Abstract
Molecular and functional studies of genes in neurons in mouse models require neuron-specific Cre lines. The current available neuronal Cre transgenic or knock-in lines either result in expression in a subset of neurons or expression in both neuronal and non-neuronal tissues. Previously we identified BAF53b as a neuron-specific subunit of the chromatin remodeling BAF complexes. Using a bacteria artificial chromosome (BAC) construct containing the BAF53b gene, we generated a Cre transgenic mouse under the control of BAF53b regulatory elements. Like the endogenous BAF53b gene, we showed that BAF53b-Cre is largely neuron-specific. In both central and peripheral nervous systems, it was expressed in all developing neurons examined and was not observed in neural progenitors or glial cells. In addition, BAF53b-Cre functioned in primary cultures in a pan-neuron-specific manner. Thus, BAF53b-Cre mice will be a useful genetic tool to manipulate gene expression in developing neurons for molecular, biochemical, and functional studies.
Keywords: Mammal, Organism, Genetics
Introduction
During neural development, multipotent neural progenitors have the ability to differentiate into neurons, astrocytes, and oligodendrocytes. Despite the diversity of neuron subtypes, all neurons share common neuron-specific features; these include the permanent exit from the cell cycle and adoption of neuronal specific morphology and physiological activities (Gage and Temple, 2013; Morrison and Kimble, 2006). Genetic studies are essential for the understanding of neuronal morphogenesis and functions. Therefore, neuron-specific Cre transgenic lines are desired to enable analysis of the functions of genes in neuronal development and function.
Several neuronal Cre transgenic mouse lines have been generated and are widely used for deletions of genes in neurons. The mis-expression of Cre in other neural or non-neural cell types or the restricted expression of Cre in subsets of neurons have limited the use of existing neuronal Cre lines in studying the general function of neurons. For example, Nestin-Cre transgenic lines have been widely used to delete genes in neural progenitors and consequently all neural progenies including neurons and glial cells (Tronche et al., 1999). The CamK2-Cre is active in mature projection neurons in the forebrain but not in the developing neurons and other neuron subtypes (Chen et al., 2001; Minichiello et al., 1999; Tsien et al., 1996). Syn1-Cre and Nex-Cre are expressed in developing neurons; however, Syn1-Cre is expressed in a mosaic pattern (Zhu et al., 2001), and Nex-Cre is expressed only in principle neurons (Goebbels et al., 2006). Several other Cre lines such as Thy1-Cre, NSE-Cre, and CRE3 result in Cre activities in either specific brain regions or specific neuronal subtypes during development and in adults (Banares et al., 2005; Campsall et al., 2002; Cinato et al., 2001; Heimer-McGinn and Young, 2011; Kwon et al., 2006). Although these Cre lines are suitable for study of the functions of specific types of neurons, it remains difficult to perform certain molecular or biochemistry experiments that require the deletion of genes in all neurons as there have been no Cre mouse lines that display Cre activities in all neurons.
Primary neuronal cultures are an important tool for study of neuronal morphology and function and for molecular, biochemistry, and pharmacological experiments. Since primary cultures usually consist of both glial and neuron cells, a Cre system that enables neurons to be distinguished from other co-cultured cells would be valuable. Since most of the Cre transgenes mentioned above are only expressed in a subset of neurons in vivo, it is likely that they will only function in a subset of neuronal cells in culture.
The generation of neuronal Cre requires neuron-specific regulatory elements. The lack of pan-neuronal Cre activities is mostly due to the intrinsic properties of the driver gene or insufficient regulatory elements used in the transgenic construct. During our study of the neuron-specific chromatin remodeling complexes, we discovered that the BAF53b gene (also known as Actl6b), which encodes a subunit of the SWI/SNF-like BAF complexes, is expressed specifically in neurons and in all neurons examined (Lessard et al., 2007; Olave et al., 2002; Wu et al., 2007). Using the regulatory elements of BAF53b in a BAC construct, we generated a Cre transgene that is specifically expressed in developing and mature neurons. The pan-neuronal expression pattern of the BAF53b-Cre transgene in vivo and in culture will make this transgenic mouse line a useful tool to study gene functions in neurons.
Results and Discussion
Construction of BAF53b-Cre BAC and generation of the transgenic line
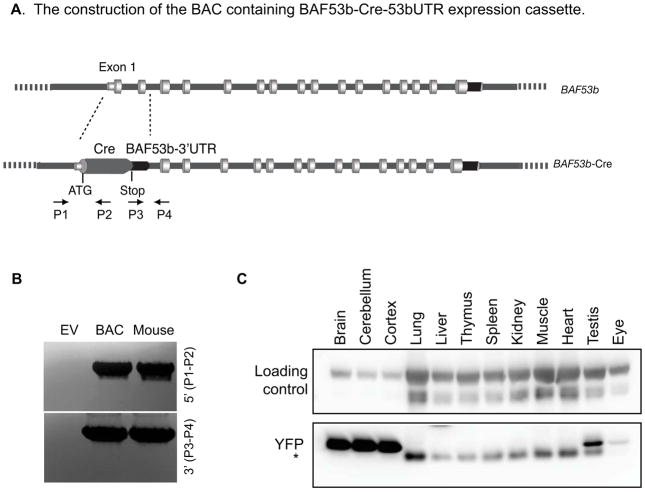
By searching NCBI Clone database, we identified a BAC clone (RP23-127E17) that contains the entire BAF53b gene and approximately 100 kb upstream and downstream (Figure 1A). Using the recombineering method (Warming et al., 2005), the BAC was first inserted with a galK selection marker replacing the first two coding exons of BAF53b gene downstream of the start codon. Then using negative selection, the galK cassette was replaced with the Cre gene coding region ligated with the BAF53b 3′ UTR (Figure 1A). The correctly recombined BACs were identified by PCR using primers surrounding the recombined junctions (Figure 1A, 1B) and further confirmed by sequencing. The BAF53b-Cre containing BAC DNA was prepared and injected into the pronucleus of fertilized FVB/N mouse eggs. The positive transgenic mice were identified using PCR (Figure 1B). One founder line was generated with germline transmission of the transgene. BAF53b-Cre hemizygotes and homozygotes are normal and fertile.
Figure 1. Generation of BAF53b-Cre BAC transgeneic mouse line.
A. Construction of the BAC-containing BAF53b-Cre-53bUTR expression cassette. The genomic structure of the BAF53b gene is shown. Recombineering methods were used to replace the first two exons of the BAF53b gene with the Cre gene and the BAF53b 3′UTR; this places the Cre gene immediately downstream of the start codon. The diagram is not drawn to scale. The positions of the primers used for cloning confirmation are shown (P1–P4).
B. PCR confirmation of the correct BAC construct and positive transgenic mouse.
C. Western blot of protein extracted from indicated tissues in P21 BAF53b-Cre R26R-YFP mouse. YFP was detected at high levels in brain tissue and at low levels in eyes and testis. YFP was not detected in other tissues examined. The asterisk indicates a non-specific band.
BAF53b-Cre is active in developing nervous system
To characterize the BAF53b-Cre activities, the BAF53b-Cre mice were crossed to two reporter mice, R26R-YFP (Srinivas et al., 2001) and R26R-tdTomato (Ai9) (Madisen et al., 2010), both containing a LoxP sites-flanked stop signal upstream of the reporter genes at the Rosa26 locus. Cre was expected to delete the stop signal and enable the expression of YFP or the tdTomato. The two reporter mice had identical expression patterns, and we used them interchangeably. Western blotting of young adult tissues indicated that BAF53b-Cre activity resulted in expression of YFP, which was detected in neural tissues such as brain and eyes (Figure 1C). Except a low expression of YFP in the testis, all the non-neural tissues examined are absent of the YFP signals (Figure 1C), indicating that BAF53b-Cre is largely neural specific.
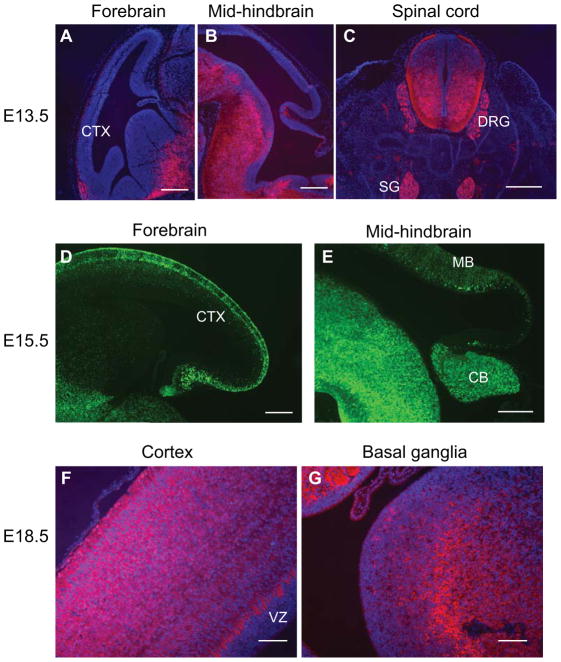
During development, BAF53b gene expression in neurons is first detectable in E12.5 brain and spinal cord (Olave et al., 2002). We observed BAF53b-Cre activities that were slightly delayed relative to this in both the developing central and peripheral nervous system. At E13.5, the reporter tdTomato expression was detected in the outermost marginal zone neurons in the cortex (Figure 2A). tdTomato was also expressed in the outer layer in mid-hindbrain where more mature neurons reside (Figure 2B). In the ventral brain regions, as well as in the spinal cord, high levels of tdTomato were detected in the differentiated areas (Figure 2C). In addition, strong tdTomato signals were detected in dorsal root ganglion, sympathetic ganglia, and nerve tracts (Figure 2C). Reporter signals were not detected in non-neural tissues (Figure 2C). At E15.5, reporter YFP signals were observed in the differentiated areas in cortex and mid-hindbrain regions (Figure 2D, 2E). In the cortex, the YFP signal was stronger in the more mature marginal zone and subplate neurons (Figure 2D). At E18.5, reporter tdTomato signals were observed in all differentiated brain areas, but were absent from undifferentiated areas such as the ventricular zone in the cortex (Figure 2F, 2G). Thus, we observed BAF53b-Cre activities in the nervous systems but not in most non-neuronal tissues.
Figure 2. BAF53b-Cre is active in developing nervous system.
A–C. Sections of E13.5 BAF53b-Cre R26R-tdTomato brain and trunk regions were stained with anti-RFP antibodies to visualize tdTomato expression. Scale bars: 200 μm.
D–E. Sections of E15.5 BAF53b-Cre R26R-YFP brain regions were stained with anti-GFP antibodies to visualize reporter YFP expression. Scale bars: 200 μm.
F–H. Sections of E18.5 BAF53b-Cre R26R-tdTomato brain regions were stained with anti-RFP antibodies to visualize tdTomato expression. Scale bars: 100 μm.
CTX: cortex, DRG: dorsal root ganglion, SG: sympathetic ganglion, MB: midbrain, CB: cerebellum, VZ: ventricular zone, EGL: external granule layer.
BAF53b-Cre is specifically active in neurons
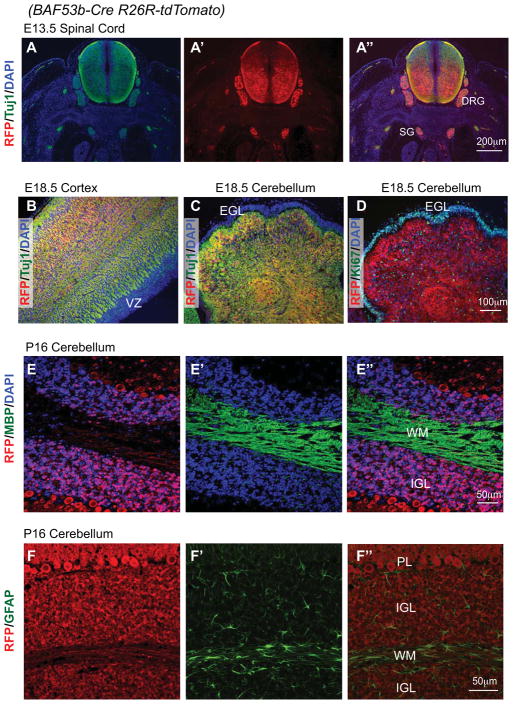
BAF53b is a neuron-specific gene that is expressed in most if not all neurons. To determine whether BAF53b-Cre is also expressed specifically in neurons, we co-stained the reporters with neural markers. In E13.5 spinal cord and peripheral ganglia/nerve tracts (Figure 3A), in E18.5 cortex, and in E18.5 cerebellum (Figure 3B, 3C), tdTomato reporter signals overlap completely with the neuronal marker beta-III tubulin detected using the Tuj1 antibody. In contrast, the tdTomato signal was mutually exclusive with the proliferation marker Ki67 detected in the external granule layer (EGL) in cerebellum (Figure 3D). In postnatal brain, we also observed differential localization of the reporter tdTomato with the oligodendrocyte marker MBP (Figure 3E––3E″) and the astrocyte marker GFAP (Figure 3F––3F″). In cerebellum, tdTomato signals were observed in both granule neurons in the internal granule layer (IGL) and in Purkinje neurons; little staining was present in the white matter where most glial cells reside (Figure 3E″, 3F″). These results indicate that BAF53b-Cre is expressed and active in all neurons examined but not in proliferating neural progenitors and glial cells.
Figure 3. BAF53b-Cre activity is neuron-specific.
Sections of BAF53b-Cre R26R-tdTomato brain and trunk regions at different developing stages were stained with anti-RFP antibodies to visualize tdTomato expression and antibodies against markers for (A–C) neurons (Tuj1), (D) neural progenitors (Ki67), (E) oligodendrocytes (MBP), and (F) astrocytes (GFAP).
A″ is the overlay of A and A′; E″ is the overlay of E and E′; F″ is the overlay of F and F′.
DRG: dorsal root ganglion, SG: sympathetic ganglion, VZ: ventricular zone, EGL: external granule layer; IGL: internal granule layer; WM: white matter, PL: Purkinje neuron layer.
BAF53b-Cre is active in the peripheral nervous system and sensory organs
The expression of reporters in the dorsal root ganglion and sympathetic ganglia (Figure 3A) suggested that BAF53b-Cre is active in peripheral nervous system. We further examined the BAF53b-Cre activities in sensory organs, peripheral nervous system and other neuronal marker expressing cells. A co-staining of tissue sections from P8 BAF53b-Cre tdTomato mice with tdTomato and the neuronal marker β-III tubulin showed an extensive overlap between BAF53b-Cre reporters and neuronal markers. In the eye, tdTomato was observed in neurons in all layers including the ganglion cell layer, inner nuclear layer and outer nuclear layer (Figure 4A). In olfactory epithelium, tdTomato was observed in olfactory sensory neurons that are Tuj1 positive (Figure 4B). In the tongue, tdTomato overlapped with Tuj1 signals in taste bud sensory neurons (Figure 4C). In the inner ear, hair cells are positive for both tdTomato and Tuj1 (Figure 4D). In the skin, Merkel cells, which were identified around a whisker follicle, were also positive for tdTomato signals (Figure 4E). In the intestine, the enteric neurons were marked by both tdTomato and Tuj1 (Figure 4F). In pancreas, beta cells express a significant number of neuronal markers (Atouf et al., 1997). Indeed, beta cells also express tdTomato reporters (Figure 4G). Thus, BAF53b-Cre is active in neurons in both the central and peripheral nervous systems.
Figure 4. BAF53b-Cre is active in the peripheral nervous system and sensory organs.
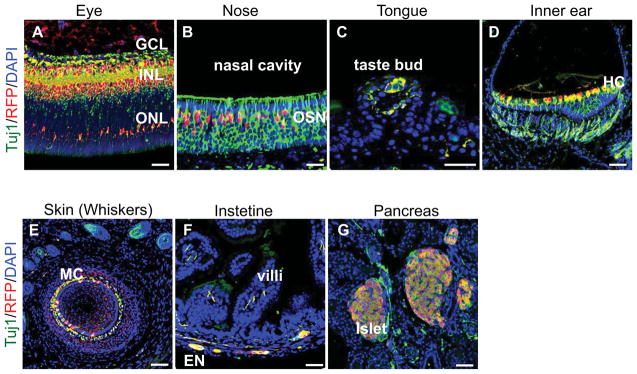
Sections of various tissues and sensory organs of P8 BAF53b-Cre R26R-tdTomato mice were co-stained with anti-RFP antibodies and the Tuj1 antibody against the neuronal marker β-III tubulin. Scale bars: 50 μm.
A. Eye. tdTomato was observed in neurons in all layers. GCL: ganglion cell layer, INL: inner nuclear layer, ONL: outer nuclear layer.
B. Nose olfactory epithelium. tdTomato was observed in Tuj1+ olfactory sensory neurons (OSN).
C. Tongue. tdTomato was observed in taste bud sensory neurons.
D. Inner ear. tdTomato was observed in hair cells (HC).
E. Skin in the whiskers area. tdTomato and Tuj1 staining overlap in the Merkel cells (MC) around a whisker follicle.
F. Intestine. tdTomato was observed in the enteric neurons.
G. Pancreas. tdTomato was observed in beta-cells that were marked by Tuj1 antibodies.
BAF53b-Cre is active in all cultured cortical and hippocampal neurons
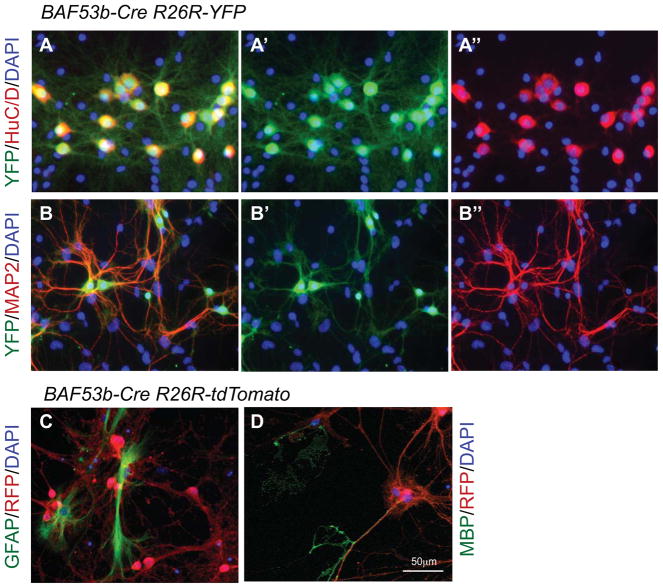
To evaluate expression of BAF53b-Cre at higher resolution, we generated primary hippocampal neural cultures from E18.5 BAF53b-Cre R26R-YFP or R26R-tdTomato embryos. In 14 day in vitro cultures, the reporter YFP signals were detected in all cultured neurons as indicated by the complete overlapping of YFP staining with staining for neuronal markers HuC/D and MAP2 in more than 500 neurons analyzed (Figure 5A––5A″, 5B–5B″). These results indicate that BAF53b-Cre is expressed specifically in neurons and in all neurons examined. Analysis of E16.5 cortical cultures also indicated exclusive expression in neurons (data not shown). Since a fraction of neurons are produced from neural progenitors in culture but all of the neurons were positive for the reporters, we concluded that BAF53b-Cre is active in neuron cultures. The neuron-specific expression of BAF53b-Cre was further confirmed by the differential staining of reporter tdTomato with astrocyte marker GFAP and oligodendrocyte marker MBP (Figure 5C, 5D).
Figure 5. BAF53b-Cre is active in cultured neurons but not in glial cells.
A–D. Cultures of E18.5 BAF53b-Cre R26R-YFP or BAF53b-Cre R26R-tdTomato hippocampal neurons were co-stained with anti-XFP antibodies and antibodies against markers for neurons (HuC/D and MAP2), astrocytes (GFAP), and oligodendrocytes (MBP). Note the coexpression of YFP and HuC/D or MAP2, and the non-overlap between tdTomato and GFAP or MBP.
In summary, using the regulatory elements of a neuronal gene BAF53b in a BAC construct, we generated a pan-neuronal Cre transgenic mouse line. Using two reporter systems, we demonstrated that BAF53b-Cre is active in all developing neurons examined. Similar to the endogenous BAF53b gene, BAF53b-Cre was expressed specifically in neurons in vivo and in cultures, but not in progenitors, glial cells, or most non-neural cells. Thus BAF53b-Cre is a useful new tool that will enable manipulation of genes in developing neurons in mice and in cultures. The pan-neuronal expression pattern and the expression in developing neurons will make BAF53b-Cre especially useful in studies of the function of genes in common neuronal morphogenesis, development and physiologies.
Methods
BAC recombineering
BAC (RP23-127E17) containing the BAF53b gene was recombined at the first two coding exons downstream of the start codon with the Cre gene using the galK-positive and galK-negative selection method as previously described (Warming et al., 2005; Yoo et al., 2009). SW106 bacterial cells were transformed with RP23-127E17 BAC, selected and further transformed with galK PCR product amplified by primers with 50-bp arms homologous to the sites to be recombined (galK-53b-FWD: 5′-TGCTGATCCCGCCGCCGCCCACGGGCCGCGAGCAGCGTAGAGAGGGCACT CCTGTTGACAATTAATCATCGGCA-3′; galK-53b-REV: 5′-TCTGCGTCCAGAGTGATGGGATTTAAAGGAACGCACTACCCTGCCCAGCT TCAGCACTGTCCTGCTCCTT-3′). Recombination was performed by heat-shocking the transformed SW106 cells, and colonies containing the recombined products were selected on minimal agarose plates plus galactose.
The galK-containing RP23-127E17 BAC was then recombined to replace galK with the Cre gene by transforming SW106 cells with the PCR product of Cre-53bUTR and homology arms. PCR products of the Cre coding region using PMC-CreN as the template (Cre-F: 5′-ATCGATAAGCTTACCATGCCCAAGAAGAAGAG-3′; Cre-R: 5′-GGTTAAGAATTCTGGCTAATCGCCATCTTCCAG-3′) and the BAF53b 3′ UTR from the stop codon to 100 bp downstream of the polyA signal (53bUTR-F: 5′-GGTTAAGAATTCAGTTGCCCCCCTCCCCCAAATAC-3′; 53bUTR-R: 5′-GGTTAAGGATCCTGAGGCCACACCCACTAGAGCAC-3′) were digested with EcoRI and ligated. The ligation was used as the template for PCR of the Cre-53bUTR product with 50-bp homologous arms on both sides (53b-Cre-F: 5′-TGCTGATCCCGCCGCCGCCCACGGGCCGCGAGCAGCGTAGAGAGGGCACTAC CATGCCCAAGAAGAAGAGGAAGG-3′; 53b-Cre-UTR-R: 5′-TCTGCGTCCAGAGTGATGGGATTTAAAGGAACGCACTACCCTGCCCAGCTGA CGGATCCGAACAAACGACCCAAC-3′). SW106 cells transformed with the Cre PCR product were grown on 2-deoxy-D-galactose-containing minimal plates for negative galK selection. Colonies with correctly recombined BACs were identified by PCR using primers surrounding the recombined junctions as illustrated in Figure 1A (P1: 5′-AGAAGCGGCTGTACCTTTAAAGC-3′; P2: 5′-GATCCCTGAACATGTCCATCAG-3′, P3: 53bUTR-F; P4: 5′-CATGAGCAGGGAGCTAGATTATTCC-3′). BAC DNA was prepared from the isolated colonies, rechecked by PCR and further confirmed by sequencing.
Generating transgenic mice
BAC-transgenic mice were generated by pronuclear injections. The BAF53b-Cre containing BAC DNA was prepared and injected into the pronucleus of fertilized FVB/N mouse eggs. The positive transgenic mice were identified by PCR. One founder line was generated that has a germline transmission of the transgene. R26R-YFP (Srinivas et al., 2001) and R26R-tdTomato (Ai9) (Madisen et al., 2010) mice were purchased from Jax Laboratory and crossed to BAF53b-Cre mice. These mice are maintained on a mixed genetic background at UT Southwestern Medical Center Animal Facility. The BAF53b-Cre mouse will be available to the research community upon acceptance of the manuscript.
Immunoblotting
For immunoblotting, mouse tissues were lysed in RIPA buffer (50 mM Tris, pH 8, 250 mM NaCl, 0.1% SDS, 0.5% DOC, 1% NP-40), and cell lysates were separated on SDS-PAGE gels. The antibody used was the mouse monoclonal against GFP (JL-8, Clontech). HRP-conjugated secondary antibodies were purchased from Jackson Immunology.
Primary hippocampal neuron culture
E18.5 hippocampal cells were cultured as previously described (Wu et al., 2007). Briefly, embryonic hippocampi were dissected, trypsinized, dissociated to single cells, and cultured on poly-L-ornithine and fibronectin-coated coverslips. Culture media contained DMEM/F12 with putrescine, 2-mercaptoethanol, transferrin, insulin, selenium, progesterone, MEM vitamin additive, and 5% FBS. Half of the medium in each well was replaced every other day. Typically 14 day cultures were used for immunostaining.
Immunofluorescent staining
Timed mouse pregnancies were determined by plugging date as day 0.5. Paraffin sections or PFA-fixed cultured neurons were stained with antibodies against RFP (rabbit, Rockland), GFP (rabbit, Molecular Probes), Tuj1 (Covance), Ki67 (BD Pharmingen), MBP (Millipore), GFAP (BD Pharmingen), MAP2 (Sigma), and HuC/D (Molecular Probes) and visualized using an Olympus BX50 microscope.
Acknowledgments
We thank Qiu Wang for mouse genotyping and technical support. This work is supported by funding from March of Dimes Foundation, American Cancer Society and NIMH (R21 MH102820 to J.I. Wu).
References
- Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J Biol Chem. 1997;272:1929–1934. doi: 10.1074/jbc.272.3.1929. [DOI] [PubMed] [Google Scholar]
- Banares S, Zeh K, Krajewska M, Kermer P, Baribault H, Reed JC, Krajewski S. Novel pan-neuronal Cre-transgenic line for conditional ablation of genes in the nervous system. Genesis. 2005;42:6–16. doi: 10.1002/gene.20117. [DOI] [PubMed] [Google Scholar]
- Campsall KD, Mazerolle CJ, De Repentingy Y, Kothary R, Wallace VA. Characterization of transgene expression and Cre recombinase activity in a panel of Thy-1 promoter-Cre transgenic mice. Dev Dyn. 2002;224:135–143. doi: 10.1002/dvdy.10092. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Cinato E, Mirotsou M, Sablitzky F. Cre-mediated transgene activation in the developing and adult mouse brain. Genesis. 2001;31:118–125. doi: 10.1002/gene.10014. [DOI] [PubMed] [Google Scholar]
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Heimer-McGinn V, Young P. Efficient inducible Pan-neuronal cre-mediated recombination in SLICK-H transgenic mice. Genesis. 2011;49:942–949. doi: 10.1002/dvg.20777. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhou J, Li Y, Kim KW, Hensley LL, Baker SJ, Parada LF. Neuron-specific enolase-cre mouse line with cre activity in specific neuronal populations. Genesis. 2006;44:130–135. doi: 10.1002/gene.20197. [DOI] [PubMed] [Google Scholar]
- Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An Essential Switch in Subunit Composition of a Chromatin Remodeling Complex during Neural Development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Olave I, Wang W, Xue Y, Kuo A, Crabtree GR. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 2002;16:2509–2517. doi: 10.1101/gad.992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]