Abstract
Purpose:
Radiotherapy remains a major treatment method for malignant tumors. Magnetic resonance imaging (MRI) is the standard modality for assessing glioma treatment response in the clinic. Compared to MRI, ultrasound imaging is low-cost and portable and can be used during intraoperative procedures. The purpose of this study was to quantitatively compare contrast-enhanced ultrasound (CEUS) imaging and MRI of irradiated gliomas in rats and to determine which quantitative ultrasound imaging parameters can be used for the assessment of early response to radiation in glioma.
Methods:
Thirteen nude rats with U87 glioma were used. A small thinned skull window preparation was performed to facilitate ultrasound imaging and mimic intraoperative procedures. Both CEUS and MRI with structural, functional, and molecular imaging parameters were performed at preradiation and at 1 day and 4 days postradiation. Statistical analysis was performed to determine the correlations between MRI and CEUS parameters and the changes between pre- and postradiation imaging.
Results:
Area under the curve (AUC) in CEUS showed significant difference between preradiation and 4 days postradiation, along with four MRI parameters, T2, apparent diffusion coefficient, cerebral blood flow, and amide proton transfer-weighted (APTw) (all p < 0.05). The APTw signal was correlated with three CEUS parameters, rise time (r = − 0.527, p < 0.05), time to peak (r = − 0.501, p < 0.05), and perfusion index (r = 458, p < 0.05). Cerebral blood flow was correlated with rise time (r = − 0.589, p < 0.01) and time to peak (r = − 0.543, p < 0.05).
Conclusions:
MRI can be used for the assessment of radiotherapy treatment response and CEUS with AUC as a new technique and can also be one of the assessment methods for early response to radiation in glioma.
Keywords: ultrasound, MRI, glioma, CEUS
1. INTRODUCTION
Glioma is the most common and deadliest form of primary brain tumor. Of these malignant tumors, glioblastoma accounts for 60%–70% and the median survival is a dismal 12–15 months.1 Currently, radiotherapy remains a major treatment method in the management of malignant gliomas.2 Radiotherapy techniques used commonly include stereotactic brachytherapy, stereotactic radiotherapy, and stereotactic radiosurgery. Stereotactic radiotherapy and stereotactic radiosurgery utilize external photon beam generated by linear accelerator. Stereotactic radiosurgery is typically a single shot and without fractionation as in stereotactic radiotherapy. Stereotactic brachytherapy, on the other hand, involves implantation of radioactive seeds such as iodine-125 or iridium-192 in brain tumors.3 Compared with external beam radiotherapy, brachytherapy enables the accurate application of highly focused necrotizing tissue dose with a steep fall-off to the target volume. Temporary implants are preferred as permanent implants bear an increased risk of prolonged edema.3
Currently, magnetic resonance imaging (MRI) is the standard modality for assessing tumor properties and treatment response in the clinic.4 The most common MRI sequences are T2-weighted (T2w), fluid-attenuated inversion recovery, and gadolinium-enhanced T1-weighted (Gd-T1w). There are numerous ongoing investigations into the ability of functional and molecular imaging techniques5,6 to assess tumor treatment effects, including diffusion imaging (using apparent diffusion coefficient or ADC),7 perfusion imaging (using blood flow or blood volume),8 and protein-based amide proton transfer (APT) imaging.9,10 The APT technique can provide contrast because of the amide proton content of endogenous mobile cytosolic proteins or tissue pH. Previous preclinical and clinical studies have indicated that malignant tumors, typically overexpressing many proteins, consistently exhibit a high APT-weighted (APTw) signal.11,12
Ultrasound has often been used in the assessment of radiotherapy responses in head and neck13 and breast cancer,14 but not widely used in brain studies as the ultrasound waves cannot pass through bones. Several techniques have been developed to overcome this issue such as transcranial ultrasound15 and intraoperative ultrasound.16,17 In transcranial ultrasound, the ultrasound wave passes through the temporal, occipital, and eye window. When a patient goes through brain surgery or stereotactic brachytherapy, craniotomy or craniectomy makes ultrasound imaging accessible.3,15,16 Clinical ultrasound is a real-time, low-cost, nonionizing, and portable imaging method that has been widely used in diagnosis and in interventional therapies. With the development of new contrast agents to visualize blood flow in the microcirculation and larger vasculatures, commonly referred to as microbubbles, contrast-enhanced ultrasound (CEUS) is becoming popular in a number of areas where perfusion is an important clinical differentiator.18,19 It does not only lead to a great improvement in diagnostic accuracy of ultrasound but also in the guidance and evaluation of responses to therapy such as in the study of focal liver lesions, follow-up of traumatic injuries of abdominal parenchymal organs, and characterization of breast lesions.20–28
Assessment of early radiotherapy treatment response is becoming increasingly important to predict overall survival, guide subsequent treatment, and customize personalized treatment.29–34 Animal studies have shown a response in glioma tumor models to radiotherapy that can be detected between 1 day and 4 days after treatment through 13C magnetic resonance spectroscopic imaging.32 Early response during radiotherapy assessed by MRI perfusion imaging has been found to be able to predict survival in high-grade glioma patients.30 Once the early identifiers for survival prediction are established, they can also be incorporated to optimize radiotherapy treatment planning for radioresistant tumor subvolumes.
In this study, we quantitatively compared the MRI and ultrasound imaging features of nonirradiated and irradiated U87 tumors in rats at several time points. We assessed multiple MRI parameters, including structural (T1 and T2), functional (ADC and blood flow), and molecular (APTw) MRI, and multiple ultrasound imaging parameters, with and without the use of contrast agents, as shown in Table I. The purposes of this study were to explore the ability of ultrasound imaging to assess glioma response to radiotherapy and to compare the use of various sensitive imaging parameters for the assessment of glioma early response to radiotherapy.
TABLE I.
US imaging and MR imaging parameters used in this work.
| Abbreviation | Nomenclature | Definition or meaning |
|---|---|---|
| US imaging | ||
| PV | Percent vascularity | Blood volume over the ROI volume |
| PE | Peak enhancement | Maximum intensity of TIC |
| AUC | Area under the TIC curve | Time integral of the gamma variate function, corresponding to blood volume |
| WiAUC | Area under the curve (wash-in) | Area under the TIC from time of arrival to the PE |
| RT | Rise time | Time from 5% to 95% of the peak intensity |
| TTP | Time to peak | Time from the arrival of the UCA to the maximum intensity value |
| mTT | Mean transit time | Average time for the blood to pass through the region of interest |
| PI | Perfusion index | Blood flow, corresponding to the ratio of AUC to mTT |
| MR imaging | ||
| T2 | Transverse relaxation time | Structural MRI parameter |
| T1 | Longitudinal relaxation time | Structural MRI parameter |
| ADC | Apparent diffusion coefficient | Functional MRI parameter, typically increases after therapy |
| CBF | Cerebral blood flow | Functional MRI parameter, typically decreases after therapy |
| APTw | Amide proton transfer-weighted | Protein-based molecular MRI parameter, typically decreases after therapy |
2. METHODS
Our goal is to measure early response using MRI and CEUS by comparing various sensitive imaging parameters before and after treatment. Figure 1 shows a block diagram of the experimental study design. Tumor inoculation was done 13 days preradiation. A thinned skull window preparation was done 6 days preradiation. Both MRI and CEUS were acquired at 1 day preradiation, 1 day postradiation, and 4 days postradiation, followed by histology and image analyses. Additional details on each step are given below.
FIG. 1.

Figure shows a timeline of the experimental study design. Tumor model inoculation was done 13 days preradiation. A thinned skull window preparation was done 6 days preradiation. Both MRI and CEUS were acquired at 1 day preradiation, 1 day postradiation, and 4 days postradiation, followed by histology and image analysis.
2.A. Tumor model inoculation
All experimental procedures were conducted with the approval of the Johns Hopkins Animal Care and Use Committee. Thirteen nude rats (male; 14–16 weeks; 300–350 g) were anesthetized by an intraperitoneal injection of 3–5 ml/kg of a solution containing ketamine hydrochloride, 25 mg/ml, and xylazine, 2.5 mg/ml. A midline scalp incision was made to expose the sagittal and coronal sutures. A small burr hole was made with an electric drill, centered 3 mm to the right of the sagittal suture and 1 mm anterior to the coronal suture. A needle was placed into the burr hole at a depth of 5 mm from the skull. U87 glioma cells (1 × 106 in 4 μl media) were then stereotactically injected over 3–4 min for each rat. The needle was withdrawn, and the skin was closed with sutures. The rats were returned to their cages and received a regular rat diet and water ad libitum.
2.B. Thinned skull window preparation
As there is no transtemporal window for ultrasound imaging with rats as in humans, a small thinned skull window is required to facilitate efficient transmission of ultrasound to the rat brain without reflection or absorption by the bone interface. Each tumor-bearing rat underwent preliminary MR imaging on several postimplantation days to determine the size of the tumor. A thinned skull window preparation was performed when the tumor reached 1–2 mm in diameter, as determined by MRI. Following the administration of anesthesia, as described previously, the skull was thinned approximately 8 mm in diameter, 6 days before radiotherapy.35 This technique leaves the dura untouched and preserves the integrity of the brain microenvironment, allowing the disease model to mimic naturally occurring physiological conditions. The skin over the window was sutured with MRI-compatible, 2-0 surgical sutures which were not removed during the study.
2.C. Radiotherapy
Seven rats were selected as the treatment group and irradiated using a small-animal radiation research platform.36 Briefly, the rats were anesthetized with 4% isoflurane for about 5 min, followed by 2%–2.5% isoflurane for maintenance during radiation. The rats were immobilized in a fixation device. A single, well-collimated x-ray beam with a dose of 8 Gy was administered to a 10 × 10 mm2 region centered at the tumor, under on-board, cone-beam CT image guidance. The rats were monitored until 22 days postimplantation.
2.D. MRI data acquisition
MRI data were acquired using a 4.7 T animal MRI system (Bruker Biospin, Billerica, MA). All 13 rats (nonirradiated and irradiated) were scanned within 1 day preradiation (baseline), and seven irradiated rats were further scanned on 1 day and 4 days postradiation. First, axial/coronal T2w images were acquired using the following parameters: repetition time (TR) = 3 s; echo time (TE) = 64 ms; five slices; thickness = 1.5 mm; field of view (FOV ) = 42/32 × 32 mm2; matrix = 256/192 × 192; number of averages (NA) = 2. Then, several quantitative MRI parameters were acquired using previously described methods,37 including T1 (inversion recovery; predelay = 3 s; TE = 30 ms; inversion recovery time = 0.05, 0.3, 0.6, 1.2, 1.8, 2.5, and 3.5 s; NA = 4), T2 (TR = 3 s; TE = 30, 40, 50, 60, 70, 80, and 90 ms; NA = 4), isotropic ADC (TR = 3 s; TE = 80 ms; b-values =0, 166.7, 333.3, 500, 666.7, 833.3, and 1000 s/mm2; NA = 8), blood flow (arterial spin labeling or ASL; 3-s labeling at a distance of 20 mm away from the imaging slice; TR = 6 s; TE = 28.6 ms), and APTw (frequency-labeling offsets of ±3.5 ppm; TR = 10 s; TE = 30 ms; saturation power =1.3 μT; saturation time = 4 s; NA = 16). These quantitative MRI sequences were acquired with the same geometry and location as one coronal T2w image slice with the maximum tumor area.
2.E. MRI image analysis
All imaging data were processed using Interactive Data Language (idl, Version 7; Exelis Visual Information Solutions, Inc., Boulder, CO). Tumor volumes were manually measured as the sum of all tumor voxels in all slices on the high-resolution T2w images. The T1 map, T2 map, and ADC map were fitted using the following equations: I = A + B exp(−TI/T1), I = I0 exp(−TE/T2), and I = I0 exp(−b ADC), respectively. The CBF map was reconstructed from images with and without labeling, using previously described methods.38 The APTw MRI signal was quantified by the MTR asymmetry at ±3.5 ppm with respect to the water signal: MTRasym(3.5 ppm) = Ssat(−3.5 ppm)/S0 − Ssat(+3.5 ppm)/S0, where Ssat and S0 are the signal intensities with and without selective radiofrequency irradiation, respectively.
2.F. Ultrasound data acquisition
Ultrasound imaging was performed on a Vevo 2100 scanner (VisualSonics, Toronto, Canada) and all images were acquired using a 21-MHz linear array transducer (MS250). 2D B-mode imaging was used to localize tumor under the thinned skull window. 3D power Doppler imaging was carried out using a step size of 0.15 mm and a 40-dB gain setting. The 3D power Doppler mode was used to measure tumor percent vascularity (PV) defined as the relative power Doppler signal within a tumor volume.
CEUS imaging with a nonlinear contrast imaging mode was performed immediately after 3D power Doppler imaging. It utilizes amplitude and pulse modulated harmonic imaging. Untargeted Vevo Micro Marker Contrast Agent (VisualSonics), which is our ultrasound contrast agent (UCA), was administered at doses of 1.0 × 109 microbubbles in 500 μl volumes of prepared bubbles through the tail vein catheter, followed by a 0.5 ml saline flush. CEUS was performed at 18 MHz, acoustic output power of 4%, and a gain of 30 dB. Two hundred second cine loop of the perfusion process was recorded right after injection of the contrast agent was administrated. The largest cross section in the rat brain was selected in each study to ensure the consistency of the imaging location.
2.G. Ultrasound image analysis
All US imaging data analysis was performed with the Vevo-CQ (VisualSonics, Toronto, Canada). In all rats, a fixed period of 200 s after the first appearance of the UCA was used to generate color-coded maps of perfusion index (PI) to facilitate the interpretation of the time-intensity curve (TIC). The fixed period selection allowed a comparable evaluation of perfusion parameters among the different animals. TIC was calculated on a predefined region of interest (ROI) and then considered for statistical analysis. The 3D power Doppler image was analyzed with the same ROI to generate PV. PV is defined as the blood volume over the ROI volume. The perfusion parameters, derived from VevoCQ software based on the bolus perfusion model, were extracted from the time-intensity curves plotted for each ROI. Peak enhancement (PE) is the maximum signal intensity reached during the transit of the contrast bolus. Area under the curve (AUC) is the time integral of the gamma variate function and is corresponding to blood volume. WiAUC is the area under the curve of the wash-in phase. Rise time (RT) is defined as the time that TIC took to increase from 5% to 95% of its peak intensity. Time to peak (TTP) is the time from the arrival of the UCA to its maximum intensity value. Mean transit time (mTT) is the width of the bolus when the intensity equals half of the peak value. PI is corresponding to blood flow and is the ratio of AUC to mTT. All the abbreviation, nomenclature, and definition of above CEUS imaging parameters in additional to the MRI parameters can be found from Table I.
2.H. Statistical analysis
All results were expressed as mean ±SD. The differences in tumor volumes measured with 3D power Doppler US imaging and multislice T2w MRI signal abnormalities are analyzed only at baseline due to the limited thinned skull window for US at later time points. All other parameters were analyzed from a similar portion of the tumor, typically at the central area. All statistical analyses were performed using the statistical package spss for Windows (Version 18, Chicago, IL). Kolmogorov–Smirnov Z test was used to determine if all the data were normally distributed. Only PV was found not normally distributed (z = 1.582, p = 0.013) and all the other parameters showed nonsignificant test results (p > 0.05). The changes in each CEUS or MRI parameter on 1 day postradiation and 4 days postradiation vs baseline were assessed. To test the significance of the changes, since PV is not normally distributed, a nonparametric sum-rank test was used. A t-test was used for all the other parameters. Statistical significance was determined using p = 0.05.
2.I. Histology
After MRI and US scanning 4 days postradiation, four out of seven irradiated animals were euthanized immediately using carbon dioxide inhalation for 7 min, and brains were excised and preserved in 4% paraformaldehyde at 4 °C. Brain samples were sectioned and histological sections were stained with hematoxylin and eosin (H&E). Brain sections were analyzed using a light microscope at 10–200 × magnification.
3. RESULTS
All rats survived the process of anesthesia, handling, CEUS, MRI, and radiotherapy. CEUS and MRI were successfully performed with all 13 rats (nonirradiated and irradiated) on 1 day preradiation. Seven rats received radiotherapy were further imaged on 1 day and 4 days postradiation. CEUS and MRI studies were not compared at the later time point for six nonirradiated rats, due to the fact that the nonirradiated tumors became much larger than the thinned skull window.
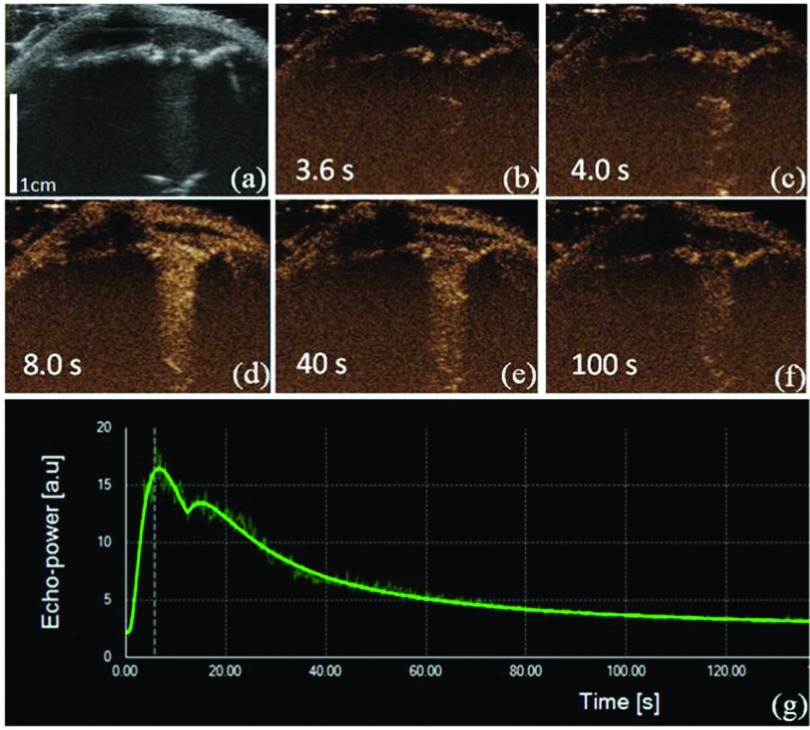
Figure 2 illustrates a typical dynamic wash-in and wash-out phase of contrast enhancement against time. In the rat U87 glioma model, all tumors showed abnormal and fast enhancement [Figs. 2(b)–2(f)] after the tail vein bolus injection of the ultrasound contrast agent. At t = 3.6 s after injection, the perfusion signal started to be seen in the central part of the tumor. The perfusion was inhomogeneous, and the signal intensity rapidly increased at t = 4.0 s. At t = 8.0 s, the entire tumor was well perfused. Figure 2(e) showed a wash-out phase of microbubble at t = 40.0 s. At t = 100.0 s, microbubble approached full wash-out. From Fig. 2(g), it can be seen that TIC showed a rapid increase of the microbubble-echo intensity and a slow decrease after it reached the peak. Thus, with the thinned skull window preparation, the glioma tumor can be imaged with both B-mode ultrasound and CEUS. By comparing the B-mode ultrasound and CEUS images, it can clearly be seen that the glioma tumor contour and size can be better detected with CEUS imaging [Figs. 2(a) and 2(d)].
FIG. 2.
Injection and perfusion of microbubbles in the U87 glioma across multiple time points including subsequent CEUS [(b)–(f)] and TIC. (a) A B-mode ultrasound image was acquired before injection. (b) After injection, a significant perfusion started to show at t = 3.6 s from CEUS. (c) A significant inhomogeneity happened at t = 4.0 s. (d) At t = 8.0 s, the entire tumor was perfused. (e) Wash-out phase of microbubbles at t = 40.0 s. (f) At t = 100.0 s, microbubble approached full wash-out. (g) TIC showed rapid increase of the microbubble echo intensity and its slow decrease after it reached the peak. Images (a)–(f) share the same scale bar as in (a).
Figure 3 shows the US images of a representative irradiated rat at three time points. The first row shows the results at baseline. The second row shows results at 1 day postradiation and the third row shows the 4 days postradiation results. The white arrows in the power Doppler image from the second column show significant decrease of regional perfusion. The third column shows the CEUS result and its change over time. The corresponding white arrow at baseline before radiation shows homogeneous enhancement. At 1 day and 4 days postradiation, tumor showed regional inhomogeneous enhancement. The TIC and its corresponding AUC also showed decrease of regional perfusion. The consistent results from the power Doppler, CEUS, AUC, and TIC from the last four columns indicate that the blood supply to the tumor significantly decreased after radiation.
FIG. 3.
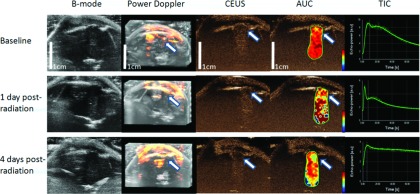
Changes in 2D B-mode, 3D power Doppler, CEUS, AUC, and TIC acquired at the different time points (baseline, 1 day postradiation, and 4 days postradiation) for a rat with a U87 glioma. The white arrows in power Doppler, CEUS, AUC are corresponding to the region showed homogeneous perfusion at baseline but with inhomogeneous and decreased perfusion at 1 day and 4 days postradiation. TIC also showed decreased perfusion for the contoured region of interest. Both 1 day postradiation and 4 days postradiation images share the same scale bar as in the baseline images.
Figure 4 presents the MRI results of a representative irradiated rat at three time points. Based on the MR images, the irradiated tumor still grew in size during 1–4 days postradiation. Compared to the contralateral brain tissue, the tumor was hyperintense on all MR images at baseline, showing slight (T2w, T2, T1, and ADC) or visible (CBF and APTw) intensity changes during 1–4 days postradiation.
FIG. 4.
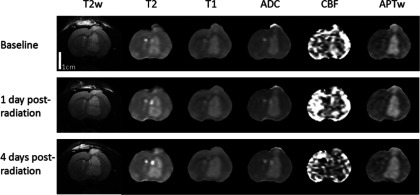
Changes in T2w, T2, T1, ADC, blood flow, and APTw images acquired at the different time points (baseline, 1 day postradiation, and 4 days postradiation) for a rat with a U87 glioma. The display windows are T2 (0–100 ms), T1 (0.5–2 s), ADC (0–2 × 10−9 m2/s), blood flow [0–200 ml/(100 g/min)], and APTw (−10% to 10% of the bulk water signal intensity). All images share the same scale bar as in the baseline T2w image.
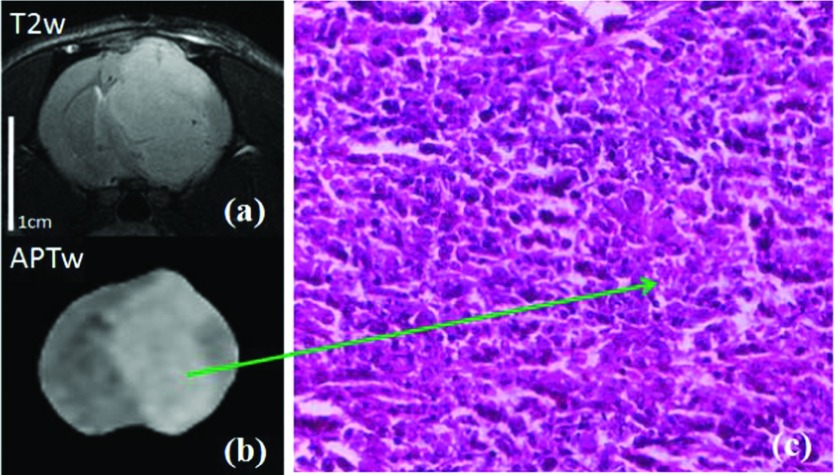
Figure 5 shows an example of T2w and APTw images and high-magnification (20×) H&E images for one rat after radiation. The H&E-stained histological sections of the irradiated area showed hypocellularity with necrosis and nuclear shrinkage.
FIG. 5.
T2w and APTw images (a) and (b) and high-magnification (20×) H&E-stained images (c) of one rat after radiation. The H&E image (c) shows hypocellularity with necrosis and nuclear shrinkage in the irradiated tumor at a later time point postradiation. Both MR images share the same scale bar as in the baseline T2w image.
Table II shows the results by comparing different imaging parameters from US and MR for assessment of the treatment response. Three different time points are compared in this study. Even though we have observed decreased perfusion based on the TIC, but among the US imaging parameters, only AUC 4 days postradiation shows significant difference compared with the baseline results (p < 0.05). All other US imaging parameters did not show significant difference. However, among MRI parameters, T2 and ADC show significant increase at 4 days postradiation compared with the results at baseline (p < 0.05 for T2 and p < 0.01 for ADC). Compared with the results at baseline, both CBF and APTw show significant decrease at 4 days postradiation. No significant difference is found between the results from baseline and 1 day postradiation for both US imaging and MRI parameters.
TABLE II.
Measured US imaging and MR imaging parameters (values ±STD) at three time points.
| Baseline (n = 13) | 1 day postradiation (n = 7) | 4 days postradiation (n = 7) | |
|---|---|---|---|
| US imaging | |||
| V (US) (mm3) | 71.0±18.6 | 70.0±18.6 | 63.1±11.4 |
| PV (%) | 3.15±3.81 | 2.25±3.36 | 1.34±2.11 |
| PE (a.u.) | 2.01±1.77 | 1.80±0.20 | 1.18±1.12 |
| WiAUC (a.u.) | 9.53±7.82 | 11.77±9.07 | 6.00±4.75 |
| RT (a.u.) | 6.50±3.42 | 8.63±6.27 | 8.96±4.30 |
| TTP (s) | 7.67±3.75 | 9.47±6.34 | 9.96±4.67 |
| AUC (a.u.) | 291.1±336.6 | 183.8±109.0 | 62.1±61.7∗ |
| MTT (s) | 226.6±182.6 | 135.3±113.9 | 118.2±45.1 |
| PI (a.u.) | 1.36±1.51 | 1.58±0.57 | 0.50±0.42 |
| MR imaging | |||
| T2 (ms) | 72.1±2.2 | 72.3±1.1 | 74.3±1.9∗ |
| T1 (s) | 1.79±0.05 | 1.79±0.01 | 1.80±0.05 |
| ADC (10−9 m2/s) | 1.09±0.03 | 1.12±0.03 | 1.13±0.02∗∗ |
| CBF [ml/(100 g/min)] | 70.6±27.5 | 58.0±19.3 | 29.2±11.3∗∗∗ |
| APTw (%) | 3.81±0.58 | 3.53±0.63 | 2.50±0.43∗∗∗ |
| V (MRI) (mm3) | 99.4±34.7 | 105.9±21.64 | 174.2±85.8∗ |
Note: The statistical significance of the difference between the parameters at baseline and those at 1 day or 4 days postradiation: *p < 0.05, **p < 0.01, ***p < 0.001. Here, a.u. is an arbitrary unit.
Table III shows the comparison between the CEUS and MRI among all the animals with data points including preradiation and postradiation results. The 3D volume of the tumor measured from the B-mode US and MRI is highly correlated (r = 0.856, p < 0.01). RT is correlated with the CBF and APTw (r = − 0.589 and r = − 0.527, p < 0.01). TTP is also correlated with the CBF and APTw (r = − 0.543, r = − 0.501, p < 0.05). PI is correlated with APTw (r = 0.458, p < 0.05).
TABLE III.
Correlation coefficients and p values for the measured ultrasound imaging and MRI parameters.
| MRI | |||||||
|---|---|---|---|---|---|---|---|
| US | T2 | T1 | ADC | CBF | APTw | V (MRI) | |
| V (US) | r value | −0.006 | 0.247 | −0.290 | −0.281 | −0.101 | 0.856a |
| p value | 0.977 | 0.244 | 0.169 | 0.183 | 0.638 | 0.000a | |
| PV | r value | −0.235 | −0.207 | −0.467 | 0.126 | 0.176 | 0.102 |
| p value | 0.268 | 0.332 | 0.021 | 0.559 | 0.412 | 0.741 | |
| CEUS | |||||||
| PE | r value | 0.015 | 0.237 | −0.212 | 0.216 | 0.342 | 0.156 |
| p value | 0.949 | 0.302 | 0.355 | 0.347 | 0.129 | 0.611 | |
| WiAUC | r value | −0.113 | 0.222 | −0.183 | −0.137 | 0.063 | 0.454 |
| p value | 0.626 | 0.333 | 0.426 | 0.553 | 0.787 | 0.119 | |
| RT | r value | −0.145 | 0.010 | 0.017 | −0.589 | −0.527 | 0.479 |
| p value | 0.543 | 0.966 | 0.944 | 0.006 | 0.017 | 0.115 | |
| TTP | r value | −0.107 | −0.040 | −0.033 | −0.543 | −0.501 | 0.315 |
| p value | 0.643 | 0.864 | 0.887 | 0.011 | 0.021 | 0.295 | |
| AUC | r value | −0.121 | 0.398 | −0.288 | 0.026 | 0.300 | 0.233 |
| p value | 0.602 | 0.074 | 0.206 | 0.909 | 0.186 | 0.443 | |
| mTT | r value | −0.088 | 0.407 | −0.291 | −0.197 | 0.066 | 0.335 |
| p value | 0.706 | 0.067 | 0.200 | 0.391 | 0.776 | 0.263 | |
| PI | r value | −0.173 | 0.127 | −0.213 | 0.362 | 0.458 | −0.142 |
| p value | 0.453 | 0.584 | 0.353 | 0.107 | 0.037 | 0.643 | |
Note: V (US), the tumor volume measured by ultrasound; V (MRI), the tumor volume measured by MRI. Statistically significant p values are in bold.
Baseline tumor volumes only due to the limited thinned skull window.
4. DISCUSSION
Malignant gliomas, such as anaplastic astrocytoma and glioblastoma, are one of the most vascularized human tumors.39 The level of angiogenesis is an important parameter for grading glioma in pathology. As the malignancy of glioma increases, the tumor is more vascularized and is associated with relatively high blood flow, larger capillary bed, and more morphological abnormalities.40 Transcranial ultrasound imaging can noninvasively detect brain lesions through temporal, occipital, and eye windows.15 The capability of transcranial ultrasound for tumor imaging can be greatly improved with the use of ultrasound contrast agent (CEUS). Compared with regular ultrasound imaging, CEUS can provide tumor perfusion information and can potentially be complementary by each other with the use of MRI, CT, MR angiography, or CT angiography as imaging method for evaluating brain lesions and vessels both in transcranial ultrasound41 and intracranial ultrasound during intraoperative procedure.16,17
The contrast agent used in CEUS, or the microbubble contrast agent, is a pure blood pool agent. It remains in the vasculature and leads to the change of TIC. This allows CEUS to evaluate angiogenesis quantitatively.42–44 Currently, CEUS is widely applied clinically and has significantly improved the measurement of tumor tissue at the microcirculation level.45–48 CEUS has also been applied to the assessment of therapy response in additional to traditional 2D anatomical or Doppler imaging. From CEUS, TIC can be used to quantitatively evaluate the tumor perfusion by measuring the flow rate change of microbubbles against time, based on the diffusion theory of the contrast agent. Lassau et al.49 reported that CEUS-based measures can be used to quantify dynamic changes in tumor vascularity as early as 4 days after targeted cancer therapy drug administration in patients with liver cancer, and the result can be used as predictor of tumor response at 2 months, progression-free survival, and overall survival. They may be potential surrogate measures of the effectiveness of antiangiogenic therapy. Another recent study has also indicated that AUC is the CEUS parameter related to blood volume at day 15 after treatment and they can predict the response of targeted cancer therapy drug for patients with metastatic gastrointestinal stromal tumors.50 In this study, the CEUS parameter, AUC, significantly decreased at 4 days postradiation, indicating that CEUS can potentially be used for evaluating radiotherapy response. Compared to other CEUS parameters, AUC may be a more comprehensive and sensitive parameter.
MRI has been the gold standard of imaging methods for clinical diagnosis and treatment response evaluation of glioma. In this study, it is shown that the T2 and ADC values significantly increased while the CBF and APTw values significantly decreased at 4 days postradiation compared with those at baseline, indicating the advantage of MRI in the early evaluation of treatment response of glioma. Previous studies showed that the increase in ADC is associated with necrosis of the tumor and the decrease in ADC is associated with the recurrence of tumor cells.51 Increased T2 and ADC values as well as decreased CBF and APTw values at 4 days postradiation observed in this study may indicate radiation-related necrosis inside the tumor.37 In radiotherapy, under the direct action of radiation, radiation is absorbed in biological material and it interacts directly with the DNA, which is thecritical target in cells. Under indirect action of radiation, the radiation primarily interacts with water to produce free radicals that further damage the DNA. Both actions cause single-strand breaks which are repaired readily and double-strand break which would lead to cell killing. In the sequence of events from the radiation to the biological damage after radiation, DNA damage occurs from minutes to hours and the cell death and DNA mutation may be expressed from hours to days.52–54 Consistent with our expectation, no parameter has significantly changed at 1 day postradiation. At the same time, MRI showed a significant tumor volume increase of the animal at 1 day and 4 days postradiation, as reported before even for a high dose of 40 Gy.37 Alternatively, US did not show such an increase of the tumor volume potentially due to the limitation of the B-mode imaging of the tumor boundary under the thinned skull window. It indicates that the traditional treatment response methods such as response evaluation criteria in solid tumors (RECIST) cannot be used at early stage after treatment. CEUS parameter AUC and MRI parameters T2, ADC, CBF, and APTw were able to detect the tumor change at 4 days postradiation.
In this study, by comparing the CEUS and MRI parameters, it was found that CEUS parameters, RT and TTP, are negatively correlated with MRI parameters, CBF and APTw, and PI is positively correlated with APTw. APT imaging is a novel molecular MRI method that can noninvasively explore the molecular properties of cancer tissue in vivo at the endogenous protein level and provide unique diagnostic information about brain cancer.55,56 Recent studies showed that APTw value in glioma is positively correlated with microvessel density (MVD) and vascular endothelial growth factor (VEGF) stained immunohistologically.57,58 Tissues with higher MVD values usually have more endothelial cells and thus higher protein concentrations and are therefore associated with high APTw signal.37 Perfusion MR imaging can be used to evaluate the distribution of the blood flow and the microcirculation in tumor. The hemodynamic perfusion index, blood volume or blood flow, may be correlated with MVD. The TIC from CEUS with parameters, RT and TTP, is normally negatively correlated with MVD, and PI is usually positively correlated with MVD.59,60 The important advantage of CEUS is its low-cost and portability, which can be used during intraoperative procedures for decision making of interventions. Our results show that perfusion of microcirculation from CEUS is correlated with MRI findings and this is consistent with previous reports.
However, there were several limitations to this study. Our primary goal of this study was to compare CEUS to MRI, which was taken as gold standard, in the assessment of glioma and glioma response to radiotherapy. Due to the fact that the tumors in the nonirradiated group grew much larger than the thinned skull window (8 mm in diameter), we were only able to perform both CEUS and MRI for postradiation studies on seven out of the total 13 animals. It would be also interesting to follow nonirradiated tumors with thinned skull window of 10–12 mm in diameter to observe the progression of the tumor growth in the future. Animal studies have shown that response of glioma tumor model to radiotherapy can be detected between 1 day and 4 days after treatment through magnetic resonance spectroscopic imaging.32 In this study, almost all rats were dead or very sick 7 days after radiation, so we were not able to perform more imaging measurements at this and later time points. Only three time points (1 day preradiation, 1 day, and 4 days postradiation) were selected for imaging. Following radiation treatment response at long term observation points such as 7 days, 14 day, and 1 month with both imaging methods is one of our particular research interests for future studies. A more comprehensive study is needed to study the long term treatment response with different radiation doses, fractionations, and time points. Table II shows significant change of tumor size before and after radiation but B-mode US showed insignificant tumor size changes. B-mode images do not show tumor boundaries clearly due to the growing tumor size and the limited thinned skull window. CEUS has better tumor visibility but it is limited to 2D in this study which prevents us from using CEUS for 3D tumor volume measurement. CEUS images may have nonlinear propagation artifacts that may reduce the contrast-to-tissue ratio. Various groups have worked on techniques to mitigate the artifacts with promising results.61 A correction algorithm from such studies can help us to improve the quantitative analysis result from CEUS.
5. CONCLUSION
To summarize, our study has shown that MRI can be used for the assessment of radiotherapy treatment response and CEUS with AUC as a new technique and can also be one of the assessment methods. Our results showed that CEUS-based AUC significantly decreased at 4 days postradiation and can potentially be used for evaluating radiotherapy response. In addition, CEUS-based RT and TTP are negatively correlated with MRI-based CBF and APTw, and PI is positively correlated with APTw. Further study will concentrate on the long time window for the effective assessment.
ACKNOWLEDGMENTS
This work was supported in part by Zhejiang Province Science and Technology General Project (Grant No. 2013C33206) and Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology, Zhejiang, China to C.Y. and the National Institutes of Health (Grant Nos. R21EB015555, R01EB009731, and R01CA166171) to J.Z. The authors thank Dr. Kelly O’Connell and Mr. Robert Haper from VisualSonics for their support during the experiment.
REFERENCES
- 1.Wen P. Y. and Kesari S., “Malignant gliomas in adults,” N. Engl. J. Med. 359, 492–507 (2008). 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- 2.Walker M. D., E. Alexander, Jr., Hunt W. E., MacCarty C. S., M. S. Mahaley, Jr., J. Mealey, Jr., Norrell H. A., Owens G., Ransohoff J., and Wilson C. B., “Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: A cooperative clinical trial,” J. Neurosurg. 49, 333–343 (1978). 10.3171/jns.1978.49.3.0333 [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S. B., Thon N., Nikolajek K., Niyazi M., Tonn J.-C., Belka C., and Kreth F.-W., “Iodine-125 brachytherapy for brain tumours—A review,” Radiat. Oncol. 7, 30–57 (2012). 10.1186/1748-717X-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiss W. D., Raab P., and Lanfermann H., “Multimodality assessment of brain tumors and tumor recurrence,” J. Nucl. Med. 52, 1585–1600 (2011). 10.2967/jnumed.110.084210 [DOI] [PubMed] [Google Scholar]
- 5.Gillies R. J., Bhujwalla Z., Evelhoch J., Garwood M., Neeman M., Robinson S. P., Sotak C. H., and van der Sanden B., “Applications of magnetic resonance in model systems: Tumor biology and physiology,” Neoplasia 2, 139–151 (2000). 10.1038/sj.neo.7900076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evelhoch J. L., Gillies R. J., Karczmar G. S., Koutcher J. A., Maxwell R. J., Nalcioglu O., Raghunand N., Ronen S. M., Ross B. D., and Swartz H. M., “Application of magnetic resonance in model systems: Cancer therapeutics,” Neoplasia 2, 152–165 (2000). 10.1038/sj.neo.7900078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenevert T. L., McKeever P. E., and Ross B. D., “Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging,” Clin. Cancer Res. 3, 1457–1466 (1997). [PubMed] [Google Scholar]
- 8.Collins D. J. and Padhani A. R., “Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges,” IEEE Eng. Med. Biol. Mag. 24, 65–83 (2004). 10.1109/MEMB.2004.1360410 [DOI] [PubMed] [Google Scholar]
- 9.Zhou J., Payen J.-F., Wilson D. A., Traystman R. J., and van Zijl P. C., “Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI,” Nat. Med. 9, 1085–1090 (2003). 10.1038/nm907 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Lal B., Wilson D. A., Laterra J., and van Zijl P., “Amide proton transfer (APT) contrast for imaging of brain tumors,” Magn. Reson. Med. 50, 1120–1126 (2003). 10.1002/mrm.10651 [DOI] [PubMed] [Google Scholar]
- 11.Wen Z., Hu S., Huang F., Wang X., Guo L., Quan X., Wang S., and Zhou J., “MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast,” NeuroImage 51, 616–622 (2010). 10.1016/j.neuroimage.2010.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G., Abaza R., Williams J. D., Zynger D. L., Zhou J. Y., Shah Z. K., Patel M., Sammet S., Wei L., Bahnson R. R., and Knopp M. V., “Amide proton transfer MR imaging of prostate cancer: A preliminary study,” J. Magn. Reson. Imaging 33, 647–654 (2011). 10.1002/jmri.22480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Tridandapani S., Beitler J. J., David S. Y., Yoshida E. J., Curran W. J., and Liu T., “Ultrasound GLCM texture analysis of radiation-induced parotid-gland injury in head-and-neck cancer radiotherapy: An in vivo study of late toxicity,” Med. Phys. 39, 5732–5739 (2012). 10.1118/1.4747526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadeghi-Naini A., Papanicolau N., Falou O., Zubovits J., Dent R., Verma S., Trudeau M., Boileau J. F., Spayne J., and Iradji S., “Quantitative ultrasound evaluation of tumor cell death response in locally advanced breast cancer patients receiving chemotherapy,” Clin. Cancer Res. 19, 2163–2174 (2013). 10.1158/1078-0432.CCR-12-2965 [DOI] [PubMed] [Google Scholar]
- 15.Bartels E., Color-Coded Duplex Ultrasonography of the Cerebral Vessels: Atlas and Manual (Stuttgart, Germany, 1999). [Google Scholar]
- 16.Kanno H., Ozawa Y., Sakata K., Sato H., Tanabe Y., Shimizu N., and Yamamoto I., “Intraoperative power Doppler ultrasonography with a contrast-enhancing agent for intracranial tumors,” J. Neurosurg. 102, 295–301 (2005). 10.3171/jns.2005.102.2.0295 [DOI] [PubMed] [Google Scholar]
- 17.Prada F., Mattei L., Del Bene M., Aiani L., Saini M., Casali C., Filippini A., Legnani F. G., Perin A., Saladino A., Vetrano I. G., Solbiati L., Martegani A., and DiMeco F., “Intraoperative cerebral glioma characterization with contrast enhanced ultrasound,” BioMed Res. Int. 484261 (2014). 10.1155/2014/484261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Oord S. C., Gerrit L., Sijbrands E. J., van der Steen A. F., and Schinkel A. F., “Effect of carotid plaque screening using contrast-enhanced ultrasound on cardiovascular risk stratification,” Am. J. Cardiol. 111, 754–759 (2013). 10.1016/j.amjcard.2012.11.033 [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi J., Sugimoto K., Moriyasu F., Kamiyama N., and Doi K., “Computer-aided diagnosis for the classification of focal liver lesions by use of contrast-enhanced ultrasonography,” Med. Phys. 35, 1734–1746 (2008). 10.1118/1.2900109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinstein S. B., Coll B., Staub D., Adam D., Schinkel A. F., Folkert J., and Thomenius K., “Contrast enhanced ultrasound imaging,” J. Nucl. Cardiol. 17, 106–115 (2010). 10.1007/s12350-009-9165-y [DOI] [PubMed] [Google Scholar]
- 21.Staub D., Partovi S., Imfeld S., Uthoff H., Baldi T., Aschwanden M., and Jaeger K. A., “Novel applications of contrast-enhanced ultrasound imaging in vascular medicine,” Vasa 42, 17–31 (2013). 10.1024/0301-1526/a000244 [DOI] [PubMed] [Google Scholar]
- 22.Tang M.-X., Mulvana H., Gauthier T., Lim A., Cosgrove D., Eckersley R., and Stride E., “Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability,” Interface Focus 1, 520–539 (2011). 10.1098/rsfs.2011.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postema M. and Gilja O. H., “Contrast-enhanced and targeted ultrasound,” World J. Gastroenterol. 17, 28–41 (2011). 10.3748/wjg.v17.i1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cagini L., Gravante S., Malaspina C. M., Cesarano E., Giganti M., Rebonato A., Fonio P., and Scialpi M., “Contrast enhanced ultrasound (CEUS) in blunt abdominal trauma,” Crit. Ultrasound J. 5, S9–S15 (2013). 10.1186/2036-7902-5-S1-S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce M., Averkiou M., and Powers J., Ultrasound Contrast in General Imaging Research (Philips Medical Systems, Eindhoven, 2007), pp. 1–20. [Google Scholar]
- 26.Malhi H., Grant E. G., and Duddalwar V., “Contrast-enhanced ultrasound of the liver and kidney,” Radiol. Clin. North Am. 52, 1177–1190 (2014). 10.1016/j.rcl.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Wan C. F., Du J., Fang H., Li F. H., Zhu J. S., and Liu Q., “Enhancement patterns and parameters of breast cancers at contrast-enhanced US: Correlation with prognostic factors,” Radiology 262, 450–459 (2012). 10.1148/radiol.11110789 [DOI] [PubMed] [Google Scholar]
- 28.Hu Q., Wang X. Y., Zhu S. Y., Kang L. K., Xiao Y. J., and Zheng H. Y., “Meta-analysis of contrast-enhanced ultrasound for the differentiation of benign and malignant breast lesions,” Acta Radiol. 56, 25–33 (2015). 10.1177/0284185113517115 [DOI] [PubMed] [Google Scholar]
- 29.Ding K., Bayouth J. E., Buatti J. M., Christensen G. E., and Reinhardt J. M., “4DCT-based measurement of changes in pulmonary function following a course of radiation therapy,” Med. Phys. 37, 1261–1272 (2010). 10.1118/1.3312210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y., Tsien C. I., Nagesh V., Junck L., Ten Haken R., Ross B. D., Chenevert T. L., and Lawrence T. S., “Clinical investigation survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT,” Int. J. Radiat. Oncol., Biol., Phys. 64, 876–885 (2006). 10.1016/j.ijrobp.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Tarnawski R., Sokol M., Pieniazek P., Maciejewski B., Walecki J., Miszczyk L., and Krupska T., “1 H-MRS in vivo predicts the early treatment outcome of postoperative radiotherapy for malignant gliomas,” Int. J. Radiat. Oncol., Biol., Phys. 52, 1271–1276 (2002). 10.1016/S0360-3016(01)02769-9 [DOI] [PubMed] [Google Scholar]
- 32.Day S. E., Kettunen M. I., Cherukuri M. K., Mitchell J. B., Lizak M. J., Morris H. D., Matsumoto S., Koretsky A. P., and Brindle K. M., “Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1–13C] pyruvate and 13C magnetic resonance spectroscopic imaging,” Magn. Reson. Med. 65, 557–563 (2011). 10.1002/mrm.22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan S., Kligerman S., Chen W., Lu M., Kim G., Feigenberg S., D’Souza W. D., Suntharalingam M., and Lu W., “Spatial–temporal [18F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy,” Int. J. Radiat. Oncol., Biol., Phys. 85, 1375–1382 (2013). 10.1016/j.ijrobp.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Tan S., Chen W., Kligerman S., Kim G., D’Souza W. D., Suntharalingam M., and Lu W., “Modeling pathologic response of esophageal cancer to chemoradiation therapy using spatial–temporal 18F-FDG PET features, clinical parameters, and demographics,” Int. J. Radiat. Oncol., Biol., Phys. 88, 195–203 (2014). 10.1016/j.ijrobp.2013.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rege A., Seifert A. C., Schlattman D., Ouyang Y., Li K. W., Basaldella L., Brem H., Tyler B. M., and Thakor N. V., “Longitudinal in vivo monitoring of rodent glioma models through thinned skull using laser speckle contrast imaging,” J. Biomed. Opt. 17, 126017 (2012). 10.1117/1.JBO.17.12.126017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong J., Armour E., Kazanzides P., Iordachita I., Tryggestad E., Deng H., Matinfar M., Kennedy C., Liu Z., Chan T., Gray O., Verhaegen F., McNutt T., Ford E., and DeWeese T. L., “High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities,” Int. J. Radiat. Oncol., Biol., Phys. 71, 1591–1599 (2008). 10.1016/j.ijrobp.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong X., Liu L., Wang M., Ding K., Fan Y., Ma B., Lal B., Tyler B., Mangraviti A., and Wang S., “Quantitative multiparametric MRI assessment of glioma response to radiotherapy in a rat model,” Neuro-Oncology 16, 856–867 (2014). 10.1093/neuonc/not245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams D. S., Detre J. A., Leigh J. S., and Koretsky A. P., “Magnetic resonance imaging of perfusion using spin inversion of arterial water,” Proc. Natl. Acad. Sci. U. S. A. 89, 212–216 (1992). 10.1073/pnas.89.1.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kargiotis O., Rao J. S., and Kyritsis A. P., “Mechanisms of angiogenesis in gliomas,” J. Neuro-Oncol. 78, 281–293 (2006). 10.1007/s11060-005-9097-6 [DOI] [PubMed] [Google Scholar]
- 40.Jain R. K., di Tomaso E., Duda D. G., Loeffler J. S., Sorensen A. G., and Batchelor T. T., “Angiogenesis in brain tumours,” Nat. Rev. Neurosci. 8, 610–622 (2007). 10.1038/nrn2175 [DOI] [PubMed] [Google Scholar]
- 41.Bogdahn U., Fröhlich T., Becker G., Krone A., Schlief R., Schürmann J., Jachimczak P., Hofmann E., Roggendorf W., and Roosen K., “Vascularization of primary central nervous system tumors: Detection with contrast-enhanced transcranial color-coded real-time sonography,” Radiology 192, 141–148 (1994). 10.1148/radiology.192.1.8208926 [DOI] [PubMed] [Google Scholar]
- 42.Caproni N., Marchisio F., Pecchi A., Canossi B., Battista R., D’Alimonte P., and Torricelli P., “Contrast-enhanced ultrasound in the characterisation of breast masses: Utility of quantitative analysis in comparison with MRI,” Eur. Radiol. 20, 1384–1395 (2010). 10.1007/s00330-009-1690-1 [DOI] [PubMed] [Google Scholar]
- 43.Williams R., Hudson J. M., Lloyd B. A., Sureshkumar A. R., Lueck G., Milot L., Atri M., Bjarnason G. A., and Burns P. N., “Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: A proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy,” Radiology 260, 581–590 (2011). 10.1148/radiol.11101893 [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Kaneko O. F., Tian L., Hristov D., and Willmann J. K., “Three-dimensional ultrasound molecular imaging of angiogenesis in colon cancer using a clinical matrix array ultrasound transducer,” Invest. Radiol. 50, 322–329 (2015). 10.1097/rli.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amarteifio E., Krix M., Wormsbecher S., Demirel S., Braun S., Delorme S., Kauczor H.-U., Böckler D., and Weber M.-A., “Dynamic contrast-enhanced ultrasound for assessment of therapy effects on skeletal muscle microcirculation in peripheral arterial disease: Pilot study,” Eur. J. Radiol. 82, 640–646 (2013). 10.1016/j.ejrad.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 46.Amarteifio E., Wormsbecher S., Demirel S., Krix M., Braun S., Rehnitz C., Delorme S., Kauczor H.-U., and Weber M.-A., “Assessment of skeletal muscle microcirculation in type 2 diabetes mellitus using dynamic contrast-enhanced ultrasound: A pilot study,” Diabetes Vasc. Dis. Res. 10, 468–470 (2013). 10.1177/1479164113484165 [DOI] [PubMed] [Google Scholar]
- 47.Clevert D., D’Anastasi M., and Jung E., “Contrast-enhanced ultrasound and microcirculation: Efficiency through dynamics–current developments,” Clin. Hemorheol. Microcirc. 53, 171–186 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Mancini M., Di Donato O., Saldalamacchia G., Liuzzi R., Rivellese A., and Salvatore M., “Contrast-enhanced ultrasound evaluation of peripheral microcirculation in diabetic patients: Effects of cigarette smoking,” Radiol. Med. 118, 206–214 (2013). 10.1007/s11547-012-0830-x [DOI] [PubMed] [Google Scholar]
- 49.Lassau N., Koscielny S., Chami L., Chebil M., Benatsou B., Roche A., Ducreux M., Malka D., and Boige V., “Advanced hepatocellular carcinoma: Early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—Preliminary result 1,” Radiology 258, 291–300 (2011). 10.1148/radiol.10091870 [DOI] [PubMed] [Google Scholar]
- 50.Lassau N., Chami L., Koscielny S., Chebil M., Massard C., Benatsou B., Bidault S., Cioffi A., Blay J.-Y., and Le Cesne A., “Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib,” Invest. New Drugs 30, 765–771 (2012). 10.1007/s10637-010-9592-2 [DOI] [PubMed] [Google Scholar]
- 51.Kono K., Inoue Y., Nakayama K., Shakudo M., Morino M., Ohata K., Wakasa K., and Yamada R., “The role of diffusion-weighted imaging in patients with brain tumors,” Am. J. Neuroradiol. 22, 1081–1088 (2001). [PMC free article] [PubMed] [Google Scholar]
- 52.Hall E. J. and Giaccia A. J., Radiobiology for the Radiologist (Lippincott Williams & Wilkins, Philadelphia, PA, 2006). [Google Scholar]
- 53.Joiner M. C. and van der Kogel A., Basic Clinical Radiobiology, 4th ed. (CRC, Boca Raton, FL, 2009). [Google Scholar]
- 54.Brenner D. J. and Carlson D. J., “Radiobiological principles underlying stereotactic radiation therapy,” in Principles and Practice of Stereotactic Radiosurgery (Springer, New York, NY, 2015), pp. 57–71. [Google Scholar]
- 55.Zhou J., Tryggestad E., Wen Z., Lal B., Zhou T., Grossman R., Wang S., Yan K., Fu D.-X., and Ford E., “Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides,” Nat. Med. 17, 130–134 (2011). 10.1038/nm.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J., Zhu H., Lim M., Blair L., Quinones-Hinojosa A., Messina A. A., Eberhart C. G., Pomper M. G., Laterra J., Barker P. B., van Zijl P. C. M., and Blakeley J. O., “Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement,” J. Magn. Reson. Imaging 38, 1119–1128 (2013). 10.1002/jmri.24067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grossman R., Tyler B., Brem H., Eberhart C. G., Wang S. L., Fu D. X., Wen Z. B., and Zhou J. Y., “Growth properties of SF188/V+ human glioma in rats in vivo observed by magnetic resonance imaging,” J. Neuro-Oncol. 110, 315–323 (2012). 10.1007/s11060-012-0974-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Togao O., Yoshiura T., Keupp J., Hiwatashi A., Yamashita K., Kikuchi K., Suzuki Y., Suzuki S. O., Iwaki T., Hata N., Mizoguchi M., Yoshimoto K., Sagiyama K., Takahashi M., and Honda H., “Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades,” Neuro-Oncology 16, 441–448 (2014). 10.1093/neuonc/not158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haris M., Gupta R. K., Singh A., Husain N., Husain M., Pandey C. M., Srivastava C., Behari S., and Rathore R. K. S., “Differentiation of infective from neoplastic brain lesions by dynamic contrast-enhanced MRI,” Neuroradiology 50, 531–540 (2008). 10.1007/s00234-008-0378-6 [DOI] [PubMed] [Google Scholar]
- 60.Noguchi T., Yoshiura T., Hiwatashi A., Togao O., Yamashita K., Nagao E., Shono T., Mizoguchi M., Nagata S., and Sasaki T., “Perfusion imaging of brain tumors using arterial spin-labeling: Correlation with histopathologic vascular density,” Am. J. Neuroradiol. 29, 688–693 (2008). 10.3174/ajnr.A0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yildiz Y. O., Eckersley R. J., Senior R., Lim A. K., Cosgrove D., and Tang M.-X., “Correction of non-linear propagation artifact in contrast-enhanced ultrasound imaging of carotid arteries: Methods and in vitro evaluation,” Ultrasound Med. Biol. 41, 1937–1947 (2015). 10.1016/j.ultrasmedbio.2015.03.014 [DOI] [PubMed] [Google Scholar]