Abstract
The etiology of major depression remains unclear, but reduced activity of the serotonin (5-HT) system remains implicated and treatments that increase 5-HT neurotransmission can ameliorate depressive symptoms. 5-HT1A receptors are critical regulators of the 5-HT system. They are expressed as both presynaptic autoreceptors that negatively regulate 5-HT neurons, and as post-synaptic heteroreceptors on non-serotonergic neurons in the hippocampus, cortex, and limbic system that are critical to mediate the antidepressant actions of 5-HT. Thus, 5-HT1A auto- and heteroreceptors have opposite actions on serotonergic neurotransmission. Because most 5-HT1A ligands target both auto- and heteroreceptors their efficacy has been limited, resulting in weak or unclear responses. We propose that by understanding the transcriptional regulation of the 5-HT1A receptor it may be possible to regulate its expression differentially in raphe and projection regions. Here we review the transcriptional architecture of the 5-HT1A gene (HTR1A) with a focus on specific DNA elements and transcription factors that have been shown to regulate 5-HT1A receptor expression in the brain. Association studies with the functional HTR1A promoter polymorphism rs6295 suggest a new model for the role of the 5-HT1A receptor in susceptibility to depression involving early deficits in cognitive, fear and stress reactivity as stressors that may ultimately lead to depression. We present evidence that by targeting specific transcription factors it may be possible to oppositely regulate 5-HT1A auto- and heteroreceptor expression, synergistically increasing serotonergic neurotransmission for the treatment of depression.
Keywords: Serotonin, repressor, enhancer, antidepressant, raphe, autoreceptor
SEROTONIN AND MAJOR DEPRESSION
Depression continues to grow as a major health challenge with a lifetime prevalence for major depressive disorder (MDD) estimated at 16 % [1, 2] (Box 1). In developed countries, MDD currently accounts for the second highest lifetime burden of disease, and is predicted to be highest by 2030 [3–7]. Reduced serotonin (5-HT) neurotransmission has been implicated in MDD and related disorders such as anxiety, obsessive-compulsive disorders, and bulimia, which show improvement when treated with 5-HT-specific reuptake inhibitors (SSRIs) such as fluoxetine (Prozac) [8–19] (Box 2). Most antidepressants, including SSRIs, monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), are thought to act in part by enhancing 5-HT neurotransmission. Oppositely, acute tryptophan depletion rapidly reduces 5-HT synthesis and can induce a relapse in 50–80% of depressed patients [20–22]. Hence the 5-HT system is implicated in susceptibility and treatment of MDD. However, current treatment strategies require several weeks for clinical improvement, and are not always effective. For example, in the STAR*D studies, only about 50% of patients responded to the initial SSRI treatment, which achieved a remission rate of only 30% [23–28]. Even with adjunctive or alternate therapy only a remission rate of 40% could be achieved. In addition, due to the long 3–6 week latency required to assess responsiveness to therapy, many patients remain ineffectively treated, with a concomitant risk of suicide that is 15% in depressed subjects [29, 30].
Box 1. Depression, a major health challenge.
Depression is a chronic condition of high incidence (15% lifetime prevalence); by comparison lifetime prevalence for schizophrenia is 1%.
Depression is associated with a high risk of suicide and decreased quality of life and direct health-care costs at Cdn $4.7 billion.
Depression is ranked 2nd among medical conditions with the greatest worldwide disease burden and 1st by 2030 (World Health Organization).
Depression is diagnosed by psychiatric tests; no genetic markers are currently available. This leads to under-diagnosis and under-medication, and increased likelihood of suicide completion.
Gene-environment interactions are thought to predispose to Depression.
Box 2. Anxiety and MDD are associated with decreased 5-HT.
Acute tryptophan depletion triggers relapses in recovered depressed subjects; triggers depressed phenotype in normals
Antidepressants target increase in 5-HT (and NE) systems, esp. SSRI
PET studies of depressed, postmortem studies of depressed suicide victims show decreases in 5-HT synthesis and in 5-HT heteroreceptors in cortex and hippocampus of depressed patients.
SSRIs are effective for many mood disorders: bipolar, OCD, anxiety; bulimia, PMDD, PPD, etc., suggesting a role for 5-HT in many mood disorders.
Animal loss-of-function models (5-HT1A−/−, 5-HT (Pet-1, TPH2), 5-HTT-knockout) indicate roles for 5-HT in anxiety models.
Case-control association studies with functional 5-HTT/5-HT1A gene polymorphisms associate hypofunction of 5-HT with depression
However, SSRI require 2–3 weeks to demonstrate effect; why? Adaptive changes!
Understanding of the etiology of MDD remains incomplete, and both genetic and environmental factors are believed to contribute to an increased predisposition to depression [19, 31–33]. Studies of hereditary transmission suggest a genetic component of up to 50% [34], however linkage analyses have been difficult as depression is likely to result from a combination of many genetic factors of small effect sizes [35, 36]. Adding to the problem of identifying genetic risk factors for depression is the heterogeneity of categories of depression that make it difficult to assess the role of specific factors or genes in this illness. Major depression is diagnosed by psychiatric tests that pool very different behavioral phenotypes [37]. For example, one can be diagnosed as having MDD based on criteria that are often opposite such as too much or too little sleep, hyper- or hypophagia, suicidal thoughts or anhedonia, anxiety or depressed thoughts, etc. Given that at least any 5 of 9 criteria need to be met, two patients diagnosed with MDD may not have a single symptom in common apart from sad mood. As a result, association studies in major depression have produced a great deal of variability and there is a lack of effective genetic markers for depression, or indeed any other mental illness. Genome-wide association studies have uncovered a number of intriguing susceptibility genes such as ion channels (CACNA1C) [38] [39], synaptic proteins [40], and transcription factors (e.g., Sp4 [41, 42]) that associate with major depression. However, these polymorphisms have very small effect sizes, limiting their usefulness as genetic markers [43]. Similarly, genome-wide associations for SSRI response have yielded several candidate polymorphisms, but replicating these results has been challenging [44]. Better phenotypic analysis of depression subtypes could greatly enhance the likelihood of identifying useful genetic markers. For example, a recent meta-analysis indicates an association of the functional 5-HT1A gene promoter polymorphism (rs6295) with major depression [45], but this association appears to be stronger in depressed patients with a comorbid anxiety disorder [46]. Unexpectedly, the only genome-wide linkage of the 5-HT1A gene is to type 1 diabetes [47], perhaps reflecting better classification criteria compared to mental illness. Further analysis of large cohorts in terms of subgroups of depression phenotypes may lead to stronger genetic associations than seen with major depression as a whole [48]. Given the above limitations in classifying mental disorders and validating risk alleles, we have focused on the roles of transcription factors in regulating the tone of the 5-HT system, with a particular focus on regulators of the HTR1A promoter (Box 3).
Box 3. Strategy for Identification of Novel Transcriptional Targets for Anxiety and Depression.
Identify gene regulatory elements of key targets for serotonin system: e.g., autoreceptor genes (eg. 5-HT1A; DRD2 genes)
Clone critical regulators (Freud 1/2-CC2D1A/B family) and identify new gene targets, mutations, and regulation for these “master switches”
Identify novel functional promoter polymorphisms that affect the expression of depression genes: HTR1A C(-1019)G (rs6295)
Identify the DNA binding proteins affected by these polymorphisms (Deaf1, HES)
Identify in vivo functions of HTR1A DNA regulators (Deaf1/HES/PET1) using animal models (eg. Deaf1 −/−; Hes1 −/−; Pet-1 −/− mice)
Target therapeutics to activate/induce or inhibit/repress specific transcription factors
THE SEROTONIN SYSTEM AND 5-HT1A RECEPTORS
The serotonin system originates from a small set of 2 × 104 neurons (0.2 billionth of the 1011 neurons in the human central nervous system) [49, 50] that are located in the raphe nuclei of the midbrain. These neurons express the neuronal form of tryptophan hydroxylase (TPH2) for the synthesis of 5-HT [51, 52]. Although small in number, 5-HT neurons send axons that project throughout the central nervous system, including to the cortex, hippocampus, septum, amygdala, hypothalamus and spinal cord, to regulate mood, stress responses, autonomic and hormonal functions [49, 50, 53–56]. Serotonin action is terminated by reuptake via the 5-HT transporter (5-HTT), and subsequent degradation by monoamine oxidase. Among the 14 different mammalian 5-HT receptors, the 5-HT1A receptor is one of the most abundant, being expressed as a postsynaptic heteroreceptor in cortex, limbic areas (septum, hippocampus, amygdala), hypothalamus and other areas, where it is implicated in a diversity of physiological, cognitive and affective functions [57–62]. In addition, the 5-HT1A receptor is expressed presynaptically on the cell body and dendrites of 5-HT neurons in the raphe nuclei, where it functions as an inhibitory autoreceptor [63, 64], acting as a key negative regulator of the activity of serotonergic neurons by mediating inhibitory feedback of 5-HT release [65–70]. The inhibitory effects of both the 5-HT1A auto- and heteroreceptors occur through activation of G-protein inward rectifying potassium channels [53] to reduce neuronal firing rate, inhibition of voltage-gated calcium channels to reduce calcium entry, and inhibition of adenylyl cyclase. In raphe cells, they also inhibit extracellular regulated kinase activation [71].
5-HT1A auto- and heteroreceptors
Since the 5-HT1A autoreceptor inhibits serotonergic activity, while the heteroreceptors mediate 5-HT action, global inhibition of the 5-HT1A receptor has opposing effects on the serotonin system. While knockout of the 5-HT1A heteroreceptor results in a depressed phenotype, specific repression of the 5-HT1A autoreceptor leads to increased serotonergic activity, a depression-resistant phenotype, and enhanced response to antidepressants [72, 73]. Because most 5-HT1A ligands target both auto- and heteroreceptors their efficacy is limited. However, some 5-HT1A partial agonists, such as pindolol, induce selective desensitization of 5-HT1A autoreceptors which may accelerate antidepressant action [74] (Fig. 1). Antidepressants such as SSRIs rapidly enter the brain yet, as mentioned above, they require at least 2–3 weeks of treatment for clinical efficacy. Acute treatment with antidepressants results in a recurrent activation of 5-HT1A autoreceptors, reducing 5-HT neuron firing and compensating for antidepressant-induced increases in 5-HT [75–77]. During chronic treatment with SSRIs, the 5-HT1A autoreceptor becomes desensitized whereas post-synaptic 5-HT receptors remain sensitive, resulting in enhanced 5-HT neurotransmission. The >2-week time period required for 5-HT1A autoreceptor desensitization is more prolonged than agonist-induced receptor phosphorylation (sec), internalization (sec-min) and even down-regulation (estimated at 2–3 days in vivo [73, 78]) and may involve decreased receptor synthesis. Recent Positron emission tomography (PET) imaging studies indicate that 5-HT1A autoreceptor density is reduced following chronic SSRI treatment [79], suggesting that long-term adaptive mechanisms such as transcriptional regulation could be involved [80] (BOX 2). Based on these findings and data from post-mortem studies showing a specific increase in 5-HT1A autoreceptor binding in depressed suicides [81], we hypothesized that transcriptional dys-regulation driven by genetic, epigenetic and environmental alterations combine to reduce serotonergic activity, predisposing individuals to depression and suicide. Thus we have developed a strategy for identification of key regulators in the serotonin system focusing on the HTR1A promoter (Box 3).
Fig. 1. Adaptive changes in 5-HT neurons upon antidepressant treatment.
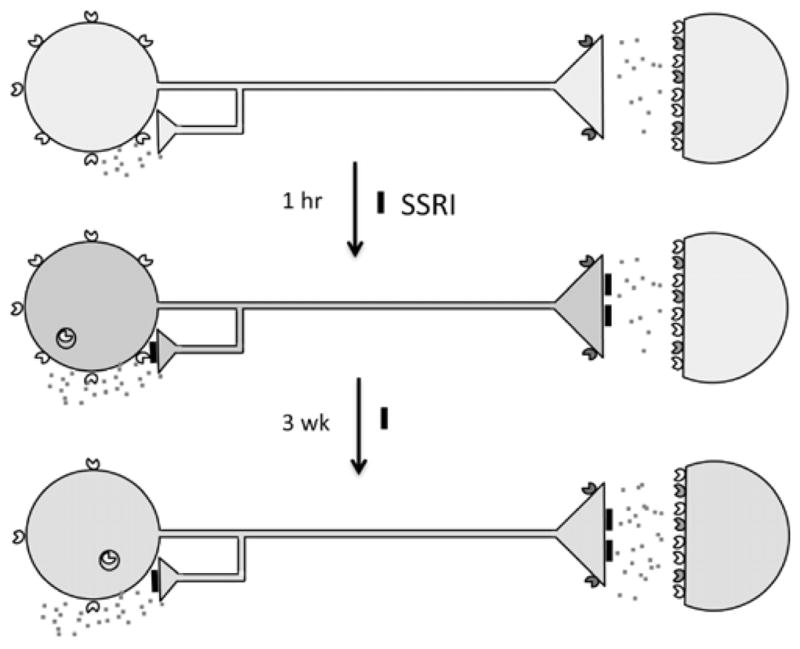
In major depression, 5-HT neurons are thought to have reduced neurotransmission (pale blue). 5-HT1A autoreceptors (yellow carets) are expressed presynaptically on 5-HT neuron cell bodies and dendrites (at left); 5-HT1B receptors (green carets) are presynaptic at the nerve terminal; 5-HT1A heteroreceptors and other 5-HT receptors are expressed postsynaptically (colored carets). The 5-HT1A receptor couples to Gi/Go protein to inhibit adenylyl cyclase (AC), open potassium channels (K+) to hyperpolarize membrane potential and reduce firing rate. Acutely, SSRI antidepressants inhibit (black blocks) the 5-HT transporter increasing synaptic 5-HT (red specks), leading to activation of 5-HT1A autoreceptors which inhibit 5-HT neuronal firing (blue), and transiently internalize. After 3 weeks of SSRI treatment, 5-HT1A autoreceptors desensitize and receptor levels are reduced, dis-inhibiting 5-HT neuronal firing and enhancing 5-HT neurotransmission (green).
Animal models of 5-HT1A receptor function
Mouse genetic and behavioural models have provided valuable insights into the role of 5-HT1A receptors in depression and anxiety BOX 2. Several groups have shown that knockout of the 5-HT1A receptor gene in mice results in increased anxiety-like behaviours [82–84]. In addition, 5-HT1A-null mice fail to respond to chronic SSRI treatment, implicating a key role for 5-HT1A receptors in mediating antidepressant action [85]. Conversely, mice that over-express the 5-HT1A receptor display reduced anxiety behaviours [86]. Similarly, mice with enhanced hippocampal 5-HT1A-G i2 signaling also display reduced anxiety [87]. In 5-HT1A knockout mice, rescue of 5-HT1A receptor expression in early postnatal forebrain restores a normal anxiety phenotype, whereas pharmacological blockade of the 5-HT1A receptor during postnatal days 13–34 induces an anxiety phenotype that emerges in the adult. These findings indicate that post-synaptic 5-HT1A receptors play a critical role during early postnatal development in the establishment of anxiety behaviour [78, 88]. However, hyperactivity of the 5-HT system induced by specific knockout of 5-HT1A autoreceptors increases anxiety, suggesting that additional 5-HT receptors, such as the 5-HT2A receptor [89], may also regulate anxiety. On the other hand, mice with a 30% increase in 5-HT1A autoreceptors in adulthood display reduced 5-HT neuron firing, reduced 5-HT release, increased depressive behaviours, and resistance to SSRI treatment, but no change in anxiety [73]. Thus, an increase in post-synaptic forebrain 5-HT1A receptors during postnatal development reduced anxiety, while an increase in presynaptic 5-HT1A autoreceptors in the adult mouse induces a depression-like phenotype and resistance to antidepressants. Similar changes in 5-HT1A auto- and heteroreceptors during human development may be induced by the C(-1019)G polymorphism and could lead to anxiety or depression phenotypes, but this remains to be assessed.
5-HT1A receptor dys-regulation in anxiety and depression
In humans, altered levels of 5-HT1A receptors have been reported in postmortem and PET imaging studies of 5-HT1A receptor levels in depression (Fig. 1). In depression, increased levels of 5-HT1A autoreceptors have been reported [81], while in some studies, a decrease in the number of 5-HT neurons and projections, as well as 5-HT1A autoreceptors, has been observed in post-mortem tissues from depressed suicides [90, 91]. Fewer 5-HT neurons may reflect a developmental dys-regulation that would reduce 5-HT1A autoreceptor levels but also decrease 5-HT neurotransmission, which has been associated with depression. In PET imaging studies of MDD [92], bipolar disorder [93], and temporal lobe epilepsy patients with depression [94], 5-HT1A binding potential was increased mainly in the raphe nuclei. In MDD patients, this increase in 5-HT1A autoreceptor levels persists following remission [95], suggesting that it represents a trait of MDD. In contrast, early PET imaging studies suggested a reduction in 5-HT1A autoreceptors in depression [96]; however, technical considerations argue for the opposite change in these studies [92, 97]. Post-synaptically, in the hippocampus and dorsolateral prefrontal cortex (DLPFC), decreases in 5-HT1A RNA and 5-HT1A signaling have been observed in depressed suicides [98, 99]. PET imaging studies of human subjects with bipolar disorder, MDD or panic disorder have shown a decrease in 5-HT1A heteroreceptor levels, particularly in DLPFC [100–102]. Reduced expression of cortical 5-HT1A heteroreceptors and increased 5-HT1A autoreceptor levels would combine to reduce 5-HT neurotransmission in these disorders.
The alterations in 5-HT1A receptor levels observed in depressed patients may have significant functional consequences. The 50% increase in 5-HT1A autoreceptor binding potential in depressed subjects [92] is similar to the 30% increase in 5-HT1A autoreceptor levels in mice that resulted in an increased susceptibility to stress-induced depression and resistance to SSRI treatment [73]. Mice with increased 5-HT1A autoreceptor levels displayed reduced basal firing of the 5-HT neurons and robust electrophysiological responses to a 5-HT1A agonist, indicating enhanced inhibition of serotonergic tone (Fig. 1). Thus, the long-term transcriptional regulation of the 5-HT1A receptor appears critical to set the tone of serotonergic activity and responsiveness to SSRI treatment [103].
5-HT1A promoter polymorphisms and mental illness
We have previously hypothesized that alterations in transcriptional activity of the 5-HT1A promoter conferred by sequence variations could predispose to MDD, and may also affect the clinical response to anti-depressants that target the serotonin system [104]. Several HTR1A polymorphisms have been identified, but many are too rare to assess their association with mental illness [105–109]. In a preliminary report we identified a common C(-1019)G 5-HT1A polymorphism [110] that is identical to the site reported as C(-1018)G [108]. Based on the consensus 5- HT1A promoter sequence, the designation C(-1019)G has been retained, and the SNP database designation is rs6295. We showed that this change was associated with MDD, and subsequently found that the G-allele and GG-genotype is also associated with completed suicide [111]. We also demonstrated that the polymorphism is functional and leads to up-regulation of 5-HT1A promoter activity, particularly in RN46A cells: 5-HT neurons that express 5-HT1A autoreceptors (Fig. 2). An increase in 5-HT1A autoreceptor binding potential is associated with the rs6295 5-HT1A promoter polymorphism in human MDD subjects [112], consistent with a genetic basis for increased 5-HT1A autoreceptors in depression. These studies provided the first evidence that a functional promoter polymorphism that alters transcription in neuronal cells could be associated with MDD.
Fig. 2. Effect of C(-1019)G polymorphism rs6295 on 5-HT1A receptor expression.
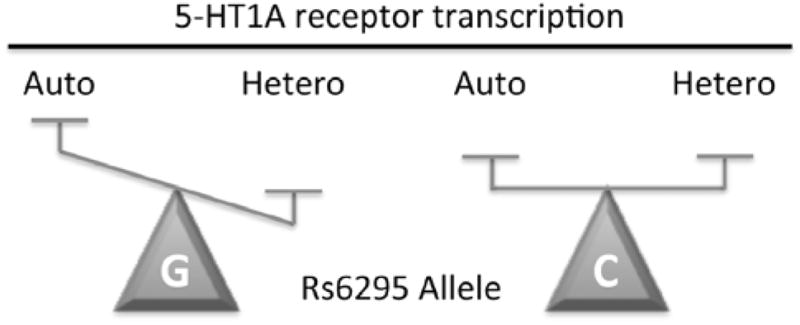
A simplistic model of the C(-1019)G change (labeled C or G), which prevents Deaf1 from recognizing its element on the 5-HT1A promoter, is presented. In 5-HT neuronal cells, Deaf1 represses 5-HT1A receptor transcription, but in certain non-serotonergic neurons it oppositely enhances 5-HT1A expression. An upregulation of 5-HT1A autoreceptor (Auto) expression in raphe neurons is predicted for the G(-1019) allele based on loss of Deaf1-mediated repression presynaptically. Conversely, in some non-serotonergic neurons a reduction in 5-HT1A heteroreceptor (Hetero) expression is predicted based on the loss of Deaf1 enhancer activity.
Since our initial study, several independent studies have shown that the G(-1019) and GG(-1019) genotype of the rs6295 HTR1A polymorphism are associated with MDD [113, 114]. A recent meta-analysis supports the association of this polymorphism (rs6295), as well as a more upstream HTR1A promoter polymorphism (rs878567), with MDD [45, 115]. Another meta-analysis indicates schizophrenia [116]. Recent studies indicate that the GG genotype is associated with increased amygdala reactivity to fearful faces [117, 118], which correlates with increased presynaptic 5-HT1A autoreceptor expression [119]. Interestingly, in bipolar depression the GG genotype was associated with smaller amygdala volume, suggesting a developmental effect [120]. The 5-HT1A receptor gene has been associated with panic disorder [121], in which the G-allele was associated with increased panic symptom severity [122], again suggesting increased fear or stress responsiveness. In normal subjects the GG genotype was associated with an impaired glucocorticoid response to social stress, similar to that associated with early life stress [123]. Together, these results suggest that the G-allele confers increased stress reactivity and reduced stress coping that may predispose individuals to depression (Fig. 3). Consistent with a genotype-stress relationship, bipolar disorder patients with the GG genotype report fewer stressful events prior to hospitalization, indicating an increased susceptibility to stress for triggering depressive episodes [124]. Similarly, a study in a Chinese population showed that the rs6295 polymorphism was associated with negative life events in 20–29 year-olds with MDD [125]. Consistent with a role for stress interacting with the HTR1A rs6295 genotype, in elderly patients who suffered a fall, the G-allele is associated with increased depressive symptoms [126]. These studies suggest that increased stress reactivity in later life may be conferred by the rs6295 polymorphism that could precipitate depression symptoms. Similarly, while the G-allele was not associated with suicide attempts, there was a trend for its association with stressful life events in suicide attempters [127]. In subjects with personality disorders, the G-allele was associated with emotional-dramatic behavior [128]. In normal subjects the GG-genotype has been found to confer an increase in impulsivity [129], as well as with neuroticism [130], although the latter has not been replicated [131]. However, no association with anxiety or depression personality traits was detected in a large study of healthy subjects [132], suggesting that these associations may be most robust in patients compared to normal subjects. Alternately, more sensitive measures of depressive or anxious behaviour may show association in normal subjects. For example, normal subjects with the GG-genotype showed reactions associated with increased negative emotionality in a reward-punishment paradigm [133]. Recent studies indicate that the presence of the G-allele in normal subjects associated with specific impairments in cognitive ability, including error and attentional processing [134, 135]. These findings in normal subjects suggest that the rs6295 polymorphism generates impairments in emotional and cognitive processing that lead to an inability to handle stressful situations, leading to an increased susceptibility to depression and anxiety (Fig. 3). Consistent with this model, recent multivariate Bayesian modeling of rs6295 association studies have suggested that an association with impulsivity may be intermediary to its association with MDD [136].
Fig. 3. Lifetime associations with the HTR1A genotype.
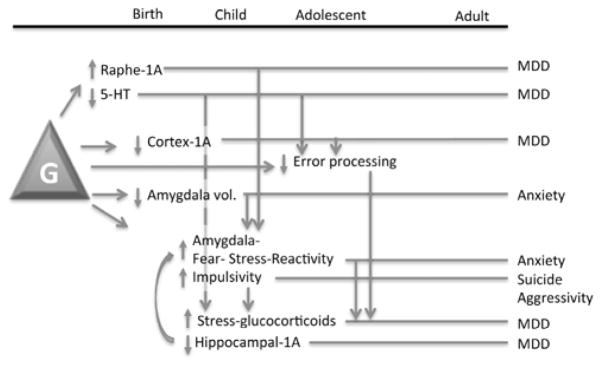
Presented is a figurative summary of association data obtained for the GG genotype (G) of the HTR1A rs6295 polymorphism and how these changes might present and interact over the course of the lifetime. Arrows indicate associations of changes with each other, as well as feed-forward consequences of the changes. Bars indicate the lifetime trajectory of the indicated changes contributing to the indicated psychopathology. Note that the GG genotype may attenuate a number of cognitive and emotional processing capabilities that could ultimately lead to psychopathology.
We also found that the HTR1A GG-genotype was associated with a reduced response to antidepressant treatment [137, 138], which has been confirmed in several studies [113, 114]. However, this remains controversial, as one study in Japanese subjects identified a greater response to SSRIs in carriers of the G-allele [139]. This may reflect a difference in ethnicity in which the G-allele is infrequent in oriental populations, or may suggest the presence of additional polymorphisms, which could potentiate the effects of rs6295 [140]. Recently, the presence of the GG(-1019) genotype was found to associate with a lack of rapid response to SSRI treatment in panic disorder patients compared to patients with the C(-1019) allele [141]. Interestingly, a report shows that the CC-genotype also confers a better response to transcranial magnetic stimulation [142], suggesting that modulation of the 5-HT1A receptor may also participate in treatments that target cortical processes, perhaps via induced expression of the 5-HT1A receptor in cortex and amygdala [143]. The GG-genotype has also been associated with weaker negative symptom improvement in schizophrenia patients treated with atypical antipsychotics [144, 145]. Taken together these studies indicate that the G-allele, which is associated with increased 5-HT1A autoreceptor levels, also associates with reduced responses to therapies that modify the 5-HT system.
5-HT1A RECEPTOR PROMOTER ARCHITECTURE
Basal promoter structure
As part of a strategy to normalize transcription of the HTR1A gene, it is important to identify discrete regulators of its transcription as potential therapeutic targets (Box 3). The 5-HT1A receptor gene (HTR1A) upstream promoter-enhancer region has been intensively studied and is regulated by several transcription factors. Although promoter regions are divergent in sequence, the proximal 5′ upstream 5-HT1A promoter region has the greatest conservation between humans and the mouse or rat, which provide useful genetic and behavioral models of anxiety and depression (Fig. 4). Within the first 715-bp, the sequences are CpG-rich and display over 70% nucleotide identity including multiple conserved MAZ and Sp1 sites (MAZ I–IV) that drive basal expression [146, 147]. These “housekeeping” gene enhancers drive strong expression in all cell types regardless of whether they endogenously express 5-HT1A receptors. Both TATA-dependent (in rat) and –independent (mouse, human) transcriptional initiation are observed, but MAZ/Sp1 regulation is conserved [146–148]. Interestingly, a possible human splice variant including a short sequence in the 5′-untranslated region (Fig. 4) has been identified from the EST database, but its prevalence is unclear. However, the coding region of HTR1A is intronless, yielding a receptor protein of the same amino acid sequence in all tissues.
Fig. 4. HTR1A promoter alignment.
Nucleotide alignment of the human (H), mouse (M) and rat (R) 5-HT1A promoter sequence is shown ending at the translational initiation ATG codon (yellow). Sequence counts are shown at right, matches are in blue, and gaps shown by dashes. A potential alternately spliced non-coding exon is shown in red. Boxes depict the proximal promoter elements (Sp1, MAZ), NFkB sites, negative glucocorticoid receptor element (nGRE), raphe-specific Pet-1 elements (PET-1), and the repressor elements for Freud-1 and Freud-2 (DRE) and REST/NRSF (RE-1); palindrome Deaf1 elements (Palindrome) for human (H), mouse M) and rat R), TATA-box and CCAAT box. Highlighted are the human C(-1019)G polymorphism (C/G) and the rat transcription start site (R-TSS). The % nucleotide identity (human vs. mouse) over the initial 800 bp was 73%. Alignment was obtained from the Ensembl database.
Inducible DNA elements
DNA elements of fundamental importance are often conserved across multiple species, especially human, rat and mouse HTR1A promoter sequences (Fig. 4). Two conserved NFκB sites (−79 bp; −350 bp) have been implicated in mitogen-induced up-regulation of the 5-HT1A receptor in immune cells (B-cells, T-cells, neutrophils and macrophages) [149, 150]. The 5-HT1A receptor is also negatively regulated by glucocorticoids, which can act indirectly through NFκB or Sp1 sites located on the promoter [147, 151], a mechanism that may be more important in immune cells than in neurons. Further upstream a novel negative glucocorticoid response element (nGRE), containing two GRE half-sites separated by six nucleotides rather than three as for a positive GRE [152], is conserved in mammalian HTR1A genes (Fig. 5A). This nGRE mediates repression by both glucocorticoid and mineralocorticoid receptors, of which the latter are ten-fold more sensitive to glucocorticoids and are highly expressed in the hippocampus. Thus, hippocampal 5-HT1A receptors are exquisitely glucocorticoid- and stress-sensitive, and implicated in stress-induced reduction of 5-HT neurotransmission in hippocampus [153].
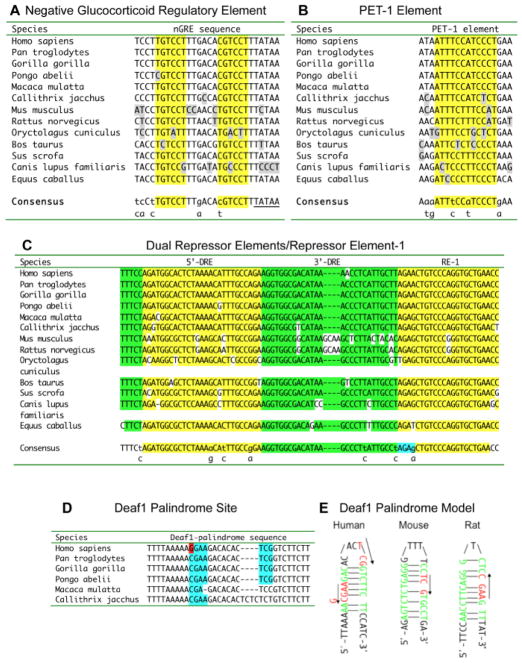
Fig. 5. Conserved HTR1A DNA elements.
The alignment of DNA elements from mammalian 5-HT1A receptor gene sequences is shown with elements of interest highlighted; below is the consensus sequence shared in three or more species; mismatches from the consensus are shown in grey.
A. Negative Glucocorticoid Regulatory Element (nGRE). The GRE half-site sequence is highlighted and a 3′-flanking TATA-box sequence of unknown function is also shown. B. PET-1 element. C. Dual repressor elements (DRE)/repressor element-1 (RE-1). The 5′DRE and RE-1 are highlighted in yellow; 3′-DRE and conserved upstream sequence in green; overlap in blue; and mismatches in white. D. Deaf1 palindrome site. Shown are conserved sequences in primate species; the minimal Deaf1 recognition sequence is in blue; the site of the rs6295 polymorphism (G-allele shown) is in red. E. Deaf1 palindrome model. Deaf1 binding sites in human, mouse and rat 5-HT1A promoters are shown as “hybridized” to highlight imperfect palindrome sequences shown in green; the consensus Deaf1 minimal TCG (forward) or CGA (reverse) is shown in red. The location of the G(-1019) rs6295 site in the human 5-HT1A palindrome is indicated.
Raphe-specific Pet-1 enhancer
Pet-1 is a transcription factor that is expressed exclusively in raphe nuclei and is required for differentiation of serotonin neurons and expression of serotonergic genes [154–156]. Several conserved Pet-1 sites are present in the 5-HT1A promoter (Fig. 4), but the upstream site (−1400 bp) shows the greatest conservation (Fig. 5B) and is the most critical for raphe-specific expression of the 5-HT1A autoreceptor [156]. Due to its exclusive expression in 5-HT neurons in the brain, inhibiting Pet-1 may be useful to reduce 5-HT1A autoreceptor levels (Box 4), but since Pet-1 also affects TPH and 5-HTT levels, inhibiting Pet-1 could undesirably reduce 5-HT levels [155]. Consistent with this, the Pet-1 knockout mice display one fifth of wild-type brain 5-HT content, as well as an increased aggressivity and anxiety phenotype [154].
Box 4. Transcriptional regulation of the 5-HT1A autoreceptor as a therapeutic opportunity.
5-HT1A autoreceptors negatively regulate tone of 5-HT system and appear to be upregulated in major depression
Increased 5-HT1A autoreceptors prevent response to 5-HT selective antidepressants; blockade or desensitization of 5-HT1A autoreceptors accelerate or permit response to antidepressants
The functional HTR1A C(-1019)G (rs6295) promoter polymorphisms is associated with increased 5-HT1A autoreceptor expression, MDD, and resistance or relapse to 5-HT selective antidepressants
DNA binding proteins inhibited by HTR1A rs6295 polymorphism (Deaf1, HES) repress 5-HT1A autoreceptor transcription in 5-HT neuronal cells
In vivo loss-of-function mouse models of HTR1A DNA regulators (eg. Deaf1 −/−; Hes1 −/−; Pet-1 −/− mice) display increased levels of 5-HT1A autoreceptor expression
Target therapeutics to activate/induce repressors of 5-HT1A autoreceptors (e.g., Deaf1, Hes1/5, Freud-1, REST)
Upstream repressor elements
Upstream of the basal promoter, we identified a region with strong repressor activity in human and rat HTR1A genes that completely silenced transcription in non-neuronal cells, but also partially repressed transcription in neuronal cells [148, 157]. This suggested that the repressor region plays a key role in regulating the basal expression of 5-HT1A receptors in neurons. Three overlapping repressor elements are functional and conserved (88% nucleotide identity to rat/mouse, Fig. 5C) [158]. These include two copies (5′ and 3′) of a novel dual repressor element (DRE, 31-bp) and an RE-1 (neuronal repressor REST binding site) [158]. The DRE is composed of two conserved elements (Fig. 5C): 5′-repressor element (FRE, 14-bp) active in 5-HT1A-positive neurons, and 3′-RE (TRE, 12-bp) active in 5-HT1A-negative cells [157]. Mutational inactivation of the FRE in the rat 5-HT1A promoter results in ten-fold induction in raphe serotonergic cells but not non-neuronal cells [157]. This indicates that FRE is a powerful repressor of 5-HT1A transcription in 5-HT-expressing neurons. By yeast one-hybrid screening we identified Freud-1 (FRE Under Dual repression binding protein)/CC2D1A, a novel protein that binds FRE and represses the 5-HT1A gene in neurons [158, 159]. Freud-2/CC2D1B is second repressor homologous to Freud-1 that binds to the 5-HT1A-TRE [160, 161]. Freud-1 and Freud-2 are powerful regulators of basal 5-HT1A receptor expression in neuronal cells, however their roles in the regulation of 5-HT1A receptor expression in vivo remain to be demonstrated. Freud-1 is strongly expressed in the raphe nuclei, suggesting that it may play an important role in regulation of the 5-HT1A autoreceptor [159], while Freud-2 is sparsely expressed in the raphe [160]. Thus, it may be possible to target Freud-1 to modify 5-HT1A autoreceptor expression (Box 4). In prefrontal cortex or hippocampus, both Freud-1 and Freud-2 are strongly expressed and colocalize with 5-HT1A receptors, suggesting that both regulate post-synaptic 5-HT1A receptor expression [160, 162]. Importantly, levels of Freud-1 and Freud-2 are altered in depression in a region-specific manner [163], suggesting that differential regulation of these transcription factors modifies 5-HT1A auto- vs. heteroreceptor expression.
Polymorphic palindrome element
In searching for DNA elements that may be important in regulating the human 5-HT1A receptor, we hypothesized that a mutation in the repressor region of the promoter could up-regulate 5-HT1A autoreceptor expression and may correlate with depression. By amplifying a 718-bp segment of the human 5-HT1A repressor region we found the novel C(-1019)G polymorphism (designated rs6295), which has been associated with several clinical populations (see above). The C-G change impairs the binding of nuclear proteins from raphe cells to a palindrome DNA element (Fig. 5D, E) located at the polymorphism [111]. Using yeast one-hybrid cloning, we cloned Deformed epidermal autoregulatory factor 1 (Deaf1) and Hairy and enhancer of split 5 (HES5), which bind and repress at the C-allele but not the G-allele of the HTR1A promoter. Since Deaf1 but not HES5 is co-expressed with 5-HT1A receptors in the adult brain, we have mainly focused on Deaf1. Both Deaf1 and HES5 repress expression at the HTR1A promoter in raphe cells, and hence the G-allele was predicted to increase 5-HT1A autoreceptor expression. Intriguingly, in some non-serotonergic neuronal cells that express 5-HT1A receptors, Deaf1 displayed opposite activity to enhance rather than repress HTR1A transcription, while the G-allele reduced HTR1A transcription [164]. The role of Deaf1 in regulation of the 5-HT1A receptor in vivo was addressed using Deaf1 −/− mice, and we found a 50% increase in 5-HT1A RNA in raphe, but a 30% decrease in prefrontal cortex [165]. Even more striking changes in 5-HT1A receptor expression were observed using dual immunofluorescence to identify 5-HT1A autoreceptors in TPH-positive 5-HT neurons. Thus, the lack of Deaf1 modifies 5-HT1A auto- and heteroreceptor expression as observed in studies of MDD and anxiety disorders (see above). The role of Deaf1 to oppositely regulate 5-HT1A auto- and heteroreceptor expression suggests that agents that activate or induce Deaf1 expression may be useful in correcting altered expression of 5-HT1A receptors in MDD or anxiety.
We also examined whether a Deaf1 element is present in the mouse 5-HT1A promoter as observed in the human HTR1A gene [165]. We defined Deaf1 elements as containing at least one minimal TCG (or reverse CGA) sequence, and located within a palindrome region (Fig. 5D, E). As shown in the alignment of human, mouse and rat HTR1A genes, unlike the conserved DNA elements identified above, the Deaf1 element was not highly conserved and did not align well with the human Deaf1 element containing the polymorphism, which is present in a region of relatively low nucleotide similarity (Fig. 4). Instead both rat and mouse 5-HT1A genes had potential Deaf1 sites located further downstream, but not aligned with each other. In addition we identified a second potential mouse Deaf1 element located close to the human polymorphism site, however this site was not functional [165]. In comparing the Deaf1 sites of different primates, there is complete conservation of the reverse CGAA Deaf1 site (CGA in macaques), as well as the overall sequence of the palindrome (Fig. 5D). However, most other mammalian species lack the TCG sequence in this region, although a relatively highly conserved Deaf1 site cluster is found further downstream. These results argue that the presence of this Deaf1 site is a late evolutionary change that is specific to primates. The polymorphism appears to be specific to humans, as we did not detect it in macaque or cynomolgus monkeys (unpublished data). Thus, had the criteria of sequence similarity been applied, the importance of the palindrome region in regulation of 5-HT1A receptor expression would not have been detected. In evolutionarily “younger” regions of high variation, it may be that common functional polymorphisms are more likely to appear.
Genetic polymorphisms, such as the C(-1019)G polymorphism, can modify the transcriptional activity of the promoter over the lifetime by affecting the binding of different transcription factors. Thus, the G(-1019) allele blocks Deaf1 binding through life, but also prevents the binding of Hes proteins, which in combination with Pet-1, control the timing of induction of 5-HT1A autoreceptor expression during differentiation of 5-HT neurons [156]. Thus the G-allele leads to a lifelong alteration in 5-HT1A autoreceptor expression to set the tone of the serotonin system, and affect the development of cognitive and emotional reactivity, thereby increasing the risk of depression or other psychopathology (Fig. 3). It is possible that by activating repressors like Freud-1 or Deaf1 it may be possible to suppress the expression of 5-HT1A autoreceptors sufficiently to mediate antidepressant activity, as even a 30% reduction may be sufficient [73] (Box 4). However, this strategy may not be as effective for anxiety-related behaviors which appear to develop in the early postnatal period, and may only respond to epigenetic strategies that can reverse environment-driven changes that set adult stress reactivity [166, 167]. The targeting of transcription factors during a period of transcriptional plasticity may lead to global re-establishment of the set point for homeostatic regulation of neurotransmitter activity.
CONCLUSIONS
As discussed above, transcriptional regulation of the 5-HT1A receptor could be an attractive target for novel therapeutic approaches (Box 4, 5). Both anxiety and MDD involve reduced activity of the serotonin system that could be driven in part by over-expression of 5-HT1A autoreceptors and/or reduced expression of 5-HT1A heteroreceptors. However, effective treatment of these disorders would require inhibition or down-regulation of 5-HT1A autoreceptors (Box 4), but activation or up-regulation of cortical and hippocampal 5-HT1A heteroreceptors (Box 5). To date, ligand-based therapeutics such as buspirone or pindolol have had limited success in treating anxiety or MDD, in part because they regulate both 5-HT1A auto- and heteroreceptors in the same way. Recently however some promising compounds may have selective activity at either 5-HT1A autoreceptors or 5-HT1A heteroreceptors, and thus may be more effective as anti-depressant or anti-anxiety compounds. Evidence from in vitro and recent in vivo studies indicates that some of the transcriptional regulators of the 5-HT1A receptor may have high selectivity for 5-HT1A auto- vs. heteroreceptors. For example, activation of Deaf1, Hes1/Hes5 or Freud-1/CC2D1A would suppress 5-HT1A autoreceptor expression. However, Deaf1 alone has the interesting property of enhancing cortical 5-HT1A heteroreceptor expression, while Freud-1/CC2D1A globally represses 5-HT1A auto- and heteroreceptors. Therefore by activating Deaf1, a dual benefit could be possible: namely reducing 5-HT1A autoreceptor expression, while increasing 5-HT1A heteroreceptor expression. However, these transcription factors could have additional gene targets, which need to be considered. In the case of Deaf1, it appears to be regulating a select type of immune response that may not lead to adverse effects. Nevertheless, targeting 5-HT1A autoreceptors selectively should enhance responsiveness to SSRIs, and mediate more rapid response and recovery from anxiety and MDD.
Box 5. Transcriptional regulation of the 5-HT1A heteroreceptor as a therapeutic opportunity.
Reductions of 5-HT1A heteroreceptors or their activity are observed in cortical regions in anxiety and MDD
In animal models, loss or blockade of forebrain 5-HT1A heteroreceptors in development increases anxiety and prevents response to 5-HT selective antidepressants
The functional HTR1A C(-1019)G (rs6295) promoter polymorphism is associated with increased stress response and anxiety phenotypes
DNA binding proteins inhibited by HTR1A rs6295 polymorphism (Deaf1) enhances 5-HT1A heteroreceptor transcription in non-5-HT neuronal cells
In vivo loss-of-function of HTR1A regulator Deaf1 reduces levels of 5-HT1A heteroreceptor expression in frontal cortex
Target therapeutics to activate/induce enhancers of 5-HT1A heteroreceptors (e.g., Deaf1) or inhibit repressors (e.g. Freud-2)
Acknowledgments
PRA was supported by funding from the Canadian Institutes of Health Research, Ontario Mental Health Foundation, and Heart and Stroke Foundation Centre for Stroke Recovery. LMF was supported by postdoctoral funding from the National Science and Engineering Research Council of Canada.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Doris A, Ebmeier K, Shajahan P. Depressive illness. Lancet. 1999;354:1369–75. doi: 10.1016/S0140-6736(99)03121-9. [DOI] [PubMed] [Google Scholar]
- 2.Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–41. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Mental Health, New Understanding, New Hope. WHO Marketing and Dissemination; Geneva, Switzerland: 2001. The World Health Report 2001. [Google Scholar]
- 5.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ustun TB, Ayuso-Mateos JL, Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–60. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 9.Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- 10.Charney DS, Krystal JH, Delgado PL, et al. Serotonin-specific drugs for anxiety and depressive disorders. Annu Rev Med. 1990;41:437–46. doi: 10.1146/annurev.me.41.020190.002253. [DOI] [PubMed] [Google Scholar]
- 11.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 12.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–65. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 13.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–13. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millan MJ. The role of monoamines in the actions of established and “novel” antidepressant agents: a critical review. Eur J Pharmacol. 2004;500:371–84. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–74. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay P, Blier P. Catecholaminergic strategies for the treatment of major depression. Curr Drug Targets. 2006;7:149–58. doi: 10.2174/138945006775515464. [DOI] [PubMed] [Google Scholar]
- 17.Jans LA, Riedel WJ, Markus CR, et al. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–43. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 18.Bhagwagar Z, Cowen PJ. ‘It’s not over when it’s over’: persistent neurobiological abnormalities in recovered depressed patients. Psychol Med. 2008;38:307–13. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- 19.Mann JJ, Arango VA, Avenevoli S, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–63. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller HL, Delgado PL, Salomon RM, et al. Acute tryptophan depletion: a method of studying antidepressant action. J Clin Psychiatry. 1992;53(Suppl):28–35. [PubMed] [Google Scholar]
- 21.Young SN. The use of diet and dietary components in the study of factors controlling affect in humans: a review. J Psychiatry Neurosci. 1993;18:235–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Moore P, Landolt HP, Seifritz E, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23:601–22. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 23.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi MH, Hollander E, Nutt D, et al. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69:246–58. doi: 10.4088/jcp.v69n0211. [DOI] [PubMed] [Google Scholar]
- 27.Blier P, Ward HE, Tremblay P, et al. Combination of Antidepressant Medications From Treatment Initiation for Major Depressive Disorder: A Double-Blind Randomized Study. Am J Psychiatry. 2010;167:281–8. doi: 10.1176/appi.ajp.2009.09020186. [DOI] [PubMed] [Google Scholar]
- 28.Deshauer D, Moher D, Fergusson D, et al. Selective serotonin reuptake inhibitors for unipolar depression: a systematic review of classic long-term randomized controlled trials. Cmaj. 2008;178:1293–301. doi: 10.1503/cmaj.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders Mol Psychiatry. 2003;8:574–91. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- 30.Arango V, Huang Y, Underwood MD, et al. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37:375–86. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ, Gould E, Manji H, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–28. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 32.Hasler G, Drevets WC, Manji HK, et al. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 33.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 34.Hyman S. Mental illness: genetically complex disorders of neural circuitry and neural communication. Neuron. 2000;28:321–3. doi: 10.1016/s0896-6273(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 35.Berrettini WH. Genetics of psychiatric disease. Annu Rev Med. 2000;51:465–79. doi: 10.1146/annurev.med.51.1.465. [DOI] [PubMed] [Google Scholar]
- 36.Johansson C, Jansson M, Linner L, et al. Genetics of affective disorders. Eur Neuropsychopharmacol. 2001;11:385–94. doi: 10.1016/s0924-977x(01)00115-8. [DOI] [PubMed] [Google Scholar]
- 37.Lam RW, Mok H. Depression. Oxford University Press Inc; New York, U.S.A.: Oxford, UK: 2008. [Google Scholar]
- 38.Bigos KL, Mattay VS, Callicott JH, et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–45. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray NR, Pergadia ML, Blackwood DH, et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan PF, de Geus EJ, Willemsen G, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–75. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi J, Potash JB, Knowles JA, et al. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry. 2011;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shyn SI, Shi J, Kraft JB, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–15. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–6. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Investigators G, Investigators M, Investigators SD. Common genetic variation and antidepressant efficacy in major depressive disorder: a meta-analysis of three genome-wide pharmacogenetic studies. Am J Psychiatry. 2013;170:207–17. doi: 10.1176/appi.ajp.2012.12020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishi T, Yoshimura R, Fukuo Y, et al. The serotonin 1A receptor gene confer susceptibility to mood disorders: results from an extended meta-analysis of patients with major depression and bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2012 doi: 10.1007/s00406-012-0337-4. [DOI] [PubMed] [Google Scholar]
- 46.Molina E, Cervilla J, Rivera M, et al. Polymorphic variation at the serotonin 1-A receptor gene is associated with comorbid depression and generalized anxiety. Psychiatr Genet. 2011;21:195–201. doi: 10.1097/YPG.0b013e3283457a48. [DOI] [PubMed] [Google Scholar]
- 47.Asad S, Nikamo P, Gyllenberg A, et al. HTR1A a novel type 1 diabetes susceptibility gene on chromosome 5p13-q13. PLoS One. 2012;7:e35439. doi: 10.1371/journal.pone.0035439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz S, Susser E. Genome-wide association studies: does only size matter? Am J Psychiatry. 2010;167:741–4. doi: 10.1176/appi.ajp.2010.10030465. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 50.Törk I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion -5. [DOI] [PubMed] [Google Scholar]
- 51.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 52.Bach-Mizrachi H, Underwood MD, Tin A, et al. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 54.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–43. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- 56.Lanfumey L, Mongeau R, Cohen-Salmon C, et al. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–84. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Albert PR, Zhou QY, Van Tol HH, et al. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–32. [PubMed] [Google Scholar]
- 58.Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 59.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–53. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) [Review] Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 61.Palchaudhuri M, Flugge G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005;321:159–72. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- 62.de Almeida J, Mengod G. Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem. 2008;107:488–96. doi: 10.1111/j.1471-4159.2008.05649.x. [DOI] [PubMed] [Google Scholar]
- 63.Sotelo C, Cholley B, SEM, et al. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–54. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 64.Riad M, Garcia S, Watkins KC, et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–94. [PubMed] [Google Scholar]
- 65.Richer M, Hen R, Blier P. Modification of serotonin neuron properties in mice lacking 5-HT1A receptors. Eur J Pharmacol. 2002;435:195–203. doi: 10.1016/s0014-2999(01)01607-7. [DOI] [PubMed] [Google Scholar]
- 66.Hjorth S, Auerbach SB. Further evidence for the importance of 5-HT1A autoreceptors in the action of selective serotonin reuptake inhibitors. Eur J Pharmacol. 1994;260:251–5. doi: 10.1016/0014-2999(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 67.Hjorth S, Bengtsson HJ, Milano S. Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or beta-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. Eur J Pharmacol. 1996;316:43–7. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- 68.Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J Neurochem. 1997;68:2593–603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu RJ, Lambe EK, Aghajanian GK. Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur J Neurosci. 2005;21:945–58. doi: 10.1111/j.1460-9568.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- 70.Bortolozzi A, Amargos-Bosch M, Toth M, et al. In vivo efflux of serotonin in the dorsal raphe nucleus of 5-HT1A receptor knockout mice. J Neurochem. 2004;88:1373–9. doi: 10.1046/j.1471-4159.2003.02267.x. [DOI] [PubMed] [Google Scholar]
- 71.Kushwaha N, Albert PR. Coupling of 5-HT1A autoreceptors to inhibition of mitogen-activated protein kinase activation via Gbetagamma subunit signaling. Eur J Neurosci. 2005;21:721–32. doi: 10.1111/j.1460-9568.2005.03904.x. [DOI] [PubMed] [Google Scholar]
- 72.Richardson-Jones JW, Craige CP, Nguyen TH, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–18. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT(1A) Autoreceptor Levels Determine Vulnerability to Stress and Response to Antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Artigas F, Romero L, de Montigny C, et al. Acceleration of the effect of selected antidepressant drugs in major depression by 5-ht1a antagonists. Trends Neurosci. 1996;19:378–83. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- 75.Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–8S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 76.Hjorth S, Bengtsson HJ, Kullberg A, et al. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14:177–85. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- 77.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–35. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 78.Gross C, Zhuang X, Stark K, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 79.Gray NA, Milak MS, Delorenzo C, et al. Antidepressant Treatment Reduces Serotonin-1A Autoreceptor Binding in Major Depressive Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albert PR, Lembo P, Storring JM, et al. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 81.Stockmeier CA, Shapiro LA, Dilley GE, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramboz S, Oosting R, Amara DA, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–81. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parks CL, Robinson PS, Sibille E, et al. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–9. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heisler LK, Chu HM, Brennan TJ, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–54. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 86.Kusserow H, Davies B, Hortnagl H, et al. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res. 2004;129:104–16. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 87.Talbot JN, Jutkiewicz EM, Graves SM, et al. RGS inhibition at G(alpha)i2 selectively potentiates 5-HT1A-mediated antidepressant effects. Proc Natl Acad Sci USA. 2010;107:11086–91. doi: 10.1073/pnas.1000003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–7. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weisstaub NV, Zhou M, Lira A, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–40. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 90.Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 91.Austin MC, Whitehead RE, Edgar CL, et al. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114:807–15. doi: 10.1016/s0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 92.Parsey RV, Ogden RT, Miller JM, et al. Higher Serotonin 1A Binding in a Second Major Depression Cohort: Modeling and Reference Region Considerations. Biol Psychiatry. 2010;68:170–8. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sullivan GM, Ogden RT, Oquendo MA, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–30. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lothe A, Didelot A, Hammers A, et al. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008;131:2765–82. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller JM, Brennan KG, Ogden TR, et al. Elevated Serotonin 1A Binding in Remitted Major Depressive Disorder: Evidence for a Trait Biological Abnormality. Neuropsychopharmacology. 2009;34:2275–84. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shrestha S, Hirvonen J, Hines CS, et al. Serotonin-1A receptors in major depression quantified using PET: controversies, confounds, and recommendations. Neuroimage. 2012;59:3243–51. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–33. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 99.Hsiung SC, Adlersberg M, Arango V, et al. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J Neurochem. 2003;87:182–94. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- 100.Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 101.Drevets WC, Frank E, Price JC, et al. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 102.Neumeister A, Bain E, Nugent AC, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–91. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albert PR, Francois BL. Modifying 5-HT1A receptor gene expression as a new target for antidepressant therapy. Front Neurosci. 2010;4:35. doi: 10.3389/fnins.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albert PR, Lembo P, Storring JM, et al. The 5-HT1A receptor: signaling, desensitization, and gene transcription [Review] Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 105.Erdmann J, Shimron-Abarbanell D, Cichon S, et al. Systematic screening for mutations in the promoter and the coding region of the 5-HT1A gene. Am J Med Genet. 1995;60:393–9. doi: 10.1002/ajmg.1320600509. [DOI] [PubMed] [Google Scholar]
- 106.Kawanishi Y, Harada S, Tachikawa H, et al. Novel mutations in the promoter and coding region of the human 5-HT1A receptor gene and association analysis in schizophrenia. Am J Med Genet. 1998;81:434–9. doi: 10.1002/(sici)1096-8628(19980907)81:5<434::aid-ajmg13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 107.Nakhai B, Nielsen DA, Linnoila M, et al. Two naturally occurring amino acid substitutions in the human 5-HT1A receptor: glycine 22 to serine 22 and isoleucine 28 to valine 28. Biochemical & Biophysical Research Communications. 1995;210:530–6. doi: 10.1006/bbrc.1995.1692. [DOI] [PubMed] [Google Scholar]
- 108.Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatr Genet. 1999;9:105–6. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 109.Del Tredici AL, Schiffer HH, Burstein ES, et al. Pharmacology of polymorphic variants of the human 5-HT1A receptor. Biochem Pharmacol. 2004;67:479–90. doi: 10.1016/j.bcp.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 110.Lemonde S, Morris SJ, Bakish D, et al. Proc Soc Neurosci; Society for Neuroscience; U.S.A.: New Orleans, LA. 2000; p. 1850. [Google Scholar]
- 111.Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neuroscience. 2003;23:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hesselgrave N, Parsey RV. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Phil Trans Roy Soc B. 2012 doi: 10.1098/rstb.2012.0004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le François B, Czesak M, Steubl D, et al. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–85. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 114.Villafuerte SM, Vallabhaneni K, Sliwerska E, et al. SSRI response in depression may be influenced by SNPs in HTR1B and HTR1A. Psychiatr Genet. 2009;19:281–91. doi: 10.1097/YPG.0b013e32832a506e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brezo J, Bureau A, Merette C, et al. Differences and similarities in the serotonergic diathesis for suicide attempts and mood disorders: a 22-year longitudinal gene-environment study. Mol Psychiatry. 2010;15:831–43. doi: 10.1038/mp.2009.19. [DOI] [PubMed] [Google Scholar]
- 116.Kishi T, Okochi T, Tsunoka T, et al. Serotonin 1A receptor gene, schizophrenia and bipolar disorder: An association study and meta-analysis. Psychiatry Res. 2011;185:20–6. doi: 10.1016/j.psychres.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Fakra E, Hyde LW, Gorka A, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mekli K, Payton A, Miyajima F, et al. The HTR1A and HTR1B receptor genes influence stress-related information processing. Eur Neuropsychopharmacol. 2011;21:129–39. doi: 10.1016/j.euroneuro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 119.Fisher PM, Meltzer CC, Ziolko SK, et al. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–3. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 120.Zetzsche T, Preuss UW, Bondy B, et al. 5-HT1A receptor gene C -1019 G polymorphism and amygdala volume in borderline personality disorder. Genes Brain Behav. 2008;7:306–13. doi: 10.1111/j.1601-183X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 121.Blaya C, Salum GA, Moorjani P, et al. Panic disorder and serotonergic genes (SLC6A4, HTR1A and HTR2A): Association and interaction with childhood trauma and parenting. Neurosci Lett. 2011;485:11–5. doi: 10.1016/j.neulet.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 122.Choi WS, Lee BH, Yang JC, et al. Association Study between 5-HT1A Receptor Gene C(-1019)G Polymorphism and Panic Disorder in a Korean Population. Psychiatry Investig. 2010;7:141–6. doi: 10.4306/pi.2010.7.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Armbruster D, Mueller A, Strobel A, et al. Predicting cortisol stress responses in older individuals: Influence of serotonin receptor 1A gene (HTR1A) and stressful life events. Horm Behav. 2011;60:105–11. doi: 10.1016/j.yhbeh.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Benedetti F, Radaelli D, Poletti S, et al. Association of the C(-1019)G 5-HT1A promoter polymorphism with exposure to stressors preceding hospitalization for bipolar depression. J Affect Disord. 2011;132:297–300. doi: 10.1016/j.jad.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 125.Zhang KR, Xu Q, Xu Y, et al. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224–31. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 126.Lenze EJ, Shardell M, Ferrell RE, et al. Association of serotonin-1A and 2A receptor promoter polymorphisms with depressive symptoms and functional recovery in elderly persons after hip fracture. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wasserman D, Geijer T, Sokolowski M, et al. The serotonin 1A receptor C(-1019)G polymorphism in relation to suicide attempt. Behav Brain Funct. 2006;2:14. doi: 10.1186/1744-9081-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacob CP, Nguyen TT, Dempfle A, et al. A gene-environment investigation on personality traits in two independent clinical sets of adult patients with personality disorder and attention deficit/hyperactive disorder. Eur Arch Psychiatry Clin Neurosci. 2010;260:317–26. doi: 10.1007/s00406-009-0079-0. [DOI] [PubMed] [Google Scholar]
- 129.Benko A, Lazary J, Molnar E, et al. Significant association between the C(-1019)G functional polymorphism of the HTR1A gene and impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:592–9. doi: 10.1002/ajmg.b.31025. [DOI] [PubMed] [Google Scholar]
- 130.Strobel A, Gutknecht L, Rothe C, et al. Allelic variation in 5-HT(1A) receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- 131.Hettema JM, An SS, van den Oord EJ, et al. Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:661–6. doi: 10.1002/ajmg.b.30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chipman P, Jorm AF, Tan XY, et al. No association between the serotonin-1A receptor gene single nucleotide polymorphism rs6295C/G and symptoms of anxiety or depression, and no interaction between the polymorphism and environmental stressors of childhood anxiety or recent stressful life events on anxiety or depression. Psychiatr Genet. 2010;20:8–13. doi: 10.1097/YPG.0b013e3283351140. [DOI] [PubMed] [Google Scholar]
- 133.Schmitz A, Kirsch P, Reuter M, et al. The 5-HT1A C(-1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. Int J Neuropsychopharmacol. 2009;12:383–92. doi: 10.1017/S1461145708009401. [DOI] [PubMed] [Google Scholar]
- 134.Beste C, Heil M, Domschke K, et al. The relevance of the functional 5-HT1A receptor polymorphism for attention and working memory processes during mental rotation of characters. Neuropsychologia. 2010;48:1248–54. doi: 10.1016/j.neuropsychologia.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 135.Beste C, Domschke K, Kolev V, et al. Functional 5-HT1a receptor polymorphism selectively modulates error-specific subprocesses of performance monitoring. Hum Brain Mapp. 2010;31:621–30. doi: 10.1002/hbm.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hullam G, Juhasz G, Bagdy G, et al. Beyond Structural Equation Modeling: model properties and effect size from a Bayesian viewpoint. An example of complex phenotype - genotype associations in depression. Neuropsychopharmacol Hung. 2012;14:273–84. [PubMed] [Google Scholar]
- 137.Serretti A, Artioli P, Lorenzi C, et al. The C(-1019)G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol. 2004;7:453–60. doi: 10.1017/S1461145704004687. [DOI] [PubMed] [Google Scholar]
- 138.Lemonde S, Du L, Bakish D, et al. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7:501–6. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- 139.Kato M, Fukuda T, Wakeno M, et al. Effect of 5-HT1A gene polymorphisms on antidepressant response in major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:115–23. doi: 10.1002/ajmg.b.30783. [DOI] [PubMed] [Google Scholar]
- 140.Neff CD, Abkevich V, Packer JC, et al. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry. 2009;14:621–30. doi: 10.1038/mp.2008.8. [DOI] [PubMed] [Google Scholar]
- 141.Yevtushenko OO, Oros MM, Reynolds GP. Early response to selective serotonin reuptake inhibitors in panic disorder is associated with a functional 5-HT1A receptor gene polymorphism. J Affect Disord. 2010;123:308–11. doi: 10.1016/j.jad.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 142.Alessia M, David R, Adelio L, et al. Role of COMT, 5-HT(1A), and SERT genetic polymorphisms on antidepressant response to transcranial magnetic stimulation. Depress Anxiety. 2011 doi: 10.1002/da.20815. [DOI] [PubMed] [Google Scholar]
- 143.Kole MH, Fuchs E, Ziemann U, et al. Changes in 5-HT1A and NMDA binding sites by a single rapid transcranial magnetic stimulation procedure in rats. Brain Res. 1999;826:309–12. doi: 10.1016/s0006-8993(99)01257-3. [DOI] [PubMed] [Google Scholar]
- 144.Reynolds GP, Arranz B, Templeman LA, et al. Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry. 2006;163:1826–9. doi: 10.1176/ajp.2006.163.10.1826. [DOI] [PubMed] [Google Scholar]
- 145.Wang L, Fang C, Zhang A, et al. The -1019 C/G polymorphism of the 5-HT1A receptor gene is associated with negative symptom response to risperidone treatment in schizophrenia patients. J Psychopharmacol. 2008;22:904–9. doi: 10.1177/0269881107081522. [DOI] [PubMed] [Google Scholar]
- 146.Parks CL, Shenk T. The serotonin 1a receptor gene contains a TATA-less promoter that responds to MAZ and Sp1. J Biol Chem. 1996;271:4417–30. doi: 10.1074/jbc.271.8.4417. [DOI] [PubMed] [Google Scholar]
- 147.Meijer OC, Williamson A, Dallman MF, et al. Transcriptional repression of the 5-HT1A receptor promoter by corticosterone via mineralocorticoid receptors depends on the cellular context. J Neuroendocrinol. 2000;12:245–54. doi: 10.1046/j.1365-2826.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- 148.Storring JM, Charest A, Cheng P, et al. TATA-driven transcriptional initiation and regulation of the rat serotonin 5-HT1A receptor gene. J Neurochem. 1999;72:2238–47. doi: 10.1046/j.1471-4159.1999.0722238.x. [DOI] [PubMed] [Google Scholar]
- 149.Abdouh M, Albert PR, Drobetsky E, et al. 5-HT1A-mediated promotion of mitogen-activated T and B cell survival and proliferation is associated with increased translocation of NF-kappaB to the nucleus. Brain Behav Immun. 2004;18:24–34. doi: 10.1016/s0889-1591(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 150.Abdouh M, Storring JM, Riad M, et al. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem. 2001;276:4382–8. doi: 10.1074/jbc.M004559200. [DOI] [PubMed] [Google Scholar]
- 151.Wissink S, Meijer O, Pearce D, et al. Regulation of the rat serotonin-1A receptor gene by corticosteroids. J Biol Chem. 2000;275:1321–6. doi: 10.1074/jbc.275.2.1321. [DOI] [PubMed] [Google Scholar]
- 152.Ou XM, Storring JM, Kushwaha N, et al. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- 153.Meijer OC, de Kloet ER. Corticosterone and serotonergic neurotransmission in the hippocampus: functional implications of central corticosteroid receptor diversity. Crit Rev Neurobiol. 1998;12:1–20. [PubMed] [Google Scholar]
- 154.Hendricks TJ, Fyodorov DV, Wegman LJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 155.Liu C, Maejima T, Wyler SC, et al. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–8. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacobsen KX, Czesak M, Deria M, et al. Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo. J Neurochem. 2011;116:1066–76. doi: 10.1111/j.1471-4159.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ou XM, Jafar-Nejad H, Storring JM, et al. Novel dual repressor elements for neuronal cell-specific transcription of the rat 5-HT1A receptor gene. J Biol Chem. 2000;275:8161–8. doi: 10.1074/jbc.275.11.8161. [DOI] [PubMed] [Google Scholar]
- 158.Lemonde S, Rogaeva A, Albert PR. Cell type-dependent recruitment of trichostatin A-sensitive repression of the human 5-HT1A receptor gene. J Neurochem. 2004;88:857–68. doi: 10.1046/j.1471-4159.2003.02223.x. [DOI] [PubMed] [Google Scholar]
- 159.Ou XM, Lemonde S, Jafar-Nejad H, et al. Freud-1: A novel calcium-regulated repressor of the 5-HT1A receptor gene. J Neuroscience. 2003;23:7415–25. doi: 10.1523/JNEUROSCI.23-19-07415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hadjighassem MR, Austin MC, Szewczyk B, et al. Human Freud-2/CC2D1B: a novel repressor of postsynaptic serotonin-1A receptor expression. Biol Psychiatry. 2009;66:214–22. doi: 10.1016/j.biopsych.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hadjighassem MR, Galaraga K, Albert PR. Freud-2/CC2D1B mediates dual repression of the serotonin-1A receptor gene. Eur J Neurosci. 2011;33:214–23. doi: 10.1111/j.1460-9568.2010.07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szewczyk B, Albert PR, Rogaeva A, et al. Decreased expression of Freud-1/CC2D1A, a transcriptional repressor of the 5-HT1A receptor, in the prefrontal cortex of subjects with major depression. Int J Neuropsychopharmacol. 2010;13:1089–101. doi: 10.1017/S1461145710000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Albert PR, Le Francois B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Czesak M, Lemonde S, Peterson EA, et al. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006;26:1864–71. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Czesak M, Le Francois B, Millar AM, et al. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in Deformed Epidermal Autoregulatory Factor-1 (Deaf-1) gene knock-out mice. J Biol Chem. 2012;287:6615–27. doi: 10.1074/jbc.M111.293027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 167.Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]