Abstract
Radiochemical results of U isotopes (234U, 235U and 238U) and their activity ratios are reported for well waters as local sources of drinking waters collected from the ten settlements around the Semipalatinsk Nuclear Test Site (SNTS), Kazakhstan. The results show that 238U varies widely from 3.6 to 356 mBq/L (0.3–28.7 μg/L), with a factor of about 100. The 238U concentrations in some water samples from Dolon, Tailan, Sarzhal and Karaul settlements are comparable to or higher than the World Health Organization’s restrictive proposed guideline of 15 μg (U)/L. The 234U/238U activity ratios in the measured water samples are higher than 1, and vary between 1.1 and 7.9, being mostly from 1.5 to 3. The measured 235U/238U activity ratios are around 0.046, indicating that U in these well waters is of natural origin. It is probable that the elevated concentration of 238U found in some settlements around the SNTS is not due to the close-in fallout from nuclear explosions at the SNTS, but rather to the intensive weathering of rocks including U there. The calculated effective doses to adults resulting from consumption of the investigated waters are in the range 1.0–18.7 μSv/y. Those doses are lower than WHO and IAEA reference value (100 μSv/y) for drinking water.
Keywords: Semipalatinsk nuclear test site, Kazakhstan, Well waters, Uranium isotopes, Annual effective dose
Introduction
Over a period of 40 years from 1949 to 1989, the former Union of Soviet Socialistic Republics (USSR) conducted more than 450 nuclear explosions at the Semipalatinsk Nuclear Test Site (SNTS), Kazakhstan; 26 of them were above ground, 87 in the atmosphere and 346 underground [1, 2]. Considerable efforts have been devoted to investigate the consequences of radiation exposures to the residents living in the area, particularly in villages contaminated heavily by fallout of the radioactive cloud [1–3].
We have also investigated the present situation of radioecology in and around the SNTS since 1994, and measured long-lived radionuclides 137Cs and Pu isotopes in large number of soil samples from various areas [4–7]. From these measurements, settlements around the SNTS we visited were found to be contaminated by 239,240Pu with levels from several to a few hundred times higher than those (40–120 Bq/m2) for global fallout observed in Japan, while 137Cs contamination is not so high [8]. Furthermore, as for an external radiation dose in the air, it has been gradually clarified that residents of Dolon, where is well known to have been highly contaminated by radioactive fallout due to the first USSR nuclear detonation on 29 August 1949, received around 0.5 Gy [9].
On the other hand, information concerning internal doses experienced by village residents is still very limited around the SNTS. Recently, Tanaka et al. [10] reported that frequencies of unstable-type chromosome aberrations and micronucleus in lymphocytes were higher in residents of contaminated areas such as Dolon, Sarzhal and Kainar than those of the non-contaminated area. They point out that such a higher incidence may be caused mainly by internal exposure, although factors such as age, habitation, smoking, drinking water, medical exposure, life style and so on must be further considered in the interpretation of data from contaminated area.
To serve as an aid to resolve such problem, the present work was aimed at clarifying the present situation of radionuclide levels in well waters as local sources of drinking waters. Among naturally occurring radionuclides, uranium belongs to the most chemical and radiological toxicity of elements for human. Here, we report the present U isotope (234U, 235U and 238U) levels in well waters collected mainly from the settlements around the SNTS and the associated annual effective doses to adults resulting from consumption of the investigated waters.
Experimental
Samples
The settlements where well waters were collected are shown in Fig. 1. These areas are semiarid plains with a low mean annual precipitation (200–300 mm). Total of 35 well water samples was collected from the contaminated settlements such as Dolon, Sarzhal and Karaul around the SNTS. The pH measurement in the water was carried out on a potable pH meter (Model-D-24, Horiba Ltd.) that was calibrated in situ before each set of measurement. The samples were taken in two 200 mL polyethylene bottles without filtration. In addition, about 100 mL of water was collected in light-tight glass bottle for measuring alkalinity; diluted mercuric chloride solution was added to the bottle to prevent decomposition of dissolved organic matter and then the bottle was tightly sealed. For comparison, river surface water sample was also collected from the Irtysh River, which is the largest one among them and flows into the Ob River after leaving this area.
Fig. 1.
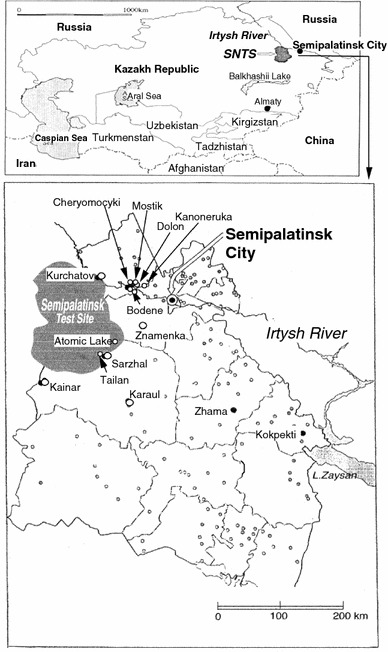
Map showing sampling locations of well waters around Semipalatinsk Nuclear Test Site, Kazakhstan
Measurement of uranium
Uranium isotopes were determined by α-particle spectrometry after radiochemical separation [11]. The sample water was at first acidified to less than pH 1 by adding a small amount of HNO3. After shaking and standing for overnight, the water was transferred into a 500 mL beaker with the addition of known amount of 232U as a yield tracer, and evaporated to dryness. The obtained residue was dissolved in 10 M HCl and the solution was passed through an anion-exchange resin column (Dowex 1 × 8 of 100–200 mesh, Cl− form, 0.8 cmϕ × 5 cm). The column was washed with a small amount of 8 M HNO3 to remove adsorbed iron and then by a sufficient amount of 10 M HCl to remove most of the other elements. Uranium was eluted from the column with 2 M HCl and the solution was evaporated to dryness. The separated U was electroplated onto a polished stainless steel disc (2 cmϕ), and its activity was measured by α-particle spectrometer with measuring time of 3–4 days (Tennelec TC256 spectrometer coupled to a 1k-channel pulse height analyzer). The counting efficiency is about 30% and the lowest limit of detection is about 0.2 mBq for 238U.
Analysis of major chemical compositions
Major dissolved ions (Na+, K+, Mg2+, Ca2+, Cl−, NO3 − and SO4 2−) of water samples were determined by ion chromatograph (Dionex ICS-1000). The alkalinity was measured by titration method with 0.1 M HCl down to pH 4.8 [12].
Results and discussion
Chemical composition
The chemical composition of 35 investigated waters is listed in Table 1. The pH of water samples ranges from 7.0 to 8.1, and is mostly neutral. The cation/anion balance ((Σcation − Σanion)/(Σcation + Σanion) in meq/L) for most of samples measured is smaller than 5%, although some samples have values over 10%. Total dissolved salt (TDS) concentrations are in the range 189–936 mg/L. As a whole, the TDS seems to be higher in Dolon, Mostik, Budene, Znamenka, Salzhal and Karaul than in other settlements. Those water samples contain large amounts of Na+, Ca2+, Mg2+, HCO3 − and SO4 2−.
Table 1.
Chemical composition of the investigated well and river waters
| Settlement | Sampling date | Ion concentration (mg/L) | Ion balance (%)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Na | K | Mg | Ca | Cl | NO3 | SO4 | HCO3 | TDS | |||
| Kanoneruka | ||||||||||||
| 00Y71 | 08.11.00 | 8.1 | 4.77 | n.d. | 6.7 | 53.23 | 51.1 | 0.75 | 38.1 | 73 | 228 | −0.4 |
| Dolon | ||||||||||||
| 05D1 | 09.20.05 | 7.7 | 93.6 | 19.3 | 24.2 | 62.7 | 33.9 | 71.2 | 114 | 260 | 679 | 5.1 |
| 05D2 | 09.20.05 | 7.4 | 120 | 4.0 | 28.4 | 76.7 | 48.6 | 114 | 132 | 293 | 817 | 3.2 |
| 04D1 | 11.11.04 | 7.6 | 92.7 | 4.6 | 21.8 | 66.0 | 39.7 | 55.7 | 116 | 273 | 670 | 1.7 |
| 04D2 | 11.11.04 | 7.2 | 116 | 3.7 | 26.9 | 81.7 | 66.0 | 104 | 138 | 236 | 773 | 5.2 |
| 04D3 | 11.11.04 | 7.2 | 93.1 | 17.3 | 22.9 | 61.3 | 46.5 | 70.2 | 119 | 269 | 699 | 0.5 |
| 04D4 | 11.11.04 | 7.4 | 81.5 | 2.7 | 17.5 | 51.0 | 28.5 | 48.8 | 97.1 | 242 | 569 | 0.0 |
| 04D5 | 11.11.04 | 7.5 | 73.6 | 4.0 | 16.7 | 49.7 | 19.9 | 10.3 | 65.2 | 301 | 541 | 0.9 |
| 00D1 | 08.11.00 | 7.4 | 61.5 | n.d. | 23.2 | 107 | 100 | 11.5 | 129 | 266 | 699 | −0.8 |
| 00D2 | 08.11.00 | 7.4 | 134 | n.d. | 30.9 | 121 | 94.1 | 15.5 | 253 | 294 | 942 | 5.2 |
| Mostik | ||||||||||||
| Y69 | 08.12.04 | 7.8 | 14.3 | n.d. | 17.4 | 122 | 95.2 | 17.4 | 62.4 | 199 | 528 | 4.2 |
| Cheryomocyki | ||||||||||||
| 03CH1 | 10.20.03 | 7.4 | 14.3 | 0.3 | 10.8 | 21.2 | 7.1 | 5.0 | 17.2 | 113 | 189 | 1.8 |
| 03CH2 | 10.20.03 | 7.5 | 37.0 | 29.5 | 21.5 | 38.3 | 34.7 | 25.6 | 48.2 | 129 | 363 | 14.6 |
| 03CH3 | 10.20.03 | 7.5 | 20.8 | 6.3 | 18.9 | 37.2 | 15.9 | 17.1 | 29.5 | 117 | 262 | 15.8 |
| 03CH4 | 10.20.03 | 7.6 | 29.1 | 6.5 | 12.1 | 22.9 | 11.1 | 5.3 | 21.3 | 121 | 229 | 11.8 |
| Bodene | ||||||||||||
| 03BW1 | 10.22.03 | 7.5 | 201 | 1.9 | 61.1 | 47.6 | 111 | 39.5 | 211 | 174 | 847 | 19.0 |
| 03BW2 | 10.22.03 | 7.9 | 231 | 1.3 | 38.0 | 30.0 | 121 | 13.0 | 261 | 180 | 874 | 10.1 |
| 03BW3 | 10.22.03 | 8.1 | 219 | 1.4 | 38.0 | 19.1 | 109 | 7.2 | 280 | 176 | 851 | 6.8 |
| Znamenka | ||||||||||||
| 05Z1 | 09.22.05 | 7.0 | 111 | 1.4 | 37.5 | 41.7 | 50.4 | 106 | 130 | 208 | 686 | 4.1 |
| Tailan | ||||||||||||
| W-7 | 10.10.99 | n.a. | 226 | 8.8 | 35.7 | 130 | 118 | n.d. | 399 | n.a. | ||
| Sarzhal | ||||||||||||
| 05S1 | 09.22.05 | 7.6 | 114 | 2.4 | 29.9 | 70.3 | 59.2 | 18.7 | 228 | 214 | 736 | 3.6 |
| 05S2 | 09.22.05 | 7.8 | 117 | 2.3 | 40.7 | 92.9 | 84.6 | 65.5 | 222 | 271 | 896 | 2.4 |
| 99S1 | 10.10.99 | n.a. | 131 | n.d. | 40.8 | 142 | 102 | 3.5 | 252 | n.a. | ||
| 99S2 | 10.10.99 | n.a. | 102 | 10.8 | 35.2 | 119 | 72.5 | n.d. | 189 | n.a. | ||
| 95S1 | 10.05.95 | n.a. | 95.5 | 5.3 | 40.1 | 131 | 104 | 36.2 | 203 | n.a. | ||
| 95S2 | 10.05.95 | n.a. | 140 | 5.2 | 58.8 | 147 | 162 | 81.6 | 272 | n.a. | ||
| Kainar | ||||||||||||
| 99KA1 | 10.11.99 | n.a. | 16.3 | 4.6 | 7.1 | 73.7 | 10.5 | n.d. | 50.4 | n.a. | ||
| 99KA2 | 10.11.99 | n.a. | 25.6 | 10.4 | 10.7 | 87.8 | 25.4 | 2.4 | 53.1 | n.a. | ||
| Karaul | ||||||||||||
| 04K1 | 11.12.04 | 7.9 | 84.3 | 6.1 | 37.6 | 132 | 84.2 | 177 | 198 | 216 | 936 | 2.4 |
| 04K2 | 11.12.04 | 7.7 | 62.9 | 2.3 | 31.4 | 110 | 44.6 | 69.0 | 185 | 262 | 767 | 1.5 |
| 04K3 | 11.12.04 | 8.0 | 23.7 | 2.1 | 16.2 | 76.4 | 9.1 | 7.9 | 111 | 203 | 450 | 1.6 |
| 04K4 | 11.12.04 | 7.2 | 35.7 | 3.2 | 22.3 | 101 | 28.1 | 47.1 | 109 | 223 | 569 | 6.5 |
| 04K5 | 11.12.04 | 7.5 | 30.8 | 2.6 | 20.1 | 86.0 | 16.6 | 18.6 | 114 | 242 | 531 | 1.7 |
| 04K6 | 11.12.04 | 7.9 | 23.7 | 2.1 | 16.1 | 76.2 | 9.2 | 8.0 | 112 | 210 | 457 | 0.5 |
| 99K1 | 10.10.99 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| Irtysh River | ||||||||||||
| 05R1 | 09.20.05 | 8.0 | 20.0 | 1.5 | 7.3 | 31.9 | 21.2 | 1.3 | 20.7 | 140 | 244 | −4.4 |
| 04R1 | 08.12.04 | 8.3 | 11.6 | n.d. | 8.2 | 58.9 | 24.7 | n.d. | 52.0 | 110 | 266 | 6.9 |
Error shows 1σ standard deviation from counting statistics
a(Σcation − Σanion)/(Σcation + Σanion) × 100
n.d. not detected, n.a. not analyzed
Uranium levels and isotopic ratios
The result of the uranium analysis of well water samples is summarized in Table 2, together with those of river water samples from the Irtysh River. It is apparent from Table 2 that concentrations of 238U in the investigated waters vary in a wide range 3.6–356 mBq/L (0.3–28.7 μg/L), with a factor of about 100. The lowest concentration (3.6 mBq/L) of 238U was detected in the well water from Kainar and the highest concentration (356 mBq/L) of 238U was observed in the well water samples from Karaul. In Dolon and Karaul, the 238U contents change widely even within the area of each settlement. For waters from other settlements, the concentrations of 238U do not change largely. Such variation of 238U concentrations may be connected with the different local, geological and hydrological conditions of the original places of the investigated waters, although the 238U levels seem to increase with increasing mineralization as a whole. Literature values of 238U in freshwater have been reported in the range 0.002–5 μg/L, and the median was 0.4 μg/L [13]. Global means, 0.04 and 2.0 μg/L, were reported [14]. The 238U contents found here are several to several tens of times higher than the reported values. Kawabata et al. [15, 16] have also observed similarly high 238U concentrations for well water samples collected from some areas in Kazakhstan and Uzbekistan. It is worth noting that the 238U concentrations in some well samples from Dolon, Tailan, Sarzhal and Karaul settlements are comparable to or higher than the World Health Organization’s restrictive proposed guideline of 15 μg (U)/L [17]. Most of the measured 234U/238U activity ratios are higher than 1, and vary from 1.5 to 7.9, with most from 1.5 to 3. Those higher ratios of 234U/238U may be explained by preferential leaching of 234U due to the α-recoil effect [11, 18]. No clear relationship is observed between 238U concentration and 234U/238U activity ratio. On the other hand, all of the measured 235U/238U activity ratios show the value of around 0.046 with a relatively large counting error of about 10%. It is probable that the elevated concentration of 238U found in some settlements around the SNTS is not due to the close-in fallout from nuclear explosions at the SNTS, but rather to the intensive weathering of rocks including U there.
Table 2.
Results of uranium measurements of the investigated well and river waters
| Settlement | 238U concentration | Activity ratio | ||
|---|---|---|---|---|
| (mBq/L) | (μg/L) | 234U/238U | 235U/238U | |
| Kanoneruka | ||||
| 00Y71 | 11.4 ± 0.7 | 0.92 | 1.63 ± 0.12 | 0.047 ± 0.008 |
| Dolon | ||||
| 05D1 | 189.9 ± 6.8 | 15.3 | 1.63 ± 0.03 | 0.044 ± 0.002 |
| 05D2 | 157.2 ± 8.7 | 12.6 | 1.64 ± 0.06 | 0.043 ± 0.002 |
| 04D1 | 99.8 ± 5.0 | 8.03 | 1.44 ± 0.05 | |
| 04D2 | 194.6 ± 12.1 | 15.7 | 1.54 ± 0.06 | |
| 04D3 | 197.9 ± 11.4 | 15.9 | 1.48 ± 0.05 | |
| 04D4 | 45.1 ± 1.4 | 3.62 | 1.65 ± 0.05 | |
| 04D5 | 51.4 ± 2.8 | 4.13 | 1.51 ± 0.07 | |
| 00D1 | 135.6 ± 6.3 | 10.9 | 1.42 ± 0.06 | 0.040 ± 0.005 |
| 00D2 | 117.1 ± 5.4 | 9.42 | 1.36 ± 0.06 | 0.048 ± 0.004 |
| Mostik | ||||
| Y69 | 49.2 ± 2.2 | 3.96 | 1.54 ± 0.07 | 0.050 ± 0.010 |
| Cheryomocyki | ||||
| 03CH1 | 42.3 ± 1.9 | 3.40 | 1.36 ± 0.06 | 0.047 ± 0.006 |
| 03CH2 | 25.1 ± 0.7 | 2.02 | 1.44 ± 0.05 | |
| 03CH3 | 22.6 ± 0.7 | 1.81 | 1.38 ± 0.05 | 0.043 ± 0.006 |
| 03CH4 | 28.9 ± 0.7 | 2.33 | 1.53 ± 0.04 | |
| Bodene | ||||
| 03BW1 | 144.3 ± 4.8 | 11.6 | 2.93 ± 0.06 | 0.048 ± 0.005 |
| 03BW2 | 114.2 ± 4.9 | 9.19 | 2.98 ± 0.08 | 0.043 ± 0.005 |
| 03BW3 | 139.9 ± 5.2 | 11.2 | 2.67 ± 0.06 | 0.049 ± 0.006 |
| Znamenka | ||||
| 05Z1 | 36.5 ± 2.5 | 2.94 | 6.80 ± 0.38 | 0.045 ± 0.005 |
| Tailan | ||||
| W-7 | 274.0 ± 10.9 | 22.0 | 1.97 ± 0.06 | 0.049 ± 0.003 |
| Sarzhal | ||||
| 05S1 | 144.4 ± 6.1 | 11.6 | 2.42 ± 0.06 | 0.044 ± 0.003 |
| 05S2 | 213.9 ± 12.0 | 17.2 | 2.41 ± 0.07 | 0.043 ± 0.003 |
| 99S1 | 206.4 ± 14.1 | 16.6 | 2.35 ± 0.13 | 0.048 ± 0.003 |
| 99S2 | 127.8 ± 6.9 | 10.3 | 2.19 ± 0.11 | 0.052 ± 0.006 |
| 95S1 | 156.1 ± 8.3 | 12.6 | 2.15 ± 0.10 | 0.050 ± 0.006 |
| 95S2 | 172.4 ± 10.0 | 13.9 | 2.26 ± 0.12 | 0.045 ± 0.005 |
| Kainar | ||||
| 99KA1 | 3.56 ± 0.59 | 0.29 | 7.88 ± 1.28 | |
| 99KA2 | 4.14 ± 0.67 | 0.33 | 5.51 ± 0.87 | |
| Karaul | ||||
| 04K1 | 82.8 ± 4.9 | 6.66 | 2.34 ± 0.10 | |
| 04K2 | 90.8 ± 6.6 | 7.30 | 2.16 ± 0.11 | 0.048 ± 0.005 |
| 04K3 | 52.1 ± 3.6 | 4.19 | 2.53 ± 0.14 | |
| 04K4 | 116.8 ± 7.4 | 9.39 | 1.51 ± 0.07 | 0.045 ± 0.004 |
| 04K5 | 97.6 ± 5.1 | 7.85 | 1.73 ± 0.07 | 0.048 ± 0.005 |
| 04K6 | 48.6 ± 2.8 | 3.91 | 2.35 ± 0.11 | |
| 99K1 | 355.6 ± 23.4 | 28.6 | 1.09 ± 0.06 | 0.047 ± 0.009 |
| Irtysh River | ||||
| 05R1 | 31.0 ± 1.3 | 2.49 | 1.77 ± 0.07 | 0.044 ± 0.005 |
| 04R1 | 37.0 ± 1.5 | 2.98 | 1.65 ± 0.06 | |
Error shows 1σ standard deviation from counting statistics
The 238U concentrations for river water samples collected from the Irtysh River are 31–37 mBq/L (2.5–3.0 μg/L). The levels in the Irtysh River are close to the values in well water samples from Mostik and Cheryomocyki. The 234U/238U activity ratios (1.7–1.8) are nearly the same as those found at settlements around the Irtysh River.
Radiological annual dose
Assuming that a man drinks 2 L of water per day, the annual effective doses (D) resulting from consumption of the investigated waters can be calculated using the following formula:
where Ii is the concentration of the given U isotopes (Bq/day), and geometric mean values of 238U and 234U concentrations were used as representative values for each settlement. The 235U contents were estimated by using the value (0.046) of 235U/238U activity ratio for natural uranium. The values of Fi are the ingestion dose coefficients (dose equivalent per intake of unit activity, Sv/Bq) reported by the International Commission on Radiological Protection [19]; 4.4 × 10−8 for 238U, 4.9 × 10−8 for 234U and 4.9 × 10−8 for 235U. In this case, fractional transfer to blood is assumed to be 0.02 for all uranium isotopes. The effective dose for adults caused by ingestion of uranium isotopes of the investigated well waters are presented in Table 3, except the settlements where only one well water sample was measured. The calculated effective doses vary from 1.0 to 18.7 μSv/y. The dose (18.7 μSv/y) estimated for the adults living in the Sarzhal region is comparable to the value of 16 μSv/y (range 9–20 μSv/y) reported recently by Vintró et al. [20]. Those doses are lower than WHO and IAEA reference value (100 μSv/y) for drinking water [21, 22].
Table 3.
Annual effective dose to adult arising from U ingestion (consuming 2 L of water daily) through well water in each settlement
| Settlement | Analyzed number of samples | Range | Geometric mean | Effective dose (mSv/y) | ||
|---|---|---|---|---|---|---|
| 238U (mBq/L) | 238U (mBq/L) | 234U (mBq/L) | 235Ua (mBq/L) | |||
| Dolon | 9 | 45.1–197.9 | 117.3 | 177.9 | 5.4 | 10.3 |
| Cheryomocyki | 4 | 22.6–42.3 | 28.9 | 41.1 | 1.3 | 2.4 |
| Bodene | 3 | 114.2–144.3 | 132.1 | 377.4 | 6.1 | 18.0 |
| Sarzhal | 6 | 127.8–213.9 | 167.3 | 365.4 | 7.7 | 18.7 |
| Kainar | 2 | 3.56–4.14 | 3.8 | 25.3 | 0.2 | 1.0 |
| Karaul | 7 | 48.6–355.6 | 94.6 | 181.9 | 4.4 | 9.8 |
aContents of 235U were calculated by using the value (0.046) of 235U/238U for natural U
Conclusion
The concentrations of 238U in the well water samples from some settlements around the SNTS vary in a wide range 3.6–356 mBq/L (0.3–28.7 μg/L). The 238U concentrations in some samples from Dolon, Tailan, Sarzhal and Karaul settlements are comparable to or higher than the World Health Organization’s restrictive proposed guideline of 15 μg (U)/L. The measured 234U/238U activity ratios are higher than 1, and are mostly from 1.5 to 3. The 235U/238U activity ratios show the value of around 0.046, indicating that U in the wells is of natural origin. It is probable that the higher 238U concentrations are not due to the close-in fallout from nuclear explosions at the SNTS, but rather to the intensive weathering of rocks including U there. The calculated annual effective doses arising from the ingestion of U isotopes (234U, 235U and 238U) are in the range 1.0–18.7 μSv/y, and are lower than the recommended value of 100 μSv/y for drinking water.
Acknowledgments
We would like to express our gratitude to the research staff of the Kazakh Scientific Research Institute of Radiation Medicine and Ecology for their help with sampling. This work was supported by a Grand-in-Aid (M. Y. and M. H.: 1995–2005) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Gusev BI, Abylkassimova ZN, Apsalikov KN. Radiat Environ Biophys. 1977;39:201. doi: 10.1007/s004110050072. [DOI] [PubMed] [Google Scholar]
- 2.Gordeev K, Vasilenko I, Lebedev A, Bouville A, Luckyanov N, Simon SL, Shinkarev S, Anspaugh L. Radiat Environ Biophys. 2002;41:61. doi: 10.1007/s00411-001-0139-y. [DOI] [PubMed] [Google Scholar]
- 3.Grosche B, Land C, Bauer S, Pivina LM, Abylkassimova ZN, Gusev BI. Radiat Environ Biophys. 2002;41:75. doi: 10.1007/s00411-001-0136-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Tsumura A, Katayama Y, Tsukatani T. Radiochim Acta. 1996;40:209. [Google Scholar]
- 5.Yamamoto M, Tsukatani T, Katayama Y. Health Phys. 1996;71:142. doi: 10.1097/00004032-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Hoshi M, Takada J, Sakelbaev AK, Gusev BI. J Radioanal Nucl Chem. 1999;242:63. doi: 10.1007/BF02345895. [DOI] [Google Scholar]
- 7.Yamamoto M, Hoshi M, Takada J, Sakaguchi A, Apsalikov KN. J Radioanal Nucl Chem. 2004;261:19. doi: 10.1023/B:JRNC.0000030931.74889.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M, Komura K, Sakanoue M. J Radiat Res. 1983;24:237. doi: 10.1269/jrr.24.237. [DOI] [PubMed] [Google Scholar]
- 9.Imanaka T, Fukutani S, Yamamoto M, Sakaguchi A, Hoshi M. J Radiat Res. 2006;47 Suppl:A121. doi: 10.1269/jrr.47.A121. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Iida S, Takeichi N, Chaizhunusova NJ, Gusev BI, Apsalikov KN, Inaba T, Hoshi M. J Radiat Res. 2006;47 Suppl:A159. doi: 10.1269/jrr.47.A159. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Sato T, Sasaki K, Hama K, Nakamura T, Komura K. J Radioanal Nucl Chem. 2003;255:369. doi: 10.1023/A:1022573208205. [DOI] [Google Scholar]
- 12.Tomita J, Satake H, Sasaki K, Sakaguchi A, Inoue M, Hamajima Y, Yamamoto M. J Hot Spring Sci. 2008;58:244. [Google Scholar]
- 13.Bowen HL (1979) In: Environmental chemistry of the elements. Academic press, New York, p 1611
- 14.United Nations Scientific Committee on the Effects of Atomic Radiation (1988) In: Sources, effects, and risks of ionizing radiation. New York
- 15.Kawabata Y, Aparin V, Nagai M, Yamamoto M, Shiraishi K, Katayama Y. J Radioanal Nucl Chem. 2008;278:459. doi: 10.1007/s10967-008-0904-3. [DOI] [Google Scholar]
- 16.Kawabata Y, Yamamoto M, Aparin V, Ko S, Shiraishi K, Nagai M, Katayama Y. J Radioanal Nucl Chem. 2006;270:137. doi: 10.1007/s10967-006-0320-5. [DOI] [Google Scholar]
- 17.Guidelines for drinking water quality, 3rd edn. World Health Organization (WHO), Geneva, Switzerland (2004)
- 18.Kigoshi K. Science. 1971;173:47. doi: 10.1126/science.173.3991.47. [DOI] [PubMed] [Google Scholar]
- 19.International Commission of Radiological Protection (1994) Dose coefficient for intake of radionuclides by workers. In: Replacement of ICRP Publication 61. Pergamon Press ICRP Publication 68, Oxford
- 20.Vintró LL, Mitchell PJ, Omarova A, Burkitbayev M, Jiménez Nápoles H, Priest ND. J Environ Radioact. 2009;100:308. doi: 10.1016/j.jenvrad.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines for drinking water quality, Chapter 9, 3rd edn. World Health Organization (WHO), Geneva, Switzerland (2003)
- 22.Radionuclide content in commodities not requiring regulation for the purposes of radiation protection. In: Draft safety guide DS161. IAEA, Vienna, p 14 (2002)


