Abstract
Encephalopathy of prematurity (EoP) encompasses the central nervous system (CNS) abnormalities associated with injury from preterm birth. Although rapid progress is being made, limited understanding exists of how the cellular and molecular CNS injury from early birth manifests as the myriad of neurological deficits in children who are born preterm. More importantly, this lack of direct insight into the pathogenesis of these deficits hinders both our ability to diagnose those infants who are at risk in real-time and could potentially benefit from treatment, and our ability to develop more effective interventions. Current barriers to clarifying the pathophysiology, developmental trajectory, injury timing and evolution include preclinical animal models that only partially recapitulate the molecular, cellular, histological and functional abnormalities observed in the mature CNS following EoP. Inflammation from hypoxic-ischemic and/or infectious injury induced in utero in lower mammals, or actual prenatal delivery of more phylogenetically-advanced mammals, are likely to be the most clinically relevant EOP models, facilitating translation to benefit infants. Injury timing, type, severity and pathophysiology need to be optimized to address the specific hypothesis being tested. Functional assays of the mature animal following perinatal injury to mimic EoP should ideally test for the array of neurological deficits commonly observed in preterm infants including gait, seizure threshold, cognitive and behavioral abnormalities. Here, we review the merits of various preclinical models, identify gaps in knowledge that warrant further study and consider challenges that animal researchers may face in embarking on these studies. While no one model system is perfect, insights relevant to the clinical problem can be gained with interpretation of experimental results within the context of inherent limitations of the chosen model system. Collectively, optimal use of multiple models will address a major challenge facing the field today – to identify the type and severity of CNS injury these vulnerable infants suffer in a safe and timely manner, such that emerging neuro-interventions can be tailored to specifically address individual reparative needs.
Keywords: cerebral palsy, chorioamnionitis, encephalopathy of prematurity, hypoxia-ischemia, inflammation, lipopolysaccharide, perinatal brain injury, preclinical models, preterm, subplate
Introduction
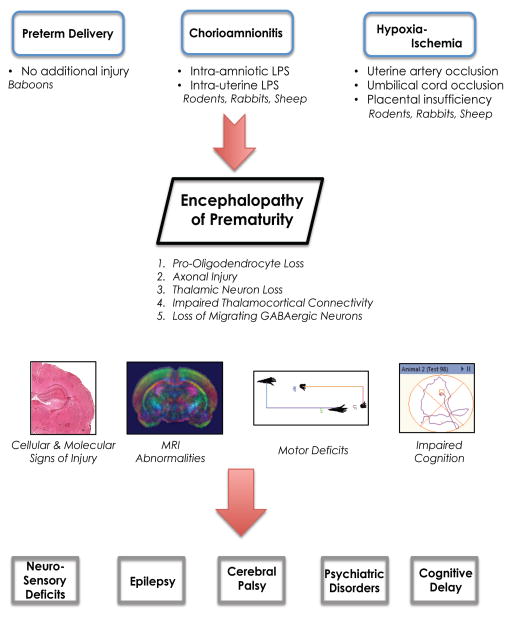
Encephalopathy of prematurity (EoP) encompasses the central nervous system (CNS) abnormalities that are associated with CNS injury from preterm birth[1]. While white matter injury of periventricular leukomalacia (PVL) was previously the most obvious finding on neonatal cranial ultrasounds, over time it has become evident that perinatal complications associated with preterm birth affect not only the developing cerebral white matter but also the entire CNS[1,2]. In the past two decades in regions with advanced neonatal intensive care unit (NICU) care, a decline in white matter injury severity has occurred with a shift from cystic PVL to the now more common diffuse white matter gliosis. A concomitant shift in improvement in cognitive and behavioral deficits, however, has not been observed[3], which supports the concept that EoP involves more than white matter injury. EoP encompasses white matter injury with loss of immature oligodendrocytes and axons, premature loss of subplate, loss of thalamic neurons and impaired thalamocortical connections, and loss of migrating γ-aminobutyric acid (GABAergic) neurons[2]. Despite rapid progress in neuroscience, we still have a relatively limited understanding of how the cellular and molecular CNS injury from preterm birth manifests as the myriad of chronic neurological deficits in children who are born preterm. Causes of perinatal brain injury (PBI) from preterm birth vary in type and severity, and individual variation in the susceptibility to those diverse insults creates additional complexity. Moreover, a wide gulf exists between the patterns of CNS injury observed emerging from advanced NICUs and the general population of preterm infants worldwide. A major challenge facing the field today is identifying the extent of CNS injury these vulnerable infants are suffering on a molecular level in real-time, such that infants can be stratified and receive emerging neuro-interventions in specifically tailored and indicated regimens, rather than a single regimen for all. Given these challenges of repairing the injured developing brain, models used to test specific hypotheses in preclinical studies to advance the care of these children need to be carefully chosen to adequately mimic the human condition of EoP (Figure 1).
Figure 1.
Preclinical models of encephalopathy of prematurity (EoP) include preterm deliveries in non-human primates, and in other mammals chorioamnionitis induced by intra-amniotic or intrauterine endotoxin and/or hypoxia-ischemia mimicking placental underperfusion. These primary approaches to recapitulating CNS injury associated with preterm birth lead to loss of oligodendroglial lineage cells and axonal injury, premature subplate loss, loss of thalamic neurons, and loss of migrating GABAergic neurons, the key components of EoP. Cumulatively, the cellular and molecular abnormalities manifest as imaging abnormalities, motor deficits, propensity to seizures, and impaired cognition and behavior in the mature brain. EoP leads to cerebral palsy, epilepsy, cognitive deficits, and behavioral abnormalities such as anxiety, attention-deficit disorder and autism.
Encephalopathy of Prematurity: Clinical Considerations
In 2010, there were approximately 15 million preterm births (gestation <37 weeks) worldwide, and direct complications from preterm birth accounted for approximately 35% of the world’s neonatal deaths[4]. Beyond the neonatal mortality, preterm birth extensively alters the trajectory of neurodevelopment and overall health for these children. In the USA, approximately 25% of cerebral palsy (CP) results from preterm birth. As gestational age (GA) decreases, the risk of CP and related co-morbidities increases. Indeed, 14% of infants born before 27 weeks GA develop cerebral palsy (CP) compared to 0.2% of the general population[5]. As former preterm infants become children and young adults, further neurological deficits become manifest. Besides CP and the related but milder developmental coordination disorder, impairments of learning, cognition, memory, executive function, vision and hearing, as well as epilepsy, and psychiatric disorders are often diagnosed. The risks of these neurological deficits specifically increase for infants born extremely preterm (<28 weeks GA), with up to 50% experiencing cognitive delay and behavioral problems[3,6]. Together, these deficits contribute to lifelong prematurity-related burden of chronic disease and generate significant cumulative individual, familial, social and economic impact.
Encephalopathy of prematurity has multiple causes[2], and systemic perinatal inflammation from infection and/or hypoxia-ischemia (HI) likely act in concert to potentiate CNS damage from preterm birth. The risks of neurological deficits are higher in preterm infants who are also small for gestational age (SGA, weight < 10% of expected for GA)[6,7], or who have associated perinatal infection[6–9]. Detailed analyses of placentas from extremely preterm infants show inflammation is more commonly associated with spontaneous early birth[10], and chorioamnionitis is now recognized as a major cause of spontaneous preterm delivery[11]. While the impact of isolated prenatal hypoxia-ischemia is challenging to document in humans born preterm, placental perfusion defects compound damage from chorioamnionitis[12].
Improving Translational Science and Therapeutic Target Identification in EoP
To identify and refine targets for new, effective therapeutic interventions for EoP, it is essential to improve our understanding of how insults in utero and in the perinatal period translate to impaired neurodevelopment. EoP is a constellation of abnormalities that includes diffuse white matter damage and oligodendrocyte loss, axonal injury, premature subplate loss, and neuronal injury to the cerebral cortex, thalamus, basal ganglia, cerebellum, and brain stem[2,13]. Post-mortem samples from human infants with diffuse white matter gliosis show loss of markers of cerebral GABAergic signaling[14], and loss of expression of the neuron-specific potassium chloride cotransporter 2 (KCC2) compared to brains without evidence of white matter injury[15]. Cerebral cortical layer IV KCC2 upregulation and GABAergic subunit maturation is dependent upon subplate neuronal survival[16]. The subplate also contributes to cerebral circuit development and white matter tract refinement[17,18]. Excess subplate gliosis[19] and thalamic neuronal loss[20] have been observed in specimens from human preterm infants with white matter injury, and deficient thalamocortical connections were evident with advanced magnetic resonance imaging (MRI)[21]. On a cellular level, EoP affects the development of all neural elements including neurons, oligodendrocytes, astrocytes, microglia and the neurovascular unit, and related components including the extracellular matrix, circuit and network formation. The recent demonstration that myelination of cortical pyramidal neuron axons is likely involved in cerebral network development[22] reinforces the concept that integrated development of all neural elements is crucial to cerebral cortical network formation.
Because of the complex interaction of etiologies, neurodevelopmental time course, intricacy of human network formation, overlapping mechanisms and diverse phenotypes of CNS injury manifest in human preterm infants, EoP is difficult to model well in animals (Table 1). As discussed above, EoP is also challenging to model because multiple cell types are injured, intersecting pathophysiological pathways converge and overlap, and the immune response is also undergoing maturation. There are cell-type specific vulnerabilities (i.e. immature oligodendrocytes)[23], and diverse developmentally-regulated pathways (i.e. subplate, antioxidants, transporters and receptor subunits)[24–28]. Preclinical models of EoP need to replicate the human condition as much as possible by including a prenatal insult that incorporates the maternal-placental-fetal unit, the heterogeneity of mechanisms of CNS injury observed in the preterm infant, and evaluation of both gray and white matter damage and recovery. EoP models should also mimic the pathophysiology at the regional, cellular and molecular levels, including the related development of the neuroimmune system. Thus, to best advance translational objectives, ideal preclinical models of EoP need to include similar mechanisms of prenatal global injury observed in humans, the interaction of various neural cell types through critical developmental periods, address the multiple components of injury evident throughout the CNS, and produce a similar spectrum of functional deficits in the mature animal (Figure 1). No one preclinical model is ideal to test every hypothesis (Table 1).
Table 1.
Advantages, disadvantages, and controversies involved in selecting animal models of encephalopathy of prematurity.
| Species | Advantages | Disadvantages | Controversies |
|---|---|---|---|
| Mouse |
|
|
|
| Rat |
|
|
|
| Rabbit |
|
|
|
| Piglet |
|
|
|
| Sheep |
|
|
|
| Non-Human Primates |
|
|
|
EEG; electroencephalogram; EoP, encephalopathy of prematurity; HI, ypoxia-ischemia; LPS, lipopolysaccharide, MCA, middle cerebral artery; MRI, magnetic resonance imaging; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome.
Because EoP is difficult to model, it is essential that the experiments be well designed and include proper controls. For example, sham animals should undergo comparable anesthetic and surgical exposure. Especially because of gender differences observed in human clinical studies of outcomes for preterm infants[29], both sexes of animals should be analyzed for functional outcomes, including both sex-specific effects and the effects on both sexes combined. The challenges inherent to EoP that need to be addressed in models include repair of multiple neural cell types, restoration of the microenvironment to support neural cell development, re-establishment of homeostasis, and realignment of multiple developmental trajectories. Similar to the human condition, individual variation in injury exists between animals, and to some degree represents an integral component of an accurate preclinical model. Phenotypic variation following early CNS injury in preclinical models is advantageous because individual variation occurs in humans, such as spasticity versus dystonia or mixed motor deficits, and varying degrees of cognitive and behavioral impairments. In humans and animals, individual variation in severity, recovery, repair and treatment response can impact efficacy of novel therapeutics.
Species: Large or Small Mammals
The choice of animals species used to model EoP impacts the interpretation of experimental data in the context of the inherent limitations posed by the species (Table 1). In the simplest terms, birth does not equate to similar points of CNS development across species[30]. Injury to the developing brain from systemic insults and brain injury as a result of early birth are not necessarily the same entity. For example, the rodent CNS at birth, depending on strain (i.e. Long Evans vs. Sprague Dawley), cell type (i.e. neurons vs. oligodendrocytes) or pathway or protein being examined (i.e. receptor subunit expression), is immature compared to the human CNS at birth, while the human CNS at birth is approximately equivalent to the P10 rodent.
The timing of injury during gestation has a crucial role in the neurodevelopmental trajectory of the offspring. The spatiotemporal regulation of neural cell developmental stages of proliferation, migration and differentiation differ amongst various mammals[30–32] and these cell-specific developmental programs influence the vulnerability to injury. For example, the overlap of the timing of oligodendrocyte lineage and GABAergic neuronal development with the timing of preterm birth makes these cells particularly susceptible to perinatal insults[14,33,34]. In rodents, oligodendrocytes proliferate, migrate and differentiate and myelinate primarily from late gestation through the first two postnatal weeks [35], whereas in humans, oligodendrocytes arise during the third trimester and neonatal period and myelination persists over 2–3 years[36]. Oligodendroglial lineage development can be assessed to through stage specific antigens and although the development of human and animal brains differ in varying degrees of complexity and myelinogenesis, these stage-specific antibodies label developmentally and biologically similar cells across species[23]. Loss of either oligodendrocyte precursor cells or immature oligodendrocytes impacts subsequent myelin formation[37]. With respect to neurons, human GABAergic neurons migrate through the developing white matter and subplate in the third trimester[14,34], and thus are also vulnerable to insults from preterm birth. Thalamic neuronal development is also altered [20], although few mechanistic details regarding thalamic neuronal loss have been elucidated thus far. Thus, to test a specific hypothesis regarding the developmental impact of an injury, maturation of the neural cell type or pathway should be investigated over a broad developmental window as well as in the mature CNS.
In addition to these cellular and developmental considerations, the type of outcome measures being investigated also impacts the choice of species used because functional maturation also varies amongst mammals[30]. While rodents offer practical advantages with respect to available molecular tools, behavioral assessments and resources, larger mammals have gyrencephalic brains with a more prominent subplate, improved gray to white matter ratio and a clinical course of illness more similar to human preterm infants. Studies using genetic approaches and outcome measures such as hippocampal-dependent memory, cognitive and behavioral assessment post-injury are more suitable for rodents. By contrast, larger animals can be instrumented, ventilated and have severity of illness and gray to white matter ratio more comparable to human preterm neonates, but larger mammals often require full veterinary NICUs and significant resources. Other important considerations include, but are not limited to, the plasticity of CNS or neural cell at the stage of development being investigated, the pace of recovery, and injury to other systemic organs that also impact CNS development and repair. Models in sheep can be advantageous to study the brain injury associated with preterm birth[38]. Sheep afford the opportunity to perform clinically relevant studies of white matter, cerebral blood flow, neuroimaging and electroencephalography that are more difficult in smaller mammals. In addition, chronic instrumentation and vascular access also replicate NICU interventions in human infants. However, functional assessment and outcome in the mature ovine brain has been limited, the ovine genome has not been fully characterized and the molecular tools available to study ovine brain injury are limited. Specifically, it is unclear whether ovine models recapitulate the motor and cognitive deficits commonly diagnosed in former preterm infants, and whether cellular and functional damage is sustained in the mature ovine CNS.
Similarly, porcine models may also offer advantages in mimicking the anatomy and physiology of preterm newborns, but preterm porcine models of CNS injury have not yet been fully characterized. Piglet models have been very useful in the study of resuscitation, hemodynamics, hypoxic-ischemic encephalopathy and term brain injury[24,39]. Piglets can also be delivered preterm via Cesarean section between 0.79 and 0.98 GA, with 0.70 GA being approximately equivalent to a 25–27 week GA human. Like sheep, very young piglets require extensive neonatal care. Not only are full Cesarean sections required, but intact animal NICUs are necessary as piglets born before 0.87 GA are thermo- and hemodynamically unstable and have immature lungs that require ventilation and surfactant[40]. Preterm pigs born less than 95% gestation also have many of the same organ immaturities as the preterm human infant, including increased sensitivity to necrotizing enterocolitis, respiratory distress syndrome and chronic lung disease of prematurity[41]. These similarities accurately recapitulate the needs of preterm human infants, especially in the early neonatal period.
Non-human primate models of the brain injury associated with preterm birth have revealed numerous similarities with preterm human infants. Baboons delivered at 125d gestation (0.68 GA) show histological and MRI evidence of white matter loss and ventriculomegaly, concomitant with imaging abnormalities similar to those observed in human preterm infants at term age equivalent including T2 signal changes, elevated apparent diffusion coefficient (ADC), lower relative fractional anisotropy (FA) and higher radial diffusivity values[42]. Importantly, the preterm baboon model differs from other existing animal models in that there is no direct insult applied to the developing brain other than that associated with standard neonatal intensive care and ventilatory support[43]. Thus, the baboon model is unlike rodent and other mammal models that subject the developing animals to additional insults such placental insufficiency induced uterine artery occlusion, or inflammation induced by an endotoxin. The combination of having CNS injury evident without an additional insult plus the complex gyral formation of a higher order species makes this model compelling[43], especially as diffuse white matter injury without cystic lesions is the most common finding, with hippocampal and gray matter cell loss, EEG and MRI abnormalities. Thus, all of the mammals offer prenatal models of injury and advanced imaging with MRI (Table 1). Moving forward a reasonable strategy would be to validate injury mechanism and therapeutic interventions in multiple animal models. Rodent models are ideal to provide for rapid, cost-effective access to cellular and molecular mechanisms and target validation, which could then be subsequently confirmed, as necessary, in large animal models such as the instrumented sheep, preterm piglet, or non-human primate.
Cross-Talk within the Maternal-Placental-Fetal Unit
Infants who are born preterm are at risk for a combination of hypoxia-ischemia (HI) and/or infectious inflammatory insults throughout the perinatal period, and both infection and HI induce inflammatory signaling, both systemically and in the CNS. Many existing animal models can be categorized according to the location of the injury in relation to the maternal-placental-fetal unit. Models in rats, mice, rabbits, sheep, pigs, and non-human primates have contributed information from each level of the maternal-placental-fetal system. However, historically and despite recent advances in understanding the pathophysiology of EoP, injury models in postnatal rodents have been used, even though in utero inflammation or placental underperfusion is not recapitulated. Many, but not all postnatal rodent models, demonstrate focal stroke-like pathophysiology accompanied by cystic lesions in white and gray matter, or severe cell death in the middle cerebral artery (MCA) vascular territory, in contrast to the diffuse injury and gliosis observed throughout the brain that is commonly observed in preterm infants in current advanced NICUs. Models of in utero inflammation from inactivated-infectious agents and/or placental hypoxia-ischemia that show cellular, histologic and function deficits similar to those observed in humans, however, exist in rodents[35,44–46], rabbits[47,48], sheep [38,49]. These models mimic the complex maternal-placental-fetal insults that occur in human infants born preterm, which is crucial because chorioamnionitis, placental and amniotic membrane inflammation and placental underperfusion comprise such a large component of human preterm pathophysiology that leads to EoP. Despite these improvements, it is difficult to have preclinical models that are fully representative of every component of EoP. Structurally, rabbits, rats, mice and higher order primates like humans, have hemochorial placentation with discoid placentas. Sheep on the other hand, have cotyledonary and epitheliochorial placentas that differ significantly in structure, blood flow, maternal-placental interface and development. Additionally, actually recapitulating the common clinical scenario in humans of ascending bacterial infections that weaken the amnion and precipitate premature rupture of membranes and/or placental perfusion defects that stress the placental interface and disrupt placental homeostasis is challenging. However, strides have been made to involve multiple components of the maternal-placental-fetal unit and involve in utero inflammation to various degrees. The type of bacterial or viral infection, along with timing during gestation, may impact the neurodevelopmental consequences. As noted above, similar stimuli may induce different CNS phenotypes depending on the timing, severity and repetition of injury administration, and different stimuli may induce very similar CNS phenotypes. This complexity in preclinical models is similar to the wide array of deficits observed in humans who are born very preterm and suffer from a myriad of perinatal insults.
In animals, numerous strategies have been employed to induce inflammation from infectious agents or hypoxia-ischemia. At the maternal level, inactivated-infectious inflammation has been produced with systemic gram negative bacterial endotoxin lipopolysaccharide (LPS) administration via intraperitoneal injections, application directly to the cervix, intrauterine injection or by intrauterine infusion either alone[47,50], or in combination with HI[51]. As LPS does not cross the placenta, intraperitoneal injections in dams are less likely to produce the same fetal inflammatory response and fetal membrane and vessel involvement as the various forms of intrauterine administration. LPS has the advantage over direct infection with typical intrauterine bacteria in that it activates inflammatory signaling through toll-like receptor 4 without causing active bacterial infection and the associated risk of pathogen spread. Similar to studies using LPS as a Gram negative bacterial analog to induce activate inflammatory signaling, laboratories have also investigated maternal inflammation with intraperitoneal injections of inactivated group B streptococcus, which causes placental and neuropathological abnormalities, and autistic-like behavior in rats [52]. Similarly, Ureaplasma lipoprotein multiple-banded antigen can simulate Ureaplasma species infection, the most common cause of human chorioamnionitis[53], but preclinical studies of CNS injury using this agent have not been performed yet. As more inactivated-infectious agents become available, it will be informative to determine how they differentially impact neurodevelopment and the efficacy of neuro-reparative interventions.
In rodents, steps have been taken to make improvements in delivery of an inflammatory stimulus in the intra-uterine environment. We recently developed an in utero rat model of intra-amniotic LPS administration without or with HI that induces molecular, histologic and functional abnormalities in mature animals, including a spasticity-like motor phenotype that includes toe-walking, ataxia, shorter stride length, gait variability and inconsistency[54]. Although gait abnormalities are present in mature rats, more prominent early postnatal increases in muscle tone are observed in rabbits exposed to prenatal LPS[47], illustrating differences amongst preclinical models. Although prenatal HI alone in rats produces motor phenotype with cognitive deficits[44] and a lower seizure threshold[55], the addition of LPS-induced inflammation encompasses a component of in utero injury commonly found in extremely preterm births[10,11]. Thus, an inactivated-infectious stimulus in the uterus can promote processes that lead to fetal CNS damage[8]. Specifically, multiple pathogens can initiate the intrauterine inflammatory process and support the hypothesis that microorganisms isolated from the uterus increases the risk of white matter damage in preterm infants[8]. Further study is necessary to clarify the degree of placental involvement across species and the relationship between injury observed, placentation, inherent placental structure and vascular supply to the fetus.
Precipitating Insult: Inflammation from Infectious Stimuli and/or Hypoxia-ischemia
The induction, evolution and resolution of inflammation in the developing CNS are exceedingly complex and multifactorial. The preterm newborn is capable of intermittent or sustained systemic inflammation that contributes to adverse neurodevelopmental outcomes[56]. Specifically, prolonged, poorly regulated neuroinflammation and inadequate repair are major factors that contribute to chronic deficits[56]. Inflammation in the perinatal brain may persist because inflammation fails to resolve, developmental regulation is insufficient, positive feedback loops between innate and adaptive immune systems are immature, and/or epigenetic mechanisms[56]. Notably, preterm newborns are deficient in selected serum anti-inflammatory proteins[57], and have neutrophils resistant to apoptosis that persist and propagate inflammatory signals[58]. The abnormal immune reaction may persist. Monocytes from former preterm children with cerebral palsy show a heightened response to an LPS challenge compared to monocytes from their preterm peers who have no neurological deficits[59]. Along with limited anti-inflammatory responsiveness, this capacity to sustain inflammation places preterm infants at an increased risk for CNS damage during perinatal development.
In both large and small animal models, the role of inflammation from inactivated-infectious agents alone, or in combination with hypoxia-ischemia, has been controversial. The difficulty arises largely because multiple variables related to experimental design, such as preconditioning and sensitization, alter the observed outcomes[60]. Experimental design factors include the species and age of animals used, type and severity of precipitating insult including dose, route of administration, dosing regimen, and experimental endpoints. These various factors make direct study-to-study comparisons challenging[51]. The experimental endpoints chosen are important because acute and chronic changes differentially affect outcome with respect to myelination, neural cell survival, cellular inflammatory response, motor function[54]. Undoubtedly, the temporal relationship between LPS and HI insults can be critical[60,61]. Similarly, differences in dosage can elicit divergent results. Low concentrations of LPS have been reported to reduce damage prior to HI, whereas higher doses (300μg/kg) have been reported to potentiate HI damage[62]. The reason for this variability may be related to cellular inflammatory responses and individual changes in microglial activation and reactivity. None of the above mentioned models in rodents are clinically relevant to EoP, however, as they occur in postnatal rodents and involve carotid ligation plus systemic hypoxia that results in focal stroke-like pathology that is uncommon in human preterm infants. In addition, none of these models recapitulate the complexities of the maternal-placental-fetal abnormalities observed in human infants. These models may be useful, however, to address important questions regarding systemic inflammation and the molecular mechanisms of cellular sensitization and preconditioning in the immature neuro-immune environment.
Although sheep have a different type of maternal-placental-fetal unit than humans, sheep do allow more precise studies of physiological manipulation than rodents. In preterm fetal sheep a regimen of acute on chronic inflammation is associated with white matter injury and a fetal inflammatory response[63]. By contrast, in fetal sheep at 0.7 GA (broadly equivalent to 28–32 weeks of human development), chronic LPS infusion prior to a period of asphyxia resulted in reduced white matter injury compared to asphyxia alone[63]. Together, these data emphasize the importance of the LPS dosing regimen, time course of injury and route of administration. To facilitate clinical translation it may be most beneficial to use models that incorporate the placental unit and inflammation from either HI and/or infectious stimuli to better resolve the contribution of chorioamnionitis to brain injury in human preterm birth. A key characteristic of human EoP is that the developing CNS appears to experience a sustained and multiple waves of inflammation due to cumulative insults[56,64]. Animal models of intra-uterine inflammation from inactivated-infectious agents reveal insight into the molecular mechanisms underlying fetal CNS injury, and may be especially relevant to the clinical scenario. Chronic intra-amniotic administration of LPS to fetal sheep induces microglial activation and subcortical white matter injury, with hippocampal and white matter injury occurring independent of gross T2 volumetric MRI changes[49]. Interestingly, a single intra-amniotic bolus of LPS in the prenatal ovine also leads to microglial activation, astrocyte proliferation and increased apoptosis, associated with functional EEG changes[65]. Thus, both isolated and repetitive LPS dosages in utero can induce a sustained pattern of CNS inflammation and injury that mimics the damage observed in human preterm infants.
Similarly, the amount of hypoxic-ischemic injury can also be varied. In sheep, Rees and her colleagues have investigated HI in numerous contexts and paradigms including acute and chronic placental insufficiency[66,67]. Similar to the gradation of injury found with prenatal transient systemic HI in rats[35], a relatively prolonged interval of 25min of umbilical cord occlusion at 0.7 gestation produces a more severe, global HI injury with more robust microglial activation and proliferation, together with white matter injury, EEG suppression and neutrophil influx in to the brain[68]. Likewise, we found increasing intervals of transient systemic hypoxia-ischemia (TSHI) on E18 in pregnant Sprague-Dawley rat dams induces a graded placental underperfusion defect associated with increasing CNS damage[35]. This timing recapitulates the intrauterine global prenatal insult that occurs in human infants prior to extremely preterm birth at 23–25 weeks gestation. Previously, we showed this injury consistently results in white matter astrogliosis, oligodendrocyte loss and axonal disruption in white matter in cortex similar to alterations observed in human postmortem samples[35]. In this model following injury, O4-immunoreactive immature oligodendrocytes are most affected and their loss correlates with decreased survival and maturation[69], with the most notable reductions in O4+ and O1+ stages of the lineage[35], consistent with previous reports by other investigators[70]. This model also demonstrates premature loss of the subplate, reduced KCC2 expression, and a lower seizure threshold and impaired gait[25,26,55]. Detailed analyses of other components of EoP including loss of thalamic and GABAergic neurons, and the contribution of placental pathology is underway. The sustained period of perinatal inflammation and gradation of injury observed in humans, as well as in both small and large mammals from various HI and/or LPS insult regimens, supports the need to identify accurate biomarkers to quantify the extent of ongoing CNS injury in real-time in both preclinical models of EoP and preterm infants.
To better mimic the high incidence of chorioamnionitis in spontaneous very early preterm birth[10,11], and the related impact of infectious stimuli on CNS development, in utero inflammation from intra-amniotic LPS was added to HI during the E18 laparotomy[54]. Acute postnatal ventriculomegaly and subacute gliosis and white matter loss is observed in pups subjected to dual TSHI+LPS and TSHI alone in the first two postnatal weeks[54]. Interestingly, sustained myelin basic protein (MBP) loss and axonal damage was more prominent in postnatal day 28 juvenile rats from prenatal TSHI alone, compared to animals with prenatal TSHI+LPS. Sophisticated, computerized treadmill gait analyses revealed significant motor impairment with increased ataxia, decreased paw area consistent with toe-walking, reduced stride length and increased step-to-step variability in all P28 animals subjected to LPS alone, TSHI alone or TSHI+LPS. Limitations of this model include potential variation in the extent of HI and reperfusion injury from transient uterine artery occlusion, although all fetuses become evenly dusky after 60 minutes of TSHI. Also, while no effect was noted with sterile intra-amniotic saline injection used as a control for the LPS, potential loss of amniotic fluid at the time of LPS injection could potentially occur. Although much more work remains, the prenatal TSHI and TSHI+LPS models meet several of the criteria of an EoP model including causing loss of neurons, oligodendrocytes and axons, loss of subplate, and functional deficits in adults that mimic those observed in children born extremely preterm.
Preclinical models with prenatal injury in rabbits have also reported gait abnormalities with significant spasticity and abnormalities in tone [47,48]. Similar to the rat models of prenatal injury[35,44,46,54], the rabbit model is advantageous because it is a fetal model, involves global CNS injury, spares the dam significant effects, and yields large litter sizes via normal delivery[48]. Rabbits develop and retain motor deficits over the first three postnatal weeks. Reduced fractional anisotropy and microstructural abnormalities are also observed with advanced MRI techniques[71,72]. Although these rabbit models induce similar motor deficits to the rodent models, testing of cognition and behavior, the most burdensome deficits for children who are born preterm, remains challenging in rabbits. Similarly, the contribution of loss of subplate, thalamic and GABAergic neurons following late gestation injury has yet to be investigated well in rabbits or larger mammals. In considering similarities between inflammation induced by HI and infectious insults leading to gait deficits, it is possible that mechanistic pathways diverge in some respects and converges on others[54,73]. For example, it is possible that HI produces a primary injury to oligodendrocytes and neurons, followed by secondary microglial activation; whereas LPS may activate microglia as the primary event, followed by secondary injury to surrounding neural cells and the neurovascular niche[47].
Dissecting how the specific injuries, alone or cumulatively impact the multiple components of injury in EoP are essential to refining the targets for intervention. In the future, investigations using preclinical models could lead to substantive mechanistic advances in pathophysiology, and guide therapeutic strategies with stratification of preterm infant populations.
Conclusion
Our limited understanding of the mechanisms underlying in utero insults that adversely affect neurodevelopment following extremely preterm birth hinders the rational design and use of neonatal interventions to mitigate early CNS injury. Human studies of preterm birth reveal a strong correlation between intrauterine and postnatal inflammation, primarily postnatal hypoxia-ischemia and subsequent impaired CNS development. Human studies also suggest that different types of injury, alone or in combination, impact the developing CNS via unique mechanisms, and thus preterm infants most likely will require tailored neonatal cocktails of emerging neuro-reparative interventions. Given the immense complexity of the human developing CNS and the myriad of insults that affect the maternal-placental-fetal unit, there is no perfect animal model of EoP. However, significant strides have been made over recent years to understand the pathogenesis of EoP. While some models may be more or less relevant to the clinical scenario of EoP, each study has advanced the field and improved our understanding of the pathophysiology, and potentially viable and safe therapeutic targets such as erythropoietin, N-acetylcysteine and melatonin[74]. As one looks forward, an optimal paradigm will include both rodents and larger animals, and subsequent target validation in humans. Preclinical studies provide an essential foundation for carefully conducted clinical studies[38]. Accordingly, progress relies on consistency and selection of the most appropriate model or experimental paradigm for the hypothesis at hand. Given the potential for overlapping mechanisms of infection and HI-related brain injury, placental inflammation, ischemic and inflammatory tolerance are important considerations. Cross-talk and convergent points in the pathophysiology such as toll-like receptors, cytokines, and inflammatory cell activation likely exist, as do mechanisms for divergence such as EPOR receptor expression, repair inflammatory phenotypes and cerebral blood flow autoregulation. Thus, evaluation of the CNS microenvironment and integrated neural components is crucial, as an effective therapeutic strategy or cocktail of interventions for EoP will likely hinge on improvement of all neural cells in order to normalize function and repair deficits that prevent children with brain injury associated with prematurity from leading healthy, productive, and independent adult lives.
Acknowledgments
Funding Sources: NIH-NINDS R01 NS060765 (SR), Boston Children’s Hospital Translational Research Program (SR + LJ), University of New Mexico P30 CoBRE Pilot Program (LJ), University of New Mexico Child Health Signature Program (LJ).
Literature Cited
- 1.Volpe J. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- 2.Volpe J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neuro. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson PJ. Neuropsychological outcomes of children born very preterm. Sem Fet Neonatal Med. 2014;19:90–96. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J Born Too Soon Preterm Birth Action G. Born too soon: The global epidemiology of 15 million preterm births. Reproductive Health. 2013;10 (Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev. 2013;89:781–786. doi: 10.1016/j.earlhumdev.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, Hack M. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J International Neuropsych Soc : JINS. 2011;17:1067–1079. doi: 10.1017/S135561771100107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leviton A, Fichorova RN, O’Shea TM, Kuban K, Paneth N, Dammann O, Allred EN, Investigators ES. Two-hit model of brain damage in the very preterm newborn: Small for gestational age and postnatal systemic inflammation. Ped Res. 2013;73:362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’Shea TM, Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. The ELGAN study Ped Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, Leviton A, Investigators ES. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun. 2013;29:104–112. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trivedi S, Joachim M, McElrath T, Kliman HJ, Allred EN, Fichorova RN, Onderdonk A, Heitor F, Chaychi L, Leviton A, Majzoub JA Extremely Low Gestational Age Newborns Study Investigators. Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstetr Gynec. 2012;206:236, e231–238. doi: 10.1016/j.ajog.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, Walsh MC, Das A, Hale EC, Newman NS, Higgins RD. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168:137–147. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Ped Res. 2006;59:478–483. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- 13.Robinson S. Systemic prenatal insults disrupt telencephalon development: Implications for treatment. Epilepsy Beh. 2005;7:345–363. doi: 10.1016/j.yebeh.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson S, Li Q, DeChant A, Cohen M. Neonatal loss of gamma amino butyric acid pathway expression after human perinatal brain injury. J Neurosurgery: Pediatrics. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson S, Mikolaenko I, Thompson I, Cohen M, Goyal M. Loss of cation-chloride cotransporter expression in preterm infants with white matter lesions: Implications for the pathogenesis of epilepsy. J Neuropath Exp Neurol. 2010;69:565–572. doi: 10.1097/NEN.0b013e3181dd25bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanold P, Shatz C. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Kostovic I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 19.Pogledic I, Kostovic I, Fallet-Bianco C, Adle-Biassette H, Gressen P, Verney C. Involvement of the subplate zone in preterm infants with periventricular white matter injury. Brain Pathol. 2014;24:128–141. doi: 10.1111/bpa.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, Kinney HC. Thalamic damage in periventricular leukomalacia: Novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Ped Res. 2009;65:524–529. doi: 10.1203/PDR.0b013e3181998baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2013;49:1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Back S, Han B, Luo N, Chricton C, Xanthoudakis S, Tam J, Arvin K, Holtzman D. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantzie LL, Cheung PY, Johnson ST, Bigam DL, Todd KG. Cerebral amino acid profiles after hypoxia-reoxygenation and N-acetylcysteine treatment in the newborn piglet. Neonatology. 2010;97:195–203. doi: 10.1159/000252972. [DOI] [PubMed] [Google Scholar]
- 25.Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu066. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Mol Cell Neurosci. 2014;61:152–162. doi: 10.1016/j.mcn.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. Developmental expression of n-methyl-d-aspartate (nmda) receptor subunits in human white and gray matter: Potential mechanism of increased vulnerability in the immature brain. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht246. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkerth R, Haynes R, Borenstein N, Belliveau R, Trachtenberg F, Rosenberg P, Volpe J, Kinney H. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropath Exp Neurol. 2004;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 29.Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol. 2014;254:54–67. doi: 10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Kelsom C, Lu W. Development and specification of gabaergic cortical interneurons. Cell Biosci. 2013;3:19. doi: 10.1186/2045-3701-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinney H, Back S. Human oligodendroglial development: Relationship to periventricualr leukomalacia. Semin Pediatr Neuro. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Broadbelt KG, Haynes RL, Folkerth RD, Borenstein NS, Belliveau RA, Trachtenberg FL, Volpe JJ, Kinney HC. Late development of the gabaergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson S, Petelenz K, Li Q, Cohen M, Buczek M, Lust D, Miller R. Developmental changes induced by prenatal hypoxia-ischemia insult in rats models human perinatal brain injury. Neurobiol Dis. 2005;18:568–581. doi: 10.1016/j.nbd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Back S, Luo N, Borenstein N, Levine J, Volpe J, Kinney H. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Lewis R, Miller RH. Interactions between oligodendrocyte precursors control the onset of cns myelination. Dev Biol. 2011;350:127–138. doi: 10.1016/j.ydbio.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuypers E, Ophelders D, Jellema RK, Kunzmann S, Gavilanes AW, Kramer BW. White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: Lessons from experimental ovine models. Early Hum Dev. 2012;88:931–936. doi: 10.1016/j.earlhumdev.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Jantzie LL, Cheung PY, Obaid L, Emara M, Johnson ST, Bigam DL, Todd KG. Persistent neurochemical changes in neonatal piglets after hypoxia-ischemia and resuscitation with 100%, 21% or 18% oxygen. Resuscitation. 2008;77:111–120. doi: 10.1016/j.resuscitation.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, Keates HL, Lumbers ER, Colditz PB, Lingwood BE. A pig model of the preterm neonate: Anthropometric and physiological characteristics. PloS One. 2013;8:e68763. doi: 10.1371/journal.pone.0068763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. Acta Paediatr. 2010;99:201–208. doi: 10.1111/j.1651-2227.2009.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith JL, Shimony JS, Cousins SA, Rees SE, McCurnin DC, Inder TE, Neil JJ. Mr imaging correlates of white-matter pathology in a preterm baboon model. Ped Res. 2012;71:185–191. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S. The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropath Exp Neurol. 2004;63:1297–1309. doi: 10.1093/jnen/63.12.1297. [DOI] [PubMed] [Google Scholar]
- 44.Delcour M, Russier M, Amin M, Baud O, Paban V, Barbe M, Coq J. Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Beh Brain Res. 2012;232:233–244. doi: 10.1016/j.bbr.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 45.Sab IM, Ferraz MM, Amaral TA, Resende AC, Ferraz MR, Matsuura C, Brunini TM, Mendes-Ribeiro AC. Prenatal hypoxia, habituation memory and oxidative stress. Pharmacol Biochem Beh. 2013;107:24–28. doi: 10.1016/j.pbb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Olivier P, Baud O, Evrard P, Gressens P, Verney C. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- 47.Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: A link between prenatal infection and cerebral palsy. Am J Obstetr Gynecol. 2008;199:651, e651–657. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derrick M, Luo N, Bregman J, Jilling T, Ji X, Fisher K, Gladson C, Beardsley D, Murdoch G, Back S, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor defcits in the neonatal rabbit: A model for cerebral palsy. J Neuroscience. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuypers E, Jellema RK, Ophelders DR, Dudink J, Nikiforou M, Wolfs TG, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Saito M, Newnham JP, Jobe AH, Kallapur SG, Kramer BW. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PloS One. 2013;8:e81644. doi: 10.1371/journal.pone.0081644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai Z, Pan Z-L, Pang Y, Evans O, Rhodes P. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Ped Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Rousset C, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Sem Fetal Neonatal Med. 2006;11:343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Bergeron JD, Deslauriers J, Grignon S, Fortier LC, Lepage M, Stroh T, Poyart C, Sebire G. White matter injury and autistic-like behavior predominantly affecting male rat offspring exposed to group b streptococcal maternal inflammation. Dev Neurosci. 2013;35:504–515. doi: 10.1159/000355656. [DOI] [PubMed] [Google Scholar]
- 53.Uchida K, Nakahira K, Mimura K, Shimizu T, De Seta F, Wakimoto T, Kawai Y, Nomiyama M, Kuwano K, Guaschino S, Yanagihara I. Effects of ureaplasma parvum lipoprotein multiple-banded antigen on pregnancy outcome in mice. J Reprod Immunol. 2013;100:118–127. doi: 10.1016/j.jri.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Jantzie LL, Corbett CJ, Berglass J, Firl DJ, Flores J, Mannix R, Robinson S. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J Neuroinflam. 2014;11:131. doi: 10.1186/1742-2094-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazur M, Miller R, Robinson S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J Neurosurg Pediatrics. 2010;6:206–221. doi: 10.3171/2010.5.PEDS1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Ped Res. 2014;75:376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, Whyte MK. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax. 2003;58:961–967. doi: 10.1136/thorax.58.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CY, Chang YC, Wang ST, Lee TY, Lin CF, Huang CC. Altered inflammatory responses in preterm children with cerebral palsy. Ann Neurol. 2010;68:204–212. doi: 10.1002/ana.22049. [DOI] [PubMed] [Google Scholar]
- 60.Hagberg H, Dammann O, Mallard C, Leviton A. Preconditioning and the developing brain. Semin Perinatol. 2004;28:389–395. doi: 10.1053/j.semperi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Ped Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Sameshima H, Ikeda T, Ikenoue T. Lipopolysaccharide administration enhances hypoxic-ischemic brain damage in newborn rats. J Obstet Gynaecol Res. 2004;30:142–147. doi: 10.1111/j.1447-0756.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 63.van den Heuij LG, Mathai S, Davidson JO, Lear CA, Booth LC, Fraser M, Gunn AJ, Bennet L. Synergistic white matter protection with acute-on-chronic endotoxin and subsequent asphyxia in preterm fetal sheep. J Neuroinflam. 2014;11:89. doi: 10.1186/1742-2094-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Steenwinckel J, Schang AL, Sigaut S, Chhor V, Degos V, Hagberg H, Baud O, Fleiss B, Gressens P. Brain damage of the preterm infant: New insights into the role of inflammation. Biochem Soc Trans. 2014;42:557–563. doi: 10.1042/BST20130284. [DOI] [PubMed] [Google Scholar]
- 65.Gavilanes AW, Gantert M, Strackx E, Zimmermann LJ, Seeldrayers S, Vles JS, Kramer BW. Increased eeg delta frequency corresponds to chorioamnionitis-related brain injury. Front Biosci (Schol Ed) 2010;2:432–438. doi: 10.2741/s76. [DOI] [PubMed] [Google Scholar]
- 66.Duncan JR, Cock ML, Loeliger M, Louey S, Harding R, Rees SM. Effects of exposure to chronic placental insufficiency on the postnatal brain and retina in sheep. J Neuropathol Exp Neurol. 2004;63:1131–1143. doi: 10.1093/jnen/63.11.1131. [DOI] [PubMed] [Google Scholar]
- 67.Rees S, Breen S, Loeliger M, McCrabb G, Harding R. Hypoxemia near mid-gestation has long-term effects on fetal brain development. J Neuropathol Exp Neurol. 1999;58:932–945. doi: 10.1097/00005072-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Jellema RK, Lima Passos V, Zwanenburg A, Ophelders DR, De Munter S, Vanderlocht J, Germeraad WT, Kuypers E, Collins JJ, Cleutjens JP, Jennekens W, Gavilanes AW, Seehase M, Vles HJ, Steinbusch H, Andriessen P, Wolfs TG, Kramer BW. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J Neuroinflam. 2013;10:13. doi: 10.1186/1742-2094-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jantzie LL, Miller RH, Robinson S. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Ped Res. 2013;74:658–667. doi: 10.1038/pr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drobyshevsky A, Jiang R, Derrick M, Luo K, Tan S. Functional correlates of central white matter maturation in perinatal period in rabbits. Exp Neurol. 2014;261:76–86. doi: 10.1016/j.expneurol.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, Chugani D. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol. 2009;24:1179–1189. doi: 10.1177/0883073809338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hickey E, Shi H, Van Arsdell G, Askalan R. Lipopolysaccharide-induced preconditioning against ischemic injury is associated with changes in toll-like receptor 4 expression in the rat developing brain. Ped Res. 2011;70:10–14. doi: 10.1203/PDR.0b013e31821d02aa. [DOI] [PubMed] [Google Scholar]
- 74.Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, Derrick M, Back SA, Valdez RC, Northington F, Gunn AJ, Mallard C. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatrics. 2012;160:544–552. e544. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]