Abstract
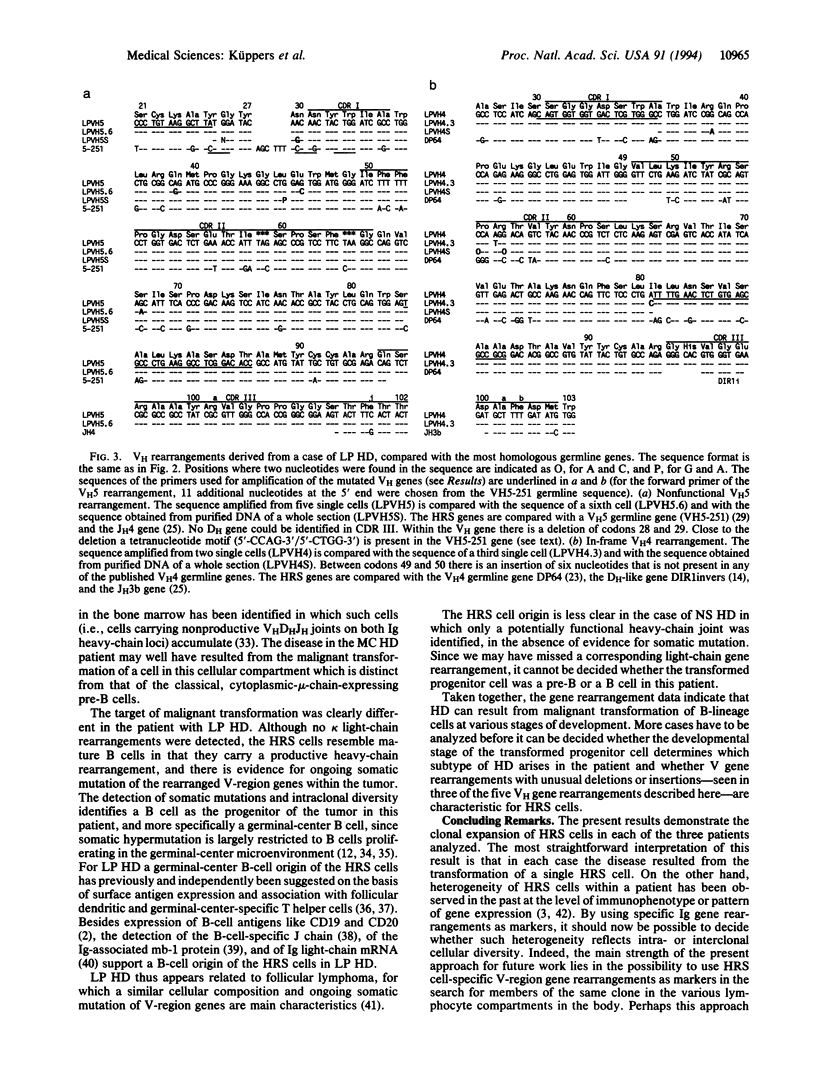
Hodgkin disease (HD) is characterized by a small number of putative malignant cells [Hodgkin and Reed-Sternberg (HRS) cells] among a background of lymphocytes and histiocytes. The lineage of HRS cells is still elusive and a clonal origin of these rare cells has not formally been demonstrated. We isolated HRS cells by micromanipulation from histological sections of three cases of Hodgkin lymphoma (each representing a distinct subtype of the disease) and analyzed individual cells for immunoglobulin variable (V) gene rearrangements by PCR. In each of the three cases a single heavy-chain V (VH) (and in one case, in addition, a kappa light-chain) gene rearrangement was amplified from the HRS cells, identifying these cells as members of a single clone. A potentially functional VH rearrangement was obtained from a case of nodular sclerosis HD. Somatic mutations and intraclonal diversity in the VH genes indicate a germinal center B-cell origin of the HRS cells in a case of lymphocyte-predominant HD, whereas in a case of mixed-cellularity HD the sequence analysis revealed only nonfunctional V gene rearrangements, suggesting a pre-B-cell origin. This indicates that HRS cells can originate from B-lineage cells at various stages of development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavaikko M. J., Hansmann M. L., Nebendahl C., Parwaresch M. R., Lennert K. Follicular dendritic cells in Hodgkin's disease. Am J Clin Pathol. 1991 Feb;95(2):194–200. doi: 10.1093/ajcp/95.2.194. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos I., Herbst H., Niedobitek G., Stein H. Demonstration of monoclonal EBV genomes in Hodgkin's disease and Ki-1-positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood. 1989 Aug 1;74(2):810–816. [PubMed] [Google Scholar]
- Bargou R. C., Mapara M. Y., Zugck C., Daniel P. T., Pawlita M., Döhner H., Dörken B. Characterization of a novel Hodgkin cell line, HD-MyZ, with myelomonocytic features mimicking Hodgkin's disease in severe combined immunodeficient mice. J Exp Med. 1993 May 1;177(5):1257–1268. doi: 10.1084/jem.177.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Morrison S. L. A common sequence motif near nonhomologous recombination breakpoints involving Ig sequences. J Immunol. 1993 Jun 15;150(12):5350–5360. [PubMed] [Google Scholar]
- Chu W. S., Abbondanzo S. L., Frizzera G. Inconsistency of the immunophenotype of Reed-Sternberg cells in simultaneous and consecutive specimens from the same patients. A paraffin section evaluation in 56 patients. Am J Pathol. 1992 Jul;141(1):11–17. [PMC free article] [PubMed] [Google Scholar]
- Diehl V., von Kalle C., Fonatsch C., Tesch H., Jüecker M., Schaadt M. The cell of origin in Hodgkin's disease. Semin Oncol. 1990 Dec;17(6):660–672. [PubMed] [Google Scholar]
- Drexler H. G. Recent results on the biology of Hodgkin and Reed-Sternberg cells. I. Biopsy material. Leuk Lymphoma. 1992 Nov;8(4-5):283–313. doi: 10.3109/10428199209051008. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Martin V., Müller W., Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994 Jul 1;4(7):573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Graninger W. B., Goldman P. L., Morton C. C., O'Brien S. J., Korsmeyer S. J. The kappa-deleting element. Germline and rearranged, duplicated and dispersed forms. J Exp Med. 1988 Feb 1;167(2):488–501. doi: 10.1084/jem.167.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Hell K., Pringle J. H., Hansmann M. L., Lorenzen J., Colloby P., Lauder I., Fischer R. Demonstration of light chain mRNA in Hodgkin's disease. J Pathol. 1993 Oct;171(2):137–143. doi: 10.1002/path.1711710211. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Szabolcs M. J., Yee H. T., Corradini P., Cesarman E., Knowles D. M. Detection of immunoglobulin gene rearrangement of B cell non-Hodgkin's lymphomas and leukemias in fresh, unfixed and formalin-fixed, paraffin-embedded tissue by polymerase chain reaction. Lab Invest. 1993 Jun;68(6):746–757. [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Kanzaki T., Kubonishi I., Eguchi T., Yano S., Sonobe H., Ohyashiki J. H., Ohyashiki K., Toyama K., Ohtsuki Y., Miyoshi I. Establishment of a new Hodgkin's cell line (HD-70) of B-cell origin. Cancer. 1992 Feb 15;69(4):1034–1041. doi: 10.1002/1097-0142(19920215)69:4<1034::aid-cncr2820690434>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R. Immunoglobulin heavy chain gene VH-D junctional diversity at diagnosis in patients with acute lymphoblastic leukemia. Blood. 1993 Feb 1;81(3):775–782. [PubMed] [Google Scholar]
- Kuzu I., Delsol G., Jones M., Gatter K. C., Mason D. Y. Expression of the Ig-associated heterodimer (mb-1 and B29) in Hodgkin's disease. Histopathology. 1993 Feb;22(2):141–144. doi: 10.1111/j.1365-2559.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M. L., Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993 Dec 15;12(13):4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Levy S., Cleary M. L., Carroll W., Kon S., Bird J., Sklar J. Somatic mutation in human B-cell tumors. Immunol Rev. 1987 Apr;96:43–58. doi: 10.1111/j.1600-065x.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E. Cutting and closing without recombination in V(D)J joining. EMBO J. 1991 Dec;10(12):3631–3639. doi: 10.1002/j.1460-2075.1991.tb04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Ford A. M., Wiedemann L. M., Chan L. C., Furley A. J., Greaves M. F., Molgaard H. V. Rearrangement of immunoglobulin heavy chain genes in human T leukaemic cells shows preferential utilization of the D segment (DQ52) nearest to the J region. EMBO J. 1986 Dec 20;5(13):3467–3473. doi: 10.1002/j.1460-2075.1986.tb04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Victor K., Lelsz D., Spellerberg M. B., Hamblin T. J., Thompson K. M., Randen I., Natvig J., Capra J. D., Stevenson F. K. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991 Jun 15;146(12):4385–4391. [PubMed] [Google Scholar]
- Poppema S., Kaleta J., Hepperle B. Chromosomal abnormalities in patients with Hodgkin's disease: evidence for frequent involvement of the 14q chromosomal region but infrequent bcl-2 gene rearrangement in Reed-Sternberg cells. J Natl Cancer Inst. 1992 Dec 2;84(23):1789–1793. doi: 10.1093/jnci/84.23.1789. [DOI] [PubMed] [Google Scholar]
- Poppema S. Lymphocyte-predominance Hodgkin's disease. Int Rev Exp Pathol. 1992;33:53–79. doi: 10.1016/b978-0-12-364933-1.50008-4. [DOI] [PubMed] [Google Scholar]
- Reed T. J., Reid A., Wallberg K., O'Leary T. J., Frizzera G. Determination of B-cell clonality in paraffin-embedded lymph nodes using the polymerase chain reaction. Diagn Mol Pathol. 1993 Mar;2(1):42–49. [PubMed] [Google Scholar]
- Roth J., Daus H., Trümper L., Gause A., Salamon-Looijen M., Pfreundschuh M. Detection of immunoglobulin heavy-chain gene rearrangement at the single-cell level in malignant lymphomas: no rearrangement is found in Hodgkin and Reed-Sternberg cells. Int J Cancer. 1994 Jun 15;57(6):799–804. doi: 10.1002/ijc.2910570607. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Segal G. H., Wittwer C. T., Fishleder A. J., Stoler M. H., Tubbs R. R., Kjeldsberg C. R. Identification of monoclonal B-cell populations by rapid cycle polymerase chain reaction. A practical screening method for the detection of immunoglobulin gene rearrangements. Am J Pathol. 1992 Dec;141(6):1291–1297. [PMC free article] [PubMed] [Google Scholar]
- Stein H., Hansmann M. L., Lennert K., Brandtzaeg P., Gatter K. C., Mason D. Y. Reed-Sternberg and Hodgkin cells in lymphocyte-predominant Hodgkin's disease of nodular subtype contain J chain. Am J Clin Pathol. 1986 Sep;86(3):292–297. doi: 10.1093/ajcp/86.3.292. [DOI] [PubMed] [Google Scholar]
- Teerenhovi L., Lindholm C., Pakkala A., Franssila K., Stein H., Knuutila S. Unique display of a pathologic karyotype in Hodgkin's disease by Reed-Sternberg cells. Cancer Genet Cytogenet. 1988 Sep;34(2):305–311. doi: 10.1016/0165-4608(88)90277-4. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Trainor K. J., Brisco M. J., Wan J. H., Neoh S., Grist S., Morley A. A. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991 Jul 1;78(1):192–196. [PubMed] [Google Scholar]
- Trümper L. H., Brady G., Bagg A., Gray D., Loke S. L., Griesser H., Wagman R., Braziel R., Gascoyne R. D., Vicini S. Single-cell analysis of Hodgkin and Reed-Sternberg cells: molecular heterogeneity of gene expression and p53 mutations. Blood. 1993 Jun 1;81(11):3097–3115. [PubMed] [Google Scholar]
- Waldmann T. A. The arrangement of immunoglobulin and T cell receptor genes in human lymphoproliferative disorders. Adv Immunol. 1987;40:247–321. doi: 10.1016/s0065-2776(08)60241-2. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Chang K. L. Molecular biologic studies of Hodgkin's disease. Semin Diagn Pathol. 1992 Nov;9(4):272–278. [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Warnke R. A., Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989 Feb 23;320(8):502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]