Abstract
Synaptic roles for neurofilament proteins have rarely been considered. Here, we establish all four neurofilament subunits as integral resident proteins of synapses. Compared to the population in axons, neurofilament subunits isolated from synapses have distinctive stoichiometry and phosphorylation state, and respond differently to perturbations in vivo. Completely eliminating neurofilament proteins from brain by genetically deleting three subunits (α-internexin, NFH and NFL) markedly depresses hippocampal LTP induction without detectably altering synapse morphology. Deletion of NFM in mice, but not the deletion of any other neurofilament subunit, amplifies dopamine D1-receptor-mediated motor responses to cocaine while redistributing postsynaptic D1-receptors from endosomes to plasma membrane, consistent with a specific modulatory role of NFM in D1-receptor recycling. These results identify a distinct pool of synaptic neurofilament subunits and establish their key role in neurotransmission in vivo, suggesting potential novel influences of neurofilament proteins in psychiatric as well as neurological states.
Keywords: Neurofilament, synaptic plasticity, D1 receptor, stimulant drug, cocaine, LTP
INTRODUCTION
Neurofilaments (NFs), the intermediate filaments of mature neurons, are among the most abundant proteins in brain. NFs expand the calibers of large myelinated axons to support nerve conduction1, 2 and support dendrites of large motoneurons 3, 4. Unlike the intermediate filaments (IF) of other cell types, CNS neurofilaments are hetero-polymers composed of NFL, NFM, NFH and α-internexin 5, 6. NFM, NFL, and NFH subunits also differ from most other IF proteins in being regulated by phosphorylation at many sites by multiple protein kinases7. The complex hetero-polymeric structure and dynamically changing phosphate topography of NF proteins suggest that they might serve additional biological roles beyond static structural support of axon caliber 8, 9. Notably, NF proteins have been reported to be altered in certain neuropsychiatric states although a mechanistic understanding of their involvement is lacking.
Synapses have long been considered to be degradative sites for NF reaching terminals by axonal transport. A functional role for NF proteins within synapses, however, has rarely been considered although scattered observations hint at this possibility 8, 9. For example, NF subunits can be transported to synaptic terminals in oligomeric form, including hetero-dimers 10. Individual NF subunits or their fragments have been detected in synaptic fractions and bound to specific isolated synaptic proteins 7 although contamination by axonal NF has not been excluded 11. A splice variant of the NMDA receptor subunit NR1 has been reported to bind specifically through its cytoplasmic C-terminal domain to the NFL subunit in vitro and in non-neuronal cells transfected with NF proteins 8. Evidence is lacking, however, for a direct interaction between NFL and NR1 in brain. NFM was shown to bind via its C-terminus to the third cytoplasmic loop of the dopamine D1 receptor (D1R) in vitro when both proteins were co-transfected in non-neuronal cells, which lowered D1R cell surface occupancy and sensitivity of the cells to D1R agonists 9. D1R is a primary site of action of stimulant drugs like cocaine and amphetamine and, interestingly, chronic exposure to drugs of abuse in humans 12 and in animal models of drug addiction 13, 14 selectively lowers levels of NF proteins and alters their phosphorylation states. Cocaine administration also activates extracellular signal- regulated kinase (ERK), a known NF protein kinase 15, by promoting its phosphorylation 16–18, which is coupled to increased NF phosphorylation12, 16. The functional significance of these cytoskeletal changes and their implications for synaptic function remain unclear.
In this study, we used multiple independent approaches to establish that all four NF subunits of the mature CNS are integral proteins of synapses, particularly the post-synaptic area. This synaptic population can be distinguished from NFs within the axonal cytoskeleton, thus excluding the possibility of artifactual NF contamination as a basis for their presence in synapses. We further observed that eliminating NF proteins from the CNS profoundly disrupts synaptic plasticity without altering the structural integrity of synapses. Finally, using a large panel of genetic mouse models in which individual NF subunits were selectively deleted, we establish in vivo a specific functional role of post-synaptic NFM subunits in modulating D1 receptor-mediated behavior. Our findings indicate that distinctive pools of NF proteins within synapses perform roles in neurotransmission quite different from the conventional role of NF in axons. Thus, they provide insight into the functional significance of selective NF subunit alterations observed in certain neuropsychiatric disorders, in addition to the better known alterations associated with neurodegenerative diseases 19–23.
MATERIALS AND METHODS
Generation of transgenic animals, drugs and antibodies
Please see supplemental information.
Analytical methods
Our published methods were used for all the procedures. Please see supplemental information.
RESULTS
Identification of a unique population of NF proteins within synapses
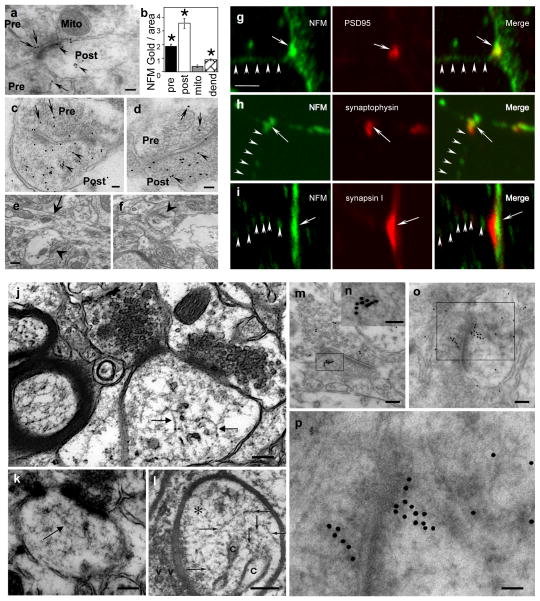
Although NF subunits have diverse ligands, including several structural and receptor proteins enriched in synaptic terminals 8, 9, 24, 25, it is not known whether or not NF proteins are actually stable constituents of synapses or simply axonal NF contaminants. To resolve this issue, we applied multiple complementary analytical approaches, beginning with investigations of NF subunits in synapses using immunogold electron microscopy. We first performed quantitative immunogold analyses on NFM in synaptic terminals of mouse striatum with monoclonal anti-NFM RMO44 using a post-embedding labeling procedure. In analyses of >100 digitized micrographs using Bioquant software, we quantified the densities of gold particles contained within the cytoplasm of pre-synaptic or post-synaptic bouton areas, respectively identified by clear synaptic vesicles or a post-synaptic density, and separately analyzed the dendrites adjacent to synaptic terminals. To assess non-specific labeling, we analyzed grids incubated without primary antibodies or with primary antibody pre-absorbed as a negative control (Figure 1e, f) and, as a second negative control, we also compared immunogold labeling over synaptic mitochondria, which should lack specific labeling. Comparisons of gold particles per unit area for these morphological structures (Figure 1a,b) revealed a significant NFM enrichment in post-synaptic bouton areas compared to pre-terminal dendritic areas or pre-synaptic terminals. Immunogold particle density for NFM in post-synaptic boutons was 8-fold higher (p<0.0001, Mann-Whitney test) than that associated with mitochondria and 4 fold greater than that in adjacent pre-terminal regions of dendrites (p<0.0001, Mann-Whitney test). NFM immunogold density in pre-synaptic terminals, while 4 fold higher than non-specific mitochondrial labeling (p<0.0001, Mann-Whitney test) was much lower than that in adjacent axon preterminal regions, contrasting with the NFM enrichment in postsynaptic boutons relative to pre-terminal dendritic areas.
Figure 1.
Presence of NFM proteins in synapses and 10nm NF polymers in synapses. Post-embedding EM using anti-NFM antibody (RMO-44)(a) shows immunogold decoration of pre-synaptic (a,” Pre”, arrows) and post-synaptic regions (a, “Post”, arrowheads). Mitochondria (Mito), as a background control, exhibit little or no labeling. (b) Morphometry of NFM-gold particles shows 8-fold more immunogold in post-synaptic regions (P<0.0001) per unit area than in mitochondria and 4-fold more than in pre-terminal dendrites where the presence of NFM is established (P<0.0001). Gold particles in the pre-synaptic region are 4-fold more numerous than in mitochondria. (c, d) Two examples illustrating the greater immunogold labeling in post-synaptic regions than in pre-synaptic regions (” Pre”, arrows and “Post”, arrowheads). Negative controls without primary antibody (e) or with primary antibody pre-absorbed (f) show negligible labeling. Arrow points to a dendrite in (e) and arrowheads to synapses in (e, f). Double-immunolabeled primary neurons show colocalization of NFM and the post-synaptic marker, PSD95. (g) A focal region of strong NFM immunofluorescence is apposed to a region containing strong immunofluorescence for pre-synaptic markers synaptophysin (h) or synapsin I (i). Although not abundant, NF structures could be visualized in synapses of human (j) and mouse brain (k). The presence of 9–10 nm filaments was previously reported in dendritic spines of mouse brain prepared by rapid-freezing technique (Landis & Reese, JCB, 1983;97;1169–1178, reproduced by permission) (l). Linear structures in the synapse were also decorated by anti-NFM antibody visualized by immunogold labeling (m–p). Arrows point to 10nm filaments. n and p shows higher magnifications of m and o, respectively. Scale bar, 100 nm in a; 50 nm in c, d; 200 nm in e, f and 5 μm in g-i; 100 nm in j, k, l, m and o; 50 nm in n and p.
We then confirmed NFM enrichment in post-synaptic boutons by double-immunofluorescence analysis of primary cortical neurons derived from wild-type C57BL/6J mice at E17.5 days. Neurons (21 DIV) immunostained with mouse mAb RMO-44 or anti-NFM rabbit polyclonal antibody displayed concentrations of NFM immunofluorescence that colocalized with the post-synaptic marker, PSD95, identified by the monoclonal antibody from Sigma, and were apposed to presynaptic terminals labeled with antibodies to synaptophysin (monoclonal, Sigma) or synapsin I (polyclonal, Sigma) (Figure 1g–i). NFM immunofluorescence in the post-synaptic terminal was stronger than in the pre-terminal dendrite. We could observe short 10nm filaments in occasional synapses, which in some cases, were linearly decorated by gold particles after reaction with anti-NFM antibody in immunoEM analyses (Figure 1j–p). The images indicate that at least some synaptic NF subunits exist in polymerized form. Finally, we also confirmed the immuno-gold labeling of NFM was 150kDa intact subunit and not the degradation products of this subunit by immunoblot analysis, which showed that full-length NFM is the major NFM-related protein species of synaptosomes (Supplementary Figure S1).
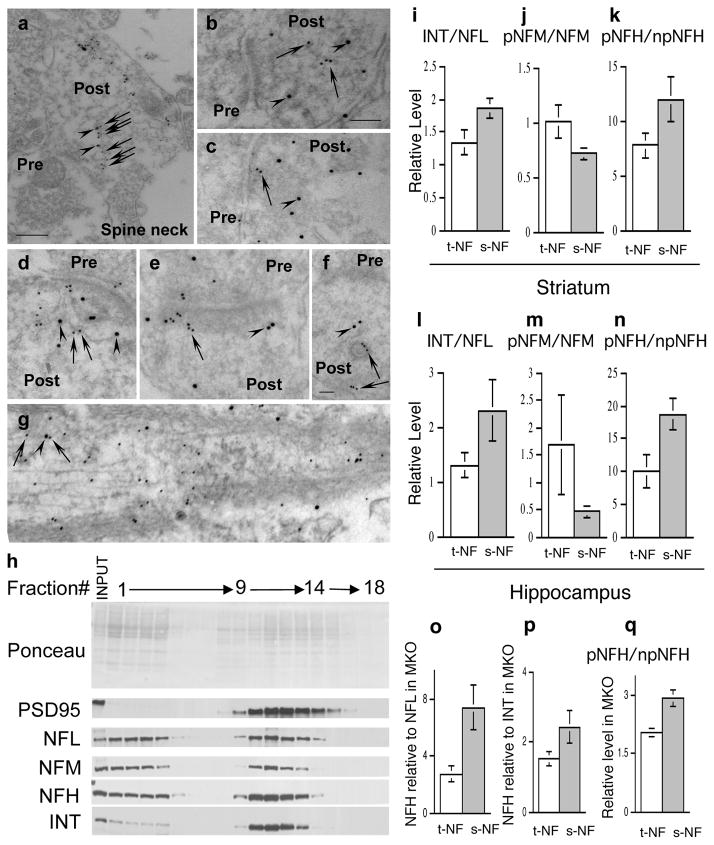
We also performed pre-embedding immunogold EM labeling to investigate the presence of all 4 NF subunits in synapses of WT mouse striatum (Supplementary Figure S2a–e) and hippocampus (Supplementary Figure S2f–h). With each antibody, we analyzed, as negative controls, brain sections from mice lacking all NF proteins through knockout of α-internexin, NFH and NFL (triple IHL-TKO mice, described in detail in Figure 3) to assess the specific binding of antibodies to each NF subunit (Supplementary Figure S2c–d). Under the conditions used in our labeling studies, morphometric analyses showed that each NF subunit antibody negligibly labeled IHL-TKO striatum (Figure 2c–d) or hippocampus (not shown) in contrast to the strong decoration of the abundant NFs in myelinated axons of WT mice, which served as a positive control (Supplementary Figure S2a–b). Morphometric analysis of gold particles associated with each of the four NF subunits, NFM (Supplementary Figure S2e), NFL (Supplementary Figure S2f), NFH (Supplementary Figure S2g) and α-internexin (Supplementary Figure S2h), showed significantly higher labeling density in wild-type than in IHL-TKO mice (Supplementary Figure S2i) (p<0.0001, Mann-Whitney test, mean ± SEM, n=10–26). Similar to NFM labeling, more labeling of NFL, NFH and α-internexin was seen in post-synaptic areas than in pre-synaptic areas. To further establish the identity of NF assemblies in synapses, we performed immuno-gold double-labeling of NFL with NFM or NFL with NFH. The labeling of two subunits was clearly aligned along single filaments or associated with a loose filamentous web within the postsynaptic bouton (Figure 2a–g).
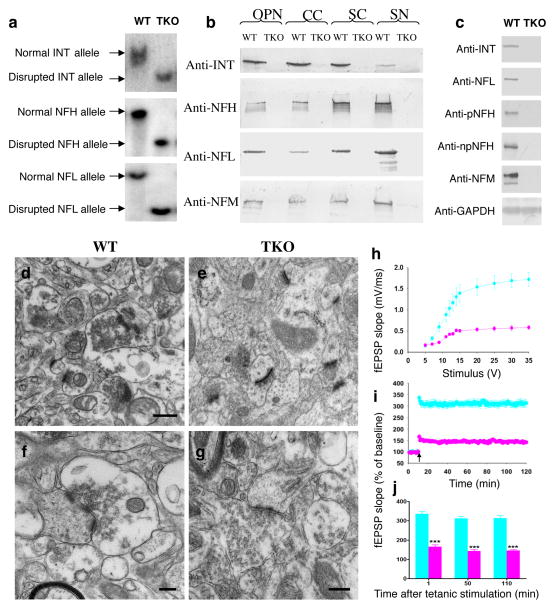
Figure 3.
Construction and characterization of NF triple knockout homozygous mice and impaired CA1 LTP in IHL-TKO mice. NF triple knockout homozygous mice were generated by cross-breeding α-internexin knockout mice with NFH knockout mice and then with NFL knockout mice. a. Southern blot analysis of genomic tail DNA digested with enzymes and hybridized with 32P-labeled probes. Targeted disruption of all three genes was confirmed. b. Western blot analysis of CNS and PNS nerve extracts of the triple knockout mice. Protein extracts prepared from optic nerves, corpus callosum, spinal cord and sciatic nerve were separated on a 10% SDS polyacrylamide gel and transferred to nitrocellulose membrane. Membranes were probed with different antibodies. Absence of the three proteins was confirmed with corresponding antibodies. Note that NFM was undetectable in optic nerves, corpus callosum, and spinal cord and limited (3% of normal level) in sciatic nerves. c. NFM was also undetectable in isolated hippocampal synaptosomes from TKO mice compared with wild-type mice. There was no neurodegeneration of synapses in the brain of TKO mice (e, g) compared with wild-type mice (d, f). OPN: optic nerve; CC: corpus callosum; SC: spinal cord; SN: sciatic nerve. TKO: α-internexin, NFH and NFL triple knockout mice. h, A summary graph showing the field I/O relationship for WT (light blue) and IHL-TKO (pink) mice. i, A time course of the average of the fEPSP slopes from slices obtained from WT and IHL-TKO mice. The fEPSP slopes were normalized to the average value during the 10 min prior to stimulation in each experiment. An arrow shows the time of tetanic stimulation (4 pulses at 100 Hz with bursts repeated at 5 Hz, and each tetanus including 3 10-burst trains separated by 15 s). j, A combined plot of the averages of fEPSP slopes at several time points. Each point is the mean ± SEM (n=5 mice per group, 12 slices per group). Two-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001. Scale bars, 500 nm in d, e, f and 200 nm in g.
Figure 2.
Distinct NF populations in synapses and axons. Ultrastructural colocalization of NFL and NFH (a, b, c) or NFL with NFM (d, e, f) on the same NF or on a filamentous web within the postsynaptic terminal by pre-embedding (a) or post-embedding immunoelectron microscopy (b–g). Paraformaldehyde-fixed samples were incubated with mouse anti-NFL or rabbit anti-NFH or NFM antibodies and probed with goat anti-mouse IgG and goat anti-rabbit IgG conjugated to 15 and 10 nm gold beads. a shows an enlarged spine containing NF decorated by both NFL (arrowheads) and NFH (arrows). b shows a filament decorated by only NFH (arrows). d shows a filament decorated by both NFL (arrowheads) and NFM (arrows). f shows two linear structures decorated by NFM (arrows) and one of them is in close contact with a vesicle (arrow). d shows a NF decorated by both NFL (arrowhead) and NFM (arrows) in an axon. Scale bars, 200nm in a; 100nm in b, c, d, e, g; 50nm in f. Pre, presynaptic terminal; Post, postsynaptic terminal. Western blot profiles of fractions 1–18 from 0–30% Optiprep gradient separation of hippocampal post-nuclear supernatant showing NF proteins are associated with synaptosomes (j). j Ponceau S stain and immunoblots stained with corresponding antibodies. Total NF (mainly axonal) and synaptosomal NF were isolated from striatum (i, j, k) or hippocampus (l, m, n) and quantified after immunoblotting with corresponding antibodies. (i) relative ratios of α-internexin to NFL in fractions of total NF and synaptosomal NF. j relative ratio of phosphorylated NFM (RMO55) to total NFM(RMO44) in total NF and synaptosomal NF. (k) relative ratio of phosphorylated NFH (SMI31) to non-phosphorylated NFH (SMI33) in total NF and synaptosomal NF. (l) relative ratios of α-internexin to NFL in fractions of total NF and synaptosomal NF. (m) relative ratio of phosphorylated NFM (RMO55) to total NFM(RMO44) in total NF and synaptosomal NF. (n) relative ratio of phosphorylated NFH (SMI31) to non-phosphorylated NFH (SMI33) in total NF and synaptosomal NF. (o) proportion of NFH relative to NFL increased in the synaptic pool compared to the total pool in striatum of MKO mice. (p) proportion of NFH relative to α-internexin increased in the synaptic pool compared to the total pool in striatum of MKO mice. (q) relative ratio of phosphorylated NFH (SMI31) to non-phosphorylated NFH (SMI33) in total NF and synaptosomal NF from striatum of MKO mice. ANT: α-internexin; PSD95: postsynaptic density protein 95; WT, wild-type; TKO, α-internexin, NFH and NFL triple knockout mice. IHL-TKO, α-internexin, NFH and NFL triple knockout mice. t-NF, total NF; s-NF, synaptic NF; MKO, NFM knockout mice.
In further confirmation of the immunoEM results, western blot profiles of fractions from 30% Optiprep gradients demonstrated that all four NF subunits co-fractionate in significant quantities with vesicular fractions containing the synaptosomal marker PSD95 (Figure 2h, fractions 9–14). Supporting the uniqueness of the NF subunit assemblies in synaptosomes, the ratio of α-internexin to NFL in synaptosomes (Supplementary Figure S3) from striatal tissue was significantly higher than that in Triton-insoluble NF fractions (p<0.05, n=3, Student’s t test) (Figure 2i). Moreover, we observed a significant difference in phosphorylation states of the long C-terminal tails of synaptic NFM and NFH compared to their counterparts in the total tissue (mainly axonal) NF population, as evidenced by significantly less phosphorylation of NFM (p<0.01, n=3, Student’s t-test) (Figure 2j) and greater phosphorylation of NFH (p<0.01, n=3, Student’s t-test) (Figure 2k). We confirmed that the same pattern exists in hippocampal synaptosomes (Figure 2l–n).
When NFM was deleted from mice, the proportion of NFH relative to NFL increased 107% in the synaptic pool compared to the total pool (p = 0.05, n=3, Student’s t test) while the NFH to α-internexin ratio showed a similar though non-significant trend (p =0.16, Student’s t test, n=3)(Figure 2o–q). Moreover, in the absence of NFM, phosphorylation of synaptic NFH increased relative to that of total (mainly axonal) NF (p=0.02, n=3, Student’s t test). These results indicate a differential effect of NFM loss on axonal and synaptic NF pools.
Elimination of neurofilaments impairs hippocampal LTP induction
IHL-TKO mice were further characterized and used to investigate the importance of NF proteins for synaptic function. In the absence of the three subunits from these mice, NFM was also undetectable in the CNS (Figure 3a,b), including in synaptosomal preparations from hippocampus (Figure 3c). Despite the complete absence of NF, CNS neurons maintain normal structural appearance with no evident neurodegenerative changes (Figure 3d–g). In electrophysiological studies, we determined the input/output (I/O) responses of fEPSP in the Schaffer collateral pathway of the hippocampal slices prepared from control and IHL-TKO animals. Increasing stimulus intensity evoked robust I/O responses of fEPSP in wild-type mice while the fEPSP slope was significantly reduced in IHL-TKO mice (Figure 3h). Before tetanic stimulations, the baseline fEPSP was recorded in 60 s intervals for 10 min with stimulation at an intensity equivalent to about 35% of the maximum evoked response. The tetanic stimulation 26, 27 evoked a typical LTP of fEPSP in slices from wild-type mice. These responses were stable over 120 min. However, tetanic stimulation evoked a significantly reduced fEPSP slopes in IHL-TKO slices (n =12 slices/5 mice/group, p<0.001, Two-way ANOVA with Bonferroni’s post hoc test) (Figure 3i–j). NF proteins are, therefore, required for synaptic plasticity. Consistent with this result IHL-TKO mice showed social interaction deficits using the 5-trial social memory test (Supplementary Figure S4), indicating that mice lacking NF proteins failed to develop normal social memory.
NFM specifically influences dopamine D1 receptor mediated behavior in vivo
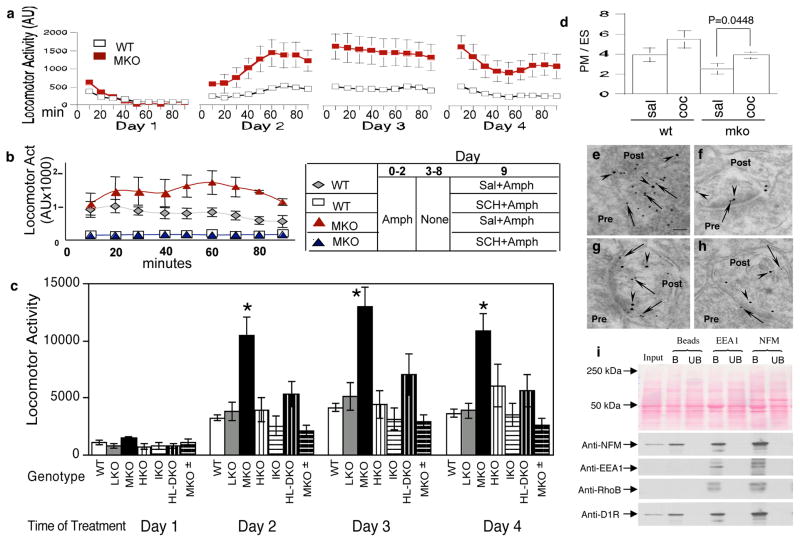
We evaluated the effect of genetically deleting the NFM subunit on a classic D1 receptor-mediated behavior -- locomotor activity response to cocaine administration. Baseline locomotor activity, calculated as total ambulatory counts over 14 hours of nighttime activity in 30-min segments, was 33% higher in MKO mice than in wild-type (WT) controls (25217±3058 vs 18861±3525, n=10, p=0.19, Student’s t test). To achieve sensitization to cocaine, we then injected the mice with saline on day one followed by daily cocaine injections (25 mg/kg) for 3 days. Activity measurements over the first 90 min after each administration showed that the repeated injections induced sensitization in both mouse groups, as evidenced by higher activity after the second cocaine injection compared with the first cocaine injection (Figure 4a). We obtained similar results after injecting amphetamine, another dopamine-releasing drug (Figure 4b). In analyses of the pooled data from 24 MKO and 66 wild-type controls, onset of a motor response was faster (within the first 10 min) after the second cocaine injection than after the first injection in both MKO (582 ± 132 vs 1636 ± 349, n=24, p=0.0024, Mann-Whitney test) and WT controls (237 ± 29 vs 536 ± 78, n=66, p =0.0002, Mann-Whitney test). Additional WT and MKO mice were later studied and the pooled data from 59 MKO and 157 WT mice showed a significantly higher locomotor activity in MKO mice than WT controls after cocaine injection on days 2, 3 and 4 (p<0.0001, Mann-Whitney test) (Figure 4a). We showed that these enhanced responses to stimulant drugs in MKO mice were D1R-mediated by measuring stimulant drug responses in the presence of the specific D1R antagonist SCH-23390. On days 1–3, MKO and wild-type mice received 4 mg/kg amphetamine; half of each group was also treated with 0.5 mg/kg SCH-23390 for 30 min before amphetamine. On day 9 (6-day abstinence), mice were challenged with 1 mg/kg amphetamine (no SCH-23390 given). In the absence of SCH-23390, MKO mice exhibited a higher degree of sensitization than wild-type controls; however, in SCH-23390-treated MKO and WT mice, the sensitization responses were markedly attenuated to the same low level (Figure 4b).
Figure 4.
Enhancement of cocaine-induced locomotor activity in MKO mice. Mice given a saline injection on day 1 followed by cocaine (25 mg/kg, sc) on days 2–4, were analyzed for locomotor activity during 90 min after each injection. Sensitization occurred in both MKO and wild-type controls after repeated injection as evidenced by higher activity after the second cocaine injection compared with the first cocaine injection (a). Statistical analyses of the pooled data from 24 MKO and 66 wild-type controls indicate faster onset of activity (the first 10 min) with the second compared with the first cocaine injection both in MKO (582 ± 132 vs 1636 ± 349, n=24, p=0.0024, Mann-Whitney Test) and in wild-type controls (237 ± 29 vs 536 ± 78, n=66, p=0.0002, Mann-Whitney test). Data are presented as mean ± SEM, n=24–66. Wild-type : open square; MKO: filled square. Locomotor activity was 2 to 3-fold higher than wild-type control levels (p<0.0001, Mann-Whitney test) in MKO mice. Dependence of increased locomotor responses in MKO mice on dopamine D1 receptor activation (b). Mice injected with 4 mg/kg amphetamine on days 1–3 were divided into two groups: one treated with 0.5 mg/kg D1 receptor antagonist SCH23390 30 min before amphetamine and the second given saline vehicle. On day 9 (6-day abstinence) each group of mice was challenged with 1 mg/kg amphetamine (no SCH23390 given) and locomotor activity was measured during a 90 min interval immediately after injection. MKO mice (no SCH23390, filled square) show more sensitization compared to wild-type (no SCH23390, open diamond). These sensitization responses were completely blocked by SCH23390-treatment in MKO (open square) and wild-type mice (filled diamond). Measurements are mean ± SEM from at least 8 animals for each group. (c) Enhanced cocaine-induced locomotor activity after deletion of NFM, but not NFL, NFH or α-internexin, from mice. Mice given a saline injection on day 1 followed by cocaine (25 mg/kg, sc) on days 2–4, were analyzed for locomotor activity during 90 min after each injection. Locomotor activity was 3-fold higher than wild-type control levels (p<0.0001, Mann-Whitney test, as indicated by stars) in mice lacking NFM but not significantly altered in mice lacking NFL, NFH or α-internexin or containing 1 gene copy of NFM. Measurements are mean ± SEM from at least 8 animals for each group. NFM depletion leads to recycling of the D1 receptor from endosomes to plasma membrane in response to cocaine administration (d). Plasma membrane-enriched and endosome-enriched fractions of mouse striata were isolated following subcutaneous injections of cocaine (25mg/kg/day) or saline for 7 days. Analysis of SCH23390 binding to synaptic membranes or endosomes showed increased D1R localization in striatal synaptosomal plasma membrane (PM) versus endosomes (ES) in response to cocaine in MKO mice (d). Measurements are mean ± SD (n=5, p=0.0448, Student’s t- test). sal: saline; coc: cocaine. Ultrastructural colocalization of D1R and NFM in the postsynaptic terminal by post-embedding immunogold electron microscopy (e–h). Paraformaldehyde-fixed samples were incubated with mouse anti-D1R and rabbit anti-NFM antibodies and probed with goat anti-mouse IgG and goat anti-rabbit IgG conjugated to 10 and 15 nm gold beads. D1R (arrows) was located both on the plasma membrane and inside postsynaptic terminal while NFM (arrowheads) was mainly inside the postsynaptic terminal and some in close contact with D1R (e, f). Co-immunoprecipitation of NFM and endosome markers (EEA1 and Rho B) with D1R from mouse synaptosomal preparations (i). EEA1, early endosome antigen 1; RhoB, Rho-related GTP-binding protein RhoB; B, bound; UB, unbound.
To determine whether or not these enhanced cocaine responses were selective for NFM among the four CNS NF subunits, we also analyzed mice lacking either NFH, NFL, or α-internexin 5. In contrast to MKO mice, these three mouse lines exhibited normal responses to cocaine (Figure 4c). Replacing half the normal level of NFM in MKO (MKO ±) mice by cross-breeding them with wild-type mice restored normal cocaine responses (Figure 4c), indicating that the effect on D1R-mediated locomotor behavior was related to NFM (Supplementary Figure S5) and could be achieved at half the normal NFM gene dosage.
In mice receiving daily cocaine injections for 7 days, p-ERK immunoreactivity was stronger in neurons of MKO mice than in WT controls (Supplementary Figure S6). HPLC-electrochemical detection of dopamine, serotonin, and their metabolites revealed no differences in levels between WT and MKO mice (Supplementary Figure S7), thus excluding a change in biogenic amine levels as an explanation for the enhancement of cocaine-induced locomotor activity in MKO mice. In addition, total D1R levels in striatum, determined by quantitative immunoblot analysis, were not significantly affected by NFM deletion (Supplementary Figure S8). To investigate this relationship in neurons in vivo in the context of cocaine sensitization, we isolated striata from 12 MKO and 12 WT mice injected daily for 7 days with saline or cocaine to induce sensitization and assesses D1R distribution within plasma membrane-enriched and endosome-enriched subcellular fractions. Western blot analyses of the plasma membrane marker ATPase or the endosome marker EEA1 confirmed the substantial enrichment of plasma membranes and endosomes, respectively (Supplementary Figure S9). As predicted, cocaine treatment caused more D1R to shift from endosomes (ES) to plasma membranes (PM) in MKO mice as demonstrated by SCH ligand binding (p<0.05, Student’s t- test) (Figure 4d). To further establish the relationship of D1R-positive endosomes to NFM, we performed immuno-gold double labeling of D1R and NFM, which demonstrated co-localization of D1R and NFM in postsynaptic boutons (Figure 4e–h). Moreover, in synaptosomal fractions, we could show that co-immunoprecipitation of NFM and endosomal markers also pulls down D1R further indicating in vivo interaction between NFM and D1R-containing endosomes (Figure 4i).
DISCUSSION
Although NF proteins have been detected in synaptic fractions and bound to synaptic proteins in vitro 7, they are usually considered to be axonal NF contaminants and their presence, let alone their possible function, in synapses has been controversial 11, 28. Using multiple independent approaches, we have shown unequivocally for the first time that a unique population of NF proteins is present and functional in synapses and relatively abundant in the postsynaptic area relative to terminal dendritic branches. NF proteins within the synapses not only exhibit different relative subunit ratios than the ones in axons, but they are also differently phosphorylated, indicating that NF proteins in synapses may serve different functions from those in axons. The relatively high proportions of α-internexin and lowered phosphorylation state of NFM in synaptic NF assemblies would be expected to confer greater plasticity to the cytoskeleton and allow increased interaction with binding partners.
Although NF proteins are present in both pre- and postsynaptic areas, 10nm NF polymers are not abundant in synaptic areas compared to myelinated axons, estimated by conventional electron microscopy. Using a rapid-freezing technique, Landis and Reese described infrequent 9–10nm filaments in dendritic spines of mouse brain (Figure 1l) but interpreted these structures as actin filaments due to their periodicity 29. NF also co-isolated with PSD 30 but have generally been regarded as contaminants 11. The infrequency of conventional long intermediate-sized polymers in synapses indicates that most NF subunits in synapses are probably in the form of oligomeric structures (protofilaments or protofibrils). Consistent with this interpretation, transport of NFM in non-filamentous, but oligomeric form, has been reported in vivo 10, 31. It is also possible that NF polymers in synapses are more susceptible than axonal NF to disassembly and /or degradation. Our findings that NF proteins within the synapses contain more α-internexin and a less phosphorylated form of NFM support the notion that NF proteins within the synaptic cytoskeleton network are in a relatively immature state as in developing neurons, making the cytoskeletal structure more dynamic and susceptible to disassembly. Because NFL and NFM monomers are inefficiently transported along axons 5, 10, NF proteins in synapses are at least oligomeric in form and can be transported as such at least in axons, providing one possible basis for their postsynaptic location. It is also possible that NF proteins in postsynaptic boutons are synthesized locally because mRNAs of NFL32, 33 and α-internexin34 together with mRNAs of β-actin35 and tubulin34 were reported to be present in dendrites and polyribosomes are preferentially localized under the base of dendritic spines36. Furthermore, BDNF-induced synaptic plasticity from hippocampal slices is blocked by inhibitors of protein synthesis and Schaffer-collateral CA1 synapses that were isolated from their pre- and postsynaptic cell bodies still exhibited protein synthesis-dependent plasticity, suggesting a local dendritic source of protein synthesis37. In this regard, evidence indicates that NF proteins identified in nerve terminals isolated from squid brain are generated by local protein synthesis 38. Therefore, by either mechanism, assembly states and ratios of NF subunits could be regulated by the local concentration and phosphorylation of individual subunits. In our triple knockout mice lacking α-internexin, NFH, and NFL, or a second mouse line lacking α-internexin, NFH and NFM (not shown), the content of NFM or NFL proteins in the CNS, respectively, is negligible suggesting that single subunits are unlikely to be a functional form of NF proteins in synapses. Collectively, our data suggest that a platform minimally of oligomeric NF assemblies or short 10nm NF polymers may be needed for function at synapses.
Our gene deletion studies demonstrated for the first time that lack of NFM leads to changes of D1R-mediated LTP and D1R-mediated behavior. Our findings are consistent with a model (Figure 5) in which NFM, within a synaptic cytoskeletal structure, anchors D1R-containing endosomes within synapses, thereby establishing a reservoir of receptors available for rapid recycling to the synaptic plasma membrane following dopamine agonist stimulation. Without NFM, recycling back to the surface is accentuated favoring hypersensitivity to D1R agonists. NFM is one of two neurofilament subunits with exceptionally long carboxyl-terminal tail domains but it seems to play a much more important role than its larger counterpart, NFH, in regulating the structure and function of neurofilaments in axons 5, 10. The diversity of roles for NFM in neuronal function is consistent with its complex regulation by phosphorylation, which involves second-messenger regulated kinase regulation of up to 36 sites on the head domain and 12 sites on the C-terminal tail of human NFM, which are regulated by at least four potential proline-directed kinases and multiple protein phosphatases 39–41. The D1 receptor has been shown to interact with the C-terminal tail of NFM and it will be important for future studies to address the role of phosphorylation of this domain in regulating D1R binding.
Figure 5.
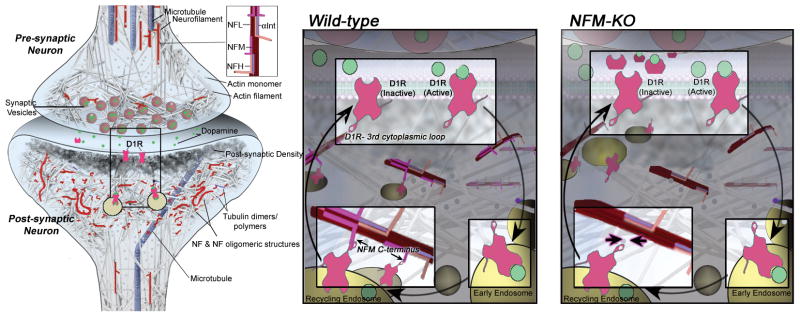
Model of D1R-containing endosomes anchored on NFM-containing cytoskeletal assemblies. Based on collective findings on NF scaffolding functions and our D1R data on NF subunit null mice, we propose a model by which NFM acts in synaptic terminals to anchor D1R-containing endosomes formed after agonist-induced internalization of membrane D1R. Retention of D1R in a readily available internal pool within the synapse would favor desensitization to D1R stimulation: in the absence of NFM, the greater recycling back to the plasma membrane surface would favor hypersensitivity to D1R agonists, as observed in our in vivo studies.
Our demonstrations of NFM involvement in D1R-mediated LTP and D1R-mediated behavior provide evidence that a unique population of NF assemblies is functional in synapses and that the observed effects on synaptic function are not a result of abnormal transport or abnormal effects on nerve conduction or axonal diameter. Only deletion of NFM but not other NF subunits markedly exaggerated D1R-mediated response to cocaine, even though deletions of NFL or NFH markedly alter caliber and conduction or conduction, respectively. Besides NFM, it might be predicted that α-internexin, NFH and NFL also have specific functions in synapses because deletion of all NFs has profound effects on synaptic plasticity and social memory. Supporting this view, our further observations also indicate that NFL selectively influences glutamate receptor function (Veeranna/Basavarajappa et al. unpublished data). Moreover, depressed hippocampal LTP induction is also NF subunit-selective: maintenance of LTP is deficient in NFH-null mice while basal neurotransmission and induction of LTP are normal in NFM-null mice (not shown).
Binding of NFM to D1R has been previously shown in non-neuronal cells of epithelial origin, although both NFM and D1R were over-expressed and foreign to these cultured human embryonic kidney cells 8, 9. Our study demonstrates in vivo functional significance of this molecular interaction at synapses. These findings strongly support the view that NF proteins are integral components of synapses, providing insight into the causes and functional significance of NF subunit alterations observed in neuropsychiatric diseases 21–23, 42, 43.
Supplementary Material
Acknowledgments
We thank Corrine Peterhoff for assistance with figures and Nicole Gogel for manuscript preparation. This work was supported by Grant 5R01AG005604 (R.A.N.) from the National Institutes on Aging. B.S.B. is supported by NIH grant (R01 AA019443).
Footnotes
AUTHOR CONTRIBUTIONS
A.Y., H.S., V, B.S.B., R.A.N. designed research; A.Y., H.S., V., B.S.B., A.K., A.H., M.B., J-H. L. and Y.S. performed research; A.Y., H.S., V., B.S.B., M.B., A.K., A.H., M.V.R, V. D., J-P.J. and R.A.N. analyzed data; P.S.M. provided reagents; V. M-Y. L. provided reagents and critical advice; A.Y. H.S. and R.A.N. wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp).
References
- 1.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970;167(4):379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong J, Tung VW, Aghajanian J, Xu Z. Antagonistic roles of neurofilament subunits NF-H and NF-M against NF-L in shaping dendritic arborization in spinal motor neurons. J Cell Biol. 1998;140(5):1167–1176. doi: 10.1083/jcb.140.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Casey DM, Julien JP, Xu Z. Normal dendritic arborization in spinal motoneurons requires neurofilament subunit L. J Comp Neurol. 2002;450(2):144–152. doi: 10.1002/cne.10306. [DOI] [PubMed] [Google Scholar]
- 5.Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, et al. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26(39):10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, et al. Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J Neurosci. 2012;32 (25):8501–8508. doi: 10.1523/JNEUROSCI.1081-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125(Pt 14):3257–3263. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers MD, Fung ET, O’Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18(2):720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22(14):5920–5930. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA. Neurofilament transport in vivo minimally requires hetero-oligomer formation. J Neurosci. 2003;23(28):9452–9458. doi: 10.1523/JNEUROSCI.23-28-09452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matus A, Pehling G, Ackermann M, Maeder J. Brain postsynaptic densities: the relationship to glial and neuronal filaments. J Cell Biol. 1980;87(2 Pt 1):346–359. doi: 10.1083/jcb.87.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beitner-Johnson D, Guitart X, Nestler EJ. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci. 1992;12(6):2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimon J. Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. Neuroreport. 1997;8(7):1561–1565. doi: 10.1097/00001756-199705060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sbarbati A, Bunnemann B, Cristofori P, Terron A, Chiamulera C, Merigo F, et al. Chronic nicotine treatment changes the axonal distribution of 68 kDa neurofilaments in the rat ventral tegmental area. Eur J Neurosci. 2002;16(5):877–882. doi: 10.1046/j.1460-9568.2002.02167.x. [DOI] [PubMed] [Google Scholar]
- 15.Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, et al. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18(11):4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16(15):4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8(2):212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 19.Rosengren LE, Karlsson JE, Sjogren M, Blennow K, Wallin A. Neurofilament protein levels in CSF are increased in dementia. Neurology. 1999;52(5):1090–1093. doi: 10.1212/wnl.52.5.1090. [DOI] [PubMed] [Google Scholar]
- 20.Bajo M, Yoo BC, Cairns N, Gratzer M, Lubec G. Neurofilament proteins NF-L, NF-M and NF-H in brain of patients with Down syndrome and Alzheimer’s disease. Amino Acids. 2001;21(3):293–301. doi: 10.1007/s007260170015. [DOI] [PubMed] [Google Scholar]
- 21.Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IR, et al. alpha-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol. 2004;164(6):2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9(12):3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- 23.Reines A, Cereseto M, Ferrero A, Bonavita C, Wikinski S. Neuronal cytoskeletal alterations in an experimental model of depression. Neuroscience. 2004;129(3):529–538. doi: 10.1016/j.neuroscience.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Hirao K, Hata Y, Deguchi M, Yao I, Ogura M, Rokukawa C, et al. Association of synapse-associated protein 90/ postsynaptic density-95-associated protein (SAPAP) with neurofilaments. Genes Cells. 2000;5(3):203–210. doi: 10.1046/j.1365-2443.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 25.Frappier T, Stetzkowski-Marden F, Pradel LA. Interaction domains of neurofilament light chain and brain spectrin. Biochem J. 1991;275 (Pt 2):521–527. doi: 10.1042/bj2750521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99(20):13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience. 2012;206:122–135. doi: 10.1016/j.neuroscience.2011.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouimet CC, da Cruz e Silva EF, Greengard P. The alpha and gamma 1 isoforms of protein phosphatase 1 are highly and specifically concentrated in dendritic spines. Proc Natl Acad Sci U S A. 1995;92(8):3396–3400. doi: 10.1073/pnas.92.8.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landis DM, Reese TS. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol. 1983;97(4):1169–1178. doi: 10.1083/jcb.97.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeGiorgis JA, Galbraith JA, Dosemeci A, Chen X, Reese TS. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biol. 2006;35(4–6):239–250. doi: 10.1007/s11068-007-9017-0. [DOI] [PubMed] [Google Scholar]
- 31.Terada S, Nakata T, Peterson AC, Hirokawa N. Visualization of slow axonal transport in vivo. Science. 1996;273(5276):784–788. doi: 10.1126/science.273.5276.784. [DOI] [PubMed] [Google Scholar]
- 32.Paradies MA, Steward O. Multiple subcellular mRNA distribution patterns in neurons: a nonisotopic in situ hybridization analysis. J Neurobiol. 1997;33(4):473–493. doi: 10.1002/(sici)1097-4695(199710)33:4<473::aid-neu10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17(6):1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 34.Villace P, Marion RM, Ortin J. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res. 2004;32(8):2411–2420. doi: 10.1093/nar/gkh552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, et al. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23(8):3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273(5280):1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 38.Crispino M, Capano CP, Kaplan BB, Giuditta A. Neurofilament proteins are synthesized in nerve endings from squid brain. Journal of neurochemistry. 1993;61 (3):1144–1146. doi: 10.1111/j.1471-4159.1993.tb03632.x. [DOI] [PubMed] [Google Scholar]
- 39.Sihag RK, Nixon RA. Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments. Evidence for regulation by second messenger-dependent protein kinases. J Biol Chem. 1990;265(7):4166–4171. [PubMed] [Google Scholar]
- 40.Sihag RK, Jaffe H, Nixon RA, Rong X. Serine-23 is a major protein kinase A phosphorylation site on the amino-terminal head domain of the middle molecular mass subunit of neurofilament proteins. Journal of neurochemistry. 1999;72(2):491–499. doi: 10.1046/j.1471-4159.1999.0720491.x. [DOI] [PubMed] [Google Scholar]
- 41.Pant HC, Rudrabhatla P. Topographic Regulation of Neuronal Intermediate Filament Proteins by Phosphorylation: In Health and Disease. In: Nixon RA, Yuan A, editors. Cytoskeleton of the Nervous System. Vol. 3. Springer New York; New York, NY: 2011. pp. 627–656. [Google Scholar]
- 42.Clark D, Dedova I, Cordwell S, Matsumoto I. Altered proteins of the anterior cingulate cortex white matter proteome in schizophrenia. Proteomics Clin Appl. 2007;1(2):157–166. doi: 10.1002/prca.200600541. [DOI] [PubMed] [Google Scholar]
- 43.Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13(12):1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.