Abstract
Secretory azoospermia is a severe form of male infertility caused by unknown factors. DAX-1 is predominantly expressed in mammalian reproductive tissues and plays an important role in spermatogenesis because Dax-1 knockout male mice show spermatogenesis defects. To examine whether DAX-1 is involved in the pathogenesis of secretory azoospermia in humans, we sequenced all of the exons of DAX-1 in 776 patients diagnosed with secretory azoospermia and 709 proven fertile men. A number of coding mutations unique to the patient group, including two synonymous mutations and six missense mutations, were identified. Of the missense mutations, our functional assay demonstrated that the V385L mutation caused the reduced functioning of DAX-1. This novel mutation (p. V385L) of DAX-1 is the first to be identified in association with secretory azoospermia, thereby highlighting the important role of DAX-1 in spermatogenesis.
Introduction
The incidence of infertility is approximately 15% to 20% for couples of childbearing age, and about half of cases of infertility are caused by male factors [1, 2]. Secretory azoospermia, characterized by the absence of spermatozoa in semen, is a severe form of male infertility that affects approximately 1% of adult men in the general population [1, 2]. Previous studies have shown that genetic factors play an important role in IA [3].
DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region on the X chromosome, gene 1; also called NROB1) is predominantly expressed in both male and female reproductive tissues [4–6]. Mutations in DAX-1 cause X-linked adrenal hypoplasia congenita (AHC), a disorder characterized by primary adrenal failure, hypogonadotropic hypogonadism and azoospermia [7]. Accumulating evidence from studies of Dax-1 knockout mice and those of AHC patients indicate that mutations in DAX-1 may directly cause abnormalities in spermatogenesis [8–12]. In particular, previous studies have shown that gonadotropin is unsuccessful for the treatment of azoospermia patients with classic X-linked AHC, suggesting a direct effect of DAX-1 on spermatogenesis [13–16].
DAX-1 is a member of the orphan nuclear receptor family of transcription factors [17]. Previous in vitro and in vivo studies have shown that it represses the activity of androgen receptor (AR) by directly interacting with it [18–20]. As a result, certain sequence changes in DAX-1 may affect its interaction with AR and eliminate its repression of AR activity, ultimately resulting in abnormal spermatogenesis.
In the present study, we sequenced the exons of DAX-1 in 776 secretory azoospermia patients and 709 fertile men to estimate the association of this gene with secretory azoospermia. Two synonymous mutations and six missense mutations were identified that were unique to the patient group. Among them, the DAX-1 V385L mutation was present in one patient, resulting in the significantly reduced inhibition of the transcriptional activity of AR. Thus, we propose that this mutation in DAX-1 eliminates its repression of AR and that it potentially contributed to the onset of secretory azoospermia in this patient.
Materials and Methods
Patient samples
A total of 1,880 azoospermic patients were recruited for this study from the Center of Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology from Jan 2007 to Oct 2011 [21]. Among of them, 776 Han Chinese patients fulfilled the following criteria for secretory azoospermia diagnosis: (1) no sperm detected in the pellets of semen samples on three different occasions; (2) no obstruction, inflammation or injury of the reproductive system or pelvic cavity; and (3) no karyotypic abnormality or Y chromosome microdeletion. A total of 709 fertile Han Chinese men from the Center of Physical Examination, Peking University Shenzhen Hospital were recruited as controls who had fathered at least one child without assisted reproductive techniques, such as IVF, ICSI or IMSI. After a panel re-sequencing study and quality control steps, 776 patients aged 24–46 years (average of 30.6 years) and 709 fertile men aged 29–51 years (average of 35.6 years) were available for further analysis.
Ethics statement
Informed written consent was obtained from each subject, and the study was approved by the ethics committee of Peking University Shenzhen Hospital. All clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Panel re-sequencing study
Five micrograms of genomic DNA isolated from peripheral blood samples were sent to Beijing Genomics Institute at Shenzhen for exonic capture and sequencing. The capture procedure was performed in solution with a NimbleGen custom array (Roche NimbleGen, Madison, WI, USA) that is capable of enriching the exonic sequences of 654 infertility- or subfertility-related genes [21]. Most of these genes have been reviewed by Matzuk and Lamb [3]. Moreover, we selected other genes that have been shown to cause male reproductive defects in mouse models in studies published between November 2008 and December 2010. Panel re-sequencing was performed with an Illumina platform with 90 bp pair-end reads.
Fastq sequence files were aligned against the human reference genome (NCBI build 37.1, hg19) with SOAPaligner software (2.21). Duplicated paired-end reads were removed from the merged data sets. Single nucleotide variants that were not present in different from the hg19 reference genome were filtered out if they met any of the following criteria: a Phred-like quality score of ≤ 20, overall depth of ≤ 8×, estimated copy number of ≥ 2 or genomic distance between two adjacent variants of < 5 bp. In addition, the quality scores of both the major and minor alleles at heterozygous loci were at least 20. The variants were then annotated using an in-house functional prediction tool and were compared with dbSNP132 and 1000 Genomes databases (as of August 2010).
Validation of novel missense mutations by Sanger sequencing
To validate the novel missense mutations identified by deep sequencing, PCR amplifications were carried out, and the PCR products were sequenced in both directions with a 3730 DNA analyzer (Applied Biosystems). The primers for PCR and Sanger sequencing validation of the DAX-1 gene are listed in S1 Table.
Western blot analysis and immunoprecipitation
For Western blot analysis, cells were washed once in PBS, resuspended in cell lysis buffer (38733, Sigma, Shanghai, China) with a protease inhibitor mixture containing PMSF and Cocktail, and then incubated on ice for 30 min. The lysates were then cleared by centrifugation at 12,000 rpm for 5 min, and the total protein was boiled for 5 min with SDS sample buffer.
Equal amounts of each protein sample were then separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in 5% skim milk with 0.5% Tween-TBS (TBST) and then probed with an anti-AR (1:1000; sc-13062X, Santa Cruz, CA, USA) or anti-HA (1:1000; H3663; Sigma, Shanghai, China) antibody overnight at 4°C in TBST containing 5% skim milk. After being washed 3 times for 5 min with TBST, the membranes were incubated at room temperature for 1 h with secondary antibodies (1:5000, SA00001-1/SA00001-2, Proteintech, Chicago, IL, USA) diluted in 5% skim milk. Following three 5 min washes in TBST, the proteins were visualized by ECL (WBKLS0500, Millipore).
For immunoprecipitation, cells were lysed in cell lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton, 1 mM EGTA, 1 mM Na2EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4 and 1 mg/ml leupeptin) for 1 h at 4°C and then centrifuged at 12,000 rpm for 20 min. The supernatant was precleared with protein A/G, followed by incubation with 2 ml of primary antibody overnight at 4°C. Thirty microliters of protein A/G bead slurry (GE Healthcare) was added for an additional hour prior to an extensive wash in cell lysis buffer. After being washed, the pellets were boiled in SDS sample buffer for 5 min, and the immunoprecipitates were analyzed by Western blot as described above.
Plasmid construction and site-directed mutagenesis
Human DAX-1 complementary DNA (cDNA) was amplified from human testicle cDNA (636533, Takara, Japan) by PCR with the following primers: 5’- GGAATTCGCCACCATGGCGGGCGAGAACCACCA -3’ (forward) and 5’- CTTGTGGATCCCACATGACTTTATATCTTTGTACAG -3’ (reverse). The PCR product was subcloned into the EcoR1/BamH1 sites of a pcDNA3.1-HA expression vector (Invitrogen, Carlsbad, CA, USA). Site-directed mutagenesis was performed to generate DAX-1 expression plasmids bearing the R51K, C104W, A242V, E256Q, V385L, or I427V mutation, as described previously [22]. DNA sequencing was performed to confirm the introduced mutations. The PCR primers used for site-directed mutagenesis and plasmid construction are shown in S2 Table.
Luciferase assay
Luciferase analysis was performed as described previously with some modifications [23]. HeLa cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C, 95% humidity and 5% CO2. Cells were seeded in 24-well tissue culture plates for 24 h prior to transfection. Equivalent amounts (100 ng) of DAX-1 (wild type [WT] or mutant) expression plasmids were cotransfected with mouse mammary tumor virus long terminal repeat (pMMTV-LUC) plasmids (100 ng) and an AR expression vector (10 ng) into HeLa cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. Cells were treated with or without 100 nM testosterone after 6 h of transfection and harvested at 24 h after treatment. Firefly and Renilla luciferase expression was assessed using a Dual Luciferase Reporter Assay System (E1910, Promega, Madison, WI). Renilla luciferase activity was normalized to that of firefly luciferase. After normalization for transfection efficiency, induction factors were calculated as the ratio of the average of the luciferase value for the testosterone-stimulated samples vs. the non-testosterone-stimulated (ethanol vehicle-treated) samples.
Statistical analysis
All experiments were repeated at least three times. Data were expressed as the mean ± SD. SPSS 17.0 statistical software was used for statistical analysis. Student’s t-test was used to compare the difference in means between the two groups. A p < 0.05 was considered to be statistically significant.
Results
Identification of DAX-1 mutation in patients with secretory azoospermia
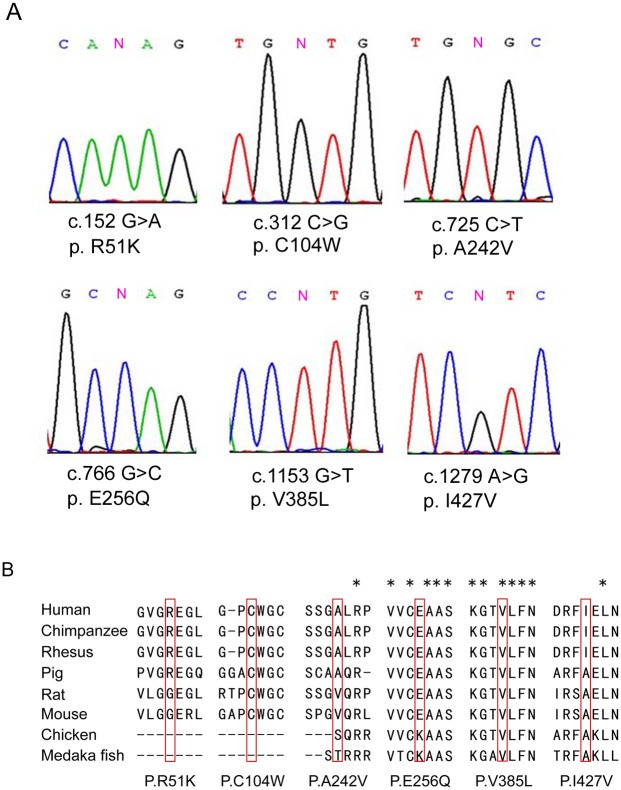
To examine whether DAX-1 genetic defects are associated with secretory azoospermia, we screened for DAX-1 exonic mutations in 776 secretory azoospermia patients and 709 men with proven fertility using massively parallel sequencing technology. As shown in Table 1, six missense mutations and six synonymous mutations were detected in DAX-1. All of the mutations except for c.498 G>A, c.376 G>A and c.114 C>T were not present in either dbSNP135 database or 1000 Genome Project dataset and were not identified in the 709 normal controls. The six novel missense mutations (c.152 G>A, c.321 C>G, c.725 C>T, c.766 G>C, c.1153 G>T and c.1279 A>G) were further confirmed by Sanger sequencing (Fig 1A). Alignment of the amino acid sequence of DAX-1 to its orthologs in different species showed that the V385L mutation affected a highly conserved amino acid (Fig 1B). Bioinformatic assessment of the variants indicated that the mutations C104W, E256Q and V385L were possibly damaging to the protein predicted by both Polyphen 2.0[24] and MutationTaster[25] (Table 2).
Table 1. DAX-1 mutations and SNPs identified in the secretory azoospermia patients and controls.
| No. | Position | Nucleotide change | Amino Acid Change | Patient (n = 776) | Fertile men (n = 709) | dbSNP135 |
|---|---|---|---|---|---|---|
| Missense mutations | ||||||
| 1 | 30322830 | c.1279 A>G | p.I427V | 1 | 0 | |
| 2 | 30326328 | c.1153 G>T | p.V385L | 1 | 0 | |
| 3 | 30326715 | c.766 G>C | p.E256Q | 1 | 0 | |
| 4 | 30326756 | c.725 C>T | p.A242V | 2 | 0 | |
| 5 | 30327169 | c.312 C>G | p.C104W | 1 | 0 | |
| 6 | 30327329 | c.152 G>A | p.R51K | 3 | 0 | |
| Synonymous mutation | ||||||
| 7 | 30326903 | c.578 C>G | None | 0 | 1 | |
| 8 | 30326983 | c.498 G>A | None | 550 | 539 | rs2269345 |
| 9 | 30327105 | c.376 G>A | None | 26 | 26 | rs193205940 |
| 10 | 30327319 | c.162 G>A | None | 1 | 0 | |
| 11 | 30327367 | c.114 C>T | None | 99 | 83 | rs6150 |
| 12 | 30327383 | c.98 G>T | None | 1 | 0 | |
Fig 1. Six missense mutations in DAX-1 identified in patients with secretory azoospermia.
(A) Chromatogram traces from Sanger sequencing, showing the validated missense mutations. (B) Evolutionary conservation of amino acids affected by the missense mutations. Multiple protein alignments were performed with MegAlign (Demonstration System DNASTAR, Inc.). The identification numbers of the DAX-1 protein were as follows: human (NP_000466.2), chimpanzee (XP_520991.2), rhesus (XP_002806222.1), pig (NP_999552.1), rat (NP_445769.1), mouse (NP_031456.1), chicken (NP_989924.1), and Medaka fish (NP_001104259.1). The mutant alleles are boxed, and the star (*) indicates the conserved residue.
Table 2. List of missense mutations predicted by PolyPhen 2.0 and Mutation Taster.
| No. | Nucleotide Change | Amino Acid Change | Polyphen | MutationTaster |
|---|---|---|---|---|
| 1 | c.152 G>A | p.R51K | Possibly damaging | polymorphism |
| 2 | c.312 C>G | p.C104W | Probably damaging | disease causing |
| 3 | c.725 C>T | p.A242V | Benign | polymorphism |
| 4 | c.766 G>C | p.E256Q | Probably damaging | disease causing |
| 5 | c.1153 G>T | p.V385L | Possibly damaging | disease causing |
| 6 | c.1279 A>G | p.I427V | Benign | polymorphism |
Interaction between DAX-1 mutants and AR
DAX-1 inhibits the transcriptional activity of AR through protein-protein interactions [18–20]. To determine whether the identified missense mutations in DAX-1 affect its ability to bind to AR, WT and mutated DAX-1 constructs were transfected into HeLa cells with an AR plasmid. The results showed that all DAX-1 R51K, C104W, A242V, E256Q, and I427V mutants co-immunoprecipitated with AR similar to DAX-1 WT but that V385L was weakly bound to AR (Fig 2). Collectively, these results indicated that the V385L mutation affected the interaction between DAX-1 and AR.
Fig 2. Interactions between DAX-1 mutants and AR.
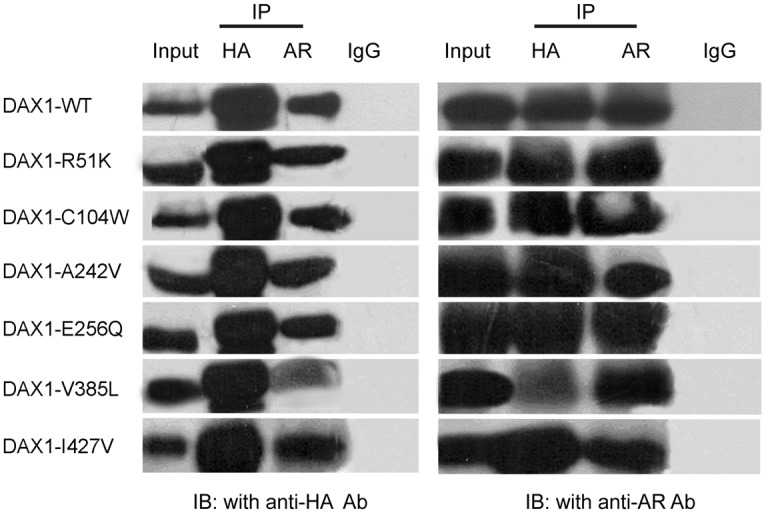
HeLa cells were transfected with expression vectors for AR, DAX-1 WT, DAX-1 R51K, DAX-1 C104W, DAX-1 A242V, DAX-1 E256Q, DAX-1 V385L, and DAX-1 I427V as indicated. DAX-1 and AR were immunoprecipitated (IP) with an anti-HA antibody and AR antibody, respectively. Then, the immunocomplexes were analyzed by SDS-PAGE and Western blotting (WB) analysis using anti-HA and anti-AR antibodies as indicated.
Effects of DAX-1 mutations on AR function
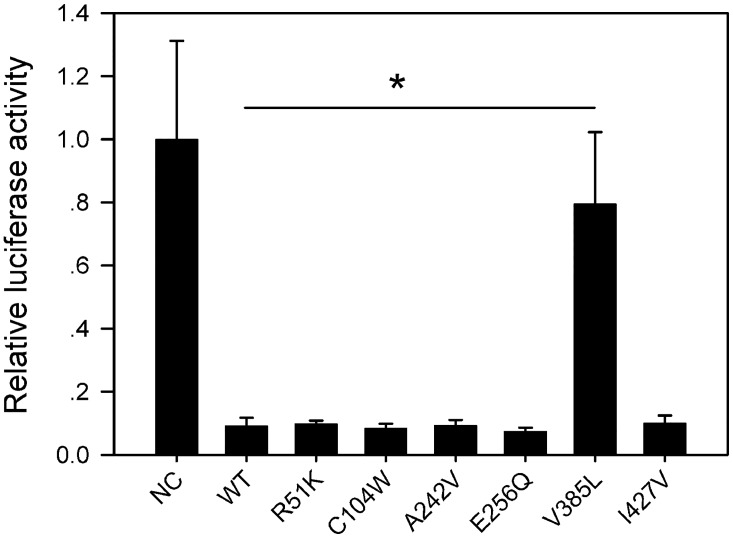
To test whether the six identified missense mutations in DAX-1 eliminate its repression of AR, HeLa cells were transfected with DAX-1 expression vectors containing these mutations along with AR plasmids. We used an androgen-responsive luciferase reporter construct, namely pMMTV. In the absence of DAX-1, AR activated this reporter in an agonist-dependent fashion. The inhibition of AR by DAX-1 V385L was significantly reduced, while no changes were observed for the other mutants compared with the WT (Fig 3). These results indicated that V385L eliminated the repression of AR by DAX-1.
Fig 3. The effect of the DAX-1 V385L variant on AR function.
A WT or mutant DAX-1 expression vector was cotransfected with an AR expression vector and a testosterone-inducible pMMTV-LUC plasmid into HeLa cells, and luciferase activity was measured with or without testosterone treatment. Compared with the WT and the other mutants, the DAX-1 V385L mutant failed to repress the transcriptional activity of AR in the presence of testosterone. The results are presented as the fold-change of testosterone treated relative to that in the vehicle-treated control. NC: pcDNA3.1-HA were cotransfected with an AR expression vector and a testosterone-inducible pMMTV-LUC plasmid into HeLa cells. (* p < 0.05).
Clinical data
The clinical information of the patients with DAX-1 missense mutations is shown in Table 3. The patient with the V385L mutation had normal hormone levels and no relevant reproductive family history. The histology of the testes of this patient confirmed the diagnosis of non-obstructive azoospermia and showed an arrest of spermatogenesis at the spermatocyte stage (Fig 4).
Table 3. Clinical information of the patients with missense mutation in DAX-1 .
| Nucleotide Change | Patient No. | Age | Testicular volume (ml) | FSH(mIU/ml) [1.5–12.5mIU/ml] | LH(mIU/ml) [1.7–8.6mIU/ml] | T(ng/ml) [2.5–8.0ng/ml] |
|---|---|---|---|---|---|---|
| c.152 G>A | 24 | 37 | 6 | NA | NA | NA |
| c.152 G>A | 566 | 30 | 3 | 2.94 | 3.41 | 3.07 |
| c.152 G>A | 698 | 32 | 6 | 2.58 | 1.80 | 1.02 |
| c.312 C>G | 505 | 44 | NA | 2.82 | 3.95 | 1.32 |
| c.725 C>T | 33 | 34 | 2 | NA | NA | NA |
| c.725 C>T | 251 | 33 | 2 | 4.82 | 5.04 | 3.26 |
| c.766 G>C | 570 | 32 | 5 | 25.38 | 12.13 | 2.64 |
| c.1153 G>T | 628 | 31 | 15 | 3.24 | 4.28 | 2.84 |
| c.1279 A>G | 520 | 38 | 5 | 10.57 | 4.22 | 2.57 |
NA: Not available.
Fig 4. Testicular histology analysis of the patient with the V385L mutation by hematoxylin and eosin staining (Magnification: 400x).
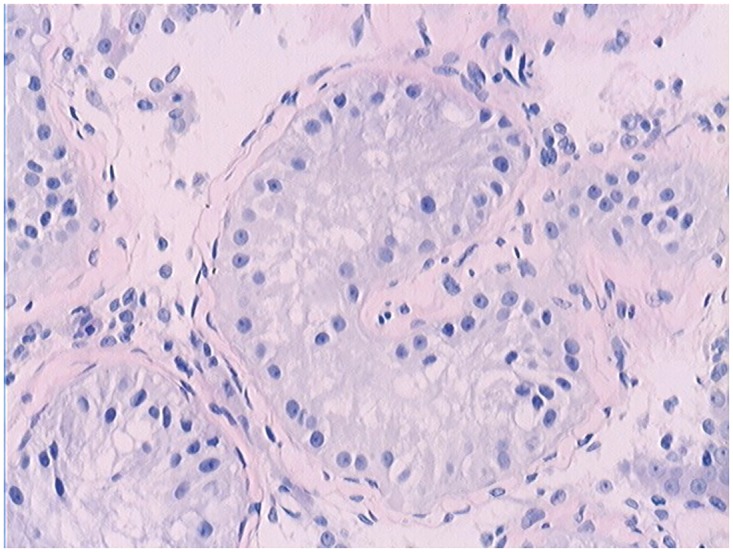
The spermatogenesis process was mainly blocked at the spermatocyte stage.
Discussion
It is known that humans carrying mutations in the DAX-1 locus often exhibit primary adrenal failure, hypogonadotropic hypogonadism and azoospermia. Dax-1 knockout mice exhibit dysgenesis and degeneration of the testicular germinal epithelium until the complete loss of germ cells after 14 weeks, while the serum hormone levels, including the LH and FSH levels, in DAX-1 knockout mice are indistinguishable from those of wild-type mice, suggesting primary testicular failure rather than dysfunction at the pituitary level [8]. Therefore, it is possible that somatic mutations in DAX-1 might be present in a subset of patients with secretory azoospermia, normal hormone levels, and no relevant reproductive family history. In a previous study, no DAX-1 mutations were detected in 15 testicular biopsy samples from men with secretory azoospermia because of the limited sample size [26]. To date, no causative mutation has been identified in DAX-1 for secretory azoospermia.
Previous Studies have shown that DAX-1 directly interacts with AR and represses its activity. AR is present in the cytoplasm in the absence of its ligand, but it is transported to the nucleus upon ligand binding to carry out its functions. Holter et al. have shown that cytoplasmic DAX-1 tethers AR in the cytoplasm in the presence of its ligand, preventing its translocation to the nucleus. Because both AR and DAX-1 are located on the X chromosome, all mutations affecting their functions yield a phenotype [18].
In this study, six novel missense mutations were detected in DAX-1 in the patients with secretory azoospermia. Of these mutations, V385L affected the inhibition of transcriptional AR activation by DAX-1. In vitro transient transfection assays showed that this mutation affected the ability of DAX-1 to repress transcription of AR compared with wild-type DAX-1. These results indicate that aberrations of DAX-1 might be intolerable and that the V385L mutation of DAX-1 might be involved in impairment of human spermatogenesis. Unfortunately, the family of this patient was not available for genotype–phenotype correlations. Other variants of DAX-1 may also be pathogenic, causing malfunctions by restricting the inductive activities of testis-promoting factors, such as SRY and SF-1, thereby modulating their effects during testicular development [27, 28]. Thus, the additional variants of DAX-1 assessed in this study may also be pathogenic and cause malfunction due to the following reasons: (1) there are functional limitations of the pMMTV-LUC promoter, which is only responsive to more severe mutations but is not informative for mild mutations [29]; and (2) DAX-1 also represses the inductive activities of testis-promoting factors, such as SRY and SF-1, thereby modulating their effects during testicular development [27, 28].
In summary, a novel mutation (p. V385L) in DAX-1 was detected in a patient with secretory azoospermia with no personal or family history of X-linked adrenal hypoplasia congenita or adrenal insufficiency. This mutation potentially contributed to the onset of secretory azoospermia in this patient.
Supporting Information
(DOC)
(DOC)
Acknowledgments
The authors thank the patients and their family members for their cooperation during the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (grant number 31271244 to YG, 81200465 to LM) and the Shenzhen Foundation of Science and Technology (grant number GJHZ20140414170821192 to NX, JCYJ20140414170821337 to NX, GJHZ20130412153906740 to NX). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jarvi K, Lo K, Fischer A, Grantmyre J, Zini A, Chow V, et al. CUA Guideline: The workup of azoospermic males. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2010;4(3):163–7. Epub 2010/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirsh A. Male subfertility. BMJ. 2003;327(7416):669–72. Epub 2003/09/23. 10.1136/bmj.327.7416.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nature medicine. 2008;14(11):1197–213. Epub 2008/11/08. 10.1038/nm.f.1895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bae DS, Schaefer ML, Partan BW, Muglia L. Characterization of the mouse DAX-1 gene reveals evolutionary conservation of a unique amino-terminal motif and widespread expression in mouse tissue. Endocrinology. 1996;137(9):3921–7. 10.1210/endo.137.9.8756567 . [DOI] [PubMed] [Google Scholar]
- 5. Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, et al. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Molecular endocrinology. 1996;10(10):1261–72. 10.1210/mend.10.10.9121493 . [DOI] [PubMed] [Google Scholar]
- 6. Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nature genetics. 1996;12(4):404–9. 10.1038/ng0496-404 . [DOI] [PubMed] [Google Scholar]
- 7. Jadhav U, Harris RM, Jameson JL. Hypogonadotropic hypogonadism in subjects with DAX1 mutations. Molecular and cellular endocrinology. 2011;346(1–2):65–73. Epub 2011/06/16. 10.1016/j.mce.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nature genetics. 1998;20(4):353–7. 10.1038/3822 . [DOI] [PubMed] [Google Scholar]
- 9. Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nature genetics. 2003;34(1):32–3. Epub 2003/04/08. 10.1038/ng1141 . [DOI] [PubMed] [Google Scholar]
- 10. Wu CM, Zhang HB, Zhou Q, Wan L, Jin J, Ni L, et al. Two novel DAX1 gene mutations in Chinese patients with X-linked adrenal hypoplasia congenita: clinical, hormonal and genetic analysis. Journal of endocrinological investigation. 2011;34(8):e235–9. Epub 2011/01/29. 10.3275/7484 . [DOI] [PubMed] [Google Scholar]
- 11. Raffin-Sanson ML, Oudet B, Salenave S, Brailly-Tabard S, Pehuet M, Christin-Maitre S, et al. A man with a DAX1/NR0B1 mutation, normal puberty, and an intact hypothalamic-pituitary-gonadal axis but deteriorating oligospermia during long-term follow-up. European journal of endocrinology / European Federation of Endocrine Societies. 2013;168(4):K45–50. Epub 2013/02/07. 10.1530/EJE-12-1055 . [DOI] [PubMed] [Google Scholar]
- 12. Ponikwicka-Tyszko D, Kotula-Balak M, Jarzabek K, Bilinska B, Wolczynski S. The DAX1 mutation in a patient with hypogonadotropic hypogonadism and adrenal hypoplasia congenita causes functional disruption of induction of spermatogenesis. Journal of assisted reproduction and genetics. 2012;29(8):811–6. Epub 2012/05/09. 10.1007/s10815-012-9778-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seminara SB, Achermann JC, Genel M, Jameson JL, Crowley WF Jr. X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. The Journal of clinical endocrinology and metabolism. 1999;84(12):4501–9. 10.1210/jcem.84.12.6172 . [DOI] [PubMed] [Google Scholar]
- 14. Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, et al. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. The Journal of clinical investigation. 2000;105(3):321–8. 10.1172/JCI7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantovani G, Ozisik G, Achermann JC, Romoli R, Borretta G, Persani L, et al. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. The Journal of clinical endocrinology and metabolism. 2002;87(1):44–8. 10.1210/jcem.87.1.8163 . [DOI] [PubMed] [Google Scholar]
- 16. Ozisik G, Mantovani G, Achermann JC, Persani L, Spada A, Weiss J, et al. An alternate translation initiation site circumvents an amino-terminal DAX1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. The Journal of clinical endocrinology and metabolism. 2003;88(1):417–23. 10.1210/jc.2002-021034 . [DOI] [PubMed] [Google Scholar]
- 17. Mullertz A, Schmedes A, Holmer G. Separation and detection of phospholipid hydroperoxides in the low nanomolar range by a high performance liquid chromatography/ironthiocyanate assay. Lipids. 1990;25(7):415–8. Epub 1990/07/01. . [DOI] [PubMed] [Google Scholar]
- 18. Holter E, Kotaja N, Makela S, Strauss L, Kietz S, Janne OA, et al. Inhibition of androgen receptor (AR) function by the reproductive orphan nuclear receptor DAX-1. Molecular endocrinology. 2002;16(3):515–28. 10.1210/mend.16.3.0804 . [DOI] [PubMed] [Google Scholar]
- 19. Agoulnik IU, Krause WC, Bingman WE 3rd, Rahman HT, Amrikachi M, Ayala GE, et al. Repressors of androgen and progesterone receptor action. The Journal of biological chemistry. 2003;278(33):31136–48. 10.1074/jbc.M305153200 . [DOI] [PubMed] [Google Scholar]
- 20. Jouravel N, Sablin E, Arnold LA, Guy RK, Fletterick RJ. Interaction between the androgen receptor and a segment of its corepressor SHP. Acta crystallographica Section D, Biological crystallography. 2007;63(Pt 11):1198–200. 10.1107/S0907444907045702 . [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Huang Y, Li H, Hu J, Liu X, Jiang T, et al. Excess of Rare Variants in Genes that are Key Epigenetic Regulators of Spermatogenesis in the Patients with Non-Obstructive Azoospermia. Sci Rep. 2015;5:8785 10.1038/srep08785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Li C, Xiao W, Yuan D, Wan G, Ma L. Site-directed mutagenesis by combination of homologous recombination and DpnI digestion of the plasmid template in Escherichia coli. Analytical biochemistry. 2008;373(2):389–91. Epub 2007/11/27. 10.1016/j.ab.2007.10.034 . [DOI] [PubMed] [Google Scholar]
- 23. Mou L, Zhang Q, Wang Y, Zhang Q, Sun L, Li C, et al. Identification of Ube2b as a novel target of androgen receptor in mouse sertoli cells. Biology of reproduction. 2013;89(2):32 10.1095/biolreprod.112.103648 . [DOI] [PubMed] [Google Scholar]
- 24. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–9. Epub 2010/04/01. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods. 2014;11(4):361–2. Epub 2014/04/01. 10.1038/nmeth.2890 . [DOI] [PubMed] [Google Scholar]
- 26. Mantovani G, Mancini M, Gazzano G, Spada A, Colpi GM, Beck-Peccoz P, et al. Somatic mutational analysis of DAX1 in testes from men with idiopathic azoospermia. Fertility and sterility. 2005;84(5):1542–4. 10.1016/j.fertnstert.2005.05.037 . [DOI] [PubMed] [Google Scholar]
- 27. Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391(6669):761–7. Epub 1998/03/05. 10.1038/35799 . [DOI] [PubMed] [Google Scholar]
- 28. Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Molecular and cellular biology. 1997;17(3):1476–83. Epub 1997/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zuccarello D, Ferlin A, Vinanzi C, Prana E, Garolla A, Callewaert L, et al. Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility. Clinical endocrinology. 2008;68(4):580–8. Epub 2007/11/01. 10.1111/j.1365-2265.2007.03069.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.