Abstract
Objective
Dyslipidemia is implicated in abdominal aortic aneurysms (AAAs) in humans and angiotensin (Ang)II-infused mice. This study determined effects of major lipoprotein classes on AngII-induced AAAs using multiple mouse strains with dietary and pharmacological manipulations.
Approach and Results
Western diet had minor effects on plasma cholesterol concentrations and the low incidence of AngII-induced AAAs in C57BL/6J mice. Low incidence of AAAs in this strain was not attributed to protection from HDL, since apolipoprotein (apo)AI deficiency did not increase AngII-induced AAAs. ApoAI deletion also failed to alter AAA occurrence in hypercholesterolemic mice. Low density lipoprotein (LDL) receptor−/− mice fed normal diet had low incidence of AngII-induced AAAs. Western diet feeding of this strain provoked pronounced hypercholesterolemia due to increased apoB-containing lipoproteins with attendant increases of atherosclerosis in both genders, but AAAs only in male mice. ApoE−/− mice fed normal diet were modestly hypercholesterolemic, whereas this strain fed Western diet was severely hypercholesterolemic due to increased apoB-containing lipoprotein concentrations. The latter augmented atherosclerosis, but did not change the high incidence of AAAs in this strain. To determine whether reductions in apoB-containing lipoproteins influenced AngII-induced AAAs, ezetimibe was administered at a dose that partially reduced plasma cholesterol concentrations to apoE−/− mice fed Western diet. This decreased atherosclerosis, but not AAAs. This ezetimibe dose in apoE−/− mice fed normal diet significantly decreased plasma apoB-containing lipoprotein concentrations and reduced AngII-induced AAAs.
Conclusions
ApoB-containing lipoproteins contribute to augmentation of AngII-induced AAA in male mice. However, unlike atherosclerosis, AAA occurrence was not correlated with increases in plasma apoB-containing lipoprotein concentrations.
Keywords: angiotensin, aortic aneurysms, lipoproteins, hypercholesterolemia
INTRODUCTION
Abdominal aortic aneurysms (AAAs) are permanent dilations that portend the devastating consequence of aortic rupture. AAA prevalence and severity have been associated with increased plasma cholesterol concentrations.1,2 Several studies demonstrated that prevalence of AAAs was correlated positively with plasma low-density lipoprotein (LDL)-cholesterol concentrations and negatively correlated with plasma high-density lipoprotein (HDL)-cholesterol concentrations.3–8 However, other studies failed to demonstrate a relationship between either LDL- or HDL-cholesterol concentrations and AAAs.6,9–11 Overall, hypercholesterolemia has a poorly defined role in human AAAs and further clarification is needed on the function of specific lipoprotein fractions. Regardless of an undefined association between hypercholesterolemia and AAAs, statin administration is common for patients afflicted with AAA to reduce aortic expansion, which has beneficial effects in preventing progression of AAAs or improving related cardiovascular events.12,13
Chronic subcutaneous infusion of angiotensin II (AngII) into mice is a commonly used model of AAAs.14 Initial studies using this model were performed in hypercholesterolemic mice that were either LDL receptor−/− fed a Western diet15 or apolipoprotein (apo)E−/− fed either a normal or Western diet.16,17 In addition to a substantial number of studies of AngII-induced AAAs using hypercholesterolemic mice,18 a small number of studies have demonstrated that AngII infusion induces AAA formation in normocholesterolemic mice,12,19–23 albeit at much lower incidence than in hypercholesterolemic mice. Despite the potential role of hypercholesterolemia in formation of AngII-induced AAAs, effects of specific lipoprotein fractions in experimental AAA development have received sparse attention. One study reported that increased plasma HDL-cholesterol concentrations by daily subcutaneous administration of human HDL (10 mg/kg/day of apoAI) reduced AngII-induced AAAs in apoE−/− mice.24 Injection of reconstituted HDL, composed of human apoAI and phosphatidylcholine, also decreased AAA formation in both AngII-infused hypercholesterolemic apoE−/− mice and calcium chloride administered normocholesterolemic C57BL/6 mice.24 Findings from this study implicate potential associations between apoAI and AAAs.
To determine roles of specific lipoprotein fractions in development of AngII-induced AAAs, we used multiple mouse strains with dietary and pharmacological manipulations that resulted in different forms and severity of hypercholesterolemia. The occurrence of AngII-induced AAAs in these mice was contrasted to the formation of atherosclerosis, which has been strongly associated with plasma apoB-containing lipoprotein concentrations in mouse studies. These studies demonstrated that development of AngII-induced AAAs was augmented by elevation of apoB-containing lipoproteins, but not by deficiency of apoAI, the major structural apolipoprotein of HDL. However, unlike atherosclerosis, there was no simple concentration-dependent association of plasma apoB-containing lipoproteins with AngII-induced AAAs.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
Western Diet Had No Effects on AngII-induced AAA Formation in Male C57BL/6 Mice
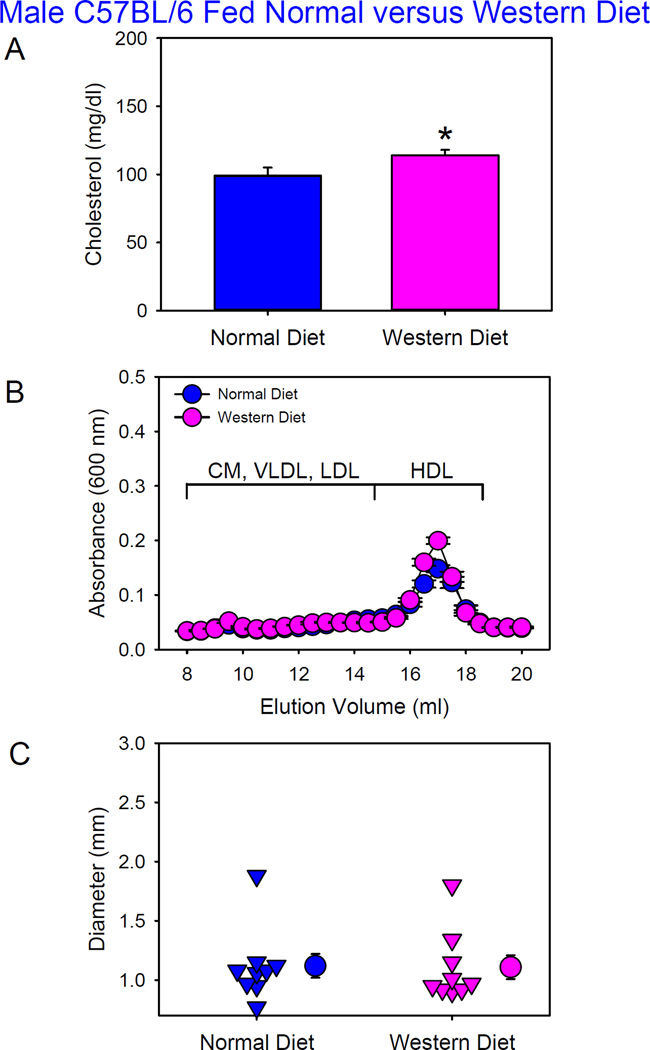
Prolonged feeding of a high fat diet (60% calories from saturated fat) augments AngII-induced AAAs in normocholesterolemic mice that have become obese.25 Therefore, in the initial experiment we determined whether Western diet (42% calories from milk fat) per se had effects on AngII-induced AAAs in wild-type C57BL/6J mice. Male C57BL/6J mice were fed either a normal or Western diet and infused with AngII (1,000 ng/kg/min) for 4 weeks. Western diet feeding started 1 week prior to AngII infusion and was maintained during AngII infusion. There was no significant body weight gain difference between mice fed normal versus Western diet. Western diet feeding modestly increased plasma total cholesterol concentrations in C57BL/6 mice (Figure 1A). With no overt presence of apoB-containing lipoproteins, HDL was the predominant lipoprotein in these mice fed either diet as defined by size exclusion chromatography (Figure 1B). There were no differences of LDL/HDL ratio between C57BL/6 mice fed normal versus Western diet (Table I in Online-only Data Supplement). No discernable atherosclerotic lesions were detected in these mice. One of 10 mice (10%) from each group died of aortic rupture. There were no significant differences in maximal outer diameter of suprarenal aortas between mice fed these two diets (Figure 1C).
Figure 1. Western diet did not augment AngII-induced AAA formation in male C57BL/6 mice.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM. * denotes P=0.03 by Mann-Whitney Rank Sum Test. N=9 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=6–8 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Maximal outer diameters of suprarenal aortas. Triangles are values from individual mice. Circles represent means and error bars are SEM. P=0.6 by Mann-Whitney Rank Sum Test. N=9 per group.
Deficiency of ApoAI Did Not Exacerbate AngII-induced AAA Formation
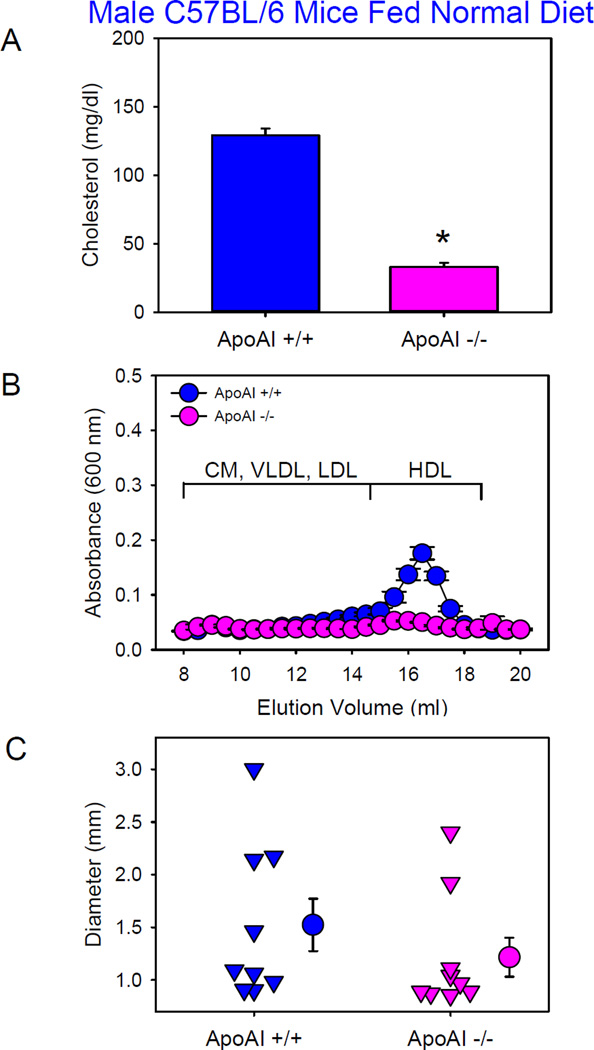
HDL is the major lipoprotein fraction in plasma of male C57BL/6 mice (Figure 1B), and apoAI is the predominant structural apolipoprotein of HDL. To determine whether low HDL augmented AngII-induced AAAs, we compared AngII-induced AAA formation between male apoAI+/+ and −/− mice in a C57BL/6 background fed the normal laboratory diet and infused with AngII (1,000 ng/kg/min) for 4 weeks. Deficiency of ApoAI led to significant reductions of plasma cholesterol concentrations (Figure 2A) due to reductions of HDL-cholesterol concentrations (Figure 2B). One of 10 mice (10%) from each group died of aortic rupture. Deficiency of ApoAI did not augment AngII-induced AAAs in C57BL/6 background (Figure 2C).
Figure 2. Deficiency of ApoAI in male C57BL/6 mice did not exacerbate AngII-induced AAA formation.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM. * denotes P<0.001 by Student’s t test. N=9 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=4 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Maximal outer diameters of suprarenal aortas. Triangles are values from individual mice. Circles represent means and error bars represent SEM. P=0.2 by Mann-Whitney Rank Sum Test. N=9 per group.
Effects of apoAI deficiency were also studied in male LDL receptor−/− mice. Since apoAI deficiency was hypothesized to enhance AngII-induced AAA formation, infusion rates of AngII were selected to create a low incidence of AAAs in apoAI mice to enable demonstration of enhanced AAAs in apoAI−/− mice. In the first experiment, mice were infused with 1,000 ng/kg/min of AngII and fed the normal laboratory diet. Plasma total cholesterol or apoB-containing lipoprotein concentrations were not significantly different between the two apoAI genotypes (Figure IA and IB in the Online-only Data Supplement), whereas plasma HDL-cholesterol was barely detectable in mice with apoAI deficiency fed the normal laboratory diet (Figure IB in the Online-only Data Supplement). Atherosclerotic lesions were minimal and not significantly different between the two genotypes (Figure IC in the Online-only Data Supplement). Consistent with findings in C57BL/6 mice, apoAI deficiency in LDL receptor−/− mice had no effects on AngII-induced AAA formation (Figure ID in the Online-only Data Supplement). Subsequently, we compared AngII-induced AAAs using an infusion rate of 500 ng/kg/min between apoAI+/+ and −/− mice with LDL receptor−/− background that were fed the Western diet. ApoAI deficiency led to profound reductions of plasma cholesterol concentrations with barely detectable HDL (Figure IIA and B in the Online-only Data Supplement). Atherosclerotic lesions were modestly reduced in mice with apoA-I deficiency (Figure IIC in the Online-only Data Supplement). In agreement with the other studies described above, apoAI deficiency in LDL receptor−/− mice fed the Western diet did not exacerbate AngII-induced AAA formation (Figure IID in the Online-only Data Supplement). One of 12 mice that were wild type for apoAI died of abdominal aortic rupture, whereas no apoAI−/− mice died of aortic rupture.
Hypercholesterolemia Augmented AngII-induced AAA in Male LDL Receptor −/− Mice
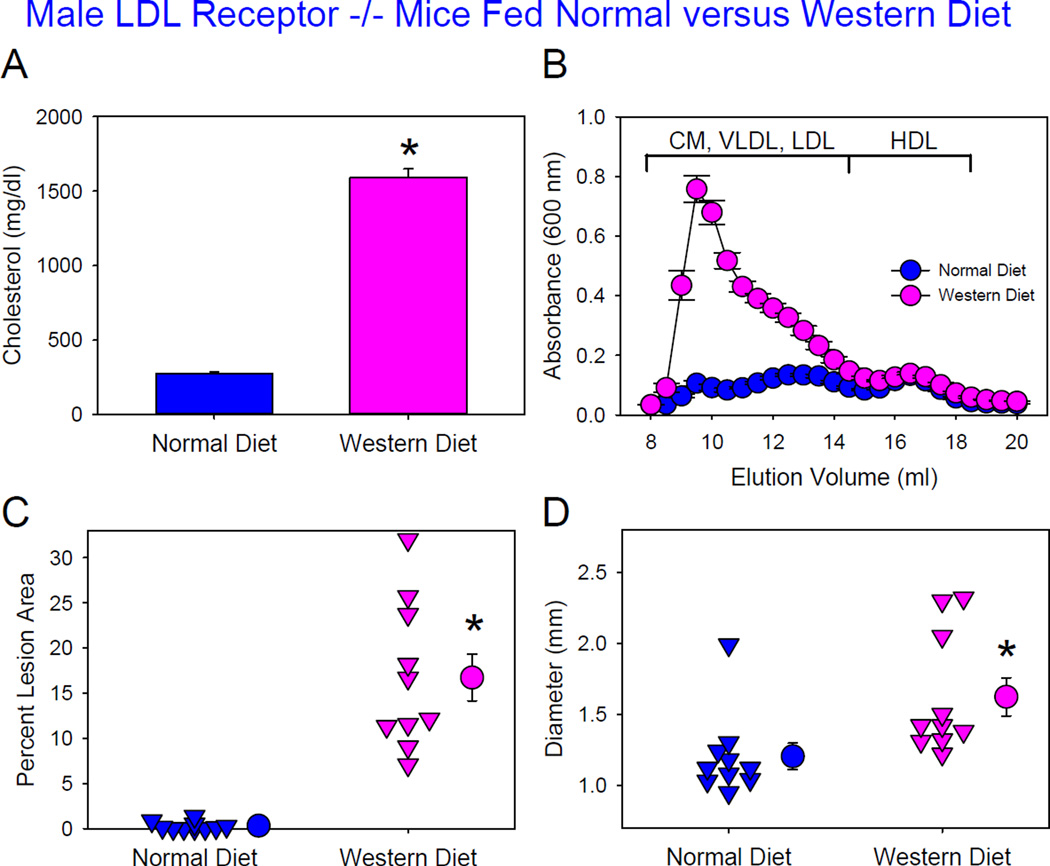
LDL receptor−/− mice fed normal laboratory diet are modestly hyper-cholesterolemic with comparable cholesterol distributions between apoB-containing lipoproteins and HDL. Previous studies have reported high susceptibility of this mouse model fed Western diet to AngII-induced AAAs.23,25,26 However, AngII-induced AAAs have not been reported in this mouse model fed normal laboratory diet. To determine whether augmented hypercholesterolemia influenced AAA formation in LDL receptor−/− background, male mice of this genotype were fed either normal or Western diet and infused with AngII 1,000 ng/kg/min for 4 weeks. Western diet feeding greatly increased plasma total cholesterol concentrations compared to mice fed normal laboratory diet (Figure 3A). These increases were solely attributed to increased apoB-containing lipoproteins that included chylomicrons, chylomicron remnants, very low density lipoprotein (VLDL), and LDL (Figure 3B). LDL/HDL ratios were increased in male LDL receptor−/−mice fed Western diet, compared to those fed a normal laboratory diet (Table I in Online-only Data Supplement). As expected, the greatly augmented hypercholesterolemia promoted by Western diet feeding profoundly increased atherosclerotic lesions (Figure 3C). Western diet also significantly increased AAA formation, as defined by maximal outer diameters of suprarenal aortas (Figure 3D). No deaths due to aortic rupture occurred in these mice.
Figure 3. Hypercholesterolemia increased AngII-induced AAA formation in male LDL receptor −/− mice.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM. * denotes P<0.001 by Mann-Whitney Rank Sum Test. N=10 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=10 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Atherosclerosis in the aortic arch region. Triangles are values from individual mice. Circles represent means and error bars represent SEM. * denotes P<0.001 by Mann-Whitney Rank Sum Test. N=10 per group. (D) Maximal outer diameters of suprarenal aortas. Triangles are values from individual mice. Circles represent means and error bars represent SEM. * denotes P=0.002 by Mann-Whitney Rank Sum Test. N=10 per group.
Male sex has been demonstrated to enhance AngII-induced AAA formation in apoE−/− mice.27–29 To determine whether hypercholesterolemia has similar effects in both sexes, female LDL receptor−/− mice fed either normal or Western diet were infused with AngII 1,000 ng/kg/min for 4 weeks. As with males, Western diet feeding profoundly increased plasma cholesterol concentrations due to increased apoB-containing lipoprotein cholesterol concentrations (Figure IIIA and B in the Online-only Data Supplement). Consistent with data from male LDL receptor−/− mice, Western diet feeding augmented atherosclerotic lesions (Figure IIIC in the Online-only Data Supplement). Despite equivalent elevations in plasma cholesterol concentrations as male mice, Western diet feeding did not augment AngII-induced AAAs in female LDL receptor−/− mice (Figure IIID in the Online-only Data Supplement). No deaths due to aortic rupture occurred in female mice.
Comparable AngII-induced AAA Formation in Male ApoE−/− Mice Fed Normal versus Western Diet
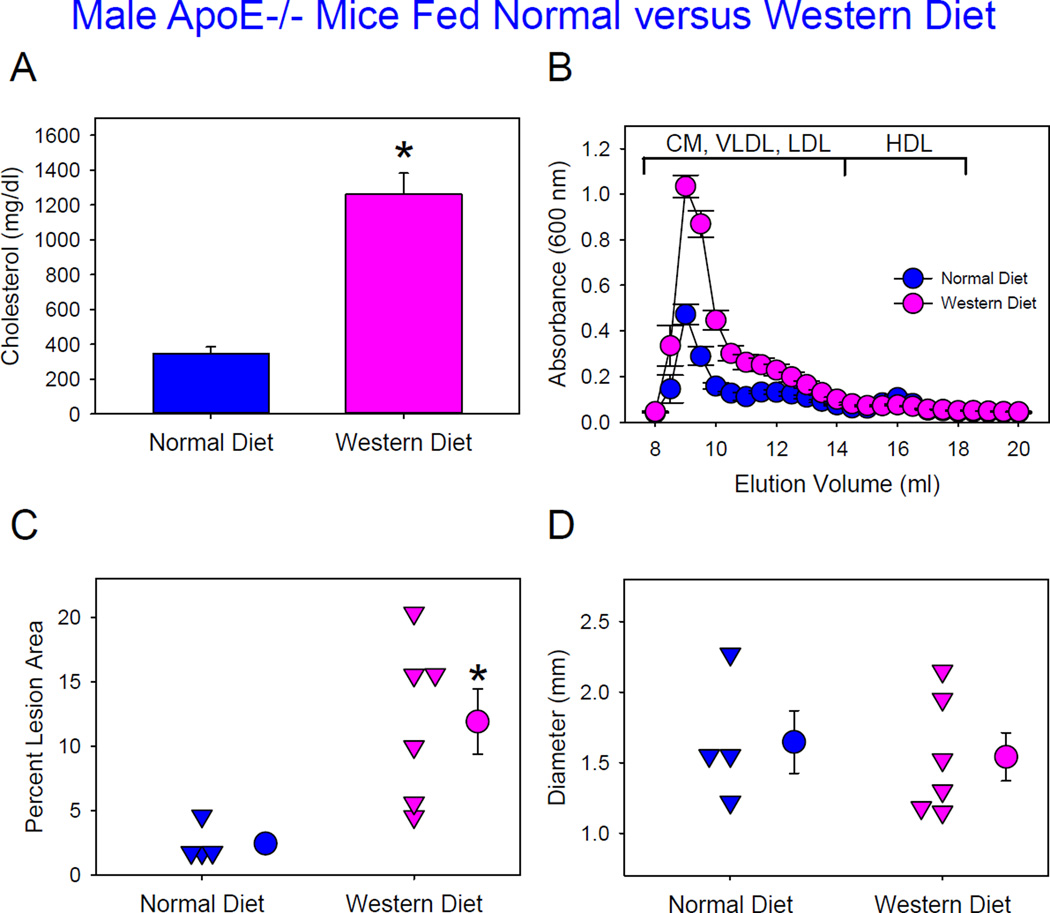
Although there have been no direct comparisons, it is inferred from the literature that AngII-induced AAAs are equivalent between apoE−/− mice fed normal versus Western diet.16,17 To compare directly, male apoE−/− mice were fed either normal or Western diet and infused with AngII 1,000 ng/kg/min. ApoE−/− mice fed a normal laboratory diet had plasma cholesterol concentrations of 347 ± 38 mg/dl, and Western diet feeding led to profound increases (1260 ± 121 mg/dl) due to increased apoB-containing lipoprotein cholesterol concentrations (Figure 4A and B). In agreement with findings in LDL receptor−/− mice, Western diet feeding significantly increased atherosclerotic lesions (Figure 4C). However, there was no difference in AngII-induced AAAs (Figure 4D) or death due to aortic rupture (60% versus 40%) between mice fed normal and Western diets.
Figure 4. Modest and severe hypercholesterolemia had equivalent effects on AngII-induced AAA formation in male ApoE−/− mice.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM. * denotes P=0.01 by Mann-Whitney Rank Sum Test. N=4–6 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=4–5 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Atherosclerosis in aortic arch region. Triangles are values from individual mice. Circles represent means and error bars represent SEM. * denotes P=0.02 by Mann-Whitney Rank Sum Test. N=4–6 per group. (D) Maximal outer diameters of suprarenal aorta. Triangles are values from individual mice. Circles represent means and error bars represent SEM for each group. P=0.7 by Student’s t test. N=4–6 per group.
Reduction of Plasma ApoB-containing Lipoproteins Attenuated AngII-induced AAA Formation in Male ApoE −/− Mice Fed Normal Diet
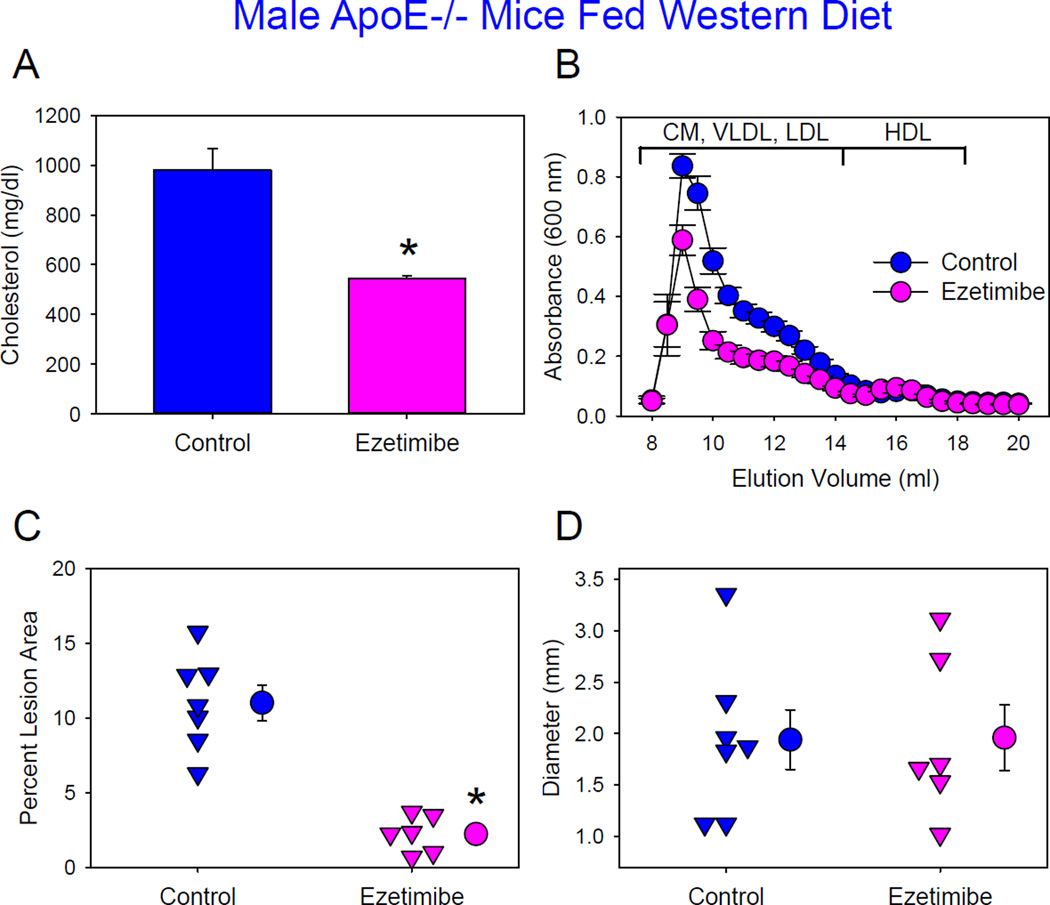
Results from LDL receptor−/− mice fed Western diet and apoE−/− mice fed either diet implicate that high concentrations of plasma apoB-containing lipoproteins contribute to augmentation of AngII-induced AAAs. ApoE−/− mice are endogenously hypercholesterolemic even when fed the normal laboratory diet. Therefore, to determine whether reduced apoB-containing lipoprotein concentrations attenuate development of AngII-induced AAAs in male apoE−/− mice, we administered an intestinal cholesterol absorption inhibitor, ezetimibe, started one week prior to initiating AngII infusion (1,000 ng/kg/min). In male apoE−/− mice fed Western diet, plasma cholesterol concentrations were reduced compared to mice not administered the drug. However, this concentration (546 mg/dl) was still high, with a predominance of high plasma apoB-containing lipoproteins (Figure 5A and B). This reduction was associated with attenuation of atherosclerosis (Figure 5C), whereas dilation of suprarenal aortas (Figure 6D) and death due to aortic rupture (control versus ezetimibe: 30% versus 40%) were not significantly different.
Figure 5. Administration of ezetimibe reduced atherosclerosis, but not AngII-induced AAA formation in male ApoE−/− mice fed Western diet.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM for each group. * P=0.001 by Mann-Whitney Rank Sum Test. N=6–7 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=4–7 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Atherosclerosis in the aortic arch region. Triangles are values from individual mice. Circles represent means and error bars represent SEM. N=6–8 per group. (D) Maximal outer diameters of suprarenal aortas. Triangles are values from individual mice. Circles represent means and error bars represent SEM. *P=0.965 by Student’s t test. N=6–8 per group.
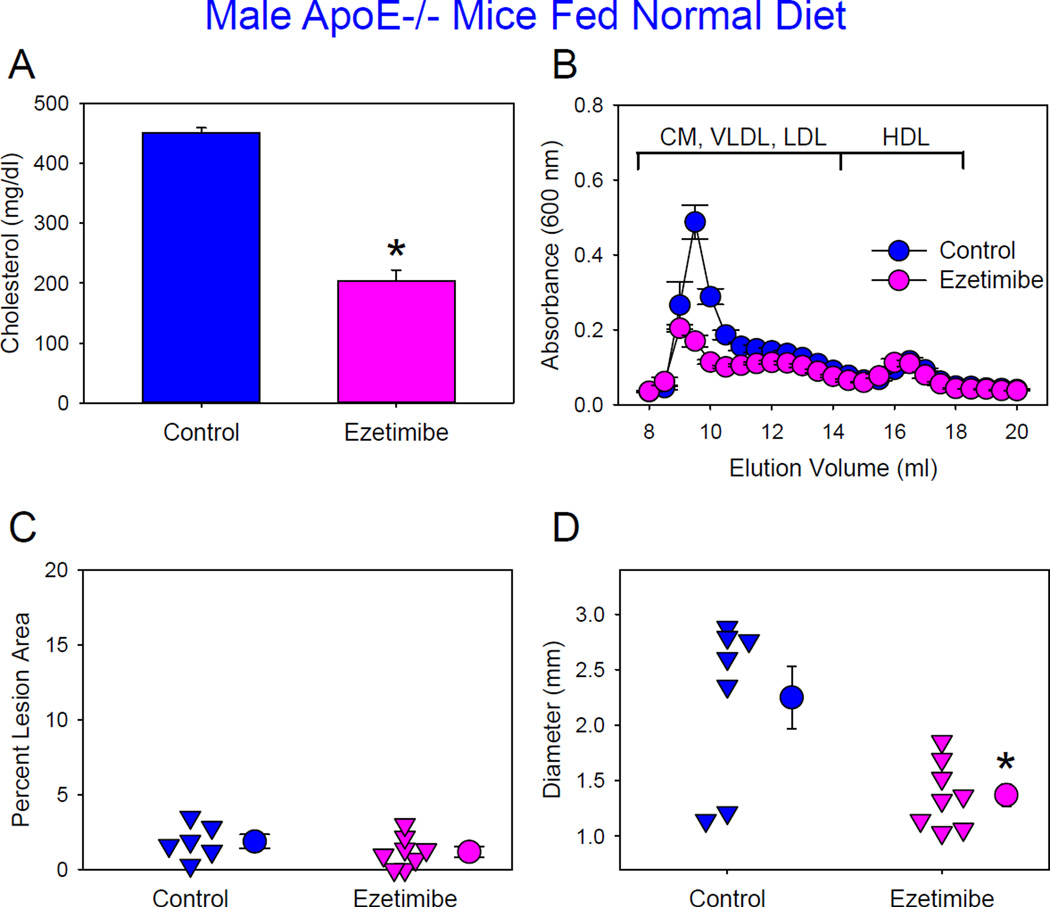
Figure 6. Administration of ezetimibe reduced AngII-induced AAA formation in male ApoE−/− mice fed normal laboratory diet.
(A) Plasma cholesterol concentrations. Histobars are means and error bars represent SEM. * denotes P<0.001 by Student’s t test. N=6–8 per group. (B) Plasma lipoprotein distributions were resolved by size exclusion chromatography. Circles and error bars are means ± SEM. N=4–7 per group. CM: chylomicrons, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, and HDL: high-density lipoprotein. (C) Atherosclerosis in aortic arch regions. Triangles are values from individual mice. Circles represent means and error bars represent SEM. P=0.256 by Student’s t test. N=6–8 per group. (D) Maximal outer diameters of suprarenal aortas. Triangles are values from individual mice. Circles represent means and error bars represent SEM. P=0.009 by Student’s t test. N=6–8 per group.
The same dose of ezetimibe decreased plasma cholesterol concentrations from 451 ± 8 mg/dl to 204 ± 18 mg/dl in male apoE−/− mice fed a normal laboratory diet (Figure 6A) and infused with AngII (1,000 ng/kg/min). This is similar to plasma cholesterol concentrations in LDL receptor−/− mice fed the same diet. This reduction was due to decreased plasma apoB-containing lipoproteins (Figure 6B). Atherosclerotic lesion sizes were minimal in both groups. Aortic rupture rate was comparable between two groups (control versus ezetimibe: 30% versus 20%). However, administration of ezetimibe decreased AngII-induced AAA formation as determined by maximal outer diameters of suprarenal aortas (Figure 6D).
DISCUSSION
Aberrant metabolism of specific lipoprotein fractions, particularly LDL and HDL, is associated with atherosclerotic diseases and modulation of their plasma concentrations is a tenet of therapeutic strategies.30,31 Dysfunctional lipoprotein metabolism has also been implicated in human AAA formation, although associations have not been studied extensively.1 In this study, AngII-induced AAAs in multiple mouse models with different plasma lipoprotein distributions were used to determine whether facets of dyslipidemia directly associated with AAA formation. We were unable to define any effect of reduced HDL-cholesterol concentrations, promoted by apoAI deficiency, on AngII-induced AAA formation in two mouse strains with dietary manipulations. However, our findings clearly demonstrated that hypercholesterolemia augmented the development of AngII-induced AAAs in mice. Changes in large sized lipoprotein particles were associated with augmentation of AngII-induced AAAs. The only feature in common for these lipoproteins was the presence of apoB. However, unlike atherosclerosis in which plasma apoB-containing lipoprotein concentrations closely correlated with lesion size, AAAs were augmented with modestly hypercholesterolemic states, but not further enhanced with progressive increases in hypercholesterolemia.
HDL-cholesterol has been associated with AAAs in humans in a limited number of observational studies.3,4,6,32–34 Recent experimental evidence consistent with this association demonstrated that administration of exogenous native or reconstituted HDL reduced AngII-induced AAAs.24 We performed the converse study in which plasma HDL concentrations were reduced markedly by genetic deficiency of apoAI. ApoAI deficiency did not augment AngII-induced AAAs in C57BL/6 mice fed a normal laboratory diet. This lack of effect was also demonstrated in LDL receptor−/− mice fed either normal or Western diet. Therefore, depletion of endogenous apoAI had no discernable effect on AngII-induced AAAs. The mechanisms by which exogenously delivered apoAI and its endogenous reduction have differential effects on AngII-induced AAAs are unclear. A similar contrast of HDL supplementation versus genetic deficiency has been observed in atherosclerosis studies, with overexpression of apoAI reducing atherosclerosis,35–37 but apoAI deficiency did not augment atherosclerosis.38,39 ApoAI deficiency was reported to be associated with40 or have no correlations with41 coronary heart disease in humans. Although a few studies suggested that low plasma apoAI concentrations were associated with AAAs in patients,6,34,42 no studies reported whether apoAI deficiency was associated with the development of AAAs.
Chronic AngII infusion into normocholesterolemic mice induces AAAs at a rate that has ranged from 5% to 40%.19–23,25 In contrast, the incidence of AngII-induced AAAs in hypercholesterolemic mice has routinely been over 70%.19,20,23,25,43 Hypercholesterolemic mice in these previous studies have included LDL receptor−/− mice fed Western diet15 or apoE−/− mice fed either normal or Western diet.16,17 Ezetimibe lowered plasma cholesterol concentrations in apoE −/− mice fed normal laboratory diet to approximately 200 mg/dl. This reduction in plasma apoB-containing lipoproteins significantly attenuated abdominal aortic dilation. Ezetimibe also reduced the extent of hypercholesterolemia in apoE−/− mice fed Western diet. While this reduction decreased atherosclerosis, it did not attenuate aortic dilation. This result demonstrated that reduced AAAs in apoE−/− mice administered ezetimibe and fed normal laboratory diet was not attributed to a direct effect of the drug. These findings from male LDL receptor−/− or apoE−/− mice fed normal versus Western diet, coupled with results using ezetimibe, demonstrate that substantial reductions in apoB-containing lipoproteins are required to attenuate AngII-induced AAAs in mice. Consistent with our findings, increased concentrations of plasma LDL, a major class of apoB-containing lipoproteins, have been associated with AAAs in humans.1,3,44–47
ApoB-containing lipoproteins encompass a heterogeneous mix of lipoprotein particles with a range of lipid and apolipoprotein compositions. One study reported that LDL incubation with cultured human smooth muscle cells increased AT1 receptor mRNA and inferred this was the cause of increased response to AngII in hypercholesterolemic patients.48 However, although whole body depletion of AT1a receptors ablates AngII-induced increases of AAAs in hypercholesterolemic mice,49 deletion of AT1a receptors specifically in smooth muscle cells had no effects on AngII-induced AAAs.50,51 One mechanism of AngII-induced AAAs is that AngII promotes leukocyte infiltration both systemically and locally into the aortic wall.43,52,53 Hypercholesterolemia also promotes leukocyte infiltration in arteries,54 or facilitates AngII-induced leukocyte mobilization into the arterial wall.55 In our future studies, it will be important to explore mechanisms by which hypercholesterolemia and AngII have synergistic effects on leukocyte infiltration to promote AAA formation.
We have demonstrated previously that AngII-induced AAAs in apoE−/− mice have a strong sexual dimorphism with males being much more susceptible to aortic expansion.56 This dimorphism is ablated by orchidectomy.27–29 The current study demonstrated this dimorphism also exists in LDL receptor−/− mice and that Western diet feeding increased plasma cholesterol concentrations to a similar magnitude in both sexes and led to equivalent atherosclerotic development. However, there was no difference on AngII-induced AAA formation in female mice fed the Western versus normal diet. Mechanisms of male gender preference in hypercholesterolemic mice remain to be defined.
In summary, this study demonstrated that increased plasma concentrations of apoB-containing lipoproteins are associated with augmentation of AngII-induced AAAs in a male-gender specific manner. Most interestingly, unlike atherosclerosis, AngII-induced AAAs were not further augmented with progressive increases of plasma apoB-containing lipoprotein concentrations.
Supplementary Material
SIGNIFICANCE.
Associations of AAAs with dyslipidemia have been inferred in human studies. To determine effects of specific lipoproteins on AAA formation, AngII was infused into mice with different extents of hypercholesterolemia and lipoprotein characteristics that were generated in several mouse strains under selected dietary and pharmacological manipulations. This study has demonstrated that (1) reductions of HDL attributed to ApoAI deficiency have no effects on AngII-induced AAAs; (2) ApoB-containing lipoproteins contribute to augmentation of AngII-induced AAAs in male, but not female mice; and (3) unlike atherosclerosis, this contribution to augmentation of AngII-induced AAA does not depend on plasma apoB-containing lipoprotein concentrations. These findings provide new insights into understanding associations of AAAs with dyslipidemia.
ACKNOWLEDGMENTS
We thank Merck & Company, Inc for providing ezetimibe, Dr. Harry Davis (Merck) for providing information of ezetimibe dosage in mice, and Manal Zabalawi (Wake Forest University School of Medicine) for generating breeding pairs of apoA-I +/+ and −/−×LDL receptor −/− mice.
SOURCES OF FUNDING
This research work was supported by grants (HL107319 to Alan Daugherty, HL107326 to Lisa Cassis, and HL112270 and HL112276 to Mary Sorci-Thomas) from the National Institutes of Health of the United States of America. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AAAs
Abdominal aortic aneurysms
- AngII
Angiotensin II
- Apo
Apolipoprotein
- HDL
High density lipoprotein
- LDL
Low density lipoprotein
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 3.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 4.Norrgard O, Angquist KA, Johnson O. Familial aortic aneurysms: serum concentrations of triglyceride, cholesterol, HDL-cholesterol and (VLDL + LDL)-cholesterol. Br J Surg. 1985;72:113–116. doi: 10.1002/bjs.1800720215. [DOI] [PubMed] [Google Scholar]
- 5.Bradley DT, Hughes AE, Badger SA, et al. A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet. 2013;6:498–504. doi: 10.1161/CIRCGENETICS.113.000165. [DOI] [PubMed] [Google Scholar]
- 6.Simoni G, Gianotti A, Ardia A, Baiardi A, Galleano R, Civalleri D. Screening study of abdominal aortic aneurysm in a general population: lipid parameters. Cardiovasc Surg. 1996;4:445–448. doi: 10.1016/0967-2109(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study - The Tromso study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 8.Wanhainen A, Bjorck M, Boman K, Rutegard J, Bergqvist D. Influence of diagnostic criteria on the prevalence of abdominal aortic aneurysm. J Vasc Surg. 2001;34:229–235. doi: 10.1067/mva.2001.115801. [DOI] [PubMed] [Google Scholar]
- 9.Louwrens HD, Adamson J, Powell JT, Greenhalgh RM. Risk factors for atherosclerosis in men with stenosing or aneurysmal disease of the abdominal aorta. Int Angiol. 1993;12:21–24. [PubMed] [Google Scholar]
- 10.Blann AD, Kirkpatrick U, Devine C, Naser S, McCollum CN. The influence of acute smoking on leucocytes, platelets and the endothelium. Atherosclerosis. 1998;141:133–139. [PubMed] [Google Scholar]
- 11.Rizzo M, Krayenbuhl PA, Pernice V, Frasheri A, Battista Rini G, Berneis K. LDL size and subclasses in patients with abdominal aortic aneurysm. Int J Cardiol. 2009;134:406–408. doi: 10.1016/j.ijcard.2007.12.082. [DOI] [PubMed] [Google Scholar]
- 12.Van Kuijk JP, Flu WJ, Witteveen OP, Voute M, Bax JJ, Poldermans D. The influence of statins on the expansion rate and rupture risk of abdominal aortic aneurysms. J Cardiovasc Surg (Torino) 2009;50:599–609. [PubMed] [Google Scholar]
- 13.de Ceniga MV, Blanco-Colio LM, Tunon J, Egido J, Martin-Ventura JL. Statin use in aortic aneurismal disease to prevent progression and cardiovascular events: review of experimental and clinical data C. Curr Vasc Pharmacol. 2013;11:299–304. doi: 10.2174/1570161111311030004. [DOI] [PubMed] [Google Scholar]
- 14.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 15.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Daugherty A, Lu H. Angiotensin II and abdominal aortic aneurysms: an update. Curr Pharm Design. 2015 doi: 10.2174/1381612821666150826093318. In press. [DOI] [PubMed] [Google Scholar]
- 19.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 20.King VL, Trivedi D, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–1143. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 21.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida HA, Poduri A, Subramanian V, Cassis LA, Daugherty A. Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:2845–2852. doi: 10.1161/ATVBAHA.111.234997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torsney E, Pirianov G, Charolidi N, Shoreim A, Gaze D, Petrova S, Laing K, Meisinger T, Xiong W, Baxter BT, Cockerill GW. Elevation of plasma high-density lipoproteins inhibits development of experimental abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2012;32:2678–2686. doi: 10.1161/ATVBAHA.112.00009. [DOI] [PubMed] [Google Scholar]
- 25.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens AP, 3rd, Passam FH, Antoniak S, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 28.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–e85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Daugherty A. Atherosclerosis: cell biology and lipoproteins. Curr Opin Lipidol. 2015;26:152–153. doi: 10.1097/MOL.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo M, Pernice V, Frasheri A, Di Lorenzo G, Rini GB, Spinas GA, Berneis K. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clin Endocrinol (Oxf) 2009;70:870–875. doi: 10.1111/j.1365-2265.2008.03407.x. [DOI] [PubMed] [Google Scholar]
- 33.Golledge J, van Bockxmeer F, Jamrozik K, McCann M, Norman PE. Association between serum lipoproteins and abdominal aortic aneurysm. Am J Cardiol. 2010;105:1480–1484. doi: 10.1016/j.amjcard.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 34.Ahnstrom J, Gottsater A, Lindblad B, Dahlback B. Plasma concentrations of apolipoproteins A-I, B and M in patients with abdominal aortic aneurysms. Clin Biochem. 2010;43:407–410. doi: 10.1016/j.clinbiochem.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Major AS, Dove DE, Ishiguro H, Su YR, Brown AM, Liu L, Carter KJ, Linton MF, Fazio S. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI((−/−)) mice. Arterioscler Thromb Vasc Biol. 2001;21:1790–1795. doi: 10.1161/hq1101.097798. [DOI] [PubMed] [Google Scholar]
- 36.Valenta DT, Bulgrien JJ, Banka CL, Curtiss LK. Overexpression of human ApoAI transgene provides long-term atheroprotection in LDL receptor-deficient mice. Atherosclerosis. 2006;189:255–263. doi: 10.1016/j.atherosclerosis.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Su YR, Blakemore JL, Zhang Y, Linton MF, Fazio S. Lentiviral transduction of apoAI into hematopoietic progenitor cells and macrophages: applications to cell therapy of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1439–1446. doi: 10.1161/ATVBAHA.107.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SH, Reddick RL, Avdievich E, Surles LK, Jones RG, Reynolds JB, Quarfordt SH, Maeda N. Paradoxical enhancement of atherosclerosis by probucol treatment in apolipoprotein E-deficient mice. J. Clin. Invest. 1997;99:2858–2866. doi: 10.1172/JCI119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plummer MR, Hasty AH. Atherosclerotic lesion formation and triglyceride storage in obese apolipoprotein AI-deficient mice. J Nutr Biochem. 2008;19:664–673. doi: 10.1016/j.jnutbio.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Yokota H, Hashimoto Y, Okubo S, Yumoto M, Mashige F, Kawamura M, Kotani K, Usuki Y, Shimada S, Kitamura K, Nakahara K. Apolipoprotein A-I deficiency with accumulated risk for CHD but no symptoms of CHD. Atherosclerosis. 2002;162:399–407. doi: 10.1016/s0021-9150(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 41.Ikewaki K, Matsunaga A, Han H, Watanabe H, Endo A, Tohyama J, Kuno M, Mogi J, Sugimoto K, Tada N, Sasaki J, Mochizuki S. A novel two nucleotide deletion in the apolipoprotein A-I gene, apoA-I Shinbashi, associated with high density lipoprotein deficiency, corneal opacities, planar xanthomas, and premature coronary artery disease. Atherosclerosis. 2004;172:39–45. doi: 10.1016/j.atherosclerosis.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Burillo E, Lindholt JS, Molina-Sanchez P, et al. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb Haemost. 2015;113 doi: 10.1160/TH14-10-0874. In press. [DOI] [PubMed] [Google Scholar]
- 43.Owens AP, 3rd, Rateri DL, Howatt DA, Moore KJ, Tobias PS, Curtiss LK, Lu H, Cassis LA, Daugherty A. MyD88 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation independent of signaling through toll-like receptors 2 and 4. Arterioscler Thromb Vasc Biol. 2011;31:2813–2819. doi: 10.1161/ATVBAHA.111.238642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Plasma tissue plasminogen activator and abdominal aortic aneurysm presence: a systematic review and meta-analysis. Ann Vasc Surg. 2010;24:686–689. doi: 10.1016/j.avsg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Hobbs SD, Claridge MW, Quick CR, Day NE, Bradbury AW, Wilmink AB. LDL cholesterol is associated with small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;26:618–622. doi: 10.1016/s1078-5884(03)00412-x. [DOI] [PubMed] [Google Scholar]
- 46.Harrison SC, Smith AJ, Jones GT, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34:3707–3716. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naydeck BL, Sutton-Tyrrell K, Schiller KD, Newman AB, Kuller LH. Prevalence and risk factors for abdominal aortic aneurysms in older adults with and without isolated systolic hypertension. Am J Cardiol. 1999;83:759–764. doi: 10.1016/s0002-9149(98)00985-0. [DOI] [PubMed] [Google Scholar]
- 48.Strehlow K, Wassmann S, Bohm M, Nickenig G. Angiotensin AT1 receptor over-expression in hypercholesterolaemia. Ann Med. 2000;32:386–389. doi: 10.3109/07853890008995944. [DOI] [PubMed] [Google Scholar]
- 49.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 50.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One. 2012;7:e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 53.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE(−/−) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tall AR, Yvan-Charvet L, Westerterp M, Murphy AJ. Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol. 2012;32:2547–2552. doi: 10.1161/ATVBAHA.112.300134. [DOI] [PubMed] [Google Scholar]
- 55.Mellak S, Ait-Oufella H, Esposito B, Loyer X, Poirier M, Tedder TF, Tedgui A, Mallat Z, Potteaux S. Angiotensin II Mobilizes Spleen Monocytes to Promote the Development of Abdominal Aortic Aneurysm in Apoe−/− Mice. Arterioscler Thromb Vasc Biol. 2015;35:378–388. doi: 10.1161/ATVBAHA.114.304389. [DOI] [PubMed] [Google Scholar]
- 56.Manning MW, Cassis LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med. 2002;7:45–54. doi: 10.1191/1358863x02vm413ra. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.