Abstract
Background
Oxidative stress and the specific impairment of peri-somatic GABA circuits are hallmarks of the schizophrenic brain and its animal models. Proper maturation of these fast-spiking inhibitory interneurons normally defines critical periods of experience-dependent cortical plasticity.
Method
Here, we link these processes by genetically inducing a redox dysregulation restricted to such parvalbumin-positive cells and examined the impact on critical period plasticity using the visual system as a model (3–6mice/group).
Results
Oxidative stress was accompanied by a significant loss of perineuronal nets, which normally enwrap mature fast-spiking cells to limit adult plasticity. Accordingly, the neocortex remained plastic even beyond the peak of its natural critical period. These effects were not seen when redox dysregulation was targeted in excitatory principal cells.
Conclusion
A cell-specific regulation of redox state thus balances plasticity and stability of cortical networks. Mis-timed developmental trajectories of brain plasticity may underlie in part the pathophysiology of mental illness. Such prolonged developmental plasticity may in turn offer a therapeutic opportunity for cognitive interventions targeting brain plasticity in schizophrenia.
Keywords: visual cortex, oxidative stress, parvalbumin, perineuronal net, GABA, schizophrenia
Introduction
Schizophrenia is increasingly recognized as a neurodevelopmental disorder that involves alterations in brain circuits, including dysfunction of parvalbumin (PV)-positive, fast-spiking GABA neurons (1, 2). Mounting evidence also indicates that “redox dysregulation,” an imbalance between oxidative stress and antioxidant defense systems, may play a role in the pathogenesis (reviewed in ref. (3)). Reduced antioxidant enzymes are reported in schizophrenic patients (4–8), and several divergent genetic (e.g. Gclc, Gclm, Disc1, PRODH, G72, NRG1) (9–17) or external factors (e.g. stress, social isolation, ketamine, neonatal ventral hippocampal lesion) (18–24) collectively lead to redox dysregulation. Animal models of globally disrupted redox state exhibit defects in fast-spiking interneurons (16, 23, 25) consistent with physiological consequences on excitatory-inhibitory (E–I) circuit balance during neurodevelopment.
Notably, the postnatal maturation of PV-positive GABA neurons has been found to define critical periods of experience-dependent cortical plasticity (26). These windows in infancy and early childhood reflect heightened circuit rewiring to adaptively match sensory maps and complex behaviors to the surrounding environment, and are then gradually consolidated into adulthood. Accordingly, disruption of E-I balance powerfully shifts the timing and quality of critical period development, as seen in the primary visual cortex (V1) (27–29). High metabolic demands render fast-spiking interneurons particularly vulnerable to oxidative stress (23). Here, we examined whether late developing redox dysregulation within PV-cells alone would directly impact the profile of cortical plasticity.
Methods and Materials
Animals
Wild-type (C57 Bl/6J; Charles River USA) and CamK2-Cre transgenic (c/o Dr A Nagy, Univ Toronto)(30), PV-ires-Cre (c/o Dr S Arber, FMI Basel) (31), or Gclc-f/f mice (32) crossed to generate CamK2-Gclc or PV-Gclc knockout (KO)/wild-type (WT) mice raised from birth on a 12-hour light/dark cycle to various postnatal ages. To rule out potential ectopic effects of Cre expression (33, 34), we were careful to compare only to homozygous Cre+/+ animals as controls.
In situ hybridization
Probes for mouse Gclc and Cre were synthesized using T3/T7 RNA polymerase (Roche) labeled with digoxigenin or fluorescein and hybridized to frozen sections. Gclc probe was generated against a pCMV-SPORT6 plasmid containing the full length mouse Gclc cDNA sequence between Not1 and Sal1 restriction enzyme sites (clone ID: 4193582, Invitrogen). Cre probe was generated using the template pBluescript2SK(−) plasmid including the full sequence of Cre subcloned from pCAG-Cre (Addgene plasmid 13775: between EcoR1 and Cla1 restriction enzyme sites). TSA-Plus DNP System (PerkinElmer Life Sciences) in combination with fast red staining was used to amplify the signal for double FISH.
Immunohistochemistry
Mice were perfused transcardially with 0.9% saline and 4% para-formaldehyde, then brains were removed into 30% sucrose and transferred to ethylene glycol solution for cryoprotection. Brains were cut in coronal section (30 or 40 μm) on a freezing microtome. Sections were rinsed in PBS, then incubated overnight at 4°C in monoclonal antibody against Parvalbumin (Rabbit,1:500, Swant), 8-oxodG (Mouse, 1:350, Trevigen), NeuN (Mouse, 1:400, Millipore), CD68 (rat;1:400, Serotec), biotin-conjugated lectin Wisteria Floribunda Agglutinin (WFA; 1:400 in PBS, Vectorlabs), Otx2 (CD4, 1:50 gift from Dr. A. Prochiantz) and secondary antibodies (anti-IgG-Alexa-488, 594, 633/streptavidin-Alexa 488; 1:400, invitrogen). Fluoro-Jade C (Millipore) staining was performed according to previously described procedure (35).
Quantification of PV+ cells with strong 8-oxodG immunoreactivity was performed by combining threshold (between the values of 20 and 256) and particle analysis (particle size above 60 pixel^2) modules of ImageJ software to distinguish strongly labeled cells from background signal. PV+/WFA+ cell counts were quantified using the spot module of IMARIS 7.1 software (Bitplane AG, Switzerland) as described (25). Briefly, we analyzed the amount of colocalization of immunostained voxels, using all immunostained voxels with fluorescence intensity above 0.03 (arbitrary unit, a.u.) and the Coloc module of Imaris software. Immunostained voxels with fluorescence intensity below 0.03 (a.u.) were neglected, as these were within the background fluorescence intensity level. Three to five mice were used per experimental condition.
Monocular deprivation (MD) procedure
Eyelid margins were trimmed by iris scissor and eyes sutured shut under isoflurane anesthesia. Eyes were closed for 4–5 days for short term MD from P25 (during the critical period) or after (P50).
Extracellular recording in vivo
Electrophysiological recordings were performed under nembutal/chlorprothixene anesthesia using standard techniques in mice (28). Ocular dominance (OD) in the binocular zone (V1b) from each mouse was calculated as a contralateral bias index (CBI): [(n1−n7)+2/3(n2−n6)+1/3(n3−n5)+N]/2N, where N=total number of cells and nx=number of cells corresponding to ocular dominance score of x. The CBI approaches 1.0 as contralateral (contra) eye input dominates and is reduced when the ipsilateral (ipsi) eye becomes stronger. OD score was computed on cells with a complete peri-stimulus time histogram analysis of peak and baseline spiking activity, by alternately covering either eye. OD score was defined as {[Peak(ipsi)−baseline(ipsi)] − [Peak(contra)−baseline(contra)]}/{[Peak(ipsi)−baseline(ipsi)] + [Peak(contra) − baseline(contra)]} (29). For statistical comparison of OD distributions, normalized OD scores of individual neurons were computed, and plotted as a cumulative distribution for each experimental group. CBI values across groups of mice were compared by One way ANOVA or Student’s t test.
Results
Modeling genetic redox dysregulation within PV cells in vivo
To perturb redox balance specifically within PV interneurons, we conditionally deleted the Gclc (glutamate cysteine ligase catalytic subunit) gene – the rate limiting enzyme that produces the primary endogenous antioxidant and redox regulator, glutathione (3) (Figure 1A). Importantly, the activity of glutathione is lower in schizophrenic patients, and the GAG-repeat of the Gclc promoter is associated with the illness (9). As systemic Gclc deletion is lethal (36), we crossed mice with the Gclc gene flanked by loxP sites (32) to animals expressing Cre recombinase under control of the PV-promoter (31). To rule out potential ectopic effects of Cre expression (33, 34), we were careful to compare PV-Cre+/+ ;Gclcf/f (PV-Gclc KO) mice to homozygous PV-Cre+/+ (PV-Gclc WT) controls.
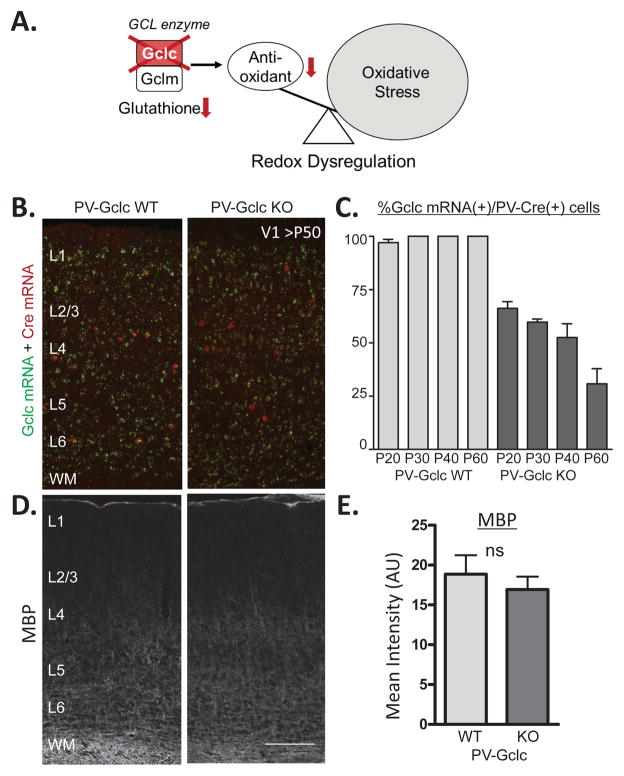
Figure 1. Modeling genetic redox dysregulation within PV cells in vivo.
(A) Gclc normally forms a complex with Gclm as the rate limiting enzyme for producing the major brain antioxidant and redox regulator, Glutathione. Deletion of the Gclc gene induces redox dysregulation by lowering Glutathione levels. (B) Double in situ hybridization for Gclc (green) and Cre (red) recombinase in mature V1 (> P50) of WT (left) and PV-Gclc KO (right) mice. (C) Quantification of co-localization reveals progressive developmental loss of Gclc expression in PV-Cre interneurons from 34% (P20) to 70% (P60) in PV-Gclc KO mice (dark grey bars) (1way ANOVA: p<0.05), as compared to WT mice in which almost 100% of Cre-positive cells constantly express Gclc. (D) Immunohistochemical visualization of myelin basic protein (MBP) density in mouse V1 (>P50) of WT (left) and PV-Gclc KO (right) mice. (E) Quantification of mean MBP intensity shows no significant difference between genotypes (p>0.3, t-test). Scale bar, 150 μm.
This yielded a progressive Gclc deletion in cortical PV-cells from 34% at postnatal day P20 to 70% in adulthood (>P50), as evaluated by double in situ hybridization for Gclc and Cre mRNA (Figure 1B,C). The cell-specific manipulation was subtle and did not cause myelin deficits (Figure 1D,E) typically observed upon global redox dysregulation (3, 37). Instead, a cell-autonomous enhancement of oxidative stress was observed in the majority (69%) of PV-cells when staining for a product of mitochondrial DNA oxidation, 8-oxo-7,8-dihydro-20-deoxyguanine (8-oxo-dG) (Figure 2A,B). This allowed us to further test the impact of PV cell-specific oxidative stress on post-adolescent cortical plasticity. Note that we cannot rule out the possibility of mild non-cell autonomous increase of oxidative stress below detection of our 8-oxo-dG staining threshold set to capture only those cells with enhanced oxidative stress beyond the basal levels of physiological stress.
Figure 2. Redox dysregulation and loss of perineuronal nets (PNNs) enwrapping PV-cells.
(A) Double-labeling for 8-oxo-dG (green) and PV (red) in mature V1 (>P50) of WT (left) and PV-Gclc KO (right) mice. Higher magnification (inset) confirms elevation of oxidative stress in PV cells of PV-Gclc KO mice. (B) (left) Quantification (%) of PV+ cells also expressing high levels of 8-oxo-dG in PV-Gclc mice (69.3% KO vs 7.2% WT; P<0.000,1 t-test). (right) Total number of PV (+) cells, **p<0.01 t-test. (C) Double immunolabeling of PNNs with WFA (green) and PV (red) in mature V1 (>P50) of WT (left) and PV-Gclc KO (right) mice. Scale bar, 100 μm. (D) (left) Quantification of the number of WFA+ cells reveals loss of PNNs in PV-Gclc KO mice (vs WT; ***p<0.001, t-test). (right) Percentage of PV-cells enwrapped by WFA+ PNN also reduced in KO mice (**p<0.01, t-test).
Redox dysregulation decreases perineuronal nets enwrapping PV-cells
As PV-cells mature through their critical period, they are increasingly enwrapped by a peri-neuronal net (PNN) of extracellular matrix. Enzymatic removal of PNNs can reopen plasticity in adulthood (38, 39). In the primary visual cortex of PV-Gclc KO mice, we observed significantly fewer PV-immunoreactive cells and PNNs, as revealed by WFA staining (Figure 2). The loss of PNNs was quite pronounced (Figure 2C), and the percentage of PV-cells bearing PNNs was also reduced (Figure 2D). Compromised PNNs prevent the persistent uptake of Otx2 homeoprotein into the enwrapped PV-cells needed for their maturation (including PNN maintenance itself) (27, 40, 41). Consistent with this, the percentage of Otx2+/PV+ cells was significantly reduced in PV-Gclc KO mice, especially in those cells enwrapped with PNNs (Supplemental Figure S3).
PV-Gclc KO mice further exhibited a significant loss of perisomatic PV+ puncta surrounding their target pyramidal cell bodies in layer 2/3 of adult visual cortex (Supplemental Figure S1). Instead, there was no indication of neurodegeneration by Fluoro Jade C staining, suggesting that PV-cells did not die in PV-Gclc KO mice but were rather impaired (Supplemental Figure S2). PNN loss might allow the diffusion of excessive reactive oxygen species outside the PV-cell, which might signal microglial cells. In fact, a mild activation of microglia was observed by CD68 staining (Figure 3A,B).
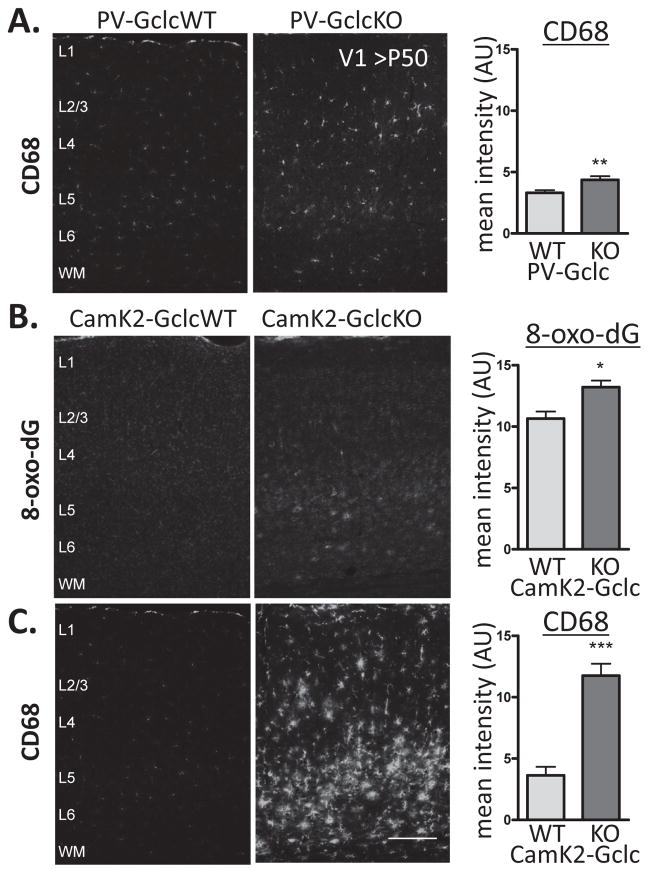
Figure 3. Microglial activation after cell-specific redox dysregulation.
(A) Immunohistochemistry for CD68, a marker of activated microglia, shows mild elevation in PV-Gclc KO (middle) compared to WT mice (left) in V1(>P50) (p<0.01, t-test). (B) Marked elevation of oxidative stress labeling (8-oxo-dG) in CamK2-Gclc KO (middle) compared to WT mice (left) (p<0.05, t-test) (C) Massive activation of microglia (CD68) in CamK2-Gclc KO (middle) compared to WT mice (left) in V1 (>P50) (p<0.01, t-test). Scale bar, 150 μm.
For comparison, we also examined the brains of mice carrying a Gclc gene deletion from principal neurons, which express the calcium/calmodulin-dependent protein kinase type 2 (CamK2) – not expressed in PV-cells (27) (Figure 3B). Consistent with a more widespread redox dysregulation across the pyramidal cell population, a massive activation of microglia (Figure 3C) and neurodegeneration (Supplemental Figure S2) was observed, indicating that this is a robust, nonspecific response to cellular stress of any origin. In contrast, immunoreactivity for PV and PNNs remained intact in the adult visual cortex of CamK2-Gclc KO mice (Supplemental Figure S4 and S5). Microglia have recently been implicated as active participants in brain plasticity per se (42–45). We next examined the status of brain plasticity in PV-Gclc KO mice, given their PNN loss – a major brake on adult cortical rewiring (38).
Prolonged critical period plasticity upon PV-cell specific redox dysregulation
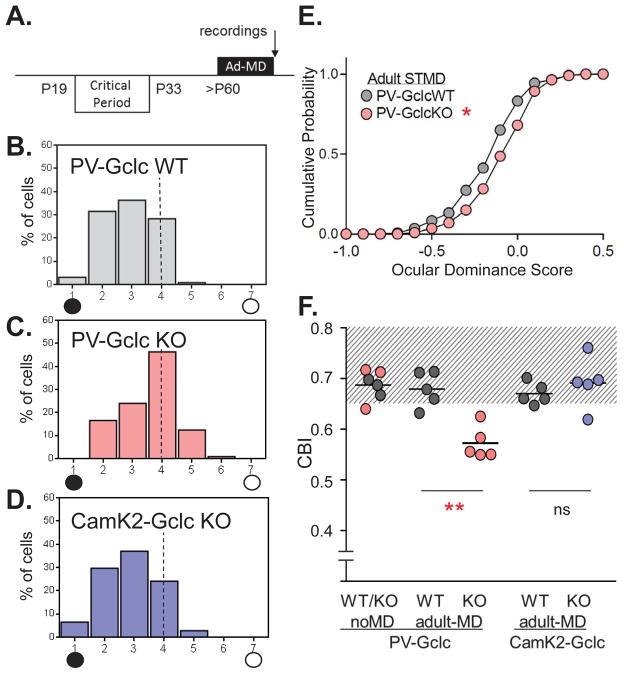
The classical loss of input from an eye briefly deprived of vision in the binocular zone of primary visual cortex is typically constrained before P50 (reviewed in ref. (46, 47)) (Fig 4A). When the majority of PV-cells lacked Gclc gene expression by this age in our conditional mutant mice (Figure 1A), extracellular single-unit recording continued to yield a significant shift away from the deprived, contralateral eye following short-term (4 day) monocular deprivation (Figure 4B,C,E) The contralateral bias index (CBI) differed significantly between genotypes (0.57 in KO vs. 0.68 in WT; age range between P54 and P63, p<0.001). This enhanced plasticity in PV-Gclc KO mice past the peak of the critical period was not corrected by concurrent administration of the benzodiazepine Diazepam (Supplemental Figure S6), suggesting an earlier window may correct the plasticity phenotype (18).
Figure 4. Prolonged cortical plasticity by PV-cell specific redox dysregulation.
(A) Contralateral eye bias assessed by single-unit electrophysiology in mouse V1 after 4 days of short-term monocular deprivation (STMD) in adulthood. (B–D) Ocular dominance distribution after STMD (5 mice each) in (B) WT (127 cells), (C) PV-Gclc KO (121 cells) and (D) CamK2-Gclc KO mice (108 cells). Age range from P54 to P63. Visual responses classified on a seven-point scale from exclusively contralateral (group 1) to ipsilateral driven (group 7). PV-Gclc KO vs WT: ***p<0.0005; PV-Gclc KO vs CamK2-Gclc KO: ****p<0.0001, χ2-test. (E) Cumulative probability of quantified spike response after adult STMD confirms shifted ocular dominance scores for PV-Gclc KO (pink filled circles), compared to WT (gray filled circles) (*p < 0.05, Kolmogorov-Smirnov test). (F) Mean contralateral bias index (CBI), starting from the same baseline values (0.68) in non-deprived mice across genotypes, is shifted only in PV-Gclc KO mice by STMD (0.57; ***p<0.001 vs WT, adjusted P value, Tukey’s multiple comparison test). No adult plasticity in CamK2-Gclc KO mice (mean CBI = 0.69 vs 0.67 in WT mice; p>0.8, adjusted P value, Tukey’s multiple comparison test, 1 way ANOVA). Shaded area indicates CBI range of non-deprived (aplastic) mice.
Consistent with a limited Gclc deletion in younger KO mice (Figure 1C) during the natural critical period, there was no difference from WT plasticity levels at that age (Supplemental Figure S7; CBI after 4 day MD = 0.49 in PV-Gclc KO vs. 0.51 in WT; p>0.2, n=3 mice each). Moreover, in CamK2-Gclc KO mice, carrying a more severe redox dysregulation restricted to pyramidal cells, no extended plasticity was seen as in typical adult WT mice (Figure 4D,E; CBI = 0.69 and 0.67, respectively; p>0.8). Given the inverse relationship of microglial activation and the level of cortical plasticity in adult PV- and CamK2-Gclc KO mice, the contribution of microglial response to cortical plasticity may be more subtle than expected or reflect an optimal range of activation.
Discussion
Our results reveal a prolonged period of brain plasticity – or a failure to stabilize cortical circuits - in young adult animals under conditions of PV-cell specific glutathione dysregulation in vivo. Mis-timed developmental trajectories of brain plasticity may therefore contribute in part to the etiology of mental illnesses involving oxidative stress, such as schizophrenia (2, 3, 18). This reflects a redox-sensitive failure to maintain PV-cells with fully enwrapped PNNs, which normally balance plasticity and stability of cortical circuits across development in a twofold manner (38, 39).
First, intact PNNs may structurally limit the robust synaptic plasticity directly upon PV-cells (48–50). Second, PNN disruption prevents the persistent uptake of Otx2 homeoprotein into the enwrapped PV-cells required for their maintenance (27, 40, 41) (Fig. S3). Subsequent inability to engage downstream molecular pathways by Otx2 within the PV-cell would retain a juvenile plastic state (41). Notably, the major endogenous source of this Otx2 has recently been identified as the choroid plexus (51), which lines the ventricles that are commonly found to be enlarged in schizophrenia.
Two of the primary PNN components, chondroitin sulfate and hyaluronan, are sensitive to excessive free radicals (52), and the relatively late timing of Gclc deletion here overlaps with their usual condensation into tight nets around PV-cells. Our recent study revealed a protective function of these PNNs as a redox sensor (53). The high metabolic requirements of the fast-spiking PV-interneuron (23) may readily generate reactive oxygen species when their antioxidant systems are impaired, leading to the breakdown of PNNs themselves (Figure 2C,D) (53). Our study suggests that cell-autonomous redox dysregulation within PV-cells is the primary contributor to a reduction of PNNs observed across mouse models and human patients sharing global redox dysregulation.
Paradoxically, redox dysregulation in schizophrenia may then act in part by transiently prolonging or reopening critical periods of developmental plasticity. An excessively plastic status may precede the progressive degenerative process as in other brain disorders (29, 54). In chronic schizophrenia patients, deficits in visual cortex and long-term potentiation-like plasticity are reported when probed by transcranial direct current stimulation (55–58). First onset patients instead do not differ significantly from healthy controls with a trend toward increased plasticity (59). Individuals at high risk show greater cognitive gains relative to adults with chronic schizophrenia in response to computer-based cognitive training (60). Our study points to the importance of studying the developmental trajectory of cortical plasticity in schizophrenia patients to understand the relation to disease progression, remission, and treatment response (61).
Prolonged developmental plasticity may then provide a diagnostic opportunity for cognitive interventions targeting brain plasticity in the prodromal stage (62–64). Unfortunately, the current study was limited before and after the age range we studied (P54–63), either by premature death of PV-Gclc KO mice by P80 (as PV is also expressed in muscle) or insufficient deletion of the Gclc gene at younger stages. Also, we cannot fully rule out the possibility that the prolonged plasticity in these mice is mediated by an as yet unknown consequence of Gclc deletion not directly related to redox dysregulation. Future studies in other animal models are required to rigorously map the trajectory of brain plasticity across the lifespan in relation to redox dysregulation and other risk factors.
Altered PNNs have been found in the human post mortem amygdala, entorhinal and prefrontal cortices of patients (65–67), and GABA-related functional deficits are seen even in their visual cortex (68–71). Pathophysiological changes (1–3) are consistent with ‘dematuration’ or a removal of ‘brakes’ on plasticity (72, 73). Several molecular brakes of relevance to schizophrenia (other than PNNs) have been identified to limit critical period plasticity, including myelin signaling through a NgR-PirB complex (2, 74, 75) or dampened nicotinic receptor signaling by Lynx1 (29, 76). Curiously, all brakes to date converge on PV-cell function as a hub of vulnerability (77). This may offer novel strategies or drug targets, in addition to antioxidants (18, 25) or Otx2 up-regulation (41, 51), for correcting aberrant brain plasticity in mental illness.
Supplementary Material
Acknowledgments
We thank M. Cuenod (Lausanne) for helpful insights, A. Prochiantz (Paris) and H. Bond for Otx2 immunostaining, M. Marcotrigiano and M. Nakamura for animal breeding and maintenance. Supported by NIH (1DP1OD003699 and 1P50MH094271 to TKH), Swiss National Science Foundation (# 31-116689), National Center of Competence in Research (NCCR) “SYNAPSY - The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (n° 51AU40_125759), Fondation Damm-Etienne (to KQD), and the Brain & Behavior Research Foundation (to HM).
Footnotes
Financial disclosures: All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 3.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Current opinion in neurobiology. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. The European journal of neuroscience. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 5.Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. Journal of affective disorders. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxidants & redox signaling. 2011;15:2011–2035. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JF, Shao L, Sun X, Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar disorders. 2009;11:523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 8.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 9.Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. American journal of human genetics. 2006;79:586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YU, Jeong J, Lee H, Mun JY, Kim JH, Lee JS, et al. Disrupted-in-schizophrenia 1 (DISC1) plays essential roles in mitochondria in collaboration with Mitofilin. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17785–17790. doi: 10.1073/pnas.1004361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, et al. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3697–3711. doi: 10.1523/JNEUROSCI.5640-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. The Journal of biological chemistry. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 14.Otte DM, Sommersberg B, Kudin A, Guerrero C, Albayram O, Filiou MD, et al. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2233–2243. doi: 10.1038/npp.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan N, Dickman MB, Becker DF. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free radical biology & medicine. 2008;44:671–681. doi: 10.1016/j.freeradbiomed.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulak A, Steullet P, Cabungcal JH, Werge T, Ingason A, Cuenod M, et al. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxidants & redox signaling. 2013;18:1428–1443. doi: 10.1089/ars.2012.4858. [DOI] [PubMed] [Google Scholar]
- 18.Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grillo CA, Piroli GG, Rosell DR, Hoskin EK, McEwen BS, Reagan LP. Region specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stress. Neuroscience. 2003;121:133–140. doi: 10.1016/s0306-4522(03)00343-9. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social Isolation Exacerbates Schizophrenia-Like Phenotypes via Oxidative Stress in Cortical Interneurons. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 24.Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biological psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-Life Insults Impair Parvalbumin Interneurons via Oxidative Stress: Reversal by N-Acetylcysteine. Biological psychiatry. 2013;73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 28.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 29.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 31.Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS biology. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, et al. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Hensch TK. Germline recombination by conditional gene targeting with Parvalbumin-Cre lines. Frontiers in neural circuits. 2013;7:168. doi: 10.3389/fncir.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harno E, Cottrell EC, White A. Metabolic pitfalls of CNS Cre-based technology. Cell metabolism. 2013;18:21–28. doi: 10.1016/j.cmet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain research. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 36.Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochemical and biophysical research communications. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 37.Do KQ, Monin A, Klaey M, Butticaz C, Cabungcal JH, Steullet P, et al. Redox dysregulation affects proliferation, differentiation of oligodendrocyte progenitors and myelination: relevance to dysconnectivity in schizophrenia. Biological psychiatry. 2012;71:4S. [Google Scholar]
- 38.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 39.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 40.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nature neuroscience. 2012;15:414–422. S411–412. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 41.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 44.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Communicative & integrative biology. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Progress in brain research. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- 47.Morishita H, Hensch TK. Critical period revisited: impact on vision. Current opinion in neurobiology. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Yazaki-Sugiyama Y, Kang S, Cateau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- 49.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, et al. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, et al. Choroid-plexus-derived otx2 homeoprotein constrains adult cortical plasticity. Cell reports. 2013;3:1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rees MD, Hawkins CL, Davies MJ. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. The Biochemical journal. 2004;381:175–184. doi: 10.1042/BJ20040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beste C, Wascher E, Dinse HR, Saft C. Faster perceptual learning through excitotoxic neurodegeneration. Current biology : CB. 2012;22:1914–1917. doi: 10.1016/j.cub.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 55.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Archives of general psychiatry. 2008;65:378–385. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- 56.McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biological psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. Biological psychiatry. 2012;71:503–511. doi: 10.1016/j.biopsych.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavus I, Reinhart RM, Roach BJ, Gueorguieva R, Teyler TJ, Clapp WC, et al. Impaired visual cortical plasticity in schizophrenia. Biological psychiatry. 2012;71:512–520. doi: 10.1016/j.biopsych.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behavioural brain research. 2011;224:15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Rauchensteiner S, Kawohl W, Ozgurdal S, Littmann E, Gudlowski Y, Witthaus H, et al. Test-performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiatry research. 2011;185:334–339. doi: 10.1016/j.psychres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Ehlers MD. Hijacking hebb: noninvasive methods to probe plasticity in psychiatric disease. Biological psychiatry. 2012;71:484–486. doi: 10.1016/j.biopsych.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaneko Y, Keshavan M. Cognitive remediation in schizophrenia. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology. 2012;10:125–135. doi: 10.9758/cpn.2012.10.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fisher M, Loewy R, Hardy K, Schlosser D, Vinogradov S. Cognitive interventions targeting brain plasticity in the prodromal and early phases of schizophrenia. Annual review of clinical psychology. 2013;9:435–463. doi: 10.1146/annurev-clinpsy-032511-143134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62:1584–1597. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Archives of general psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biological psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rokem A, Yoon JH, Ooms RE, Maddock RJ, Minzenberg MJ, Silver MA. Broader visual orientation tuning in patients with schizophrenia. Frontiers in human neuroscience. 2011;5:127. doi: 10.3389/fnhum.2011.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. The American journal of psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophrenia research. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagihara H, Ohira K, Takao K, Miyakawa T. Transcriptomic evidence for immaturity of the prefrontal cortex in patients with schizophrenia. Molecular brain. 2014;7:41. doi: 10.1186/1756-6606-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 76.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nature genetics. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 77.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Progress in brain research. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.