Abstract
Nearly one-third of patients with calcium stones have hyperuricosuria. In vitro studies and clinical trials have investigated the relationship between uric acid and calcium stones, but the association between hyperuricosuria and calcium stone formation in patients is still being debated. Uric acid appears to cause salting out of calcium oxalate in human urine. However, the importance of this in vitro phenomenon to the proposed association is not supported in cross-sectional observational studies. A small placebo-controlled randomized clinical trial showed that allopurinol decreased the rate of recurrent calcium oxalate calculi in patients with hyperuricosuria and normocalciuria. An assessment of the effect of combination therapy of allopurinol with indapamide showed no additive effect. Allopurinol may have antioxidant effects that are responsible for its reducing calcium stone formation, which are independent of xanthine oxidase inhibition. In addition, a newer xanthine oxidoreductase inhibitor, febuxostat, may also be effective in the prevention of calcium stones, as it reduces urinary uric acid excretion.
Keywords: Allopurinol, Calcium, Calculi, renal, Febuxostat, Kidney stones, Nephrolithiasis, Uric acid, Urolithiasis
Introduction
The association between calcium stones and hyperuricosuria has long been a subject of investigation. Approximately one-third of patients with calcium stones have hyperuricosuria [1]. For these patients, a long-standing hypothesis has held that tailored pharmacological therapies, rather than general preventive recommendations (such as “increase fluid intake”), may be important for minimizing recurring stones. In vitro studies and clinical trials have been paramount in investigating the relationship between uric acid and calcium stones, without necessarily achieving consensus. For instance, in vitro studies have shown that increasing amounts of dissolved urate in human urine promote crystallization of calcium oxalate [2]. Clinical studies show that urate lowering therapy, using the xanthine oxidoreductase inhibitor allopurinol, decreases urinary uric acid excretion and reduces the recurrence of calcium stones [3]. However, these data supporting the association of hyperuricosuria and calcium oxalate stones are contradicted by observational (cross-sectional) clinical data, raising doubt about whether uric acid promotes calcium oxalate stones in vivo. In this paper we review the historical data regarding hyperuricosuria and calcium stones, the effect of allopurinol on stone recurrence, and then present our recent work on febuxostat in this setting.
Does hyperuricosuria promote calcium stones?
Several theories have been proposed to explain the possible mechanism of calcium stone formation in hyperuricosuric patients. Lonsdale and Coe et al. [4, 5] suggested a role for epitaxy, the formation of one crystal on top of another, related to heterogeneous nucleation, as an explanation for calcium oxalate stone formation in the presence of hyperuricosuria. Addition of crystalline sodium urate (but not uric acid) accelerated crystallization of calcium oxalate from a metastable solution [5].
Grover et al. confirmed the occurrence of epitaxy, or heterogeneous nucleation, in aqueous inorganic solutions designed to have similar solute concentrations as human urine. However, they also recognized that uric acid may promote calcium oxalate crystallization by another mechanism in human urine [6–8]. They proposed a “salting out” mechanism [2, 9]. Salting out is a decrease in solubility of a non-electrolyte with increasing concentrations of electrolyte, causing the former to precipitate from solution. In the current example, calcium oxalate is poorly soluble and uncharged and is considered the non-electrolyte, while uric acid is the more soluble, charged electrolyte that causes precipitation of calcium oxalate [2]. The salting out mechanism was confirmed in human urine, obtained from 20 men with no history of urolithiasis [7]. The urine samples were centrifuged, filtered and treated with increasing amounts of sodium urate. Spontaneous precipitation of calcium oxalate was observed with increasing urate concentration. Increasing the urate concentration effectively halved the amount of oxalate required to provoke calcium oxalate crystallization in human urine and increased the size of particles deposited. Spontaneous precipitation of calcium oxalate did not occur in more dilute urine samples, but crystallization occurred after addition of oxalate. Observation of the precipitates by infrared, ultraviolet spectroscopic analysis, and X-ray powder diffraction, did not detect any urate crystallization. The conclusion was that epitaxy, or heterogenous nucleation, was not the phenomenon responsible for promotion of calcium oxalate precipitation by uric acid. Rather, stone formation was due to calcium oxalate being salted out of solution.
The notion that calcium stones were promoted by hyperuricosuria arose from small case series and observational studies that suggested the relationship. However, the association was not supported in a larger cross-sectional study [10]. Participants with a history of kidney stones in the Nurses’ Health Study I and II (NHS I and II), older and younger women respectively, and Health Professionals Follow-Up Study (HPFS), men, provided 24-h urine samples. There was no evidence that higher urinary uric acid excretion distinguished the prevalence of stones in the cases from the randomly selected controls. In fact, multivariable relative risk of kidney stones in the NHS II and the HPFS cohorts showed that stone risk decreased as uric acid excretion increased. The authors attempted to ensure that their results were not incorrect because of reporting artifacts related to the fact that uric acid is insoluble at low pH. Patients with urine pH <6.0 may appear to have low uric acid excretion values because their uric acid is in a crystalline, non-assayable form; laboratories should increase the pH of the urine before measurement of uric acid. To account for that possibility, they showed that uric acid was not associated with greater risk of stones at higher urine pH since uric acid excretion might have been measured more accurately at higher urine pH, and reported as falsely low at low pH values. The accuracy of stone ascertainment in these cohorts is repeatedly reported as >90 % so that false positive reports probably cannot account for the findings. In summary, these results, clearly contradicting the previous literature suggesting that hyperuricosuria increases the risk for calcium stones, led Curhan et al. to actually suggest a possible role of uric acid in prevention of stone formation in the HPFS cohort (but not in NHS I). As these studies are large, and the methods sound, it is currently unclear if uric acid excretion is a risk factor for calcium stones, despite the in vitro phenomena that appear indisputable.
Clinical trials
Ettinger [11] hypothesized that allopurinol would decrease the rate of recurrent calcium oxalate calculi in patients with hyperuricosuria and normocalciuria. Participants were selected based on calculus analysis. Subjects with >79 % calcium oxalate stone composition were selected. Half of these patients had recurrent stone disease. Subjects that were included had two or more stones within 5 years and at least one stone within the last 2 years. The urinary inclusion criteria required that participants had hyperuricosuria, defined as urinary uric acid excretion >800 mg/day in men and >750 mg/day in women, while on a self-selected diet. Only patients with normocalciuria were included, defined by <300 mg/day in men and <250 mg/day in women. Of the 102 patients eligible for the study, 72 patients were finally enrolled.
The subjects were randomly assigned 1:1 to allopurinol 100 mg three times daily or placebo. They were given no dietary advice except to increase their fluid intake. Outcomes over 39 months of treatment were determined by stone presence on plain radiograph at baseline and then yearly, as well as counting episodes of stone passage. Events in the first 6 months were ignored due to the possibility of pre-existing stones. After completing at least 6 months of treatment 60 subjects were included in the analysis. The subjects in both groups had similar age, body mass index (BMI), and serum and urine chemistries at baseline. As expected, allopurinol treatment caused a significant (p < 0.05) decline in urinary uric acid excretion compared to baseline. Mean urinary uric acid decreased by over 300 mg/24 h at 3 months after entry and finally decreased to over 400 mg/24 h from baseline at 24 months post entry. Mean serum uric acid concentration also significantly decreased by 1.7 mg/dl 3 months after entry compared to baseline in the allopurinol treated group (p < 0.001). Formation of new stones occurred in 18 participants receiving placebo and 9 participants receiving allopurinol. In addition, the allopurinol group had a significantly longer time before recurrence. The authors concluded that allopurinol would be useful clinically in patients with calcium oxalate calculi and isolated hyperuricosuria.
Borghi et al. investigated the effect of combination therapy of allopurinol with indapamide, a thiazide-like drug used in the treatment of hypertension that also lowers urinary calcium excretion [12]. This study randomized 75 patients with hypercalciuria (>300 mg/24 h in men and >250 mg/24 h in women or urine Ca >4 mg/kg or Ca/creatinine >0.20 mg/dl) to three groups: (1) diet low in calcium, sodium, purines, and oxalate and increased fluid intake; (2) the same diet with increased fluid intake and 2.5 mg of indapamide each day; (3) the same diet, increased fluid intake, with 2.5 mg of indapamide and 300 mg of allopurinol each day. The measured outcome was stone formation over 3 years of treatment. The first group did not show a significant decrease from baseline in calcium stone rates with diet and fluid intervention alone (from 0.8 ± 0.5 to 0.3 ± 0.5). The second group saw a significant (p < 0.02) decrease in stone rates with indapamide treatment (1.4 ± 1.8 to 0.1 ± 0.2). The third group with combination therapy of allopurinol and indapamide also had a significant decrease in stone rates from baseline (from 1.2 ± 1.4 to 0.04 ± 0.1, p < 0.02) but the combination therapy did not result in a significantly different change from indapamide alone. The conclusion was that allopurinol had no further effect when added to indapamide, which was relatively effective alone. Ettinger’s study excluded patients with hypercalciuria, while Borghi et al.’s study included them, but lowered urine calcium with thiazides. It therefore remains uncertain if urate-lowering therapy is effective alone in the presence of hypercalciuria and whether hyperuricosuria is an important risk factor in patients with concomitant hypercalciuria.
Allopurinol’s mechanism of lowering calcium stone occurrence may be unrelated to its uric acid lowering mechanism. The literature suggests a relationship between uric acid and calcium oxalate solubility, but the observational studies of Curhan et al. were not supportive of this hypothesis [10, 11]. This lack of consistency may suggest that allopurinol may have other effects not related to its reduction in uric acid synthesis. For instance, allopurinol may have antioxidant effects that are responsible for the decrease in calcium stone formation, which are independent of xanthine oxidase inhibition.
These other effects are exemplified by a randomized cross-over study that explored allopurinol’s ability to improve endothelial function aside from its known mechanism of lowering uric acid [13]. The investigators found that high-dose allopurinol 600 mg/day acted as an antioxidant, reducing vascular oxidative stress, and improving endothelial function [13]. To compare this result to similar levels of urate lowering, the investigators used probenecid (at 1,000 mg/day), a uricosuric drug used in the treatment of gout and hyperuricemia. Despite causing similar reduction of serum urate as allopurinol, probenecid had no effect on endothelial function. This experiment suggests that there are context specific effects of allopurinol therapy, beyond its ability to lower serum or urine uric acid. These other effects may relate to other hypotheses regarding calcium stone formation. For instance, a vascular etiology for initiation of stones has been proposed [14]. Recent data demonstrating an association of stones with coronary heart disease are also of interest [15]. The role of inflammation and reactive oxygen species in the initiation of stone disease has long been thought to be relevant as well [16]. In short, an action of allopurinol in preventing stones through an effect other than urate lowering is plausible, if currently highly speculative.
Febuxostat
A newer xanthine oxidoreductase inhibitor, febuxostat, may also be effective in the prevention of calcium stones. Febuxostat is a non-purine analog xanthine oxidoreductase inhibitor and may therefore have fewer effects than allopurinol on enzymes involved in purine and pyrimidine metabolism. The clinical significance of this specificity has not yet been demonstrated. It is predominantly metabolized by the liver and has therefore been promoted as a treatment for gout for patients with concurrent chronic kidney disease; no dose adjustment is needed for glomerular filtration rates down to 30 ml/min/1.73 m2.
We performed a randomized controlled trial to compare the efficacy of febuxostat vs. allopurinol or placebo in patients with high urinary uric acid excretion and calcium nephrolithiasis [17]. We hypothesized that febuxostat would reduce uric acid excretion, prevent calcium stone growth, and be at least equivalent to allopurinol in individuals with calcium stones and higher urinary uric acid excretion. Inclusion criteria were men and women with >700 mg/day urinary uric acid and a recent stone, with one or more calcium stones ≥3 mm in diameter on computed tomography. Some of the exclusion criteria included gout, secondary, hyperuricemia, or higher urinary calcium excretion.
The outcome was measured with 24-h urinary uric acid and computed tomography. After 6 months of treatment, patients on febuxostat had the greatest decrease in urinary uric acid from baseline compared to allopurinol and placebo (p = 0.003, Fig. 1). There was no effect on stone size and number for either xanthine oxidoreductase inhibitor, compared with placebo, after 6 months of this study. This lack of effect is not surprising since studies of calcium stone prevention that are of <2 years duration are unlikely to show efficacy [18]. We hope to perform a study of at least 3 years duration to determine whether febuxostat is effective in preventing recurrent calcium stones. The maximum dose of allopurinol approved by the US Food and Drug Administration is 800 mg per day, but 300 mg is the most commonly prescribed dose. While febuxostat 80 mg appears to lead to a greater reduction in urinary uric acid excretion than allopurinol 300 mg, higher doses of allopurinol may be equally effective.
Fig. 1.
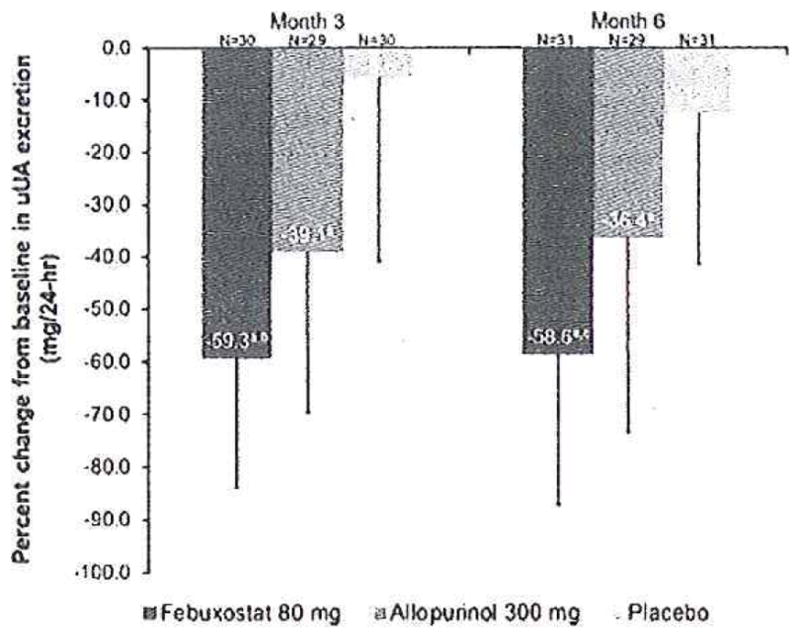
Percent change from baseline to months 3 and 6 in 24-h urinary uric acid (uUA) excretion, ap=0.001 vs. placebo; bp = 0.008 VS. allopurinol; cp = 0.003 vs. allopurinol. Error bars represent the standard deviation (SD) for each data point. Reproduced from [14] with permission
Conclusions
Uric acid clearly reduces the solubility of calcium oxalate in vitro. However, observational studies of hyperuricosuria have not convincingly demonstrated a resulting risk of calcium oxalate stones. One study of the xanthine oxidoreductase inhibitor allopurinol demonstrated benefit in patients with hyperuricosuria who did not have hypercalciuria. Another 6-month study of febuxostat and allopurinol showed no beneficial effect but was probably too short to do so. Febuxostat 80 mg lowered urinary uric acid excretion more than did 300 mg of allopurinol, but the latter could be used safely at higher doses and could probably achieve the same effect. Whether there is benefit of lowering uric acid excretion in patients with hypercalciuria has not been established. The role of hyperuricosuria in calcium stone formation remains relatively unresolved.
Footnotes
Presented in part on 3/22/13 at “Nephrolithiasis: A Systemic Disorder”, Rome, Italy.
Conflict of Interest Arowojolu: none; Goldfarb: consultant, Takeda, Astra Zeneca; owner, Ravine Group; Honoraria: Mission Pharmacal.
Contributor Information
Omotayo Arowojolu, Medicine and Physiology, NYU School of Medicine, New York, NY, USA.
David S. Goldfarb, Email: dsgold@verizon.net, Medicine and Physiology, NYU School of Medicine, New York, NY, USA. Nephrology Section, New York Harbor VA Healthcare System and NYU Langone Medical Center, NYU School of Medicine, 111G New York DVAMC 423 E. 23 St., New York, NY 10010, USA
References
- 1.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977;87(4):404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 2.Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol. 2003;10(3):271–278. doi: 10.1016/s1074-5521(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315(22):1386–1389. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 4.Lonsdale K. Epitaxy as a growth factor in urinary calculi and gallstones. Nature. 1968;217(5123):56–58. doi: 10.1038/217056a0. [DOI] [PubMed] [Google Scholar]
- 5.Coe FL, Lawton RL, Goldstein RB, Tembe V. Sodium urate accelerates precipitation of calcium oxalate in vitro. Proc Soc Exp Biol Med. 1975;149(4):926–929. doi: 10.3181/00379727-149-38928. [DOI] [PubMed] [Google Scholar]
- 6.Grover PK, Ryall RL, Marshall VR. Dissolved urate promotes calcium oxalate crystallization: epitaxy is not the cause. Clin Sci (Lond) 1993;85(3):303–307. doi: 10.1042/cs0850303. [DOI] [PubMed] [Google Scholar]
- 7.Grover PK, Ryall RL, Marshall VR. Calcium oxalate crystallization in urine: role of urate and glycosaminoglycans. Kidney Int. 1992;41(1):149–154. doi: 10.1038/ki.1992.20. [DOI] [PubMed] [Google Scholar]
- 8.Grover PK, Ryall RL, Marshall VR. Effect of urate on calcium oxalate crystallization in human urine: evidence for a promotory role of hyperuricosuria in urolithiasis. Clin Sci (Lond) 1990;79(1):9–15. doi: 10.1042/cs0790009. [DOI] [PubMed] [Google Scholar]
- 9.Grover PK, Ryall RL. Comment on epitaxial relationships between uric acid crystals and mineral surfaces: a factor in urinary stone formation. Langmuir. 2005;21(23):10898. doi: 10.1021/la0509293. [DOI] [PubMed] [Google Scholar]
- 10.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73(4):489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger B. Does hyperuricosuria play a role in calcium oxalate lithiasis? J Urol. 1989;141 (3 Pt 2):738–741. doi: 10.1016/s0022-5347(17)40998-0. [DOI] [PubMed] [Google Scholar]
- 12.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol. 1993;22 (Suppl 6):S78–S86. [PubMed] [Google Scholar]
- 13.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 14.Stoller ML, Meng MV, Abrahams HM, Kane JP. The primary stone event: a new hypothesis involving a vascular etiology. J Urol. 2004;171(5):1920–1924. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 15.Ferraro PM, Taylor EN, Eisner BH, et al. History of kidney stones and the risk of coronary heart disease. JAMA. 2013;310(4):408–415. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi S, Peck AB, Khan SR. Nadph oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxid Med Cell Longer. 2013;2013:462361. doi: 10.1155/2013/462361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldfarb DS, Macdonald PA, Gunawardhana L, Chefo S, McLean L. Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary uric acid excretion and calcium stones. Clin J Am Soc Nephrol. 2013 doi: 10.2215/CJN.01760213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an american college of physicians clinical guideline. Ann Intern Med. 2013;158(7):535–543. doi: 10.7326/0003-4819-158-7-201304020-00005. [DOI] [PubMed] [Google Scholar]


