Abstract
Objective
To examine infiltration of blood foamy monocytes, containing intracellular lipid droplets, into early atherosclerotic lesions and its contribution to development of nascent atherosclerosis.
Approach and Results
In apoE−/− mice fed western high-fat diet (WD), >10% of circulating monocytes became foamy monocytes at 3 days on WD and >20% of monocytes at 1 week. Foamy monocytes also formed early in blood of Ldlr−/−Apobec1−/− (LDb) mice on WD. Based on CD11c and CD36, mouse monocytes were categorized as CD11c−CD36−, CD11c−CD36+ and CD11c+CD36+. The majority of foamy monocytes were CD11c+CD36+, whereas most nonfoamy monocytes were CD11c−CD36− or CD11c−CD36+ in apoE−/− mice on WD. In wild-type mice, CD11c+CD36+ and CD11c−CD36+, but few CD11c−CD36−, monocytes took up cholesteryl ester–rich very-low-density lipoproteins (CE-VLDLs) isolated from apoE−/− mice on WD, and CE-VLDL uptake accelerated CD11c−CD36+–to–CD11c+CD36+ monocyte differentiation. Ablation of CD36 decreased monocyte uptake of CE-VLDLs. Intravenous injection of DiI-CE-VLDLs in apoE−/− mice on WD specifically labeled CD11c+CD36+ foamy monocytes, which infiltrated into nascent atherosclerotic lesions and became CD11c+ cells that were selectively localized in atherosclerotic lesions. CD11c deficiency reduced foamy monocyte infiltration into atherosclerotic lesions. Specific and consistent depletion of foamy monocytes (for 3 weeks) by daily intravenous injections of low-dose clodrosome reduced development of nascent atherosclerosis.
Conclusions
Foamy monocytes, which form early in blood of mice with hypercholesterolemia, infiltrate into early atherosclerotic lesions in a CD11c-dependent manner and play crucial roles in nascent atherosclerosis development.
Keywords: Atherosclerosis, monocytes, inflammation, lipoproteins
Atherosclerosis is a chronic inflammatory process characterized by accumulation of foam cells—macrophages/dendritic cells (DCs) with intracellular lipid deposition—in arterial walls.1–3 Infiltration of monocytes from blood into arterial walls, where monocytes differentiate into macrophages/DCs that take up modified lipoproteins and become foam cells, is an important step for atherogenesis.1, 3, 4 Although this process usually takes decades in humans, in genetic disorders that result in severe hypercholesterolemia, the process is dramatically accelerated, and morbidity in children can result from extensive atherosclerotic disease.5 This provides impetus to understand better the role of circulating lipoproteins in the initiation of the inflammatory axis of atherogenesis.
In our previous study, we reported that apoE−/− mice on western high-fat diet (WD), the commonly used mouse model of atherosclerosis,6, 7 had foamy monocytes—monocytes with intracellular lipid droplets—in blood.8 Foamy monocytes accounted for ~40–50% of total monocytes in blood of apoE−/− mice after WD for 12 weeks. The vast majority (≥80%) of foamy monocytes were positive for CD11c, a β2 integrin, whereas most nonfoamy monocytes in these mice were CD11c−.8 Existence of foamy monocytes in blood was confirmed by other studies in both mice and humans with hyperlipidemia.9–12 Nevertheless, it remains unknown when and how foamy monocytes are formed in blood of mice fed WD. Furthermore, the evidence for direct contributions of foamy monocytes to atherosclerosis, particularly nascent atherosclerosis in which recent studies showed that monocyte recruitment played a significant role,13, 14 is still lacking.
In the present study, we observed that foamy monocytes appeared in blood early after initiation of WD in apoE−/− mice and LDb mice15 and that CD36 played an important role in monocyte uptake of cholesteryl ester (CE)–rich very-low-density lipoproteins (CE-VLDLs), the most abundant lipoproteins from apoE−/− mice on WD. By intravenously injecting DiI-conjugated CE-VLDLs (DiI-CE-VLDLs), we selectively labeled foamy monocytes in apoE−/− mice on WD and found that they infiltrated into nascent atherosclerotic lesions in a CD11c-dependent manner. By daily intravenous injection of low-dose clodrosome, we specifically depleted foamy monocytes in apoE−/− mice on WD and found that depletion over 3 weeks effectively reduced development of nascent atherosclerosis. These studies reveal that foamy monocytes formed early in the circulation contribute to the development of nascent atherosclerosis with severe hypercholesterolemia.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Early appearance of foamy monocytes in blood of apoE−/− mice and LDb mice fed WD
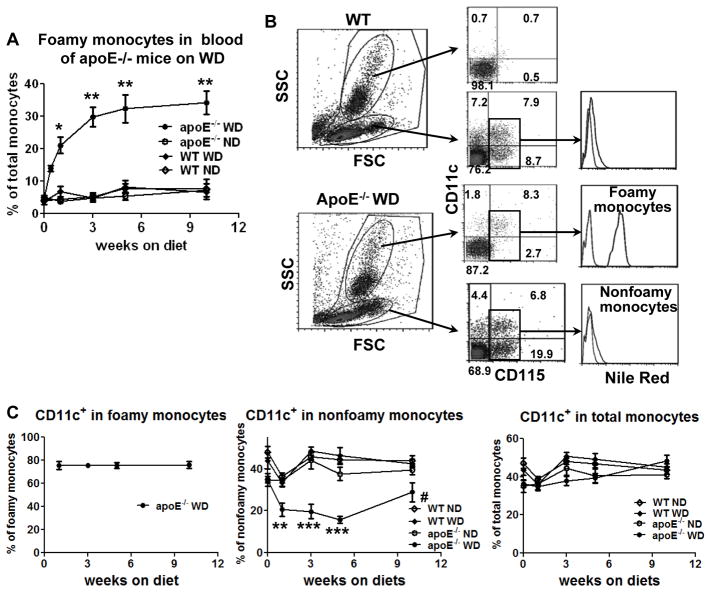
Employing flow cytometric analysis, we first examined foamy monocytes in blood of apoE−/− mice fed WD, a commonly used mouse model of atherosclerosis.6–8 Foamy monocytes were detected within the first week of WD and increased continuously through week 5 (Figure 1A). Foamy monocytes, which stained positive for Nile Red (Figure 1B) and also for oil red O,8 constituted >10% of total monocytes at 3 days, ~20% of monocytes at 1 week and ~30% of monocytes at 3 weeks after WD (Figure 1A). Appearance of foamy monocytes was accompanied by an increase in blood cholesterol (Supplemental Figure I). Consistent with our previous report,8 CD11c+ monocytes accounted for the majority of foamy monoctyes in apoE−/− mice on WD (Figure 1C). A concomitant decrease in the percentage of nonfoamy CD11c+ monocytes was observed in apoE−/− mice on WD, and the fraction of blood monocytes that were CD11c+ was maintained at ~40% over 10 weeks in apoE−/− or WT mice, regardless of diet (Figure 1C).
Figure 1. Early appearance of foamy monocytes in blood of apoE−/− mice on western high-fat diet (WD).
A, Percentages of foamy monocytes in total monocytes in blood of apoE−/− mice at various time points (from 3 days to 10 weeks) after starting WD analyzed by flow cytometry. n=6–7 mice/group. B, Representative flow cytometric examples of monocytes in WT or apoE−/− mice on WD (for 3 days) from more than 5 independent experiments with 3–7 samples/group in each experiment. C, Percentages of CD11c+ monocytes in foamy, nonfoamy and total monocytes in blood of apoE−/− and WT mice on WD or normal diet (ND). n=6–7 mice/group. *P<0.05 versus WT WD and P<0.01 versus WT ND and apoE−/− ND; **P<0.01, ***P<0.001 versus WT ND, WT WD and apoE−/− ND; #P<0.05 versus WT ND and WT WD.
It is well known that in WT mice, cholesterol is mainly transported by HDL while in apoE−/− mice on WD, cholesterol is mainly transported by VLDLs.16 We next examined monocytes in blood of LDb mice, which are deficient in Ldlr and Apobec1 and have lipoprotein profiles that mimic human hypercholesterolemia, with elevated LDL-cholesterol.15 Similarly, LDb mice displayed early appearance of foamy monocytes, which constituted ~30% of total monocytes at 2 weeks after starting WD (Supplemental Figure II). In both models, foamy monocytes emerged early and expressed CD11c, whereas most nonfoamy monocytes were CD11c− (Supplemental Figure II).
As described previously,8 we defined and quantified “foamy monocytes” by markedly increased side scatter (SSC; up to the granulocyte region), which correlated with inclusion of numerous lipid droplets. Monocytes with fewer or no droplets excluded from the granulocyte region were defined as “nonfoamy” monocytes.8 Of note, although we did not observe dramatic increases in “foamy monocytes” by this definition in blood of apoE−/− mice on normal diet (ND) as compared to WT mice, the SSC value of monocytes was higher in 28-week-old apoE−/− mice than WT mice on ND (Supplemental Figure IIIA), suggesting that monocytes in blood of apoE−/− mice on ND may also include more lipid. Consistent with these observations, monocytes from humans after a high-fat high-cholesterol diet included lipid droplets positive for oil red O staining but exhibited a minor change in SSC.10–12
Foamy monocytes in blood of apoE−/− mice fed high-fat low-cholesterol diet (HFD) or low-fat high-cholesterol diet (HCD)
WD, the most commonly used atherogenic diet, is high in saturated fat and cholesterol. To examine which constituent is predominant in early foamy monocyte formation, we fed apoE−/− mice 2 additional special diets, i.e., HFD or HCD (Supplemental Table I). As shown in Supplemental Figure IIIB, at 2 weeks on HCD, high numbers of foamy monocytes were detected in blood of apoE−/− mice. In contrast, HFD feeding for 2 weeks tended to increase the proportion of foamy monocytes compared to ND. However, the proportion of foamy monocytes was much lower than that induced by WD or HCD. Compared to ND, HCD and WD, but not HFD, markedly increased plasma levels of cholesterol, and neither HCD nor HFD significantly increased triglyceride levels, while HCD, WD and HFD all tended to increase plasma TBARS values in these mice (Supplemental Figure IIIB). These data indicate that diet-induced increases in cholesterol levels, along with enhanced lipid oxidation (also see following data on TBARS in lipoproteins), may be the major cause of foamy monocyte formation in blood of apoE−/− mice at the early stage of dietary intervention.
Three major subsets of monocytes in mouse blood
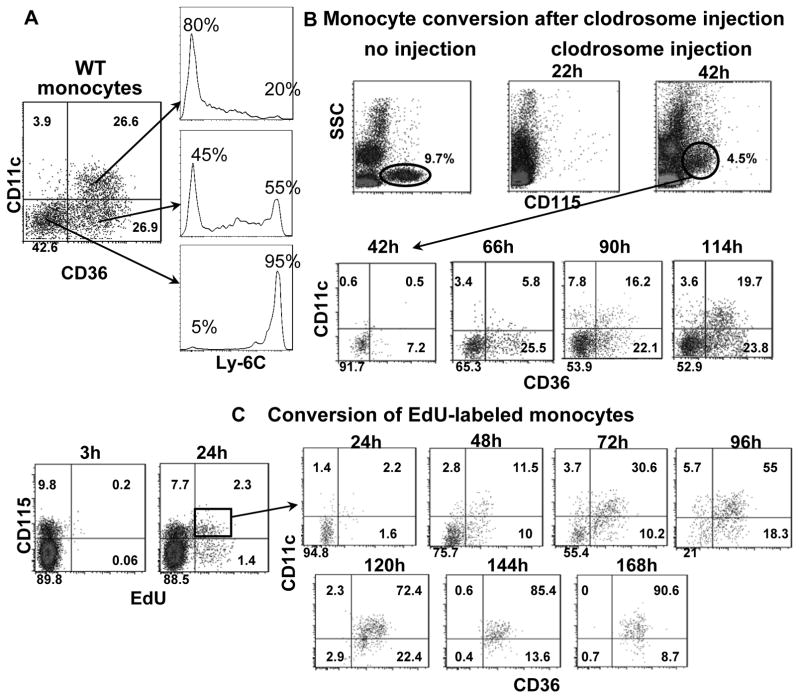
Mouse monocytes are conventionally classified into 2 major subsets based on expression of Ly-6C.8, 17 Staining monocytes for CD11c and CD36 identified 3 major subsets in WT mice: CD11c−CD36−, CD11c−CD36+ and CD11c+CD36+ (Figure 2A). Staining for Ly-6C showed that CD11c−CD36− monocytes were mainly Ly-6Chigh and CD11c+CD36+ monocytes were mainly Ly-6Clow, while CD11c−CD36+ monocytes included both Ly-6Chigh and Ly-6Clow monocytes (Figure 2A).
Figure 2. Three major monocyte subsets in mouse blood.
A, A representative sample of monocyte subsets in blood of a WT mouse showing three major subsets based on CD11c and CD36, and their relationship to Ly-6C expression, from 3 independent experiments with 3 or 4 samples in each experiment. B, A representative example of monocyte reappearance and subset conversion in blood of WT mice after monocyte depletion by intravenous injection of clodrosome (0.3 ml/mouse), from 2 independent experiments with 4 samples in each experiment. C, A representative example of subset conversion of EdU-labeled monocytes in blood of WT mice after injection with EdU, from 2 independent experiments with 3 samples in each experiment.
To examine the dynamics among the 3 distinct monocyte subsets, we depleted circulating monocytes in WT mice by injecting a bolus of clodrosome at 0.3ml/mouse and tracked each subset in blood samples. Monocyte depletion was achieved within 22 hours following clodrosome injection (Figure 2B). At 42 hours, monocytes reappeared, presumably representing those released from bone marrow. Most monocytes sampled at 42 hours were CD11c−CD36−; CD11c−CD36+ monocytes were present at 66–90 hours, and CD11c+CD36+ were detected at ~90–114 hours (Figure 2B). These data indicated that CD11c−CD36− monocytes are those newly released from bone marrow. CD11c+CD36+ monocytes emerge over time as the most mature, while CD11c−CD36+ monocytes represent an intermediate stage that appear to dynamically convert in the circulation.
To confirm this, we injected WT mice with a bolus of EdU, which is incorporated into genomic DNA during cell division.18 This allowed us to pulse-label immature monocytes undergoing proliferation in bone marrow18, 19 and to track conversion of those labeled in blood as they emerged from bone marrow. At 3 hours following EdU injection, EdU+ monocytes were observed in bone marrow (Supplemental Figure IV) but not in blood (Figure 2C). At 24 hours after EdU injection, EdU+ monocytes were present in blood and represented those monocytes newly released from bone marrow. Staining the EdU+ monocytes in blood revealed that the majority at 24 hours were CD11c−CD36−. EdU+ CD11c−CD36+ monocytes emerged within 48 hours and abundant EdU+ CD11c+CD36+ monocytes appeared by 72 hours, indicating a rapid conversion of CD11c−CD36− and CD11c−CD36+ monocytes to CD11c+CD36+ monocytes. By 120 hours, most EdU+ monocytes were CD11c+CD36+ monocytes, which remained in circulation for at least 2–3 more days (Figure 2C). These results were consistent with the depletion studies and with previous reports on the emergence of classical and nonclassical monocytes.20
Characteristics of foamy monocytes in apoE−/− mice on WD
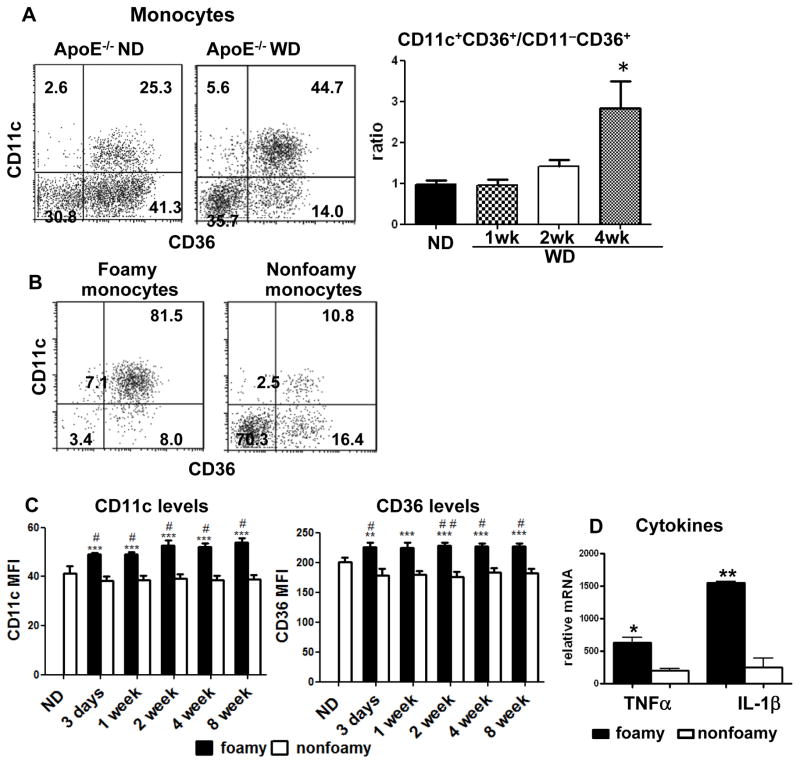
Similar to those in WT mice, 3 distinct subsets of monocytes were detected in blood of apoE−/− mice. In contrast to WT or apoE−/− mice on ND, however, apoE−/− mice on WD exhibited a gradual increase in the ratio of CD11c+CD36+ to CD11c−CD36+ subsets (Figure 3A). In apoE−/− mice on WD, the vast majority of foamy monocytes were CD11c+CD36+, whereas most nonfoamy monocytes were either CD11c−CD36− or CD11c−CD36+ (Figure 3B). CD36+CD11c+ monocytes also expressed elevated levels of CD36 and CD11c in foamy compared to nonfoamy monocytes (Figure 3C). In addition, CD11c+ (foamy) monocytes expressed higher levels of TNFα and IL-1β than did CD11c− (nonfoamy) monocytes from apoE−/− mice on WD (Figure 3D). Taken together, these data indicate that foamy monocytes upregulate surface expression of CD11c, CD36 and proinflammatory markers over days of maturation in the circulation of apoE−/− on WD.
Figure 3. Some characteristics of foamy monocytes in apoE−/− mice on WD.
A, Representative examples of monocyte subsets (left panel) and relative ratios of CD11c+CD36+ to CD11c−CD36+ monocytes (right panel) in apoE−/− mice on ND or WD. n=4–6 mice/group. B, Representative staining for CD11c and CD36 of foamy and nonfoamy monocytes in apoE−/− mice on WD (4 weeks) from more than 5 independent experiments with at least 4 samples in each experiment. C, Comparisons of CD11c and CD36 mean fluorescence intensity (MFI) levels on foamy and nonfoamy CD11c+CD36+ monocytes in apoE−/− mice on WD or ND. n=5–9 mice/group. D, mRNA levels of TNFα and IL-1β in foamy and nonfoamy monocytes from apoE−/− mice on WD. n=3 samples, each of which were pooled blood from 5 mice. *P<0.05, ***P<0.001 versus ND group (in panel A) or nonfoamy monocytes (in panels C and D); #P<0.05, ##P<0.01 versus ND group (in panel C).
Monocyte uptake of CE-VLDLs and differentiation of CD11c− to CD11c+ monocytes
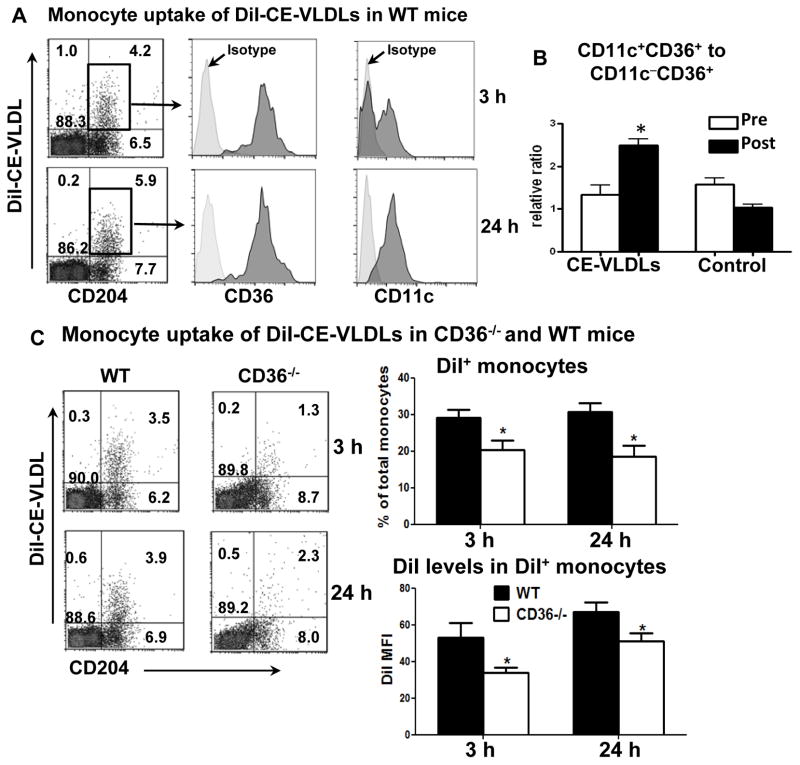
To investigate the process by which uptake of atherogenic lipoproteins directs an inflammatory monocyte phenotype, we isolated CE-VLDLs from apoE−/− mice on WD and labeled them with DiI. CE-VLDLs are the most abundant atherogenic lipoproteins in these mice and are similar to human LDLs in lipid composition.16 The level of oxidation for the isolated CE-VLDLs was confirmed as12 ± 3 TBARS value, indicating low to medium oxidation. A bolus of DiI-CE-VLDLs was injected intravenously into WT mice on ND. At 3 hours after injection, ~40% monocytes became DiI+, indicating CE-VLDL uptake (Figure 4A). Notably, the majority of DiI+ monocytes were CD36+, whereas DiI+ monocytes included both CD11c+ and CD11c− monocytes (Figure 4A), indicating that CD11c+CD36+ and CD11c−CD36+, but few CD11c−CD36−, monocytes took up CE-VLDLs. At 24 hours after DiI-CE-VLDL injection, most DiI+ monocytes became CD11c+ (Figure 4A). Given that injection of human native LDL did not induce significant CD11c−-to-CD11c+ monocyte differentiation over this period of time,8 we conclude that uptake of CE-VLDLs accelerated CD11c−CD36+–to–CD11c+CD36+ monocyte differentiation. Consistently, repetitive daily injection of CE-VLDLs (3 times) increased CD11c+CD36+–to–CD11c−CD36+ monocyte ratios in WT mice (Figure 4B). In contrast to monocyte uptake of CE-VLDLs, few monocytes took up DiI-HDL following injection (Supplemental Figure V). Furthermore, injection of an equivalent amount of free DiI did not result in appearance of DiI+ cells in mouse blood (Supplemental Figure V), confirming that DiI+ monocytes after DiI-CE-VLDL injection were the result of monocyte uptake of DiI-CE-VLDLs rather than uptake of free DiI.
Figure 4. Monocyte uptake of CE-VLDLs and subset conversion in WT and CD36−/− mice.
A, Representative flow cytometric analyses of monocytes that took up DiI-CE-VLDLs (becoming DiI+), from more than 5 independent experiments with at least 3 samples in each experiment. A bolus of DiI-CE-VLDLs was intravenously injected into WT mice on ND. At 3 and 24 hours, blood was taken for flow cytometric analysis. Gated total leukocytes (for CD204) or DiI+ monocytes (for CD36 and CD11c) are presented. B, Relative ratios of CD11c+CD36+ to CD11c−CD36+ monocytes in WT mice on ND after receiving repetitive daily injection of CE-VLDLs or PBS (control) for 3 days. n=4 mice/group. C, Representative flow cytometric analysis of monocyte uptake of DiI-CE-VLDLs, and quantification of DiI+ monocytes in total monocytes and DiI MFI levels on DiI+ monocytes, in WT and CD36−/− mice after receiving intravenous injection of a bolus of DiI-CE-VLDLs. n=11–12 mice/group. *P<0.05 versus preinjection (in panel B) or WT controls (in panel C).
The observation that CD36+, but not CD36−, monocytes took up CE-VLDLs prompted us to examine the role of CD36 in CE-VLDL uptake. First, using THP1 monocytes (without induction to macrophages) in vitro, we found that treatment with CE-VLDLs caused lipid accumulation indicated by increased SSC (Supplemental Figure VI) and Nile red staining (data not shown). This treatment also increased CD36 levels on THP1 monocytes (Supplemental Figure VI), consistent with our in vivo data showing higher CD36 levels on foamy monocytes. Along with the increase in CD36, treatment of THP1 monocytes with (unlabeled) CE-VLDLs enhanced further uptake of DiI-CE-VLDLs, indicating a positive feedback between CD36 expression and lipid uptake, by these monocytes (Supplemental Figure VI). Next, we used CD36−/− (CD36obl/obl) mice to examine a direct role of CD36 in monocyte uptake of CE-VLDLs. We injected a bolus of DiI-CE-VLDLs into CD36−/− mice and WT controls and found that CD36−/− mice exhibited ~30% reduction in the percentage of DiI(-CE-VLDL)+ monocytes and the levels of DiI at 3 and 24 hours (Figure 4C). In contrast to the reported role of TLR4 in macrophage uptake and inflammatory response to ox-LDL,21 TLR4−/− mice did not show reductions in monocyte uptake of DiI-CE-VLDLs following lipoprotein injection (Supplemental Figure VII). These data lead to the conclusion that CD36 on both CD11c−CD36+ and CD11c+CD36+ monocytes participates in CE-VLDL uptake. Further, foamy monocyte formation in apoE−/− mice on WD induced differentiation of monocytes from CD11c−CD36+ to CD11c+CD36+.
Early development of atherosclerosis in apoE−/− mice after WD
The observation of early appearance of foamy monocytes in blood of mice with hypercholesterolemia prompted us to examine whether foamy monocytes infiltrate into nascent plaque and contribute to nascent atherosclerosis. We focused on atherogenesis in apoE−/− mice fed WD for 3 weeks, the time when apoE−/− mice showed aortic atherosclerotic plaques (Supplemental Figure VIIIA). Flow cytometry showed that apoE−/− mice on WD had increased aortic macrophages/DCs, mainly CD11c+(CD11b+) cells (Supplemental Figure VIIIB), consistent with the reported changes in advanced atherosclerosis in apoE−/− mice, or in early atherosclerosis in Ldlr−/− mice.2,19 Also consistent with previous reports is the observation that CD11c+ cells selectively localized in atherosclerotic lesions (Supplemental Figure VIIIC).2, 6, 19, 22
Accompanying the increase in CD11c, IL-6, MCP-1, TNFα, IL-12 and IL-1β, but not IL-10, were increased in aortas of apoE−/− mice on WD (Supplemental Figure VIIID).
Infiltration of foamy monocytes into nascent atherosclerotic lesions
Next, we tested whether CD11c+(CD36+) foamy monocytes contributed to the early increases of CD11c+(CD11b+) cells in atherosclerotic lesions. In contrast to the response in WT mice (Figure 4A), injection of a bolus of DiI-CE-VLDLs in apoE−/− mice on WD resulted in exclusive lipoprotein uptake by CD11c+ foamy monocytes that became DiI+, and therefore specifically labeled CD11c+ foamy monocytes (Figure 5A). DiI+CD11c+ foamy monocytes were low in F4/80 (Figure 5A), which is highly expressed on macrophages (Supplemental Figure IX). At day 5 postinjection, DiI+F4/80low cells, most of which were also CD11c+, appeared in mouse aorta (Figure 5B). These data indicated that DiI+F4/80lowCD11c+ foamy monocytes infiltrated into atherosclerotic aortas and became CD11c+ cells in the lesions. Concurrently, we also observed DiI+F4/80high cells in mouse aorta (Figure 5B), which may represent lesional resident macrophages/DCs that took up DiI-CE-VLDLs.
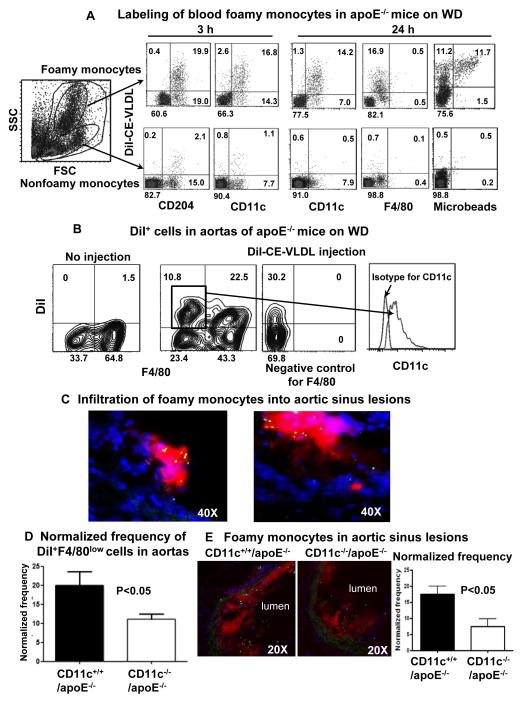
Figure 5. Labeling of foamy monocytes and infiltration of foamy monocytes into nascent atherosclerotic lesions.
A, Representative flow cytometric analysis samples showing specific labeling of foamy monocytes and phenotypes of labeled foamy monocytes in apoE−/− mice on WD (3 weeks) after intravenous injection of DiI-CE-VLDLs with or without fluorescent microbeads, from more than 5 independent experiments with at least 3 samples in each experiment. B, Representative flow cytometric analysis samples showing foamy monocytes (DiI+F4/80low) in atherosclerotic aortas with sustained expression of CD11c, from 4 independent experiments with at least 3 samples in each experiment. C, Representative histology of aortic sinus showing accumulation of microbead+ (green) cells, which were also DiI+ (red, left panel) and CD11c+ (red, right panel), in atherosclerotic lesions of apoE−/− mice on WD (3 weeks) after intravenous injection of fluorescent microbeads with (left panel) or without (right panel) DiI-CE-VLDLs. D, Normalized frequency of infiltrated foamy monocytes in aortas of CD11c−/−/apoE−/− and CD11c+/+/apoE−/− mice on WD (3 weeks). n=5–7 samples/group. E, Representative histology and quantification of foamy monocyte infiltration in aortic sinus lesions of CD11c−/−/apoE−/− and CD11c+/+/apoE−/− mice on WD (3 weeks). n=15 samples/group.
To confirm that foamy monocytes can in fact infiltrate into nascent atherosclerotic lesions, we coinjected apoE−/− mice on WD with a bolus of DiI-CE-VLDLs and/or fluorescent microbeads that are accessible only to circulating monocytes and not tissue macrophages.6, 9 After injection, the vast majority of bead+ cells in blood were DiI+ foamy monocytes, whereas ~50% of DiI+ foamy monocytes remained bead− (Figure 5A). Histological examination of aortic sinus sections revealed that DiI+bead+ cells accumulated in atherosclerotic lesions, with 12 ± 2 bead+ cells/lesion, and also expressed CD11c (Figure 5C).
Taken together, these data indicated that CD11c+ foamy monocytes infiltrated into nascent atherosclerotic lesions and became CD11c+ cells within the lesions.
Effects of CD11c deficiency on foamy monocyte infiltration into nascent atherosclerotic lesions
Deficiency of CD11c in apoE−/− mice reduced atherosclerosis.8 We therefore examined the role of CD11c in foamy monocyte infiltration into nascent atherosclerotic lesions by using CD11c−/−/apoE−/− and CD11c+/+/apoE−/− mice on WD (for 3 weeks). CD11c deficiency in apoE−/− mice did not impair DiI-CE-VLDL– or fluorescent bead–labeling of foamy monocytes in blood (Supplemental Figure X). However, at 5 days after DiI-CE-VLDL injection, foamy monocytes derived from aortic suspensions and defined by DiI+F4/80low cells were significantly lower in CD11c−/−/apoE−/− mice than in CD11c+/+/apoE−/− mice (Figure 5D). After coinjection of DiI-CE-VLDLs and fluorescent microbeads, the frequency of DiI+bead+ cells in aortic sinus lesions was 50% lower in CD11c−/−/apoE−/− mice than in CD11c+/+/apoE−/− mice (Figure 5E). Therefore, we conclude that CD11c plays a key role in foamy monocyte infiltration into nascent atherosclerotic lesions. After WD for 3 weeks, CD11c−/−/apoE−/− mice had smaller atherosclerotic lesions and less macrophage content in the lesions than control mice (Supplemental Figure XI), indicating that CD11c also contributes to development of nascent atherosclerosis, likely by participating in foamy monocyte infiltration.
Contribution of foamy monocytes to the development of nascent atherosclerosis
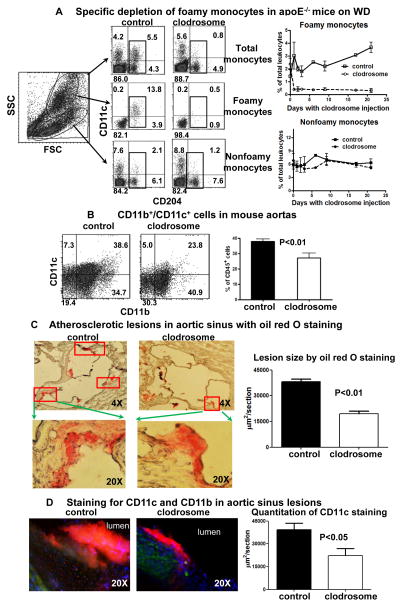
In a final set of studies, we examined whether depletion of foamy monocytes would alter the development of nascent atherosclerosis. We intravenously injected a low dose of clodrosome into apoE−/− mice daily from day 3 on WD for an additional 3 weeks and examined development of atherosclerosis following the injection. Noteworthy is the observation that the low-dose clodrosome injection consistently and specifically depleted CD11c+ foamy monocytes, with no significant alteration of the proportions of CD11c− nonfoamy monocytes, compared to PBS injection (Figure 6A). We reasoned that foamy monocytes have enhanced capacity to phagocytose clodrosome and that this leads to specific depletion of foamy monocytes. To confirm this, we injected a bolus of DiI-clodrosome into apoE−/− mice on WD, and found that at 3 hours after injection of a low dose of DiI-clodrosome, ~90% of foamy monocytes became DiI+, indicating uptake of DiI-clodrosome, whereas <10% of nonfoamy monocytes became DiI+. In contrast, injection of a larger dose (0.2 ml/mouse) of DiI-clodrosome caused ~50% of nonfoamy monocytes to take up DiI-clodrosome and >90% of foamy monocytes to take up clodrosome (Supplemental Figure XII).
Figure 6. Effects of foamy monocyte depletion on development of nascent atherosclerosis.
A, Specific depletion of foamy monocytes in apoE−/− mice on WD with daily injection of low-dose clodrosome (3 weeks). n=7 samples/group. B, CD11b+/CD11c+ cells in aortas of apoE−/− mice on WD with or without foamy monocyte depletion. n=5–7 samples/group. C, Quantification of atherosclerotic lesions, as indicated by oil red O staining, in aortic sinus of apoE−/− mice on WD with or without foamy monocyte depletion. n=5 mice/group. D, Representative staining for CD11c (red) and CD11b (green), and quantitation of CD11c staining in aortic sinus lesions of apoE−/− mice on WD with or without foamy monocyte depletion. n=5 mice/group.
Notably, specific depletion of foamy monocytes (for 3 weeks) resulted in significantly lower proportions of aortic CD11b+/CD11c+ cells compared to controls (Figure 6B). Analysis of aortic sinus lesions showed that mice with foamy monocyte depletion had smaller lesions, as assessed by oil red O staining (Figure 6C), and less CD11c (and CD11b), as assessed by immunofluorescent staining (Figure 6D).
To exclude the possibility that the clodrosome-induced changes in atherosclerosis were caused by direct depletion of lesional macrophages/DCs, we injected a larger bolus (0.2 ml/mouse) of clodrosome into apoE−/− mice on WD (3 weeks). At 22 hours postinjection, most monocytes in blood were depleted. However, the proportions of CD11b+/CD11c+ cells in aortic cell suspensions did not change (Supplemental Figure XIII), indicating that clodrosome did not alter numbers of “resident” macrophages/DCs in aortas, consistent with previous studies.14, 23 Therefore, the lower proportions of aortic CD11b+/CD11c+ cells and smaller atherosclerotic lesions in mice treated repetitively with low-dose clodrosome injections were most likely caused by decreased infiltration of circulating foamy monocytes into atherosclerotic lesions.
Discussion
Infiltration of monocytes from the circulation into atherosclerosis-prone vessels is a crucial early step in atherogenesis.1, 3, 13, 14 Genetic disorders in humans with severe increases in apoB-containing lipoproteins in the blood, such as familial hypercholesterolemia with increased LDLs and type III hyperlipoproteinemia with increased VLDLs (remnants), lead to premature atherosclerosis and xanthoma at early ages.5, 24 Based on our previous report,8 we postulate that uptake of apoB-containing CE-rich lipoproteins and foamy monocyte formation in blood plays a critical role in this process. Indeed, humans with familial hypercholesterolemia had foamy leukocytes in blood.25 In the current study, we report that foamy monocytes formed early in blood of both WD-fed apoE−/− mice, in which CE is transported by VLDLs, and LDb mice, in which CE is transported by LDLs.15 Further examination revealed upregulation of CD11c and CD36 on these monocytes and a direct correlation between monocyte uptake of CE-rich lipoproteins and upregulation of CD11c and CD36 with increased scavenger function of CD36. We also demonstrated that foamy monocytes trafficked to atherosclerotic aortas and became CD11c+ macrophages/DCs within nascent atherosclerotic lesions. The frequency of infiltrated foamy monocytes in these lesions was decreased by ~2-fold in the absence of CD11c. We conclude that CD11c may serve as a biomarker of monocyte inflammatory state in the circulation and function to enhance the capacity of monocytes to infiltrate nascent lesions and differentiate into CD11c+ macrophages/DCs.
Monocytes are a heterogeneous population in the circulation, and surface receptors are used to discriminate between several subsets.26, 27 Based on CD11c expression, we previously classified mouse monocytes into CD11c+ and CD11c− subsets.8 In the current study, detection of CD36 and CD11c expression provided discrimination of 3 distinct subsets. CD11c+CD36+ and CD11c−CD36+ monocytes took up CE-VLDLs isolated from WD-fed apoE−/− mice, and this resulted in upregulation of CD11c in the latter subset. This result is consistent with the observation that apoE−/− mice on WD, with elevated levels of CE-VLDLs, registered a 2-fold increase in the ratio of CD11c+CD36+ to CD11c−CD36+ monocytes. Our further investigation was in part motivated by previous reports of a crucial role for CD36 in macrophage uptake of modified LDL, inflammasome activation and atherogenesis.28 We now demonstrate an important role for CD36 in CE-VLDL uptake by circulating monocytes, supported by the observations that CE-VLDL uptake was prevalent in CD36+, but not CD36−, monocytes, and that the absence of CD36 reduced monocyte uptake of CE-VLDLs. The findings that CD36 levels on foamy monocytes exceeded those on nonfoamy monocytes and that treatment of THP1 monocytes with CE-VLDLs increased CD36 levels suggest that lipid accumulation itself signals a feedforward mechanism for CD36 upregulation that promotes foam cell formation. This may also explain why foamy monocytes, but few nonfoamy monocytes, in apoE−/− mice on WD took up DiI-CE-VLDLs. It is possible that elevated levels of endogenous CE-VLDLs in apoE−/− mice on WD predispose CD36+ monocytes to take up CE-VLDLs preferentially and become foamy. Consistently, CD36+ monocytes accounted for a minor portion of nonfoamy monocytes in these mice. Our current observations in mice are in agreement with previous human studies showing increased expression of CD36 and CD11c on CD16+ monocytes in patients with familial hypercholesterolemia compared with healthy controls, increased ox-LDL uptake via CD36 by CD16+ monocytes from hypercholesterolemic subjects,29 and high CD36 expression, preferential lipid accumulation and avid ox-LDL uptake in CD14++/CD16+ monocytes of patients with chronic kidney disease.30
The role of monocyte heterogeneity in human versus mouse atherogenesis has been controversial. Human studies have shown increased CD16+ monocytes, particularly CD14++/CD16+ intermediate monocytes, in hyperlipidemia and association of CD16+ monocytes with atherosclerotic cardiovascular disease.27, 30, 31 In contrast, initial studies in mouse models of hypercholesterolemia have implicated a preferential increase of Ly-6Chigh monocytes, analogous to human CD16− monocytes, in blood, and the participation of Ly-6Chigh monocytes in atherosclerosis.6, 7 However, more recent studies revealed similar ratios of Ly-6Chigh and Ly-6Clow monocytes in hypercholesterolemic mice,9, 32 contributions or infiltration of both subsets to atherosclerosis,9, 33 and higher correlation of Ly-6Clow monocyte number with lesion size.32 Furthermore, very recent studies indicated that reduced atherosclerosis was correlated with decreased recruitment of Ly-6Clow monocytes.34–36 These disparate results may be related to the fact that most previous studies focused on advanced atherosclerosis,6, 7, 34, 35 in which macrophage proliferation plays a dominant role.14 Monocyte recruitment plays a more important role in early atherogenesis.13, 14 Our data are consistent with early monocyte activation and differentiation in blood, which correlates with enhanced recruitment in early atherosclerosis. Indeed, our current study revealed early emergence of foamy monocytes in blood of mice with severe hypercholesterolemia and upregulation of CD11c on this population. Moreover, we report that CD11c+(CD36+) foamy monocytes infiltrated into early atherosclerotic lesions and maintained high expression of CD11c within the lesions. We have previously reported that CD11c represents a functional integrin on foamy monocytes in human and mouse circulation and is necessary for shear-resistant adhesion to VCAM-1 upregulated on inflamed arterial endothelium.8, 11 Induction of the high-affinity ligand-binding state of CD11c on monocytes correlates with lipid uptake and enhances monocyte recruitment to VCAM-1 through coalescence on the plasma membrane and subsequent activation of the α4β1-integrin (VLA-4).37 Significantly, we currently showed that deletion of CD11c reduced foamy monocyte infiltration into early atherosclerotic lesions, and the specific depletion of foamy monocytes decreased CD11c+ cells in atherosclerotic aortas and reduced development of early atherosclerosis. These data support a crucial role of foamy monocytes and increased expression and function of CD11c on monocytes in activation of recruitment to sites of early atherosclerosis. Also noteworthy is the observation that CD16+ monocytes from humans with familial hypercholesterolemia, with elevated levels of CD11c, showed enhanced adhesion to activated endothelium.29
Studies from Cybulsky’s group indicated that macrophages/DCs also proliferated in early atherosclerosis and that Ly-6Chigh monocytes infiltrated into early atherosclerotic lesions. Notably, most of the cells they observed in early lesions expressed CD11c.19, 38 Our findings are consistent with these reports and another study2 showing preferential increases in CD11c+/CD11b+ cells in advanced atherosclerotic aortas. Furthermore, CD11c+ cells seem to be selectively localized in atherosclerotic lesions (but rarely in adventitia where there are CD11b+/CD11c− cells)2, 6, 8, 19, 22, 38 and are the major cell type undergoing proliferation in early atherosclerotic lesions.19 Cell proliferation may contribute to the increases in CD11c+ cells in atherosclerotic lesions.19, 38 Here we demonstrated that infiltration of CD11c+ foamy monocytes contributes to the increase in CD11c+ cells in the lesions and development of early atherosclerosis. Consistent with our findings is the report of Tacke et al showing that Ly-6Clow monocytes, most of which were CD11c+, were more prone to become CD11c+ cells in atherosclerotic lesions following infiltration.6
Finally, we need to point out that our current study focused on the role of “foamy monocytes,” but not specifically “Ly-6Clow” monocytes, in nascent atherosclerosis. Deficiency of NR4A1 in apoE−/− or Ldlr−/− mice decreased abundance of Ly-6Clow monocytes, but increased39 or did not change40 the development of atherosclerosis. However, loss of NR4A1 increased lipid accumulation in circulating monocytes, also supporting a role of lipid accumulation within monocytes in atherosclerosis.39
A limitation of our current study is that we used apoE−/− mice on WD as the animal model of atherosclerosis. Although this mouse model has been widely used,2, 6, 7 these mice have severe hypercholesterolemia. Foamy monocytes in these mice may have higher lipid content than foamy monocytes in human subjects after high-fat high cholesterol diet.10–12 Nevertheless, because of the prevalence of foamy monocytes and easier identification of the early stage of atherogenesis in these mice, we believe that the data presented with this mouse model are relevant to human studies showing the presence of foamy monocyes in the circulation after high-fat high-cholesterol diet or with severe hypercholesterolemia.10–12, 25
In summary, we reveal the dynamics of inflammatory changes in monocytes in apoE−/− mice fed a WD, an established model of hypercholesterolemia and atherosclerosis. Blood monocytes take up CE-VLDLs and become foamy, and this elicits subsequent phenotypic changes including upregulation of CD11c, CD36 and proinflammatory cytokines. Foamy monocytes infiltrate into early atherosclerotic lesions and become CD11c+ cells. We show that CD11c specifically expressed on foamy monocytes supports their selective trafficking to atherosclerosis-prone lesions in aorta and differentiation into CD11c+ macrophages/DCs. Evidence from other reports19, 38 supports their proliferation within the lesions. These processes may represent the earliest events and play a major role in nascent atherosclerosis.
Supplementary Material
Significance.
Hypercholesterolemia is a major risk factor for atherosclerosis. Monocyte infiltration from the circulation into the arterial wall is a crucial early step in atherogenesis. However, effects of atherogenic lipoproteins on circulating monocytes and their consequences on early atherogenesis remain largely unknown. We previously reported foamy monocytes, containing intracellular lipid droplets, in the circulation of mice with hypercholesterolemia. We now report early emergence of foamy monocytes, with upregulation of CD11c and CD36, in blood of mice with severe hypercholesterolemia, and revealed an important role of CD36 in monocyte uptake of atherogenic lipoproteins. Specific labeling or depletion of circulating foamy monocytes demonstrated that foamy monocytes infiltrated into nascent atherosclerotic lesions in a CD11c-dependent manner and contributed to early atherogenesis. Therefore, our current studies add novel information to the current concept of development of early atherosclerosis with severe hypercholesterolemia.
Acknowledgments
The authors thank Kerrie Jara, BA, at Baylor College of Medicine for editorial assistance and Greg Foster, BS, at University of California, Davis for thoughtful comments.
Sources of Funding
This work was supported by NIH grants R01 HL098839 (to H.W.), R01 DK078847 (to C.M.B.), and R01 HL082689 (to S.I.S). L.X. was partially supported by Chinese Scholarship Council.
Non-standard Abbreviations and Acronyms
- CE
cholesteryl ester
- CE-VLDLs
cholesteryl ester–rich very-low-density lipoproteins
- DCs
dendritic cells
- HCD
high-cholesterol (low-fat) diet
- HFD
high-fat (low-cholesterol) diet
- ND
normal diet
- SSC
side scatter
- WD
western high-fat diet
Footnotes
Disclosure
None.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilheimer DW, Goldstein JL, Grundy SM, Starzl TE, Brown MS. Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984;311:1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Hartigh LJ, Connolly-Rohrbach JE, Fore S, Huser TR, Rutledge JC. Fatty acids from very low-density lipoprotein lipolysis products induce lipid droplet accumulation in human monocytes. J Immunol. 2010;184:3927–3936. doi: 10.4049/jimmunol.0903475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varela LM, Ortega A, Bermudez B, Lopez S, Pacheco YM, Villar J, Abia R, Muriana FJ. A high-fat meal promotes lipid-load and apolipoprotein B-48 receptor transcriptional activity in circulating monocytes. Am J Clin Nutr. 2011;93:918–925. doi: 10.3945/ajcn.110.007765. [DOI] [PubMed] [Google Scholar]
- 13.Ye D, Zhao Y, Hildebrand RB, Singaraja RR, Hayden MR, Van Berkel TJ, Van Eck M. The dynamics of macrophage infiltration into the arterial wall during atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Am J Pathol. 2011;178:413–422. doi: 10.1016/j.ajpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak S, Sun H, Acevedo F, Shimmin LC, Zhao L, Teng BB, Hixson JE. Differential expression of genes in the calcium-signaling pathway underlies lesion development in the LDb mouse model of atherosclerosis. Atherosclerosis. 2010;213:40–51. doi: 10.1016/j.atherosclerosis.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 18.Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW, Ballantyne CM, Wu H. Essential Role of CD11a in CD8+ T-Cell Accumulation and Activation in Adipose Tissue. Arterioscler Thromb Vasc Biol. 2014;34:34–43. doi: 10.1161/ATVBAHA.113.302077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 23.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahley RW, Weisgraber KH, Innerarity TL, Rall SC., Jr Genetic defects in lipoprotein metabolism. Elevation of atherogenic lipoproteins caused by impaired catabolism. JAMA. 1991;265:78–83. doi: 10.1001/jama.265.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Dresel HA, Via DP, Stohr M, Elchner U, Gnasso A, Postiglione A, Blin N, Augustin J, Schettler G. Observations on leukocytes from patients with severe familial hypercholesterolemia. Arteriosclerosis. 1986;6:259–264. doi: 10.1161/01.atv.6.3.259. [DOI] [PubMed] [Google Scholar]
- 26.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 27.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 28.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013 doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosig S, Rennert K, Krause S, Kzhyshkowska J, Neunubel K, Heller R, Funke H. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 2009;23:866–874. doi: 10.1096/fj.08-118240. [DOI] [PubMed] [Google Scholar]
- 30.Rogacev KS, Zawada AM, Emrich I, Seiler S, Bohm M, Fliser D, Woollard KJ, Heine GH. Lower Apo A-I and Lower HDL-C Levels Are Associated With Higher Intermediate CD14++CD16+ Monocyte Counts That Predict Cardiovascular Events in Chronic Kidney Disease. Arterioscler Thromb Vasc Biol. 2014;34:2120–2127. doi: 10.1161/ATVBAHA.114.304172. [DOI] [PubMed] [Google Scholar]
- 31.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 33.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander-Miller MA, Parks JS. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Distel E, Barrett TJ, Chung K, Girgis NM, Parathath S, Essau CC, Murphy AJ, Moore KJ, Fisher EA. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res. 2014;115:759–769. doi: 10.1161/CIRCRESAHA.115.304164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandoch M, Feldmann K, Gothert JR, Dick LS, Homann S, Bayer JK, Klatt C, Waldheim JN, Rabausch B, Nagy N, Oberhuber A, Deenen R, Kohrer K, Lehr S, Homey B, Pfeffer K, Fischer JW. Deficiency in Lymphotoxin Beta Receptor Protects from Atherosclerosis in apoE-Deficient Mice. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.116.305723. [DOI] [PubMed] [Google Scholar]
- 37.Foster GA, Gower RM, Stanhope KL, Havel PJ, Simon SI, Armstrong EJ. On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proc Natl Acad Sci U S A. 2013;110:13944–13949. doi: 10.1073/pnas.1300651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 39.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.