Abstract
Near-infrared Spectroscopy (NIRS) is a broadly utilized technology with many emerging applications including clinical diagnostics, sports medicine, and functional neuroimaging, to name a few. For functional brain imaging NIR light is delivered at multiple wavelengths through the scalp and skull to the brain to enable spatial oximetry measurements. Dynamic changes in brain oxygenation are highly correlated with neural stimulation, activation, and function. Unfortunately, NIRS is currently limited by its low spatial resolution, shallow penetration depth, and, perhaps most importantly, signal corruption due to light interactions with superficial non-target tissues such as scalp and skull. In response to these issues, we have combined the non-invasive and rapidly reversible method of mechanical tissue optical clearing (MOC) with a commercially available NIRS system. MOC utilizes a compressive loading force on tissue, causing the lateral displacement of blood and water, while simultaneously thinning the tissue. A MOC-NIRS Breath Hold Test displayed a ∼3.5 fold decrease in the time-averaged standard deviation between channels, consequentially promoting greater channel agreement. A Skin Pinch Test was implemented to negate brain and muscle activity from affecting the recorded signal. These results displayed a 2.5-3.0 fold increase in raw signal amplitude. Existing NIRS instrumentation has been further integrated within a custom helmet device to provide a uniform force distribution across the NIRS sensor array. These results showed a gradual decrease in time-averaged standard deviation among channels with an increase in applied pressure. Through these experiments, and the development of the MOC-NIRS helmet device, MOC appears to provide enhancement of NIRS technology beyond its current limitations.
Keywords: Keywords: NIRS, scalp perfusion, compression, oximetry
Introduction
NIRS is a non-invasive optical sensing technology that encompasses very broad clinical and research applications including but not limited to cancer detection [1], muscle oximetry [2], atherosclerotic plaque analysis [3], pediatric brain monitoring [4, 5], stroke physiology evaluation [6], neuro-rehabilitation [7, 8], brain-computer interfacing [8] and cognitive neuroscience [7-9]. A current and promising practice for NIRS lies in its application for functional brain imaging (fNIRS). fNIRS is currently used for analysis of brain activity relevant to medical conditions such as traumatic brain injury [5, 10, 11], Alzheimer's disease [12], and autism [13]. These applications of NIRS are made possible by the technology's ability to measure dynamic changes in cerebral blood oxygenation, which has been shown to correlate to neural activity [14].
NIR light is typically delivered from LED or fiber optic sources through the scalp and skull to the outer layers of the brain. Two wavelengths on opposite sides of the isobestic point for oxy-hemoglobin absorption are used, similar to the principle of pulse oximetry. Diffusely reflected light is then measured by detectors in contact with the skin surface at defined distances from the emitter. Measurement of the remitted light intensity and appropriate normalization enables calculation of diffuse reflectance as a percentage of input light intensity. Further calculations yield relative changes in oxy-hemoglobin (HbO2) and deoxy-hemoglobin (HHb) concentrations in tissue, as well as total hemoglobin. The relative changes in hemoglobin are compared to a baseline of measured brain activity [15].
NIRS is currently limited due to its poor spatial resolution, shallow penetration depth, and signal corruption caused by blood perfusion effects in superficial tissues such as the scalp and skull. Poor spatial resolution (typically 10-40 mm), tends to be set by the geometry of the sensor array, where separation distance between emitter-detector pairs dictates the “banana-shaped” sensing volume inherent in diffuse light transport [16, 17]. Additionally, the optical penetration depth of the NIR light in cranial tissues ranges from about 5-20 mm, with practical interrogation signals seen at depths of ∼15 mm [18-20]. These depth limitations confine interrogation of brain activity to more superficial regions such as the outer cortex. Perhaps most importantly, scalp perfusion has been shown to be a primary confounding factor in accurately determining cerebral oxygenation measurements [21-24]. In order for light to interact with brain tissue, it must pass through the scalp twice (once travelling into tissue, and again travelling out of tissue). The blood in scalp tissue causes unwanted, transiently variable absorbance of the NIR light, which corrupts the desired cerebral hemodynamic signal. While constant scalp absorption would result in a fixed attenuation that could be accounted for via normalization scalp tissue may contain different transient dynamics than those within the brain in regards to oxygenation. In addition to absorption effects, skin is highly scattering due to refractive index mismatch between interstitial water and proteins such as collagen and elastin fibers [25]. Scattering will influence penetration depth as well as the size of the volume a sensor pair is sensing [17, 26]. These factors generate difficulty in relating NIRS signal to corresponding cerebral hemodynamics. Current attempts to reduce these errors include signal processing techniques [27, 28] and increasing the number of sensors on the device to include scalp-isolating sensor pairs to assist in eliminating scalp contributions from brain-sensitive pairs [22, 29]. However, this adds significant system cost and complexity.
Tissue optical clearing is a research area that revolves around developing techniques for causing reversible changes in tissue optical properties (i.e. absorption, scattering) to allow for increased light delivery into deeper tissue levels. Appropriate modifications to optical properties allow for improved light delivery for diagnostic or therapeutic purposes. Many studies have focused on the delivery of exogenous chemicals such as glycerol or dimethyl sulfoxide to replace interstitial water content and improve refractive index matching [30-32]. However, chemical treatments may pose safety concerns for in vivo studies. Additionally, optical clearing efficacy using chemical techniques is limited by the low permeability of these agents in the tissues to which they are delivered. While methods to improve chemical species permeability in skin have been developed, including the use of sonophoresis or microneedle arrays, these techniques are either invasive or would require additional hardware to be installed in the limited space around the NIRS sensor array. Mechanical tissue optical clearing (MOC) is a rapidly reversible and non-invasive optical clearing method with benefits over chemical immersion techniques [33]. A mechanical compressive load applied to the tissue causes an increase in local interstitial pressure, which laterally displaces blood and water, thins the tissue, and alters optical properties [34-41].
Due to these effects, we hypothesize that application and integration of MOC with NIRS will result in an enhanced instrument allowing for a next-level enablement of NIRS. Additionally, MOC modification of NIRS signal rationally stands to benefit a number of NIRS applications. These applications may include, but are not limited to: cancer detection, muscle oximetry, atherosclerotic plaque analysis, pediatrics, evaluation of stroke physiology, human computer interfaces [6, 11, 42-46]. The experiments and results described in this article demonstrate a strong proof-of-concept for the proposed hypothesis.
Materials and Methods
The goal of our study was to establish proof-of-concept for MOC effect on NIRS signal. Three sets of experiments were conducted: (1) Breath Hold Test, (2) Skin Pinch Test, and (3) Helmet Device Test. These tests were all designed to establish MOC-NIRS modification of NIRS signals. All NIRS related data was obtained using the proprietary software (Cognitive Optical Brain Imaging Studio) included with the NIRS system. Protocol for the Breath Hold Test and Skin Pinch Test incorporated NIRS and MOC-NIRS measurements sequentially. For the Helmet Device Test, only the MOC-NIRS configuration was used for these trials. Between measurements, approximately 10 minutes was allowed between trial runs on each subject to enable recovery of sub dermal water/blood where compressive load had been applied.
Standard NIRS
A 16-channel NIRS device (fNIR Imager 1000, fNIR Devices; Potomac, MD) was used to perform NIRS measurements in human subjects for the three testing procedures. The device emits alternating wavelengths of 730 and 850 nm via four LEDs. Each LED emits in sequence to avoid unwanted sensor pair cross-talk, resulting in a 2.0 Hz scan rate for all 16 channels at both wavelengths. We denote this commercial device as the “Standard NIRS” device (Figure 1A) [47].
Figure 1.
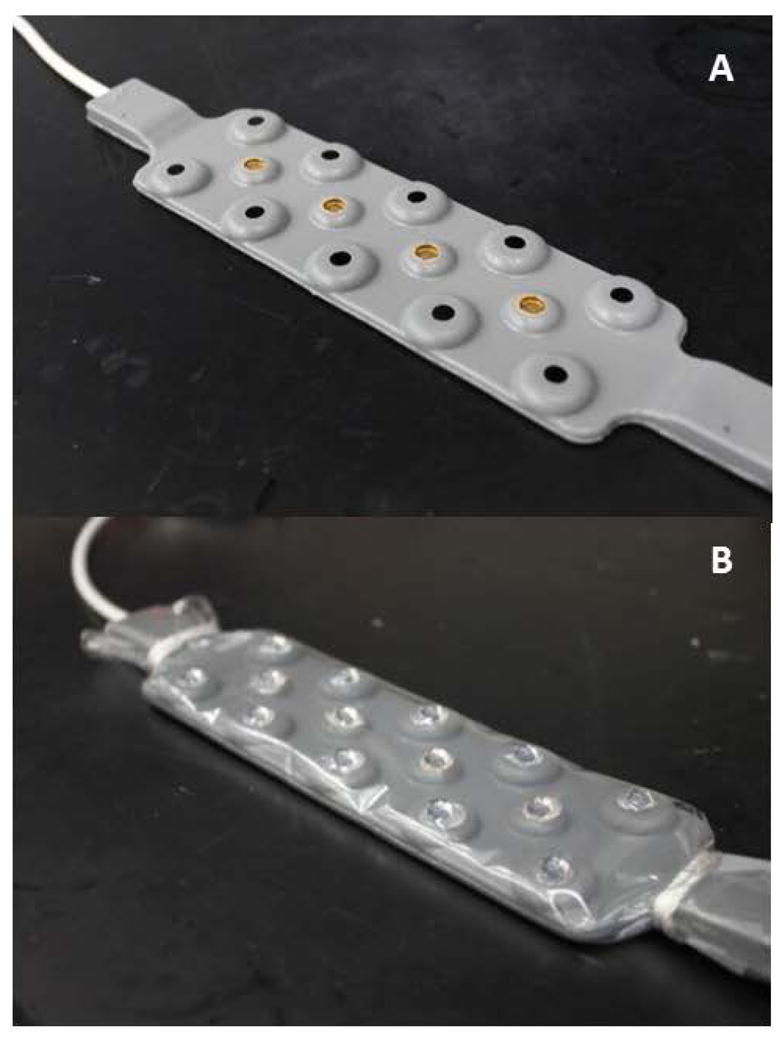
MOC-NIRS
We have modified this Standard NIRS device by adding arrays of half-ball lenses (6 mm diameter) to the sensors. These lenses doubly function as the mechanical indenters. In the initial Breath Hold Tests, the transparent film design facilitated sanitation between subjects and avoided permanent modification of the commercial NIRS sensor array. In subsequent helmet prototypes, we found that permanent bonding of the lenses to the sensor array was necessary to maintain alignment during mechanical loading. Great care was taken to ensure that no epoxy was placed in between the sensor-lens interfaces for this setup. For simplicity, we refer to both of these device configurations jointly as the “MOC-NIRS” device, since they both represent the same theoretical application of MOC. It should be noted that hemispherical indenter lenses were used instead flat lens geometries as research more strongly supports their efficiency for MOC applications [48].
Breath Hold Test
For the Breath Hold Test, the MOC-NIRS indenter lenses (borosilicate glass hemispheres) were fixed directly to a NIR-transparent polycarbonate plastic film that laid overtop the NIRS band utilizing an optical grade epoxy (F120 Fast Room Temperature Cure, Thorlabs; Sterling, VA) (Figure 1B) [47]. Breath holding generates a non-neuronal blood oxygenation level dependent (BOLD) response in gray matter uniformly throughout the brain [49]. This response allows for the significant nullification of spatial variance that would otherwise be experienced in typical cognitive testing procedures. This test provides a consistent method of testing devices with accurate and comparable data responses between subjects.
One subject was tested with both MOC-NIRS and Standard NIRS (control). Once the device was placed on the subject's forehead, a rubber backplate was placed overlying the film-covered NIRS device, and straps were used to manually apply a mechanical load of 15 Newtons to the device (Figure 2) [47]. A 10 second resting baseline was recorded. Then, the subject was asked to perform 6 cycles consisting of 15 second breath-holds followed by 15 seconds of relaxed breathing. The subject was asked to remain motionless and silent for the duration of the testing procedure.
Figure 2.

Skin Pinch Test
The MOC-NIRS indenter lenses used in the Skin Pinch Test were made of sapphire (Sapphire Half-Ball Lens, Edmund Optics Ltd; York, UK). While the Breath Hold Test provides a spatially uniform signal, any changes in signal could still be due in part to brain hemodynamics, rather than effects of MOC-NIRS acting on skin. Therefore, we additionally developed a “Skin Pinch” Test that eliminated the potential confounding effects of muscle or neural activation, ultimately removing it as a factor for the modification of NIRS signal. A single NIRS emitter-sensor pair was manually folded around two dermal locations: the base of the earlobe and excess anterior forearm dermis. Both locations were convenient for the experimental design and also incorporated the exclusion of oxygenated muscle activity. Applied compression force was measured at approximately 9.8 ± 0.66 N, which translates to 0.34 ± 0.02 MPa per indenter lens, ultimately remaining below the quantified pain threshold of 0.5-1.0 MPa [50-52]. Since the emitter and detector were facing one another on opposing sides of the skin flap, this setup required implementation of neutral density filters (OD= 4.3) to attenuate the diffuse light transmission to avoid signal saturation. A subject was tested with both MOC-NIRS and Standard NIRS. Since no BOLD response was monitored in regards to muscle activation, raw signal voltage was measured and reported instead of relative change in hemoglobin concentrations. Sensors were placed in a resting “minimal force” state on the patient. A 10 second baseline was measured. Next, recording was initiated, followed shortly by force application to the dermal fold site. Data was acquired until the signal appeared to oversaturate.
Helmet Device Test
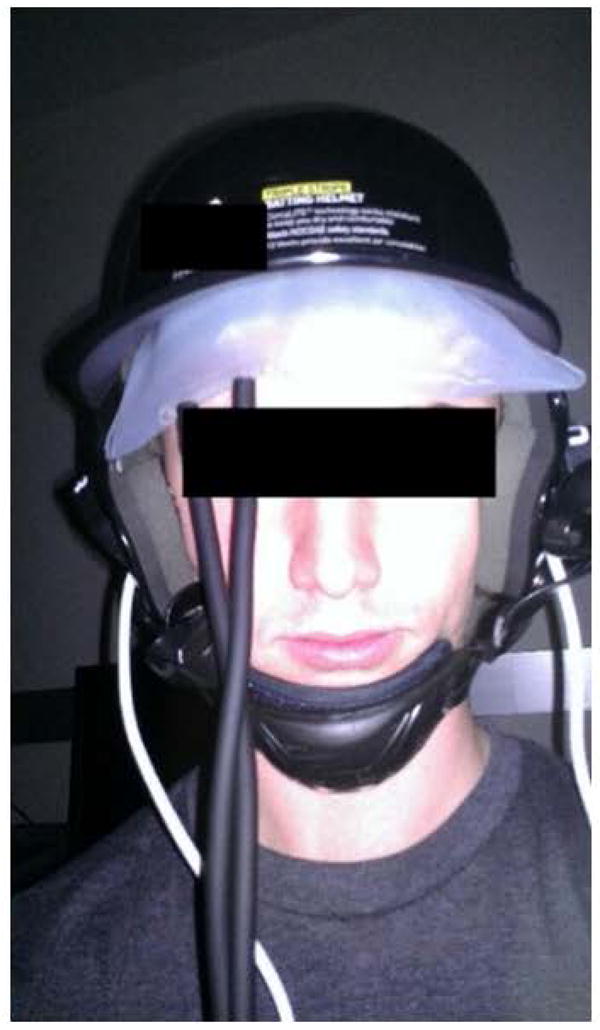
The MOC-NIRS indenter lenses used in the Helmet Device Tests were made of sapphire (Sapphire Half-Ball Lens, Edmund Optics Ltd; York, UK). The goal of the prototype and experiment (N=1) was to establish a more uniform distribution of force among individual sensor components on the MOC-NIRS device. We tested whether this force distribution would allow for improved agreement of hemodynamic signals using a procedure similar to that described in the Breath-Hold Test. A commercial baseball helmet (Triple Stripe Helmet, Adidas; Herzogenaurach, Germany) was modified and then combined with a MOC-NIRS device and an inflatable air bladder (CVS Self-Taking BP Monitor, CVS Pharmacy; Woonsocket, RI) (Figure 3). The prototype was worn by one subject, and the MOC-NIRS sensors was positioned on the forehead for measuring signals in the prefrontal cortex. The bladder was then inflated to a desired pressure value (20, 30, 40, or 50 mmHg) while performing data acquisition with the MOC-NIRS device. A 10 second baseline measurement was obtained for each pressure level before the Breath Hold Test was administered. The subject participated in 3 breath-hold cycles consisting of 20 seconds of normal/relaxed breathing, followed by a 15 second breath-hold. The bladder was then deflated and the subject was allowed an approximate rest period of 5 minutes to return to a neural baseline before continuing to the next pressure level. Trials with the inflated bladder were performed in turn as described, with ascending pressure values between trials. It is worthwhile to note that the helmet device was not removed between trials. The test was performed in a dark room to negate any potential interference from additional light sources or distractions to the subject.
Figure 3.
Results
Breath Hold Test
For subjects (N=4), we compared the performance of Standard NIRS and MOC-NIRS devices by comparing time-averaged standard deviation (STD) values across all channels for each subject. Figures 4A and 4B show all individual channels of subject 1 data for both device configurations, indicating that MOC-NIRS provides improved inter-channel agreement. Vertical dashed lines denote the beginning of a breath-hold event. Additionally, a single channel (V11) experienced a malfunction during testing and acquired no data, and was discarded from the analysis. Averaged STD values for Standard and MOC-NIRS configurations of each subject can be seen in Table 1, displaying a total average 3.24 (± 0.68) fold decrease in STD with MOC-NIRS compared to Standard NIRS. HbO2 data for subject 1 was averaged over all channels, and is shown in Figure 4C, while Figure 4D shows the standard deviation of these channel-averaged signals over time. A gradual increase in STD was observed with Standard NIRS, implying a potential time-dependent source of noise, such as motion artifacts or changes in sensor array contact quality. This trend was notably attenuated in MOC-NIRS data. Results show that MOC-NIRS reduces variability in oxygenation signals measured in human subjects.
Figure 4.
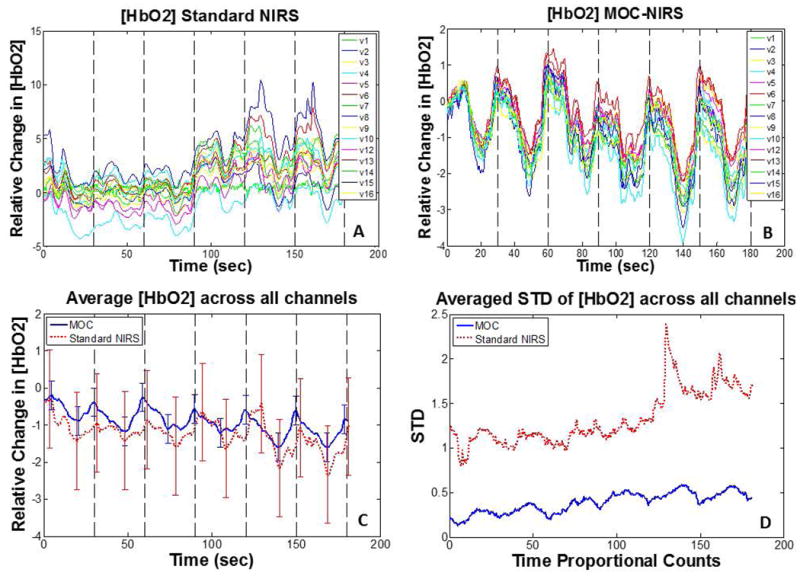
Table 1.
| Time-Average STD (HbO2) | |||
|---|---|---|---|
| Subject | Standard NIRS | MOC-NIRS | Standard NIRS:MOC-NIRS |
| 1 | 1.46 | 0.37 | 3.97 |
| 2 | 2.16 | 0.90 | 2.41 |
| 3 | 1.60 | 0.41 | 3.93 |
| 4 | 1.43 | 0.54 | 2.65 |
Skin Pinch Test
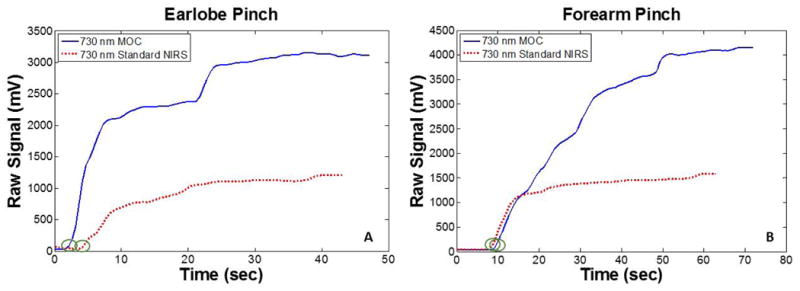
Results show a ∼2.5-3 fold increase in raw signal amplitude with MOC-NIRS over Standard NIRS. Data show that the increase in signal amplitude was not instantaneous, but rather, gradual (Figure 5). This steady increase in signal amplitude could be caused by time-dependent lateral displacement of blood and water that results from the increase of local interstitial pressures that is generated via MOC. Additionally, during the forearm pinch test specifically (Figure 5B), MOC-NIRS demonstrated an increase in signal substantial enough to cause saturation, even with the attenuated emitter-sensor pairs, indicating that the 2.5-3 fold increase is an underestimation of MOC efficacy. It is worth noting that the initial transient jump seen in the graphs are due to a stall in the manual force application after initiating device recording. The Skin Pinch Test shows that MOC-NIRS permits increased throughput of light signal through dermal tissue layers.
Figure 5.
Helmet Device Test
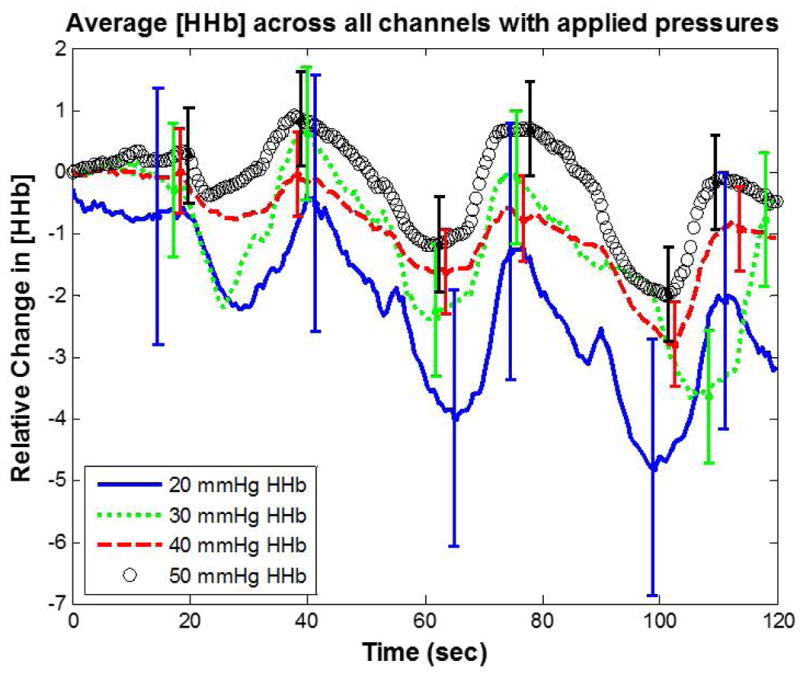
For individual inflated bladder pressure trials, we calculated the average HHb signal and STD across all measured channels at each time point. For each applied pressure we averaged the STD of HHb over a three cycle breath-hold duration (∼120 seconds). This time-averaged STD at 50 mmHg was compared to 20, 30, and 40 mmHg. The results displayed in Figure 6 show a decrease in time-averaged STD of 300%, 100%, and 60%, respectively. The error bars for each pressure level represent the channel and time-averaged STD between the measured channels. Application of the helmet device with uniform force distribution via a controllable inflatable bladder showed a drastic improvement in channel agreement with increasing bladder pressure.
Figure 6.
Discussion and Conclusion
The Breath Hold Test and Skin Pinch Test demonstrated MOC as a source for enhancing NIRS signal. In the Skin Pinch Test the transient jump seen in the data is suspected to relate to the moethod of manual force application. A more consistent protocol for force application should be used for further MOC-NIRS implementation. Still, potential transient changes in signal such as this can be negated by allowing MOC effects to stabalize before initiating data acquistion.
The Helmet Device Test demonstrated an improved agreement between NIRS channels (lower STD) proportional to bladder pressure (Figure 6). The integrated MOC and NIRS processes allow for improved light delivery and interrogation of brain hemodynamics. The increased channel agreement shown by the results of the Helmet Device Test is likely attributed to the more even force distribution across channels delivered by the inflated bladder. We speculate that the combination of MOC-NIRS technologies within the helmet addresses NIRS signal interference caused by dermal perfusion via a more uniform lateral displacement of blood and water into surrounding tissue. This trend appears to improve with increased application of force. It should be noted that it is possible that some light emitted by the source may interact with laterally displaced water. The full effect of this interaction on measured signal is currently unquantified. Previous publications pertaining to MOC have utilized OCT to help garner an understanding for MOC effects on water displacement, as well as light penetration through skin. One previous study implemented the MOC technique with half-ball lenses coupled with a 1310 nm swept-source OCT system, and showed a decrease in water volume fraction from 0.66 to 0.20 in porcine models at sites of MOC application [34]. Other research using tissue optical clearing devices on human models has shown increased OCT light penetration depth up to threefold using wavelengths of 850 and 1310 nm [38].
One significant limitation of MOC-NIRS is the pain threshold, since pain could cause subject discomfort and would negatively impact cognitive studies. While there is a slight discomfort from forces exerted by the indenter lenses on the skin, the quantified pain threshold (∼0.5-1.0 MPa) [50-52] was not exceeded in our current study. Future research will explore methods of relieving the minor discomfort experienced with MOC application. While this preliminary data supports the proof-of-concept being displayed, future work should also incorporate more robust experimentation with quantification of additional optical/mechanical parameters and higher number of trials for more established repeatability. Future work will also investigate MOC-NIRS in fNIRS applications such as cognitive neuroscience.
Acknowledgments
American Society for Lasers in Medicine and Surgery Travel Award
NIH National Heart, Lung, and Blood Institute (NHLBI), R01-HL098912
Footnotes
Disclosure: All authors have completed the ICMJE form. No authors have any conflict of interest at this time. Some figures are reproduced from SPIE Proceedings (85782K, Vogt et al, “Mechanical indentation improves cerebral blood oxygenation signal quality of functional near-infrared spectroscopy (fNIRS) during breath holding,” 2013).
References
- 1.Nioka S. NIR spectroscopic detection of breast cancer. Technology in Cancer And Research Treatment. 2005;4(1) doi: 10.1177/153303460500400504. [DOI] [PubMed] [Google Scholar]
- 2.Hamaoka T, et al. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12(6):062105–062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 3.Moreno PR, et al. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105(21):2504–2511. doi: 10.1161/01.cir.0000017265.52501.37. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, et al. Auditory-evoked cerebral oxygenation changes in hypoxic-ischemic encephalopathy of newborn infants monitored by near infrared spectroscopy. Early Hum Dev. 2002;67(1-2):113–121. doi: 10.1016/s0378-3782(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 5.Adelson PD, et al. The use of near infrared spectroscopy (NIRS) in children after traumatic brain injury: a preliminary report. Acta Neurochir Suppl. 1998;71:250–254. doi: 10.1007/978-3-7091-6475-4_72. [DOI] [PubMed] [Google Scholar]
- 6.Sakatani K, et al. Comparison of blood-oxygen-level-dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J Biomed Opt. 2007;12(6):062110–062110. doi: 10.1117/1.2823036. [DOI] [PubMed] [Google Scholar]
- 7.Arenth PM, Ricker JH, Schultheis MT. Applications of functional near-infrared spectroscopy (fNIRS) to Neurorehabilitation of cognitive disabilities. Clin Neuropsychol. 2007;21(1):38–57. doi: 10.1080/13854040600878785. [DOI] [PubMed] [Google Scholar]
- 8.Holper L, et al. Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near-infrared spectroscopy (fNIRS) J Neuroeng Rehabil. 2010;7:57–57. doi: 10.1186/1743-0003-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 10.Tachtsidis I, et al. Analysis of the Changes in the Oxidation of Brain Tissue Cytochrome-c-Oxidase in Traumatic Brain Injury Patients during Hypercapnoea. Advances in Experimental Medicine and Biology. 2011;701:9–14. doi: 10.1007/978-1-4419-7756-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amyot F, et al. Normative database of judgment of complexity task with functional near infrared spectroscopy--application for TBI. Neuroimage. 2012;60(2):879–883. doi: 10.1016/j.neuroimage.2012.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou PH, Lan TH. The role of near-infrared spectroscopy in Alzheimer's disease. Journal of Clinical Gerontology and Geriatrics. 2013;4(2):33–36. [Google Scholar]
- 13.Xiao T, et al. Response inhibition impairment in high functioning autism and attention deficit hyperactivity disorder: evidence from near-infrared spectroscopy data. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong K, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.S B, et al. Functional Near-Infrared Spectroscopy: An Emerging Neuroimaging Modality. IEEE Engineering in Medicine and Biology Magazine. 2006 [Google Scholar]
- 16.Bevilacqua F, Depeursinge C. Monte Carlo study of diffuse reflectance at source–detector separations close to one transport mean free path. Journal of the Optical Society of America. 1999;16(12):2935–2945. [Google Scholar]
- 17.Okada E, et al. Theoretical and experimental investigation of near-infrared light propagation in a model of the adult head. Appl Opt. 1997;36(1) doi: 10.1364/ao.36.000021. [DOI] [PubMed] [Google Scholar]
- 18.Minati L, et al. Intra- and extra-cranial effects of transient blood pressure changes on brain near-infrared spectroscopy (NIRS) measurements. Journal of Neuroscience Methods. 2011;197(2):283–288. doi: 10.1016/j.jneumeth.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faris F, et al. Non-invasive in vivo near-infrared optical measurement of the penetration depth in the neonatal head. Clinical Physics and Physiological MeasurementEmail alert RSS feed. 1991;12(353) doi: 10.1088/0143-0815/12/4/005. [DOI] [PubMed] [Google Scholar]
- 20.Chuan-hua C, Ting L, Hui G. Research on the Detecting Depth in Biological Tissue by Near Infrared Spectroscopy System. Journal of Biomedical Engineering Research. 2006;25(1) [Google Scholar]
- 21.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 22.Saager R, Berger A. Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy. J Biomed Opt. 2008;13(3):034017–034017. doi: 10.1117/1.2940587. [DOI] [PubMed] [Google Scholar]
- 23.Owen-Reece H, et al. The effect of scalp ischaemia on measurement of cerebral blood volume by near-infrared spectroscopy. Physiol Meas. 1996;17(4):279–86. doi: 10.1088/0967-3334/17/4/005. [DOI] [PubMed] [Google Scholar]
- 24.Davis S, et al. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol. 2006;100(1):221–224. doi: 10.1152/japplphysiol.00867.2005. (1985) [DOI] [PubMed] [Google Scholar]
- 25.Churmakov D, Meglinski I, Greenhalgh D. Influence of refractice index matching on the photon diffuse reflectance. Physics in Medicine and Biology. 2002;47(23):4271–4285. doi: 10.1088/0031-9155/47/23/312. [DOI] [PubMed] [Google Scholar]
- 26.Mansouri C, et al. Depth sensitivity analysis of functional near-infrared spectroscopy measurement using three-dimensional Monte Carlo modelling-based magnetic resonance imaging. Lasers Med Sci. 2010;25(3):431–8. doi: 10.1007/s10103-010-0754-4. [DOI] [PubMed] [Google Scholar]
- 27.Schelkanova I, Toronov V. Independent component analysis of broadband near-infrared spectroscopy data acquired on adult human head. Biomed Opt Express. 2012;3(1):64–74. doi: 10.1364/BOE.3.000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohno S, et al. Removal of the skin blood flow artifact in functional near-infrared spectroscopic imaging data through independent component analysis. J Biomed Opt. 2007;12(6):062111–062111. doi: 10.1117/1.2814249. [DOI] [PubMed] [Google Scholar]
- 29.White BR, Culver JP. Phase-encoded retinotopy as an evaluation of diffuse optical neuroimaging. Neuroimage. 2010;49(1):568–577. doi: 10.1016/j.neuroimage.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas G, et al. Use of osmotically active agents to alter optical properties of tissue: effects on the detected fluorescence signal measured through skin. Lasers Surg Med. 2001;29(3):213–220. doi: 10.1002/lsm.1110. [DOI] [PubMed] [Google Scholar]
- 31.Tuchin V, et al. Light propagation in tissues with controlled optical properties. J Biomed Opt. 1997;2(4):401–417. doi: 10.1117/12.281502. [DOI] [PubMed] [Google Scholar]
- 32.Rylander C, et al. Dehydration mechanism of optical clearing in tissue. J Biomed Opt. 2006;11(4):041117–041117. doi: 10.1117/1.2343208. [DOI] [PubMed] [Google Scholar]
- 33.Izquierdo-Roman A, et al. Mechanical tissue optical clearing technique increases imaging resolution and contrast through ex vivo porcine skin. Lasers Surg Med. 2011;43(8):814–823. doi: 10.1002/lsm.21105. [DOI] [PubMed] [Google Scholar]
- 34.Gurjarpadhye A, et al. Effect of Localized Mechanical Indentation on Skin Water Content Evaluated Using OCT. Int J Biomed Imaging. 2011;2011:817250–817250. doi: 10.1155/2011/817250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Jiang J, Xu K. The variations of water in human tissue under certain compression: Studied with diffuse reflectance spectroscopy. Journal of Innovative Optical Health Sciences. 2013;6(1) [Google Scholar]
- 36.Kirillin M, Agrba P, Kamensky V. In vivo study of the effect of mechanical compressionon formation of OCT images of human skin. 4363792J Biophotonics. 2010;3(12):752–758. doi: 10.1002/jbio.201000063. [DOI] [PubMed] [Google Scholar]
- 37.Agrba P, et al. Compression As a Method for Increasing the Informativity of Optical Coherence Tomography of Biotissues. Optics and Spectroscopy in Biomedical Investigations. 2009;107(6):853–858. [Google Scholar]
- 38.Drew C, Milner T, Rylander C. Mechanical tissue optical clearing devices: evaluation of enhanced light penetration in skin using optical coherence tomography. J Biomed Opt. 2009;14(6):064019–064019. doi: 10.1117/1.3268441. [DOI] [PubMed] [Google Scholar]
- 39.Rylander C, et al. Mechanical Tissue Optical Clearing Devices: Enhancement of Light Penetration in Ex Vivo Porcine Skin and Adipose Tissue. Lasers in Surgery and Medicine. 2008;40:688–694. doi: 10.1002/lsm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Askar'yan GA. Enhancement of transmission of laser and other radiation by soft turbid physical and biological media. Sov J Quantum Electron. 1982;12(7):877. [Google Scholar]
- 41.Ivanov AP, Makarevich SA, Khairullina AY. Radiation propagation in tissues and liquids with close particle packing. Journal of Applied Spectroscopy. 1987;47(4):1077–1082. [Google Scholar]
- 42.Moreno P, et al. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105(8):923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- 43.Arenth P, Ricker J, Schultheis M. Applications of Functional Near-Infrared Spectroscopy (fNIRS) to neurorehabilitation of cognitive disabilities. Clin Neuropsychol. 2007;21(1):38–57. doi: 10.1080/13854040600878785. [DOI] [PubMed] [Google Scholar]
- 44.Holper L, et al. Testing the potential of virtual reality with neurorehabilitation system during performance of observation, imagery, and imitation of motor actions recorded by wireless fucntional near-infrared spectroscopy (fNIRS) Journal of NeuroEngineering and Rehabilitation. 2010;7:57. doi: 10.1186/1743-0003-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari M, et al. Prefrontal Cortex Activated Bilaterally by a Tilt Board Balance Task: A Functional Near-Infrared Spectroscopy Study in a Semi-Immersive Virtual Reality Environment. Brain Topogr. 2013 doi: 10.1007/s10548-013-0320-z. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa S, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogt W, et al. Mechanical indentation improves cerebral blood oxygenation signal quality of functional near-infrared spectroscopy (fNIRS) during breath holding. Proc of SPIE. 2013;8578 [Google Scholar]
- 48.Vogt WC. Virginia Polytechnic Institute and State University (Dissertation) 2013. Development of Mechanical Optical Clearing Devices for Improved Light Delivery in Optical Diagnostics. [Google Scholar]
- 49.Stillman A, Hu X, Jerosch-Herold M. Functional MRI of brain during breath holding. Magnetic Resonance Imaging. 1995;13(6):893–897. doi: 10.1016/0730-725x(95)00037-h. [DOI] [PubMed] [Google Scholar]
- 50.Lee W, Zhang M, Mak A. Regional differences in pain threshold and tolerance of the transtibial residual limb: including the effects of age and interface material. Arch Phys Med Rehabil. 2005;86(4):641–649. doi: 10.1016/j.apmr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Fischer AA. Pressure threshold meter: its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67(11):836–838. [PubMed] [Google Scholar]
- 52.Xiong S, et al. An indentation apparatus for evaluating discomfort and pain thresholds in conjunction with mechanical properties of foot tissue in vivo. J Rehabil Res Dev. 2010;47(7):629–641. doi: 10.1682/jrrd.2009.09.0152. [DOI] [PubMed] [Google Scholar]


