Abstract
Objectives
To determine the extent to which initial therapy for nonmetastatic prostate cancer was concordant with nationally recognized guidelines using supplemented cancer registry data and what factors were associated with receipt of nonguideline-concordant care.
Methods
Initial therapy for 8229 nonmetastatic prostate cancer cases diagnosed in 2004 from cancer registries in 7 states was abstracted as part of the Centers for Disease Control’s Patterns of Care Breast and Prostate Cancer study conducted during 2007 to 2009. The National Comprehensive Cancer Network clinical practice guidelines version 1.2002 was used as the standard of care based on recurrence risk group and life expectancy (LE). A multivariable model was used to determine risk factors associated with receipt of nonguideline-concordant care.
Results
Nearly 80% with nonmetastatic prostate cancer received guideline-concordant care for initial therapy. Receipt of nonguideline-concordant care (including receiving either less aggressive therapy or more aggressive therapy than indicated) was related to older age, African American race/ethnicity, being unmarried, rural residence, and especially to being in the high recurrence risk group where receiving less aggressive therapy than indicated occurred more often than receiving more aggressive therapy (adjusted OR = 4.2; 95% CL, 3.5–5.2 vs. low-risk group). Compared with life table estimates adjusted for comorbidity, physicians tended to underestimate LE.
Conclusions
Receipt of less aggressive therapy than indicated among high-risk group men with >5-year LE based on life table estimates adjusted for comorbidity was a concern. Physicians may tend to underestimate 5-year survival among this group and should be alerted to the importance of recommending aggressive therapy when warranted. However, based on more recent guidelines, among those with low-risk disease, the proportion considered to be receiving less aggressive therapy than indicated may now be lower because active surveillance is now considered appropriate.
Keywords: prostate cancer, initial therapy, guideline care, cancer registry
Factors affecting initial prostate cancer treatment selection include life expectancy (LE), clinical stage, prostate-specific antigen (PSA) level, Gleason score, comorbidities1; effects of treatment on quality of life2; physician recommendations, and personal values.3 Treatment alternatives are commonly divided between conservative therapy (ie, androgen deprivation therapy [ADT], watchful waiting, and, more recently, active surveillance [AS]) and aggressive therapy (including radical prosta-tectomy [RP], external beam radiation therapy [EBRT], bra-chytherapy [BT], or combinations of these therapies).4
Because of the wide range of options for treatment and the role of patient and physician preference, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines for prostate cancer treatment are recommendations that “represent a current consensus regarding acceptable approaches to prostate cancer treatment, rather than a universally prescribed course of therapy” (NCCN 2002).5 The guideline goals are to optimize cancer survival while minimizing treatment-related morbidity, thus physicians may use independent judgment when developing treatment plans.
The Institute of Medicine (IOM) recommended that population-based cancer registries be used for assessing quality of cancer care and examining variations in standards of care.6 This is also an IOM goal for comparative effectiveness research.7 In response, the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) funded the Breast and Prostate Cancer Data Quality and Patterns of Care Study (POC-BP). One of the aims was to determine the extent to which care received was concordant with nationally recognized treatment guidelines and here we report on the findings related to prostate cancer treatment.
MATERIALS AND METHODS
Sample Selection and Data Collection
Conducted in 2007 to 2009, the POC-BP study included cases diagnosed in 2004 from 7 states, including 6 statewide cancer registries in Georgia, Kentucky, Louisiana, Minnesota, North Carolina, and Wisconsin, as well as regional registries in southern California (including Los Angeles and San Bernardino counties).8 The study involved supplementation of cancer registry data with reabstraction of medical records from hospitals and some outpatient facilities. Information on the demographic characteristics of the patient, clinical characteristics of the tumor, workup procedures, and the first course of cancer-directed treatment (ie, therapy that was given or planned at the time of initial cancer diagnosis, before disease recurrence or progression) was collected. The POC-BP data were linked with the 2000 US Census to obtain residential indicators. The study was approved or exempted by institutional review boards at participating institutions from the 7 states and the CDC.
From all participating registries, 11,679 cases of invasive prostate cancer (International Classification of Diseases for Oncology, 3rd ed, codes C61.9) were randomly selected across strata defined by race/ethnicity and state-specific factors such as Appalachian region, type of facility, or patient volume of the facility. Each case was the first primary cancer for the patient except for the previous diagnoses of basal or squamous cell carcinomas of the skin, benign brain tumors, or lymphohematopoietic malignancies diagnosed before 2001. Cases diagnosed at Veteran’s Administration hospitals were excluded,9 as were cases identified through death certificates or at autopsy, and those with a diagnosis of a subsequent cancer within 4 months. On the basis of records available when the reabstraction was done, 77.2% (9017/11,679) of the selected cases were reabstracted. About 13% of the reabstracted cases had missing data for ≥1 of the approximately 300 variables in the study; and when available, the original abstracted value was included for these missing values. After eliminating 33 who died within 6 months after diagnosis, 178 with missing stage information, and 577 with advanced disease, 8229 with nonmetastatic disease were included in these analyses. These cases included those with a T stage of T0, T1, or T2, excluding those with any positive lymph nodes or distant metastases.
Comorbidity Definition
The Adult Comorbidity Evaluation-27 (ACE-27) was selected to measure comorbidity burden because it is specific for cancer and has a dose-response relationship to survival.10–13 It is based on 26 comorbid conditions with 3 grades of decompensation (or severity) and excludes complications of cancer or its treatment. An overall comorbidity severity score (none, severe, moderate, or mild) was determined by the highest ranking single condition,11 unless the subject had at least 2 moderate comorbidities in different body systems when the grade was coded as severe.
Estimation of LE
Because LE is a major factor for determination of guideline care, we asked physicians to provide an estimate of the patient’s LE using intervals that were relevant to the NCCN guidelines (ie, <5, 5 to 9, 10 to 19, and 20 +). However, because physicians were not contacted for all patients or did not provide an estimate, this information was available for only 5.3% of the total. Thus, we determined the remaining years of life based on a man’s age at diagnosis from standard US life tables in 2004 for whites and African Americans,14 and adjusted it using the ACE-27 comorbidity severity score, similar to the method described in the NCCN guidelines.4 As the life table reflects the average number of remaining years of life among all men in the subgroup of the population it is based on, it is likely that those with better health would be expected to live longer than this average, whereas those with poorer health may be expected to live a shorter time than the average. To take a man’s health into account, we increased the number of remaining years obtained from the life table by multiplying it by 1.5 for those in the quartile with the lowest comorbidity severity scores (ie, those with the fewest comorbidities), and reduced life table estimate of remaining years by multiplying it by 0.5 for those in the highest quartile of the comorbidity severity scores (ie, those with the worst health). No change was made in the life table estimates for those with comorbidity severity scores in the middle 2 quartiles.
Definitions of Initial Therapies
Initial therapies received within 6 months after diagnosis were grouped into major categories to assess overall patterns of care, including RP (regardless of other therapy received), BT, EBRT (including 2D-XRT, 3D-CRT, and IMRT), combination of EBRT + BT ( ± ADT), primary ADT (PADT), and no therapy (NT) within the 6 months after diagnosis (AS, if there was specific mention of surveillance, watchful waiting, or monitoring and NT, otherwise).
A more detailed categorization used to assess guideline care was based on single versus combination therapies. Single therapies included: RP, EBRT, BT, radiotherapy type unknown (RTunk), PADT, and AS or NT. Combination therapies were defined as: RP + ADT, RP + EBRT/RTunk, RP + EBRT/RTunk + ADT, EBRT + ADT, BT + ADT, EBRT + BT, EBRT + BT + ADT, and RTunk + ADT.
Prostate Cancer Guideline Care Definition
The guidelines for therapy were based on the version 1.2002 of the NCCN Clinical Practice Guidelines in Oncology,4 which were in place in 2004 (Appendix 1). Guideline care was based on LE categories (< 5, 5 to 9, 10 to 19, and 20 + y) and NCCN risk of recurrence group (restricted to localized, nonmetastatic disease):
Recurrence risk low (T1 to T2a and Gleason score 2 to 6 and PSA < 10 ng/mL).
Recurrence risk intermediate (T2b to T2c OR Gleason score 7 OR PSA 10 to 20 ng/mL).
Recurrence risk high (T≤3 AND Gleason score 8 to 10 OR PSA > 20 ng/mL).
Receipt of guideline care was categorized as guideline concordant or guideline discordant which was categorized as follows: (1) had more aggressive therapy than indicated, (2) had less aggressive therapy than indicated, and (3) had PADT when the options were either observation or surgery/radiation therapy.
Demographic, Residential, and Clinical Variables
Specific categories for all variables are shown in Table 1. The demographic variables included age, race/ethnicity, marital status, and health insurance. Area-based measures were constructed from 2000 US Census data linked to the census tract of the patient’s residence and included percent of the population that was in an urban area, percent in the working class, percent below the federal poverty level, and percent of adults (above 25 y old) without a high school education. A 3-level socioeconomic status (SES) index was based on the poverty level and educational attainment variables: high (poverty level <20% and percent without a high school degree <25%), middle (with one of the indicators in these ranges), and low (with neither indicator in these categories).15 Clinical variables included clinical stage at diagnosis, PSA which was the highest value prior and closest to the initiation of treatment, Gleason score, comorbidity severity score, and NCCN Recurrence Risk Group as previously defined.
TABLE 1.
Demographic, Residential, and Clinical Characteristics of Men Diagnosed With Nonmetastatic Prostate Cancer, 2004, CDC Breast and Prostate Patterns of Care Study
| Variables | Total Sample | Weighted % |
|---|---|---|
| 8229 | 100.00 | |
| Demographic | ||
| Age group at diagnosis | ||
| < 60 | 2169 | 26.2 |
| 60–64 | 1444 | 17.5 |
| 65–69 | 1681 | 20.3 |
| 70–74 | 1390 | 17.1 |
| 75 + | 1545 | 18.8 |
| Race/ethnicity | ||
| White | 4822 | 73.6 |
| Black | 2444 | 17.4 |
| Hispanic | 570 | 6.5 |
| API/AI/AN | 393 | 2.5 |
| Marital status | ||
| Married | 5965 | 74.1 |
| Single/Divorced/Separated/Widowed | 1794 | 20.1 |
| Unknown | 470 | 5.8 |
| Insurance | ||
| Private | 4787 | 60.6 |
| Medicaid | 600 | 5.6 |
| Medicare, other public insurance | 2326 | 27.1 |
| None | 134 | 1.4 |
| Unknown | 382 | 5.3 |
| State | ||
| A | 1467 | 24.6 |
| B | 1729 | 16.3 |
| C | 494 | 7.4 |
| D | 1618 | 10.0 |
| E | 918 | 11.9 |
| F | 989 | 18.2 |
| G | 1014 | 11.6 |
| Residential (census tract) | ||
| Urbanization | ||
| Urban | 3985 | 50.4 |
| Rural | 1313 | 14.2 |
| Urban-rural mix | 2900 | 35.0 |
| Unknown | 31 | 0.4 |
| Working class | ||
| < 66% in working class | 3322 | 46.0 |
| 66 + % in working class | 4875 | 53.6 |
| Unknown | 32 | 0.4 |
| Poverty level | ||
| < 20% in poverty | 6185 | 82.4 |
| 20 + % in poverty | 2012 | 17.2 |
| Unknown | 32 | 0.4 |
| Educational attainment | ||
| < 25% without HS education | 5026 | 68.0 |
| 25 + % without HS education | 3171 | 31.6 |
| Unknown | 32 | 0.4 |
| Socioeconomic status | ||
| Low | 1776 | 15.1 |
| Middle | 1631 | 18.5 |
| High | 4790 | 65.9 |
| Unknown | 32 | 0.4 |
| Clinical variables | ||
| Comorbidity severity | ||
| None | 2667 | 34.1 |
| Mild | 4091 | 48.4 |
| Moderate | 887 | 10.4 |
| Severe | 309 | 3.8 |
| Unknown | 275 | 3.3 |
| Stage | ||
| Tx-T0 | 224 | 2.6 |
| T1 | 5275 | 64.1 |
| T2 | 2730 | 33.3 |
| PSA | ||
| < 4 | 901 | 11.7 |
| 4–10 | 4824 | 60.2 |
| > 10 | 2075 | 23.3 |
| Unknown | 429 | 4.8 |
| Gleason Score | ||
| 2–6 | 4249 | 52.8 |
| 7 | 2946 | 35.0 |
| 8–10 | 956 | 11.3 |
| Unknown | 78 | 0.9 |
| NCCN risk group | ||
| Low-complete data | 2966 | 38.1 |
| Low-incomplete data* | 273 | 3.2 |
| Intermediate | 3491 | 41.8 |
| High | 1499 | 16.9 |
T1-2a and either PSA < 10 OR Gleason Score <7 with the other variable unknown.
Statistical Methods
Both univariate and multivariable methods were used to identify factors associated with receipt of guideline care for initial therapy for those with nonmetastatic. The χ2 tests were used to determine univariate differences and multivariable logistic regression modeling was used to estimate significant independent predictors of use of guideline-discordant care, including variables that were significant at P < 0.10 from the univariate results. The results of the logistic regression analyses were expressed as adjusted odds ratios (ORs) with 95% confident limits (CL). All results were weighted based on the sampling fraction to provide results that represented the source population of the sampled cases using SAS Proc Survey-Logistic and SAS Proc SurveyFreq.
RESULTS
Just over a quarter of the prostate cases were younger than 60 years and between 17% and 20% were in each of the other age categories (Table 1). Nearly 3 quarters of subjects were non-Hispanic white (NHW), 17% were African American, over 6% were Hispanic, and <3% were Asian or other. Just over 60% had a form of private insurance (including Medicare + private), whereas 27% had Medicare alone or other public insurance. The proportions residing in each of the 7 states ranged from 7.4% to 24.6%. Half lived in an area classified as urban, and 15% lived in census tracts with the lowest SES ranking based on income and education.
Just over one third had no comorbidities. The most common stage at diagnosis was T1 (64%), 60% had a PSA between 4 and 10 ng/mL, and close to 53% had a Gleason score between 2 and 6. Over 41% of these nonmetastatic cases were in the low recurrence risk group, another 42% were in the intermediate risk group, and just under 17% were in the high-risk group.
LE
The effect of the comorbidity adjustment of LE varied by age group (Fig. 1). Among those below 60 years of age at diagnosis, 98% had a ≥20 years LE based on the standard life tables and after adjustment for comorbidity severity 96% did so. Among those 60 years old or over, the comorbidity adjustment resulted in no change or a greater proportion was shifted to an increased LE than to reduced LE.
FIGURE 1.
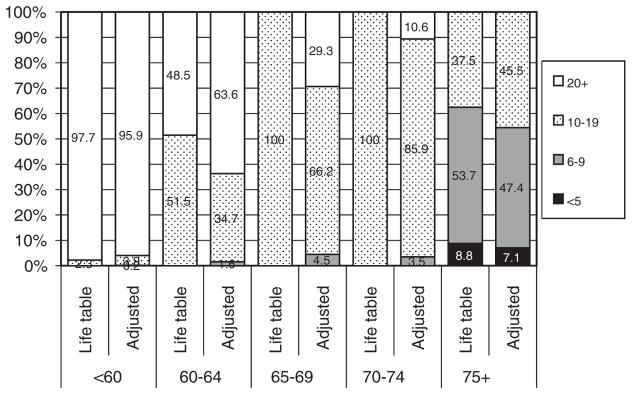
Percent distribution of years of life expectancy remaining calculated from life table and adjusted for comorbidity by age group at diagnosis: CDC POC BP Study.
We compared the physicians’ estimates of patient’s LE to the adjusted life table estimates for 476 cases and found low to moderate agreement beyond what would be expected by chance (κ = 0.28, percent agree = 50%) for the major categories required for NCCN treatment decisions (ie, <5 y, 5 to 9 y, 10 to 19, and 20 + y). Compared with the adjusted life table–based estimates, physicians were more likely to underestimate than to overestimate LE by a ratio of 2.5:1.
Initial Treatment
Among men with nonmetastatic prostate cancer, almost 80% received some form of aggressive therapy: 43.6% received RP (as a single therapy or in combination with other therapies), 19.9% received EBRT (alone), and 16.0% received BT (with or without EBRT) (Table 2). Those receiving conservative therapy included 6.9% with PADT, 4.7% with AS, and 9.0% with NT. Age at diagnosis was a major factor related to therapy choice, with RP being the most common therapy within each age group under 70. EBRT was the most common therapy among those 70 and over. Over 20% of men 65 to 74 years received BT, whereas nearly half of men 75 + years received a type of conservative therapy. NHW were the most likely to receive RP (46%), whereas African Americans were the least likely (36%). African Americans and Asians were more likely to receive EBRT than NHW and Hispanics. NHW had the highest proportion receiving BT (16.9%) compared with the other racial/ethnic groups.
TABLE 2.
Weighted Percent Distribution of Type of Initial Therapy Received for Men With Nonmetastatic Prostate Cancer by Demographic, Residential, and Clinical Characteristics; CDC Breast and Prostate Patterns of Care Study
| Selected Characteristics | Total (N [%])* | Type of Initial Therapy
|
|||||
|---|---|---|---|---|---|---|---|
| RP (%)* | EBRT (%)* | BT/BT + EBRT (%)* | PADT (%)* | AS (%)* | NT (%)* | ||
| All localized cases | 8229 (100.0) | 43.6 | 19.9 | 16.0 | 6.9 | 4.7 | 9.0 |
| Demographic variables | |||||||
| Age group | |||||||
| < 60 | 2169 (100.0) | 70.4 | 8.4 | 11.4 | 0.8 | 2.0 | 6.9 |
| 60–64 | 1444 (100.0) | 58.4 | 14.4 | 15.3 | 3.2 | 2.7 | 5.9 |
| 65–69 | 1681 (100.0) | 43.0 | 21.0 | 20.6 | 3.2 | 3.0 | 9.2 |
| 70–74 | 1390 (100.0) | 25.7 | 32.6 | 20.9 | 5.8 | 3.9 | 11.1 |
| 75 + | 1545 (100.0) | 9.5 | 28.0 | 13.8 | 23.6 | 12.7 | 12.4 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 4822 (100.0) | 46.0 | 18.5 | 16.9 | 6.1 | 4.8 | 7.7 |
| African American | 2444 (100.0) | 35.6 | 24.5 | 15.5 | 9.0 | 3.8 | 11.6 |
| Hispanic | 570 (100.0) | 39.0 | 21.9 | 8.4 | 10.2 | 5.2 | 15.2 |
| Asian/other | 393 (100.0) | 40.3 | 24.6 | 13.3 | 4.8 | 5.9 | 10.9 |
| Marital status | |||||||
| Married | 5965 (100.0) | 46.4 | 19.5 | 16.7 | 5.1 | 4.5 | 7.8 |
| Single/Divorced/Separated/Widowed | 1794 (100.0) | 35.0 | 22.5 | 16.2 | 10.4 | 4.4 | 11.5 |
| Unknown | 470 (100.0) | 37.2 | 16.1 | 6.5 | 17.3 | 7.8 | 15.0 |
| State | |||||||
| A | 1467 (100.0) | 41.2 | 22.6 | 9.9 | 8.0 | 4.9 | 13.3 |
| B | 1729 (100.0) | 33.9 | 22.3 | 28.2 | 4.0 | 2.9 | 8.8 |
| C | 494 (100.0) | 49.0 | 19.8 | 14.0 | 5.6 | 2.9 | 8.6 |
| D | 1618 (100.0) | 37.7 | 18.0 | 17.2 | 12.8 | 5.5 | 8.7 |
| E | 918 (100.0) | 55.1 | 14.0 | 12.0 | 6.1 | 6.9 | 5.9 |
| F | 989 (100.0) | 44.4 | 22.0 | 16.8 | 5.6 | 5.3 | 6.0 |
| G | 1014 (100.0) | 51.1 | 15.0 | 15.0 | 6.8 | 3.9 | 8.3 |
| Insurance coverage | |||||||
| Private | 4787 (100.0) | 51.7 | 15.8 | 16.2 | 4.0 | 3.8 | 8.4 |
| Medicaid | 600 (100.0) | 31.5 | 21.9 | 12.7 | 15.5 | 3.6 | 14.7 |
| Medicare, other public | 2326 (100.0) | 28.2 | 27.9 | 17.5 | 10.7 | 6.9 | 8.8 |
| None | 134 (100.0) | 49.0 | 23.0 | 7.9 | 8.8 | 3.0 | 8.3 |
| Unknown | 382 (100.0) | 41.3 | 22.5 | 11.3 | 10.4 | 4.8 | 9.6 |
| Residential variables | |||||||
| Urban/rural residence* | |||||||
| 100% urban | 3985 (100.0) | 43.5 | 19.1 | 15.3 | 6.8 | 5.0 | 10.2 |
| 100% rural | 1313 (100.0) | 39.2 | 23.1 | 15.5 | 7.7 | 5.6 | 8.9 |
| Rural/urban mix | 2900 (100.0) | 45.8 | 19.7 | 17.3 | 6.6 | 3.7 | 6.8 |
| Working CLASS | |||||||
| < 66% in working class | 3322 (100.0) | 48.2 | 17.5 | 16.5 | 5.0 | 4.6 | 8.1 |
| 66% + in working class | 4875 (100.0) | 39.8 | 21.9 | 15.6 | 8.5 | 4.7 | 9.4 |
| Poverty level | |||||||
| < 20% below poverty level | 6185 (100.0) | 46.1 | 19.0 | 16.4 | 5.8 | 4.6 | 8.1 |
| 20 + % in poverty level | 2012 (100.0) | 32.1 | 24.2 | 14.6 | 11.8 | 5.1 | 12.2 |
| Educational attainment | |||||||
| < 25% without HS education | 5026 (100.0) | 48.1 | 17.6 | 16.4 | 5.3 | 4.9 | 7.7 |
| 25 + % without HS education | 3171 (100.0) | 34.2 | 24.8 | 15.2 | 10.3 | 4.2 | 11.2 |
| Socioeconomic status | |||||||
| High | 4790 (100.0) | 48.3 | 17.6 | 16.5 | 5.2 | 4.8 | 7.6 |
| Middle | 1631 (100.0) | 37.9 | 23.3 | 15.4 | 8.5 | 4.2 | 10.6 |
| Low | 1776 (100.0) | 30.7 | 25.4 | 14.7 | 12.3 | 4.7 | 12.2 |
| Clinical variables | |||||||
| Comorbidity severity | |||||||
| None | 2667 (100.0) | 52.2 | 15.5 | 14.2 | 5.6 | 4.0 | 8.5 |
| Mild | 4091 (100.0) | 41.6 | 21.5 | 18.3 | 5.8 | 4.3 | 8.4 |
| Moderate | 887 (100.0) | 37.1 | 25.7 | 15.2 | 7.7 | 6.1 | 8.2 |
| Severe | 309 (100.0) | 29.2 | 20.4 | 14.8 | 11.6 | 9.9 | 14.0 |
| Unknown | 275 (100.0) | 22.2 | 22.3 | 4.7 | 26.4 | 6.5 | 17.8 |
| Stage | |||||||
| Tx-T0 | 224 (100.0) | 59.3 | 7.4 | 1.0 | 16.8 | 1.0 | 14.4 |
| T1 | 5275 (100.0) | 42.4 | 19.6 | 17.8 | 5.2 | 5.1 | 10.0 |
| T2 | 2730 (100.0) | 44.7 | 21.5 | 13.8 | 9.4 | 4.2 | 6.4 |
| PSA group | |||||||
| < 4 | 901 (100.0) | 55.8 | 11.9 | 13.5 | 3.0 | 5.8 | 10.1 |
| 4–10 | 4824 (100.0) | 46.7 | 18.9 | 19.8 | 3.6 | 4.4 | 6.6 |
| > 10 | 2075 (100.0) | 29.8 | 29.1 | 10.0 | 17.5 | 4.3 | 9.3 |
| Unknown | 429 (100.0) | 41.9 | 7.0 | 3.8 | 5.8 | 6.4 | 35.0 |
| Gleason Score group | |||||||
| 2–6 | 4249 (100.0) | 39.5 | 18.3 | 20.5 | 4.6 | 6.4 | 10.6 |
| 7 | 2946 (100.0) | 52.0 | 20.6 | 11.9 | 6.8 | 2.5 | 6.2 |
| 8–10 | 956 (100.0) | 39.6 | 25.7 | 7.5 | 17.7 | 2.0 | 7.5 |
| Unknown | 78 (100.0) | 7.6 | 10.7 | 17.7 | 8.8 | 19.8 | 35.4 |
| NCCN risk group | |||||||
| Low complete | 2966 (100.0) | 42.7 | 15.8 | 23.0 | 2.9 | 6.5 | 9.0 |
| Low missing† | 273 (100.0) | 27.9 | 5.3 | 7.3 | 3.2 | 12.4 | 43.9 |
| Intermediate | 3491 (100.0) | 49.4 | 21.8 | 13.7 | 5.4 | 3.2 | 6.5 |
| High | 1499 (100.0) | 34.3 | 27.1 | 7.6 | 19.9 | 2.7 | 8.4 |
Weighted percentages.
T1-2a and either PSA < 10 OR Gleason Score <7 with the other variable unknown.
AS indicates active surveillance; BT, brachytherapy; EBRT, external beam radiation therapy; NT, no therapy; PADT, primary androgen deprivation therapy; RP, radical prostatectomy.
The proportion receiving RP varied from 55% to 34% among the 7 states participating, whereas use of BT ranged from 28.2% to 9.9%. Men living in census tracts with the high SES index were more likely to have RP, whereas use of PADT or NT occurred more often in the low SES tracts.
Among the clinical variables, those with none or lower comorbidity severity, lower T stage, and lower PSA group were more likely to receive RP than those within higher levels of these variables. However, those with a Gleason score of “7” were more likely to receive surgery compared with those with lower or higher scores, as were those in the intermediate NCCN risk group compared with those in the low-risk or high-risk groups. There were contrasting patterns for the use of EBRT and BT (alone or with EBRT). Those with clinically more advanced disease (based on stage, PSA, Gleason score, and risk group) were more likely to receive EBRT than those within lower levels of these variables. BT was more often used among the low-risk group than in the high-risk group (23% vs. 7.6%). Similarly, PADT was used more often among those with higher PSA, Gleason score, and risk group categories, whereas AS and NT were more often used among those within lower categories of these variables.
Assessment of Receipt of Guideline Care for Initial Therapy
After applying the criteria used to assess receipt of NCCN guideline care based on risk group and LE, 79.2% of men with nonmetastatic disease received guideline-concordant care, 6.3% had more aggressive therapy than indicated, 12.5% had less aggressive therapy than indicated, and 2.0% received PADT when it was not indicated (Table 3).
TABLE 3.
Percent Receiving NCCN Guideline Care for Initial Treatment for Nonmetastatic Prostate Cancer by Type of Treatment Received: CDC Breast and Prostate Patterns of Care Study
| Total (N [%]) | NCCN Guideline-concordant Care Status (N [%])*
|
||||
|---|---|---|---|---|---|
| Guideline Concordant | Nonguideline Concordant
|
||||
| Had More Aggressive Thx Than Indicated | Had Less Aggressive Thx Than Indicated | Had PADT When AS/NT or RP/EBRT Indicated | |||
| All localized cases | 8229 (100.0) | 6373 (79.2) | 539 (6.3) | 1132 (12.5) | 185 (2.0) |
| Single therapies (all) | 5953 (100.0) | 4595 (79.6) | 41 (0.7) | 1132 (17.0) | 185 (2.7) |
| AS/NT | 1135 (100.0) | 501 (45.4) | — | 634 (54.6) | — |
| RP (only) | 2882 (100.0) | 2859 (99.1) | 23 (0.9) | — | — |
| EBRT (only) | 713 (100.0) | 665 (94.0) | 1 (0.1) | 47 (5.8) | — |
| BT (only) | 541 (100.0) | 524 (97.2) | 17 (2.8) | — | — |
| RT type unknown (only) | 22 (100.0) | 20 (81.0) | 2 (19.0) | — | — |
| PADT | 660 (100.0) | 26 (3.1) | — | 449 (68.2) | 185 (28.6) |
| Combination therapies (all) | 2276 (100.0) | 1778 (78.1) | 498 (21.9) | — | — |
| RP + ADT | 293 (100.0) | 290 (99.3) | 3 (0.7) | — | — |
| RP + EBRT/BT/RT unknown ± ADT | 198 (100.0) | — | 198 (100.0) | — | — |
| EBRT + BT | 333 (100.0) | 157 (47.9) | 176 (52.1) | — | — |
| EBRT + BT + ADT | 235 (100.0) | 134 (59.6) | 101 (40.4) | — | — |
| EBRT + ADT | 932 (100.0) | 929 (99.6) | 3 (0.4) | — | — |
| BT + ADT | 264 (100.0) | 247 (94.2) | 17 (5.8) | — | — |
| RT type unknown + ADT | 21 (100.0) | 21 (100.0) | — | — | — |
Weighted percentages.
ADT indicates androgen deprivation therapy; AS, active surveillance; BT, brachytherapy; EBRT, external beam radiation therapy; NT, no therapy; PADT, primary androgen deprivation therapy; RP, radical prostatectomy.
When RP ( ± ADT) was received as a sole therapy for localized disease, 99% of the time it was considered concordant with NCCN guidelines; however, it was considered more aggressive treatment than indicated when combined with radiation therapy (Table 3). Similarly, sole use of EBRT or BT ( ± ADT) was considered guideline concordant for 94% to 97% of those receiving it; however, the combination of EBRT + BT ( ± ADT) which was only recommended for those with intermediate risk, was considered more aggressive treatment than indicated for close to half of the patients receiving both of these therapies. Most guideline-discordant care occurred among those receiving PADT with 68.2% receiving less aggressive therapy than indicated and 28.6% receiving it when either AS/NT or RP/EBRT was indicated. In addition, among those with AS/NT, 54% were deemed to have received less aggressive therapy than indicated.
On the basis of a multivariable logistic regression model, age at diagnosis, race/ethnicity, marital status, urban/rural residence, comorbidity severity, and NCCN risk group were independent predictors of guideline-discordant care (Table 4). Men 75 years of age or older were over twice as likely to receive guideline-discordant care (especially less aggressive therapy than indicated) than those under the age of 60 (adjusted OR, 2.3; 95% CL, 1.9–2.9). African American and unmarried men were 40% more likely to receive guideline-discordant care than men in their respective reference groups. Those in the intermediate risk group were less likely to receive guideline-discordant care compared with low-risk group men (adjusted OR, 0.8; 95% CL, 0.7–0.9); however, men in the high-risk group were over 4 times as likely to receive guideline-discordant care (particularly less aggressive therapy) compared with those in the low-risk group (adjusted OR, 4.2; 95% CL, 3.5–5.2).
TABLE 4.
Weighted Percent Receiving NCCN Guideline Care for Nonmetastatic Prostate Cancer (N = 8232) by Selected Characteristics and Adjusted ORs for Nonguideline Care: CDC Breast and Prostate Patterns of Care Study
| Selected Characteristics | Total (N [%]) | NCCN Guideline-concordant Care Status
|
Adjusted ORs for Receiving Any Nonguideline Care for Localized Disease†
|
||||
|---|---|---|---|---|---|---|---|
| Guideline Concordant | Nonguideline-concordant Care (N [%])*
|
||||||
| Had More Aggressive Thx Than Indicated | Had Less Aggressive Thx Than Indicated | Had PADT When AS/NT or RP/ EBRT Indicated | Adjusted OR | 95% CL | |||
| Age group | |||||||
| < 60 | 2169 (100.0) | 1776 (83.1) | 165 (7.7) | 228 (9.2) | — | 1.0 | Reference |
| 60–64 | 1444 (100.0) | 1200 (84.3) | 107 (6.7) | 133 (8.9) | 4 (0.2) | 0.9 | 0.7–1.1 |
| 65–69 | 1681 (100.0) | 1370 (83.5) | 113 (6.2) | 185 (9.8) | 13 (0.5) | 0.9 | 0.7–1.1 |
| 70–74 | 1390 (100.0) | 1068 (79.4) | 92 (6.4) | 207 (13.0) | 23 (1.2) | 1.1 | 0.9–1.4 |
| 75 + | 1545 (100.0) | 959 (64.3) | 62 (4.0) | 379 (23.0) | 145 (8.7) | 2.3 | 1.9–2.9 |
| Race/ethnicity | |||||||
| Non-Hispanic White | 4822 (100.0) | 3855 (81.0) | 318 (6.4) | 540 (10.6) | 109 (2.0) | 1.0 | Reference |
| African American | 2444 (100.0) | 1774 (73.5) | 178 (7.2) | 434 (17.2) | 58 (2.1) | 1.4 | 1.2–1.7 |
| Hispanic | 570 (100.0) | 423 (73.2) | 23 (3.3) | 108 (21.2) | 16 (2.3) | 1.3 | 0.9–1.8 |
| Asian/Other | 393 (100.0) 100.0 |
321 (81.4) | 20 (4.6) | 50 (13.46) | 2 (0.5) | 0.8 | 0.6–1.2 |
| Marital status | |||||||
| Married | 5965 (100.0) | 4787 (81.9) | 417 (6.5) | 663 (10.2) | 98 (1.4) | 1.0 | Reference |
| Single/Divorced/Separated/Widowed | 1794 (100.0) | 1307 (73.6) | 104 (6.4) | 327 (17.2) | 56 (2.8) | 1.4 | 1.2–1.7 |
| Unknown | 470 (100.0) | 279 (64.9) | 18 (3.3) | 142 (25.8) | 31 (6.0) | 2.5 | 1.9–3.4 |
| Registry | |||||||
| A | 1467 (100.0) | 1106 (76.4) | 66 (4.8) | 270 (16.8) | 25 (1.9) | 1.0 | Reference |
| B | 1729 (100.0) | 1281 (75.4) | 240 (14.0) | 183 (9.4) | 25 (1.2) | 1.3 | 0.9–1.6 |
| C | 494 (100.0) | 419 (85.0) | 20 (4.1) | 48 (9.5) | 7 (1.4) | 0.6 | 0.5–0.9 |
| D | 1618 (100.0) | 1209 (75.7) | 66 (4.2) | 276 (16.1) | 67 (4.0) | 0.9 | 0.7–1.2 |
| E | 918 (100.0) | 747 (82.6) | 53 (6.0) | 96 (8.9) | 22 (2.4) | 0.6 | 0.5–0.9 |
| F | 989 (100.0) | 807 (82.8) | 54 (4.8) | 111 (10.7) | 17 (1.6) | 0.7 | 0.5–0.9 |
| G | 1014 (100.0) | 804 (80.7) | 40 (4.4) | 148 (13.0) | 22 (1.9) | 0.9 | 0.7–1.2 |
| Urban/rural residence | |||||||
| 100% urban | 3985 (100.0) | 3097 (78.8) | 229 (5.7) | 586 (13.6) | 73 (1,9) | 1.0 | Reference |
| 100% rural | 1313 (100.0) | 988 (77.3) | 104 (7.0) | 192 (13.8) | 29 (1.8) | 1.3 | 1.1–1.6 |
| Rural/urban mix | 2900 (100.0) | 2267 (80.7) | 204 (6.8) | 347 (10.2) | 82 (2.2) | 1.1 | 0.9–1.3 |
| Comorbidity severity | |||||||
| None | 2667 (100.0) | 2035 (77.8) | 164 (6.1) | 443 (15.1) | 25 (1.0) | 1.0 | Reference |
| Mild | 4091 (100.0) | 3257 (81.7) | 279 (6.3) | 469 (10.0) | 86 (2.0) | 0.7 | 0.6–0.8 |
| Moderate | 887 (100.0) | 700 (80.4) | 53 (6.0) | 112 (11.4) | 22 (2.2) | 0.7 | 0.5–0.9 |
| Severe | 309 (100.0) | 229 (75.2) | 34 (12.1) | 23 (7.5) | 23 (5.3) | 1.0 | 0.6–1.5 |
| NCCN risk group | |||||||
| Low complete | 2966 (100.0) | 2468 (84.3) | 232 (7.3) | 179 (5.9) | 87 (2.5) | 1.0 | Reference |
| Low missing‡ | 273 (100.0) | 220 (78.1) | 7 (2.2) | 37 (16.9) | 9 (2.7) | 1.2 | 0.8–1.9 |
| Intermediate | 3491 (100.0) | 2934 (85.4) | 81 (2.3) | 387 (10.1) | 89 (2.3) | 0.8 | 0.7–0.9 |
| High | 1499 (100.0) | 751 (52.7) | 219 (14.7) | 529 (32.6) | — | 4.2 | 3.5–5.2 |
Weighted percentages.
Model includes all variables listed in the table.
T1-2a and either PSA < 10 OR Gleason Score <7 with the other variable unknown.
Values in bold indicate significant results.
AS indicates active surveillance; CL, confident limit; EBRT, external beam radiation therapy; NT, no therapy; OR, odds ratio; PADT, primary androgen deprivation therapy; RP, radical prostatectomy.
DISCUSSION
Overall we found that the NCCN guidelines in place in 2004 were broad enough so that nearly 80% of nonmetastatic cases were guideline concordant in initial treatment, based on recurrence risk group and estimated LE. Among the non-metastatic cases deemed guideline discordant, the majority received less aggressive therapy than indicated. Although these guidelines define a consensus of expert opinion for treatment, they may not always provide the best approach for treating individual patients who may have preferences related to quality of life and specific treatment effects.16 Men may wish to avoid the substantial treatment effects associated with aggressive therapy and there is lack of definitive evidence regarding the survival benefit of treatment. Recently 15-year survival data from the Prostate Cancer Outcomes Study (PCOS) showed that men older than 60, with low or intermediate risk disease and >3 comorbidities, would have a higher probability of death from other causes and would not have a survival benefit from receiving surgery or radiation.17 Likewise, a study based on Medicare data found that a greater number of comorbidities were associated with decreased survival with adjustment for treatment having little impact.18 In contrast, a study based on Surveillance, Epidemiology, and End Results (SEER)-Medicare data for men diagnosed between 1991 and 1999 found a survival benefit associated with surgery or radiation therapy among men with low/intermediate risk disease, using a propensity score to adjust for confounding factors, including comorbidities.19
If a man has high-risk disease and 5 + years of LE, aggressive therapy is usually considered. PCOS data were used to compare long-term quality of life differences between surgery and radiation and found that there were no statistically significant differences after 15 years of follow-up.20 However, additional analyses of this same dataset, also using propensity scores to adjust for confounding factors, suggest that surgery may be associated with a better long-term survival than radiation therapy, especially among younger men.21
The overall patterns of care for nonmetastatic prostate cancer that we observed in this registry-based study involving 7 states in 2004 were very similar to a study of 14 SEER program registries in 2002.22 In each study the proportion of men treated by RP was close to 44%, 20% received EBRT, and 16% to 18% received BT ( ± EBRT). We found a slightly lower proportion receiving PADT (7% vs. 8.5%) and a higher proportion with AS/NT (13.7% vs. 9.0%).
We identified important disparities in the receipt of guideline care for nonmetastatic disease, particularly among those in the high recurrence risk group who were over 4 times as likely to receive guideline-discordant care (especially less aggressive therapy) compared with those in lower recurrence risk groups, and among older men 75 + who were over twice as likely to receive guideline-discordant care, compared with those below 60 years of age. In addition, African American men were 40% more likely to receive guideline-discordant care compared with white men, which was also indicated in the previous SEER-based study.22
There have been significant changes in the NCCN guidelines since 2004.23 Among low recurrence risk men, the percentage with guideline-concordant care would likely be higher now because AS is now an option for low-risk men with 10 + years of LE and 20% of those classified as receiving less aggressive therapy than indicated would now be guideline concordant. Likewise the combination of EBRT + BT for high-risk men is now considered guideline-concordant care, and 77% of those men categorized as receiving more aggressive therapy than indicated would be reclassified to guideline concordant.
One of the most critical criteria for determining NCCN guideline care is estimated LE. Among a small sample, we found that physicians were more likely to underestimate remaining years of life than to overestimate them compared with the life table estimates adjusted for comorbidity scores. Other studies have indicated that life table estimates, even when adjusted for comorbidity, tend to overestimate LE, whereas physician estimates may be more accurate,24 especially in determining if the patient has under or over 10 years to live.25 The accuracy of physician estimates of ± 5 years, however, is less certain. If physicians are underestimating 5-year survival, some men with high recurrence risk may not be receiving appropriate therapy. The subgroup of most concern was the nonmetastatic cases within the high recurrence risk group, where 32.6% received less aggressive therapy than indicated. These were cases with a >5-year LE (adjusted life table estimate) who received AS/NT or PADT, when aggressive therapy was indicated.
Limitations of this study include the sole use of inpatient medical records for some cases, when outpatient records were not available or were not sought when guideline care was determined from hospital records. Care received in the out-patient setting, particularly use of hormones and radiation therapy, has been shown to be underreported in registry data compared with claims information.26 In addition, despite the efforts to enhance registry data, men classified as receiving NT may be missing data on therapy received.27 The results are limited to the 2004 diagnosis year and thus do not reflect current guideline-based care. However, future efforts to use registry data to assess guideline care may be hampered by lack of data necessary to identify very low-risk disease (eg, number of positive biopsy cores, maximum percent positive within a core, and PSA density)27 although this information may be available through linkage with other databases. Finally, estimates of LE based on life tables and comorbidity may result in overestimates of LE compared with physician estimates.
In summary, we found that the majority of men with nonmetastatic disease were treated according to consensus-based NCCN guideline standards, and although the proportion may be even higher if current standards were applied, the disparities in guideline treatment that we found may be indicative of subgroups at higher risk of receiving guideline-discordant care in general. Receipt of less aggressive therapy than indicated among high-risk group men with >5-year LE based on life table estimates adjusted for comorbidity was a concern. Physicians may tend to underestimate 5-year survival among this group and should be alerted to the importance of recommending aggressive therapy when warranted.. It would be important to assess physician accuracy at estimating 5-year survival in comparison with actual survival experience. Although these standards are based on a consensus of expert opinion, the final decision for treatment of a man with prostate cancer will ultimately depend on personal preferences and his specific clinical characteristics.
Acknowledgments
The Breast and Prostate Cancer Data Quality and Patterns of Care Study was supported by the Centers for Disease Control and Prevention through cooperative agreements with the California Cancer Registry (Public Health Institute) (1-U01-DP000260), Emory University (1-U01-DP000258), Louisiana State University Health Sciences Center (1-U01-DP000253), Minnesota Cancer Surveillance System (Minnesota Department of Health) (1-U01-DP000259), Medical College of Wisconsin (1-U01-DP000261), University of Kentucky (1-U01-DP000251), and Wake Forest University (1-U01-DP000264).
APPENDIX 1
NCCN Guidelines for initial treatment (Source: Version 1.2002 of the NCCN Clinical Practice Guidelines in Oncology).
Initial Therapy
The recommended initial therapy is based on recurrence risk and LE.
-
Recurrence risk low (T1 to T2a and Gleason score 2 to 6 and PSA < 10 ng/mL).
Estimation of LE
Less than 10 years: Observation or radiation therapy (3D-CRT or BT).
10 to 20 years: Observation or radiation therapy (3D-CRT or BT) or radical prostatectomy with/without pelvic lymph node dissection.
> 20 years: Radical prostatectomy with/without pelvic lymph node dissection or radiation therapy (3D-CRT or BT).
-
Recurrence risk intermediate (T2b to T2c OR Gleason score 7 OR PSA 10 to 20 ng/mL).
Estimation of LE
Less than 10 years: Observation or radiation therapy (3D-CRT and/or BT) or radical prostatectomy with/without pelvic lymph node dissection.
> 10 years: Radical prostatectomy with/without pelvic lymph node dissection or radiation therapy (3D-CRT and/or BT).
-
Recurrence risk high (localized) (T≤3 AND Gleason score 8 to 10 OR PSA > 20 ng/mL).
Estimation of LE
Less than 5 years: Observation or androgen ablation.
> 5 years: Androgen ablation (2 to 3 y) + radiation therapy (3D-CRT) OR radiation therapy (3D-CRT) for selected patients with Gleason score of <7 and PSA < 10 ng/mL OR radical prostatectomy with pelvic lymph node dissection for selected patients with low volume and no fixation.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declare no conflicts of interest.
References
- 1.Scherr D, Swindle PW, Scardino PT National Comprehensive Cancer Network. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61(suppl 1):14–24. doi: 10.1016/s0090-4295(02)02395-6. [DOI] [PubMed] [Google Scholar]
- 2.Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep. 2003;4:185–195. doi: 10.1007/s11934-003-0068-1. [DOI] [PubMed] [Google Scholar]
- 3.Brawley O. Nonmedical factors in treatment selection. Urol Clin North Am. 2003;30:331–335. doi: 10.1016/s0094-0143(02)00186-6. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology, Prostate Cancer, Version 1.2002: PROS-1. 2002. [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) National Comprehensive Cancer Network Prostate Cancer practice guidelines in oncology, Version 2.2005. 2006. [Google Scholar]
- 6.Hewitt M, Simone J. Enhancing Data Systems to Improve the Quality of Cancer Care. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 7.IOM (Institute of Medicine) Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 8.German R, Wike J, Bauer K, et al. Quality of cancer registry data: findings from CDC-NPCR’s Breast and Prostate Cancer Data Quality and Patterns of Care Study. J Registry Manag. 2011;38:75–86. [PubMed] [Google Scholar]
- 9.Howlader N, Ries LA, Stinchcomb DG, et al. The impact of underreported veterans affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101:533–536. doi: 10.1093/jnci/djn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccirillo J, Creech C, Zequeira R, et al. Inclusion of comorbidity into oncology data registries. J Registry Manag. 1999;26:66–70. [Google Scholar]
- 11.Johnson A, Piccirillo J, Creech C, et al. Validation of a comorbidity education program. J Registry Manag. 2001;28:125–131. [Google Scholar]
- 12.Piccirillo J, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manag. 2003;30:8–14. [Google Scholar]
- 13.Piccirillo J, Tierney R, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2006;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 14.Arias E. National Vital Statistics Reports. 9. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. United States Life Tables, 2004; pp. 2016–2017.pp. 2022–2023. [PubMed] [Google Scholar]
- 15.Byers T, Wolf H, Bauer K, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer. 2008;113:585–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 16.Crawford ED. Use of algorithms as determinants for individual patient decision making: national comprehensive cancer network versus artificial neural networks. Urology. 2003;62(suppl 1):13–19. doi: 10.1016/j.urology.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Daskivich T, Fan K-H, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a US population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putt M, Long JA, Montagnet C, et al. Racial differences in the impact of comorbidities on survival among elderly men with prostate cancer. Med Care Res Rev. 2009;66:409–435. doi: 10.1177/1077558709333996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong Y-N, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 20.Resnick M, Koyama T, Fan K-H, et al. Long term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman R, Koyama T, Fan K-H, et al. Mortality following radical prostatectomy or external beam radiotherapy for localized prostate cancer. J Natl Cancer Inst. 2013;105:711–718. doi: 10.1093/jnci/djt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton A, Albertsen P, Johnson T, et al. Trends in treatment of localized prostate cancer using supplemented cancer registry data. Br J Urol Int. 2011;107:576–584. doi: 10.1111/j.1464-410X.2010.09514.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 24.Walz J, Suardi N, Shariat SF, et al. Accuracy of life tables in predicting overall survival in patients after radical prostatectomy. BJU Int. 2008;102:33–38. doi: 10.1111/j.1464-410X.2008.07614.x. [DOI] [PubMed] [Google Scholar]
- 25.Krahn MD, Bremner KE, Asaria J, et al. The ten-year rule revisited: accuracy of clinicians’ estimates of life expectancy in patients with localized prostate cancer. Urology. 2002;60:258–263. doi: 10.1016/s0090-4295(02)01712-0. [DOI] [PubMed] [Google Scholar]
- 26.Fleming S, Hamilton A, Sabatino S, et al. Treatment patterns for prostate cancer: comparison of Medicare claims data to medical record review. Med Care. 2012 doi: 10.1097/MLR.0b013e318277eba5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton A, Wu X-C, Lipscomb J, et al. Regional, provider, and economic factors associated with the choice of active surveillance in the treatment of men with localized prostate cancer. J Natl Cancer Inst Monogr. 2012;45:213–220. doi: 10.1093/jncimonographs/lgs033. [DOI] [PMC free article] [PubMed] [Google Scholar]


