. Cysteine proteases of Porphyromonas gingivalis. Crit Rev Oral Biol Med. 2001;12:192–216. doi: 10.1177/10454411010120030101. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Whiteley M, Kreth J, Lei Y, et al. The two-component system BfrAB regulates expression of ABC transporters in Streptococcus gordonii and Streptococcus sanguinis. Microbiology. 2009;155:165–173. doi: 10.1099/mic.0.023168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontology. 2000;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 67.Robertson PB, Cobb CM, Taylor RE, Fullmer HM. Activation of latent collagenase by microbial plaque. J Periodontal Res. 1974;9:81–83. doi: 10.1111/j.1600-0765.1974.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 68.Robertson PB, Lantz M, Marucha PT, Kornman KS, et al. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982;17:275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 69.Henderson B, Nair S, Pallas J, Williams MA. Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev. 2011;35:147–200. doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 70.Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun. 2011;79:3476–3491. doi: 10.1128/IAI.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Pijkeren JP, Canchaya C, Ryan KA, Li Y, et al. Comparative and functional analysis of sortase-dependent proteins in the predicted secretome of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72:4143–4153. doi: 10.1128/AEM.03023-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spirig T, Weiner EM, Clubb RT. Sortase enzymes in Gram-positive bacteria. Mol Microbiol. 2011;82:1044–1059. doi: 10.1111/j.1365-2958.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer T, Berks BC. Moving folded proteins across the bacterial cell membrane. Microbiology. 2003;149:547–556. doi: 10.1099/mic.0.25900-0. [DOI] [PubMed] [Google Scholar]
- 74.Steen A, Buist G, Leenhouts KJ, El KM, et al. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem. 2003;278:23874–23881. doi: 10.1074/jbc.M211055200. [DOI] [PubMed] [Google Scholar]
- 75.Kleerebezem M, Boekhorst J, van KR, Molenaar D, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci. 2000;25:241–246. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- 77.Len AC, Cordwell SJ, Harty DW, Jacques NA. Cellular and extracellular proteome analysis of Streptococcus mutans grown in a chemostat. Proteomics. 2003;3:627–646. doi: 10.1002/pmic.200300391. [DOI] [PubMed] [Google Scholar]


