Abstract
The manner and extent to which normal aging affects the ability to speak are not fully understood. While age-related changes in voice fundamental frequency and intensity have been documented, changes affecting the planning and articulation of speech are less well understood. In the present study, 76 healthy, cognitively normal participants aged between 18 and 93 years old were asked to produce auditorily and visually triggered sequences of finely controlled movements (speech, oro-facial, and manual movement). These sequences of movements were either (1) simple, in which at least two of the three movements were the same, or (2) complex, in which three different movements were produced. For each of the resulting experimental condition, accuracy was calculated. The results show that, for speech and oro-facial movements, accuracy declined as a function of age and complexity. For these movements, the negative effect of complexity on performance accuracy increased with age. No aging or complexity effects were found for the manual movements on accuracy, but a significant slowing of movement was found, particularly for the complex sequences. These results demonstrate that there is a significant deterioration of fine motor control in normal aging across different response modalities.
Keywords: Speech motor control, Speech response accuracy, Speech production, Elderly, Speech sequencing, Syllable production
Introduction
Speech is one of the most distinguishing features of the humankind. The act of speaking is an extremely complex behavior, both cognitively and at the sensorimotor level. It begins with an intention to communicate and continues to the translation of the message into words, which are converted in syllables that in turn need to be ordered serially (i.e., sequenced) before articulation can begin. The final output stage of this complex process requires the coordination of multiple sensorimotor components for the production of fluent speech, including the respiratory system, which provides the airflow necessary to set the vocal folds into vibration, the laryngeal muscles that convert the flow of air from the lungs into speech sounds (phonation), and, finally, the supra-laryngeal muscles that change the configuration of the vocal tract to convert the laryngeal output into sequences of vowels and consonants (articulation). In spite of this complexity, the chain of events that leads to the production of speech occurs within several hundreds of milliseconds. Indeed, adult speakers may produce as many as six to nine syllables per second (Kent 2000). Despite the importance of communication on quality of life, the manner and extent to which speech behavior, from respiration to articulation, changes throughout adulthood, as well as the nature of the cognitive, physiological, and neurobiological mechanisms that underlie these changes are not well understood.
Previous studies have shown age-related changes in voice fundamental frequency [F0] (i.e., the acoustical correlate of voice pitch, which ranges from low to high) (Decoster and Debruyne 1997; Honjo and Isshiki 1980; Hunter et al. 2012; Linville 1996; Mueller 1997; Ramig 1983b), which would begin as early as ~50 years (D'Haeseleer et al. 2011). Older adults also have higher jitter—a measure of cycle-to-cycle variation of vocal F0 (i.e., the acoustical correlate of pitch fluctuations)—compared to younger adults (Wilcox and Horii 1980). In addition to changes in F0 and jitter, voice loudness also changes (decrease) in aging (Baker et al. 2001), affecting males more than females (Goy et al. 2013). A decline in speech rate has also been reported for the repetition of words or sentences (Fozo and Watson 1998; Wohlert and Smith 1998) or directed speech (Duchin and Mysak 1987; Searl et al. 2002), even when pauses between sentences were excluded from the calculation of speech rate, suggesting that the duration of speech sounds becomes longer with age (Ramig 1983a; Ryan 1972). This is indeed consistent with the results of a few studies that have shown an age-related increase in the duration of individual speech sounds and syllables during repetition of words or sentences (Morris and Brown 1987; Ryan and Burk 1974; Smith et al. 1987). There is also limited evidence that aging affects speech intelligibility, that is, the capacity to produce speech sounds that can be recognized (Shuey 1989). In this study, participants were asked to listen and write down to a series of words embedded in a carrier phrase pronounced by young and older adults, and they misunderstood significantly more often the final consonant pronounced by older compared to younger adults, suggesting an age-related decline in speech intelligibility. Consistent with this finding, others researchers have reported that a group of 20 speech-language pathologists rated older adults (67 to 81 years old) as being less intelligible than younger adults (21 to 28 years old) in a diadochokinetic (DDK) task (Parnell and Amerman 1987).
Although it is clear that the speech system undergoes important changes with age, little is known about the nature and scope of the underlying biological aging mechanisms. One approach to uncover the nature of these mechanisms is to compare aging of speech skills to the aging of other finely controlled movements (such as finger and oro-facial movements). This is particularly relevant given the apparent relationship between speech and finger movements and between speech and oro-facial movements (Gentilucci 2003; Gentilucci et al. 2008; Tremblay and Gracco 2009, 2010). Although this is not without some controversy, behavioral studies have shown that when adults manipulate an object at the same time as they produce syllables, the size of the object manipulated influences the degree of mouth opening, demonstrating a link between hand and speech movements (Gentilucci 2003; Gentilucci et al. 2008). Recent studies also suggest that several motor preparatory mechanisms, such as motor response selection mechanisms, engage similar neural resources for speech and oro-facial movements (Tremblay and Gracco 2009, 2010) as well as for speech and manual movements (Tremblay et al. 2008). In this context, it is possible that aging of shared motor control mechanisms affects movement control in a general fashion. There is abundant literature documenting a decline of manual motor control with aging (Aoki and Fukuoka 2010; Cacola et al. 2013; Cousins et al. 1998; Jimenez-Jimenez et al. 2011; Ruiz et al. 2007). For example, it has been shown that older adults are slower in producing different kinds of finger movements, including the production of sequences of three to five finger taps triggered visually (Cacola et al. 2013) or tapping a key with one finger as rapidly as possible during 10 s (Cousins et al. 1998). A relationship has also been found between age and response time in a task requiring participants to tap their thumb with their index finger and then with each finger in rapid successions, whereby response time increased with age (Ruiz et al. 2007). Interestingly, such age effects appear to develop relatively late. For instance, it has been shown that older adults (65–92 years), but not middle-aged adults (40–63 years), were slower than younger adults (18–32 years) in producing rapid multifinger tapping movements (Cacola et al. 2013).
Despite evidence of a relationship between speech, oro-facial, and manual movements, to our knowledge, no study to date has examined whether aging mechanisms are movement-specific or domain-general. The goal of the present study was therefore to examine the effect of aging on the ability to produce sequences of fine motor actions (speech, oro-facial, and manual movements) varying in complexity levels. We hypothesized that all sequences of movements would be affected by age and by sequence complexity. We also expected to find interactions between age and sequence complexity, with more complex sequences being more affected by aging across all kinds of movements. Because response sequencing is likely a domain-general mechanism, we expected to find similar effects of age on motor sequence complexity across movement types.
Methods
Participants
Eighty-five participants were recruited to participate in the study. Of these, nine were excluded (~11 %) either due to recording problems during the experiment (n = 5), difficulty complying with task demands in a specific condition (n = 1), because they did not complete the hearing assessment (n = 1), or because they did not meet the inclusion criteria (n = 2). The final group therefore contained 76 participants (mean age 52.95 ± 19.01 SD; range, 22–93 years; 50 females). As can be seen in Table 1, this group was divided into four subgroups based on age (group 1, 22–34 years; group 2, 37–54 years; group 3, 55–69 years; group 4, 70–93 years). For group 4, all but one participant were aged between 70 and 83 years, and there was a 93-year-old participant. All participants were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield 1971), native speakers of Canadian French with a mean of 16.895 ± 4.203 years of education (range, 6–29 years). All participants had normal or corrected-to-normal vision and no self-reported speech, voice, language, psychological, neurological, or neurodegenerative disorder at the time of the study, and all were non-smokers. Participants were screened for depression using the Geriatric Depression Scale (Yesavage et al. 1982), and their cognitive level was assessed using the Montreal Cognitive Assessment scale (MOCA) (Nasreddine et al. 2003). Participants’ characteristics are reported in Table 1. The study was approved by the Institutional Ethical Committee of the Institut Universitaire en Santé Mentale de Québec (#293-2012).
Table 1.
Participants’ characteristics by age group
| Age | Years of education | MOCAa | Depression scaleb | Laterality quotientc | Right ear PTA (Hz) | Left ear PTA (Hz) | ||
|---|---|---|---|---|---|---|---|---|
| Group 1 (22–34 years) (n = 20, 13 females) | Minimum | 22 | 13 | 26 | 0 | 90 | −28,67 | −27 |
| Maximum | 34 | 24 | 30 | 8 | 100 | −16 | −12 | |
| Mean ±SD | 28.05 ± 4.17 | 17.70 ± 2.64 | 28.80 ± 1.20 | 2.50 ± 2.28 | 99.50 ± 2.24 | −20.12 ± 3.76 | −19.28 ± 3.47 | |
| Group 2 (37–54 years) (n = 17, 8 females) | Minimum | 37 | 11 | 25 | 0 | 70 | −39.33 | −36 |
| Maximum | 54 | 24 | 30 | 9 | 100 | −15.33 | −12 | |
| Mean ± SD | 45.94 ± 5.45 | 16.65 ± 4.17 | 27.64 ± 1.56 | 1.65 ± 2.50 | 97.06 ± 8.49 | −22.02 ± 6.25 | −19.39 ± 5.41 | |
| Group 3 (55–69 years) (n = 21, 17 females) | Minimum | 55 | 12 | 26 | 0 | 90 | −42.33 | −35.67 |
| Maximum | 69 | 29 | 30 | 8 | 100 | −18.67 | −13 | |
| Mean ± SD | 61.81 ± 5.17 | 18.14 ± 4.07 | 28.19 ± 1.63 | 1.48 ± 2.18 | 99.52 ± 2.18 | −25.81 ± 6.17 | −23.22 ± 6.18 | |
| Group 4 (70–93 years) (n = 18, 12 females) | Minimum | 70 | 6 | 23 | 0 | 78.95 | −48.67 | −51.33 |
| Maximum | 93 | 24 | 30 | 9 | 100 | −20.33 | −17.67 | |
| Mean ± SD | 76.89 ± 5.71 | 14.78 ± 5.19 | 26.67 ± 1.68 | 2.28 ± 2.59 | 97.07 ± 6.06 | −32.46 ± 7.79 | −32.07 ± 7.85 |
PTA pure tone average, MOCA Montreal Cognitive Assessment scale, SD standard deviation
aMax score at the MOCA is 30
bMax score for the depression scale is 30
cMax score for the laterality quotient is 100
Hearing assessment
Pure tone audiometry was performed using a clinical audiometer (AC40, Interacoustic) for each ear separately, at the following frequencies: .25, .5, 1, 2, 3, 4, 6, 8, 12, and 16 kHz. For each participant, a standard pure tone average (PTA; average of threshold at .5, 1, and 2 kHz) was computed for the left and right ear and used as a covariate in the statistical analyses. PTAs are used in clinical settings as a measure of hearing loss for speech because most speech sounds fall within this range (Stach 2010). The result of the hearing assessment is provided in Table 1.
Procedure
Participants were seated in a quiet room in front of a laptop computer (Thinkpad W510, Lenovo). Following a short practice session, participants were asked to produce sequences of speech, oro-facial, and finger movements in separate blocks. The task consisted in the production of meaningless sequences of (i) three French syllables (SPEECH), (ii) three oro-facial movements (MOUTH), and (iii) three finger movements (FINGER) (see Table 2). Trials were randomly interleaved with short intertrial intervals ranging from 500 to 1250 ms (with a mean of 875 ms). Participants’ oral responses (speech and mouth) were recorded using a high-quality multidirectional headworn microphone (Shure, Beta 53) connected to a sound card (Fast Track C400, M-audio), which was in turn connected to a laptop computer. All oral responses were recorded with the software Audacity (Open source). Finger movements were recorded using a USB response pad (Cedrus, model models RB-830). Throughout the procedure, participants’ fingers rested on the response pad (see Fig. 6a).
Table 2.
Examples of sequence for each type of stimulus
| Movement type | Stimuli | Simple sequence | Complex sequence |
|---|---|---|---|
| SPEECH | krik | krik krik krik | krik grug drad |
| drad | |||
| broub | krik krik grug | drad broub krik | |
| grug | |||
| MOUTH | pop | bec bec bec | bec pop tic |
| bec (kiss) | |||
| tic | pop tic tic | pop tic bec | |
| clac | |||
| FINGER | bleu (blue) (thumb) | vert vert vert | jaune bleu rouge |
| jaune (yellow) (index) | |||
| rouge (red) (middle finger) | jaune jaune vert | vert jaune bleu | |
| vert (green) (ring finger) |
Fig. 6.
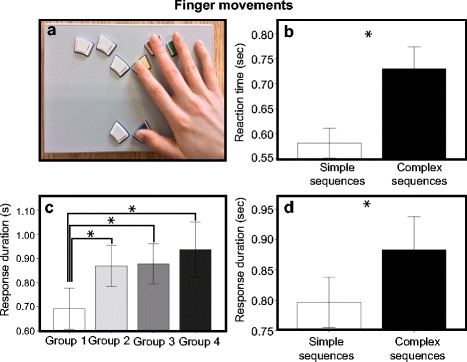
(a) Position of the fingers on USB response pad (Cedrus, model models RB-830) during the FINGER task. The bar graphs illustrate the results for the additional analyses conducted on the finger movements. Asterisks indicate significant differences. (b) Reaction time as a function of complexity; (c) response duration as function age group; (d) response duration as a function of sequence complexity
Stimuli and motor responses
The syllables used in the SPEECH condition were complex (CCVC) syllables: /krik/, /drad/, /broub/, and /grug/ (see Table 2). Meaningless syllables were used to avoid linguistic top-down effects that can facilitate speech production and because they are useful in the evaluation of maximal performance. Indeed, difficult syllable tasks could reveal a decline in maximal performance differences (reduced reserve). This is important because a reduced reserve can impair a person’s flexibility, that is, the ability to adjust speech output to different situations, and can also reveal whether the process of speaking is becoming overall more difficult (Kent et al. 1987). The oro-facial movements used in the MOUTH condition were (1) a kissing movement with the lips (kiss), (2) the production of a popping sound made with the two lips being pressed and opened (pop), (3) a sound made by pressing the tip of the tongue against the alveoles and then releasing the tongue (tic), and (4) a clapping sound also made with the tip of the tongue (clap). All movements produced a distinct sound. For the FINGER condition, participants were asked to position their right hand (thumb, index, middle, or ring finger) on the response pad and to press specific buttons when instructed.
All motor responses (SPEECH, MOUTH, and FINGER) were triggered either visually or auditory. In the auditory condition, participants were presented, through high-quality headphones (Shure, SRH440), with recordings of (1) the syllables, (2) the sounds of the oro-facial movements, and (3) the color of the button to press (red, blue, green, yellow). Auditory stimuli were read by native speaker of French Canadian in a soundproof room and recorded with Sound Studio 3.5.4 software (Felt Tip Software) at a sampling rate of 44 kHz. Stimuli were edited using Wave Pad Sound Editor 4.53 (NHC Software) to standardize their duration to 1200 ms and normalize the root-mean-square (RMS) intensity of the sound files. In the visual condition, participants were presented with (1) the syllables written on the computer screen, (2) the name of oro-facial movement (kiss, pop, tic, and clap), and (3) the name of the color of the button they needed to press (red, blue, green, yellow). All stimuli (visual and auditory) were presented using Presentation Software (Neurobehavioral System, CA, USA). The presentation of the stimuli lasted for 1800 ms and was followed, after an average of 1050 ms, by a green-colored visual response cue (✓) that remained on the screen for 3500 ms. At the end of this period, a red-colored stop cue (✖) was presented indicating to participants to stop responding. The stop cue remained on the screen until the beginning of the following trial, which occurred, on average, 875 ms later (range 750–1250 ms).
Two types of sequences were performed. The sequences were either of simple, in which at least two of the three movements were identical and performed one after the other (e.g., /krik krik krik/ or /pop pop clac/), or more complex, in which three different movements were produced (e.g. /drad krik grug/ or /tic clac kiss/). Examples of sequences are reported in Table 2. The experiment included 24 trials of each condition (3 movements × 2 complexity levels × 2 modalities) for a total of 288 trials. These trials were divided into six experimental runs (two runs for each movement modality). Within each run, the complexity of the movements was randomized while the other factors (stimuli modality and movement type) were kept constant to avoid task-switching effects not of interest in this experiment. The order of the runs was counter-balanced across participants. For all type of responses (SPEECH, MOUTH, and FINGER), the different movements (e.g., krik/, /drad/, /broub/, and /grug/) were produced a similar number of times (i.e., between 16 and 19 times) across modality and complexity level.
Behavioral data analysis
Data analyses focused on performance, measured as a percentage of errors per sequences for each condition (number of incorrect movements divided by total number of movement produced). Errors included errors of commission, errors of omission, and production of additional movements. Accuracy was calculated based on the number of runs included in the analysis. A run was kept only if at least 50 % of sequences were completed. For the analyses of SPEECH and MOUTH mistakes, a research assistant naive to the purpose of the study listened and transcribed the participants’ responses. For FINGER, the responses were recorded directly to disk and verified. The percentages of errors by condition and by age group are reported in Table 3.
Table 3.
Mean accuracy (percentage of errors) and standard deviation for each condition and each group
| Group | Group 1 (22–34 years) | Group 2 (37–54 years) | Group 3 (55–69 years) | Group 4 (70–93 years) | ||||
|---|---|---|---|---|---|---|---|---|
| Experimental condition | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| SPEECH auditory simple sequences | 12.34 | 16.65 | 9.72 | 9.56 | 9.80 | 9.62 | 18.46 | 9.72 |
| SPEECH auditory complex sequences | 21.63 | 17.47 | 24.05 | 14.44 | 28.37 | 15.96 | 39.82 | 15.05 |
| SPEECH visual simple sequences | 12.46 | 11.32 | 10.41 | 9.61 | 18.00 | 18.60 | 35.97 | 21.72 |
| SPEECH visual complex sequences | 22.44 | 14.75 | 29.43 | 14.90 | 37.33 | 21.63 | 52.71 | 23.31 |
| MOUTH auditory simple sequences | 8.00 | 8.36 | 14.69 | 7.97 | 23.38 | 13.88 | 30.94 | 18.41 |
| MOUTH auditory complex sequences | 22.76 | 13.49 | 39.78 | 13.41 | 45.40 | 10.51 | 51.62 | 13.44 |
| MOUTH visual simple sequences | 3.69 | 2.65 | 6.30 | 8.54 | 12.34 | 10.92 | 17.20 | 12.67 |
| MOUTH visual complex sequences | 3.54 | 3.97 | 11.74 | 9.54 | 19.28 | 13.88 | 25.87 | 13.33 |
| FINGER auditory simple sequences | 0.90 | 1.17 | 0.80 | 1.26 | 0.93 | 1.61 | 1.36 | 1.81 |
| FINGER auditory complex sequences | 1.27 | 1.91 | 1.17 | 2.01 | 1.04 | 1.84 | 1.57 | 1.53 |
| FINGER visual simple sequences | 0.92 | 1.23 | 1.59 | 3.35 | 1.43 | 1.32 | 2.27 | 3.45 |
| FINGER visual complex sequences | 1.79 | 1.68 | 1.58 | 2.06 | 1.94 | 3.57 | 5.52 | 11.50 |
Statistical analyses
First, a 3 × 2 × 2 × 4 analysis of covariance (ANCOVA) was run using SPSS (IBM, version 22) to analyze performance (percentage of error by sequence), with three within-subject factors (Movement [SPEECH, MOUTH, and FINGER], Complexity [simple, complex], and Modality [visual, auditory]), and one between-subject factor (Group [group 1, group 2, group 3, and group 4]). Two covariates were included in the statistical model (sex and the right PTA) to control for potential sex and hearing differences. Since a strong correlation was found between the right and the left PTA (n = 80. 876, p = 0.000), only the right PTA was included in the analyses to control for hearing sensitivity while avoiding overfitting the data. Significant effects revealed by the ANCOVA were explored using false discovery rate (FDR)-corrected post hoc tests (Benjamini and Hochberg 1995; Genovese et al. 2002) (q = 0.05, I = 25 tests).
Results
Number of errors by sequence
The four-way ANCOVA conducted on the percentage of errors revealed significant main effects of Movement (F(2,140) = 6.288, p = 0.002), Complexity (F(1,70) = 21.578, p < 0.001), and Group (F(3,70) = 9.648, p < 0.001). Interactions between Group and Movement (F(6,140) = 5.835, p < 0.001), between Complexity and Group (F(3,70) = 7.675, p < 0.001), between Movement and Modality (F(2,140) = 5.889, p = 0.004), between Movement, Modality, and Group (F(6,140) = 4.236, p = 0.001), between Movement and Complexity (F(2,140) = 8.241, p < 0.001), between Movement, Complexity, and Group (F(6,140) = 3.184, p = 0.006), and between Modality and Complexity (F(1,70) = 6.635, p = 0.012) were also found. No effects of PTA or Sex were found.
As can be seen in Fig. 1a, in general, participants were more accurate in producing simple than complex sequences (t(75) = −20.465, p < 0.001). As shown in Fig. 1b, with age, there was an overall increase in error rate. Post hoc tests revealed that the eldest participants (group 4) made more mistakes than all other groups. Performance in this group differed significantly from group 1 (t(36) = −6.584, p = 0.002), group 2 (t(33) = −4.753, p < 0.001), and group 3 (t(37) = −2.908, p = 0.009). Group 1 also made significantly fewer mistakes than group 3 (t(39) = −3.732, p = 0.001). Group 2 and group 3 did not differ from each other. The main effect of Movement is illustrated in Fig. 2, which shows that participants made less mistakes during FINGER compared to SPEECH (t(75) = 12.855, p < 0.001) and MOUTH (t(75) = 14.233, p < 0.001).
Fig. 1.
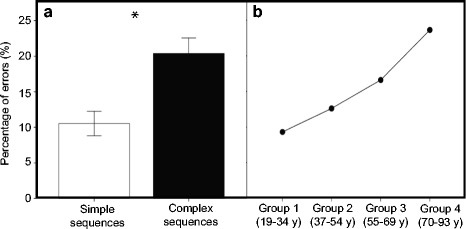
Overall percentage of errors displayed as function of (a) sequence complexity (simple and complex) and (b) age group
Fig. 2.
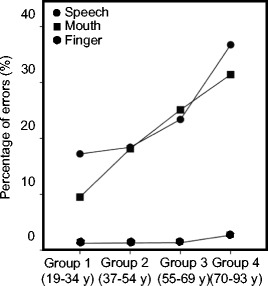
Percentage of errors displayed as function of age group for each movement
As shown in Fig. 3, for the Modality by Movement interaction, post hoc tests revealed that in the MOUTH condition, participants made more mistakes in the auditory modality than in the visual modality (t(75) = 12.500, p < 0.001). In contrast, in the SPEECH and FINGER conditions, they made more mistakes in the visual than in the auditory modality (SPEECH, t(75) = −4.022, p < 0.001; FINGER, t(75) = −2.216, p = 0.037). For the three-way interaction between Modality, Group, and Movement, post hoc tests revealed that, within each group, the direction of the stimulus Modality effect was different in the MOUTH compared to the SPEECH condition (group 1, t(19) = −3.582, p = 0.003; group 2, t(16) = −5.796, p < 0.001; group 3, t(20) = −6.034, p < 0.001; group 4, t(17) = −6.926, p < 0.001). The effect also differed between MOUTH and FINGER (group 1, t(19) = 5.551, p < 0.001; group 2, t(16) = 8.507, p < 0.001; group 3, t(20) = 5.448, p < 0.001; group 4, t(17) = 7.076, p < 0.001). The modality difference between FINGER and SPEECH was significant for group 3 (t(20) = −2.510, p = 0.027) and group 4 (t(17) = −2.704, p = 0.021). For the Modality by Complexity interaction, post hoc tests revealed that for the complex sequences, participants made more mistakes in auditory than visual modality. There was no effect of modality for the simple sequences.
Fig. 3.
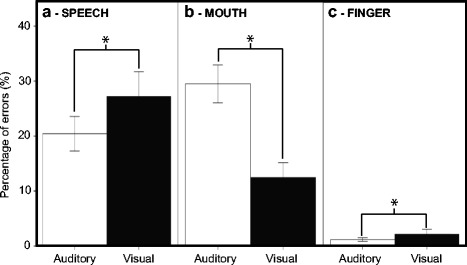
Percentage of errors displayed as function of stimulus modality (visual or auditory) for each movement: (a) SPEECH, (b) MOUTH, and (c) FINGER. Asterisks indicate significant differences
To decompose the thee-way interaction between Complexity, Group, and Movement, a series of three additional 2 × 4 ANCOVAs were run, one for each type of movement, to examine age and sequence complexity effects within each movement type, with Complexity as a within-subject factor and Group as a between subject factor. Two covariates were included in the statistical model (sex and the right PTA) to control for potential sex and hearing differences. As can be seen in Fig. 4, these analyses revealed that Complexity effects were present in the SPEECH and MOUTH conditions but not in the FINGER condition. As show in Fig. 5a, for SPEECH, the ANCOVA revealed a main effect of Complexity (F(1,70) = 18.596, p < 0.001) and Group (F(3,70) = 5.928, p = 0.001) and an interaction between Complexity and Group (F(3,70) = 6.049, p = 0.001). Analysis revealed no effect of PTA or sex. FDR-corrected post hoc t tests (Benjamini and Hochberg 1995; Genovese et al. 2002) (q = 0.05; i = 10) revealed that the difference between simple and complex sequences was significant for all groups (group 1, t(19) = −7.005, p < 0.001; group 2, t(16) = −11.737, p < 0.001; group 3, t(20) = −12.354, p < 0.001; group 4, t(17) = −8.668, p < 0.001). Moreover, the difference between simple and complex sequences was larger for group 2 (t(35) = 3.548, p = 0.002), group 3 (t(39) = 4.508, p < 0.001), and group 4 (t(36) = 3.711, p = 0.001) compared to group 1.
Fig. 4.
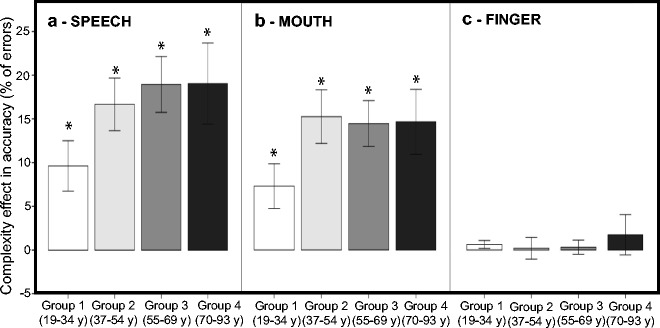
Complexity effect ([percentage of errors for complex sequences] − [percentage of errors for simple sequences]) displayed as function of age group, separately for each movement: (a) SPEECH, (b) MOUTH, and (c) FINGER. Asterisks indicate significant differences
Fig. 5.
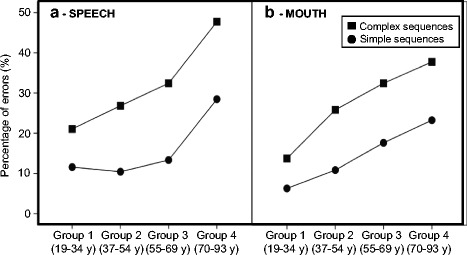
Percentage of errors for the simple and complex sequences displayed as function age group for (a) SPEECH and (b) MOUTH
As show in 5b, for MOUTH, the ANCOVA revealed a main effect of Complexity (F(1,70 = 19.075, p < 0.001), and Group (F(3,70) = 11.764, p < 0.001), and an interaction between Complexity and Group (F(3,70) = 5.868, p = 0.001). No effects of PTA or sex were found. FDR-corrected post hoc tests (q = 0.05, i = 10) (Benjamini and Hochberg 1995; Genovese et al. 2002) revealed that the difference between simple and complex sequences was significant for all groups (group 1, t(20) = −5.949, p < 0.001; group 2, t(16) = −10.503, p < 0.001; group 3, t(20) = −11.483, p < 0.001; group 4, t(17) = −8.328, p < 0.001) ). Moreover, the difference between simple and complex sequences was larger for group 2 (t(35) = 4.216, p < 0.001), group 3 (t(39) = 4.071, p < 0.001), and group 4 (t(36) = 3.489, p = 0.002) compared to group 1.
For FINGER, the ANCOVA revealed an effect of right PTA (F(1,70) = 8.044, p = 0.006) but no significant effect of complexity or age. Because we expected to find age effects on finger movements, we decided to explore the manual movements further by conducting additional analyses on reaction time (RT) and sequence duration in order to determine whether age affected finger movements in terms of timing rather than accuracy. For this analysis, we extracted RT and sequence durations only for the correct trials. Sequences that started before the response cue or that finished after the end of the trial were removed from the statistical analysis. Trials containing outliers, defined as values ±2 SD from the participant’s mean, were also removed from the analysis. One participant was excluded from the duration and RT analysis because, in the visual condition, too many sequences (73 %) were incorrect. Two separate 2 × 2 × 4 ANCOVAs were conducted on the resulting data, one for the RT and one for duration, with Complexity and Modality as the within-subject factors, and Group as the between subject factor. For RT, the ANCOVA revealed significant main effects of Modality (F(1,69) = 4.130, p = 0.046) and Complexity (F(1,69) = 6.7552, p = 0.011) but no interaction. FDR-corrected post hoc t tests (Benjamini and Hochberg 1995; Genovese et al. 2002) (q = 0.05, i = 2) revealed that participants were slower in visual than auditory condition (t(74) = −11.675, p < 0.001). As shown in Fig. 6b, in general, RTs were shorter for simple compared to complex sequences (t(74) = −13.386, p < 0.001).
For sequence duration, the ANCOVA revealed significant main effects of Complexity (F(1,69) = 4.945, p = 0.029) and Group (F(3,69) = 4.670, p = 0.005). Interactions between Complexity and Group (F(3,69) = 5.437, p = 0.002) and between Complexity and Modality (F(1,69) = 4.182, p = 0.045) were also found. FDR-corrected post hoc t tests (Benjamini and Hochberg 1995; Genovese et al. 2002) (q = 0.05, i = 12) were conducted to explore these effects. As shown in Fig. 6c, in general, simple sequences were shorter than complex sequences (t(74) = −7.935, p < 0.001). As shown in Fig. 6d, post hoc tests revealed that the youngest participants (group 1) were faster than all other groups. Performance in this group differed significantly from group 2 (t(35) = −3.070, p = 0.006), group 3 (t(39) = −3.255, p = 0.004), and group 4 (t(35) = −3.680, p = 0.002). For the Group by Complexity interaction, post hoc tests revealed a complexity effect (complex > simple) in all groups except for group 1 (group 2, t(16) = −4.556, p = 0.001; group 3, t(20) = −5.298, p < 0.001; group 4, t(16) = −5.548, p < 0.001). The youngest participants showed no complexity effect on response duration. For the Modality by Complexity interaction, post hoc tests revealed that the difference between simple sequences and complex sequences was larger in the visual condition than in auditory condition (t(74) = 5.261, p < 0.001).
Discussion
The goal of the present study was to examine the effect of aging on the ability to produce sequences of fine motor actions (speech, oro-facial, and manual movements) varying in complexity levels, while controlling for hearing, in healthy adults. Given the inherently sequential nature of speech, sequencing difficulties can be particularly detrimental to communication efficiency in older ages. Despite the importance of communication in aging, the extent and underlying causes of articulatory and speech sequencing difficulties are still unknown, i.e., whether they are related to peripheral factors such as decreased oral muscle endurance, or to neurobiological factors such as less efficient neural mechanisms or structural damage to the brain regions involved in speech production. Here, we aimed at characterizing the extent of these difficulties using a behavioral approach and by conducting an analysis of errors. As was expected, an overall age-related performance decline was observed. When movements were examined separately, differences in the effect of aging and sequence complexity were found across movement types, with speech and oro-facial movements showing age-related accuracy decline but not finger movements. For finger movements, however, additional analyses revealed an increase in response duration with age. These findings are discussed in the following paragraphs.
Speech production
In the present study, we examined accuracy during a sequential speech production task in healthy young and older adults and we found a significant decrease in performance with age and, importantly, we found that this decline was stronger for complex sequences. From group 1 to 4, a ~55 % decline was observed for the simple sequences and ~52 % decline for the complex sequences. Several prior studies have shown a decline in speech rate with age (Duchin and Mysak 1987; Fozo and Watson 1998; Ramig 1983a; Ryan 1972; Searl et al. 2002; Wohlert and Smith 1998), but few studies have examined accuracy and only a few have shown that older adults are less intelligible than younger adults (Parnell and Amerman 1987; Shuey 1989). The present results demonstrate, for the first time, a decline in speech sequencing skills in healthy adults.
The underlying causes of the decline in speech skills with aging remain unknown. In previous studies of speech rate, the stimuli used were usually sentences or words; it is therefore possible that a decline in the efficiency of linguistic processing (e.g., syntax and lexical access) may account for the observed slowing, or at least for a part of it. In contrast, in the present study, we used meaningless syllable sequences and we still observed an age-related decline in accuracy, which suggests that the decline in efficiency of linguistic processes is not the only factor contributing to the observed decline of speech skills in aging. One possibility is that physiological changes in the oro-facial sphere could be contributing to the observed decline in accuracy. For example, it has been shown that older adults exhibit decreased oral tactile sensitivity (Calhoun et al. 1992; Wohlert 1996b; Wohlert and Smith 1998), as well as decreased lip strength (Wohlert and Smith 1998), and decreased maximal tongue strength (Neel and Palmer 2012). However, one study has shown that tongue maximal strength is a poor predictor of articulation rate (Neel and Palmer 2012), suggesting limited contribution of these physiological factors. Another, more likely possibility is that decline in speech accuracy is related to a decline in the neural planning and control of speech movements. Consistent with this hypothesis, recent studies from our group have shown important age-related changes in the structure and function of brain areas involved in speech motor control, including the premotor cortex and the supplementary motor area (Bilodeau-Mercure et al. 2014; Tremblay et al. 2013). Given the importance of communication in aging, and the fact that speaking is intrinsically a sequential behavior, further research is needed to better understand the cause of these sequencing difficulties. Moreover, further research needs to establish the range of these difficulties, and whether they are modulated by factors such as syllable complexity and syllable frequency. This is necessary to establish a more complete picture of the changes that the speech system undergoes with age and help design new interventions to remediate speech difficulties based on a better understanding of the specific articulatory difficulties faced by elderly adults.
Oro-facial movements
In the present study, we observed that older adults made significantly more mistakes than younger adults when they produced sequences of oro-facial movements. This effect was significantly stronger for the complex sequences. From group 1 to 4, a ~76 % decline was observed for simple sequences and ~66 % decline for complex sequences suggesting a decline in oro-facial motor control with aging. Very few studies have explored the production of oro-facial movements in aging. In one study, age-related changes in perioral reflex movement in response to innocuous mechanical stimulation of the lip vermillion have been reported (Wohlert 1996a). In this study, older women (67–85 years) produced less reflexive responses to stimulation and their response had lower amplitude and longer latency than younger women (20–25 years). These changes in oral reflex movements can be due to physical changes like decreased lip strength (Wohlert and Smith 1998), or decreased oral tactile sensitivity (Calhoun et al. 1992; Wohlert 1996b; Wohlert and Smith 1998). In the present study, these factors could be responsible for the performance decline that we observed. However, changes in motor planning and execution are also likely to play a role in the etiology of these age-related changes. Indeed, though performance decreased with an increase in complexity in all groups, we found that the effect of the complexity increased with age consistent with a decline in the neural control of movement planning.
The finding of a similar gradual decline in speech and oro-facial movements suggests a common underlying aging mechanism, not specific to speech movements. This aging process could be related to the planning of movements involving the face and mouth or to the execution of these movements. Although only a few studies have examined the relationship between oro-facial movements and speech in adulthood and particularly in aging, a link between oro-facial movements and speech has been shown, consistent with the idea of shared mechanisms (Alcock 2006; Alcock et al. 2000; Tremblay and Gracco 2009, 2010). For example, some studies have shown that motor response selection involves similar neural resources for speech and oro-facial movements (Tremblay and Gracco 2009, 2010). Moreover, patients with speech impairments also have difficulty executing oro-facial movements (Alcock 2006; Alcock et al. 2000) suggesting shared mechanisms. In line with previous results, the present findings support the notion that speech and oro-facial movements engage common motor control mechanisms, including movement sequencing. Additional studies are needed to continue to explore the common and separate etiology of these changes, whether related to peripheral factors such as decreased oro-facial strength or sensibility, or to central factors such as motor planning, in particular motor sequencing.
Finger movements
In the present study, no effect of age or complexity was found on accuracy of finger movements. This finding was unexpected given that many studies have shown an age-related decline in the accuracy of manual movements (Chaput and Proteau 1996; Christou and Enoka 2011; Goggin and Meeuwsen 1992; Pohl et al. 1996; Yan et al. 1998). There are, however, several differences between the tasks used in these studies and the one that was used here. First, in the present study, only the fingers were used, whereas, in many others studies, participants performed more complex movements involving the whole arm (Chaput and Proteau 1996; Goggin and Meeuwsen 1992; Pohl et al. 1996; Yan et al. 1998). Moreover, in our study, accuracy was measured as the number of correct movements produced (i.e., pressing with the correct finger); it was thus a simple dichotomous dependent variable with only two possible outcomes (correct/incorrect). In other studies, in contrast, accuracy was measured continuously in terms of movement precision (Chaput and Proteau 1996; Christou and Enoka 2011; Goggin and Meeuwsen 1992; Pohl et al. 1996; Yan et al. 1998). For example, in a recent study, participants were asked to lift and lower light loads with their index finger and to stop their movements at a specific angle from the other fingers (Christou and Enoka 2011). Older adults had more difficulty stopping their movement at the specified angle compared to the younger adults, although they could still stop the movement. It is possible that, should we have measured movement trajectories, we could have found age-related differences in movement precision in our task. Consistent with this hypothesis, we conducted additional analyses of the manual movements, and we found age effects on response duration, consistent with the notion that, though globally accurate (i.e., on target), the finger movements of older adults differed from those produced by younger adults. We also show that more complex sequences of finger movements are particularly affected by age in terms of response duration. These results are consistent with previous studies that found an effect of aging on the time required to produce sequences of movements (Aoki and Fukuoka 2010; Cacola et al. 2013; Cousins et al. 1998; Ruiz et al. 2007).
Taken together, our results suggest that, in a simple finger movement (button pressing) tasks, age effects affect duration more strongly than global accuracy. Indeed, the percentage of errors was very low across conditions. This may suggest that the oro-facial tasks had a higher difficulty level compared to the finger movement task. The other possibility is that response accuracy declines more quickly in the oro-facial sphere than it does in the manual action sphere, perhaps due to the inherent complexity of speech movements, which require the coordination of several different muscles. Further studies are needed to replicate these findings and determine the extent to which the aging of speech and oro-facial movements follows a different trajectory from that of finger movements.
Stimuli modality
In the present study, we observed that stimuli modality affected performance differently depending of the type of movement produced. For SPEECH and FINGER, participants were less accurate in the visual compared to the auditory condition. In contrast, for MOUTH, participants were less accurate in the auditory than in the visual modality. Participants reported having difficulty recognizing the sounds of the oro-facial movements. In day-to-day situations, these sounds usually occur in the presence of visual information (e.g., shape of the mouth and degree of opening) or other forms of contextual information including speech, such as listening to a kissing lip movement when saying goodbye to a loved one the phone. In contrast, people frequently listen to speech without visual information, for example, during phone conversations. This may explain the difficulty related to the auditory MOUTH condition. It is important to note that, since all analyses were corrected for hearing threshold, this effect cannot be attributed to a hearing decline; otherwise, it would have affected all movements equally. The present results suggest that the modality of the movement trigger differentially affects accuracy of oro-facial and speech movements in normal aging, which may have important implications for rehabilitation.
Conclusion
This study provides important new empirical evidence that oro-facial motor control declines in cognitively healthy elderly adults (both males and females). These results suggest that the motor speech system undergoes significant decline over time that affects oro-facial movements. Here, we follow the general hypothesis that age-related speech difficulties, both perceptual and motor, have a multifactorial aetiology that includes central factors (such as speech motor planning), and possibly peripheral factors as well (such as tactile sensibility, decreased muscular endurance). Further studies are needed to continue explore the distinct impact of these different factors on the ability to communicate in aging. This is crucial since communication difficulties are considered to be of great importance by elderly adults (Jacobs-Condit 1984). Indeed, these difficulties often lead to social participation that is less diverse, is more restricted to home settings, and involves fewer relationships (Law 2002). It is therefore important that we gain a better understand of the extent to which age affects speech production and its underlying mechanisms, to be able to detect and remediate these important health issues.
Acknowledgments
This work was supported by grants from the Fonds Québécois de le Recherche – Société et Culture (FRQ-SC) and from the Fonds Québécois de le Recherche – Santé (FRQ-S) to P.T. and by a start-up grant from the Institut Universitaire en Santé Mentale de Québec (IUSMQ) to P.T. These sponsors played no role in the design, execution, analysis, and interpretation of data, or writing of the study. We thank Isabelle Deschamps for her comments on previous versions of this manuscript.
References
- Alcock K. The development of oral motor control and language. Downs Syndr Res Pract J Sarah Duffen Centre Univ Portsmouth. 2006;11:1–8. doi: 10.3104/reports.310. [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Passingham RE, Watkins KE, Vargha-Khadem F. Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain Lang. 2000;75:17–33. doi: 10.1006/brln.2000.2322. [DOI] [PubMed] [Google Scholar]
- Aoki T, Fukuoka Y. Finger tapping ability in healthy elderly and young adults. Med Sci Sports Exerc. 2010;42:449–455. doi: 10.1249/MSS.0b013e3181b7f3e1. [DOI] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Sapir S, Luschei ES, Smith ME. Control of vocal loudness in young and old adults. J Speech Lang Hear Res JSLHR. 2001;44:297–305. doi: 10.1044/1092-4388(2001/024). [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- Bilodeau-Mercure M, Lortie CL, Sato M, Guitton MJ, Tremblay P. The neurobiology of speech perception decline in aging. Brain Struct Funct. 2014 doi: 10.1007/s00429-013-0695-3. [DOI] [PubMed] [Google Scholar]
- Cacola P, Roberson J, Gabbard C. Aging in movement representations for sequential finger movements: a comparison between young-, middle-aged, and older adults. Brain Cogn. 2013;82:1–5. doi: 10.1016/j.bandc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Calhoun KH, Gibson B, Hartley L, Minton J, Hokanson JA. Age-related changes in oral sensation. Laryngoscope. 1992;102:109–116. doi: 10.1288/00005537-199202000-00001. [DOI] [PubMed] [Google Scholar]
- Chaput S, Proteau L. Aging and motor control. J Gerontol Ser B Psychol Sci Soc Sci. 1996;51:P346–P355. doi: 10.1093/geronb/51B.6.P346. [DOI] [PubMed] [Google Scholar]
- Christou EA, Enoka RM. Aging and movement errors when lifting and lowering light loads. Age (Dordrecht, Netherlands) 2011;33:393–407. doi: 10.1007/s11357-010-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Corrow C, Finn M, Salamone JD. Temporal measures of human finger tapping: effects of age. Pharmacol Biochem Behav. 1998;59:445–449. doi: 10.1016/S0091-3057(97)00443-7. [DOI] [PubMed] [Google Scholar]
- Decoster W, Debruyne F. The ageing voice: changes in fundamental frequency, waveform stability and spectrum. Acta Otorhinolaryngol Belg. 1997;51:105–112. [PubMed] [Google Scholar]
- D'Haeseleer E, Depypere H, Claeys S, Wuyts FL, Baudonck N, Van Lierde KM. Vocal characteristics of middle-aged premenopausal women. J Voice Off J Voice Found. 2011;25:360–366. doi: 10.1016/j.jvoice.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Duchin SW, Mysak ED. Disfluency and rate characteristics of young adult, middle-aged, and older males. J Commun Disord. 1987;20:245–257. doi: 10.1016/0021-9924(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Fozo MS, Watson BC. Task complexity effect on vocal reaction time in aged speakers. J Voice Off J Voice Found. 1998;12:404–414. doi: 10.1016/S0892-1997(98)80049-0. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gentilucci M. Grasp observation influences speech production. Eur J Neurosci. 2003;17:179–184. doi: 10.1046/j.1460-9568.2003.02438.x. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Dalla Volta R, Gianelli C. When the hands speak. J Physiol Paris. 2008;102:21–30. doi: 10.1016/j.jphysparis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Goggin NL, Meeuwsen HJ. Age-related differences in the control of spatial aiming movements. Res Q Exerc Sport. 1992;63:366–372. doi: 10.1080/02701367.1992.10608758. [DOI] [PubMed] [Google Scholar]
- Goy H, Fernandes DN, Pichora-Fuller MK, van Lieshout P (2013) Normative voice data for younger and older adults. J Voice 27(5):545–555. doi:10.1016/j.jvoice.2013.03.002 [DOI] [PubMed]
- Honjo I, Isshiki N. Laryngoscopic and voice characteristics of aged persons. Arch Otolaryngol (Chicago, Ill : 1960) 1980;106:149–150. doi: 10.1001/archotol.1980.00790270013003. [DOI] [PubMed] [Google Scholar]
- Hunter EJ, Kapsner-Smith M, Pead P, Engar MZ, Brown WR. Age and speech production: a 50-year longitudinal study. J Am Geriatr Soc. 2012;60:1175–1177. doi: 10.1111/j.1532-5415.2012.03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Condit LE. Gerontology and communication disorders. Rockville: American Speech-Language-Hearing Association; 1984. [Google Scholar]
- Jimenez-Jimenez FJ, et al. Influence of age and gender in motor performance in healthy subjects. J Neurol Sci. 2011;302:72–80. doi: 10.1016/j.jns.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Kent RD. Research on speech motor control and its disorders: a review and prospective. J Commun Disord. 2000;33:391–427. doi: 10.1016/S0021-9924(00)00023-X. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. J Speech Hear Disord. 1987;52:367–387. doi: 10.1044/jshd.5204.367. [DOI] [PubMed] [Google Scholar]
- Law M. Participation in the occupations of everyday life. Am J Occup Ther Off Publ Am Occup Ther Assoc. 2002;56:640–649. doi: 10.5014/ajot.56.6.640. [DOI] [PubMed] [Google Scholar]
- Linville SE. The sound of senescence. J Voice Off J Voice Found. 1996;10:190–200. doi: 10.1016/S0892-1997(96)80046-4. [DOI] [PubMed] [Google Scholar]
- Morris R, Brown WS. Age-related voice measures among adult women. J Voice. 1987;1:43. doi: 10.1016/S0892-1997(87)80022-X. [DOI] [Google Scholar]
- Mueller PB. The aging voice. Semin Speech Lang. 1997;18:159–168. doi: 10.1055/s-2008-1064070. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Chertkow H, Phillips N, Bergman H, Whitehead V (2003) Sensitivity and specificity of the Montreal cognitive assessment (MoCA) for detection of mild cognitive deficits can. J Neurol Sci 30
- Neel AT, Palmer PM. Is tongue strength an important influence on rate of articulation in diadochokinetic and reading tasks? J Speech Lang Hear Res JSLHR. 2012;55:235–246. doi: 10.1044/1092-4388(2011/10-0258). [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parnell MM, Amerman JD. Perception of oral diadochokinetic performances in elderly adults. J Commun Disord. 1987;20:339–351. doi: 10.1016/0021-9924(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Pohl PS, Winstein CJ, Fisher BE. The locus of age-related movement slowing: sensory processing in continuous goal-directed aiming. J Gerontol Ser B Psychol Sci Soc Sci. 1996;51:P94–P102. doi: 10.1093/geronb/51B.2.P94. [DOI] [PubMed] [Google Scholar]
- Ramig LA. Effects of physiological aging on speaking and reading rates. J Commun Disord. 1983;16:217–226. doi: 10.1016/0021-9924(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Ramig LA. Effects of physiological aging on vowel spectral noise. J Gerontol. 1983;38:223–225. doi: 10.1093/geronj/38.2.223. [DOI] [PubMed] [Google Scholar]
- Ruiz PJ, Bernardos VS, Bartolome M, Torres AG. Capit timed tests quantify age-related motor decline in normal subjects. J Neurol Sci. 2007;260:283–285. doi: 10.1016/j.jns.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Ryan WJ. Acoustic aspects of the aging voice. J Gerontol. 1972;27:265–268. doi: 10.1093/geronj/27.2.265. [DOI] [PubMed] [Google Scholar]
- Ryan WJ, Burk KW. Perceptual and acoustic correlates of aging in the speech of males. J Commun Disord. 1974;7:181–192. doi: 10.1016/0021-9924(74)90030-6. [DOI] [PubMed] [Google Scholar]
- Searl JP, Gabel RM, Fulks JS. Speech disfluency in centenarians. J Commun Disord. 2002;35:383–392. doi: 10.1016/S0021-9924(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Shuey EM. Intelligibility of older versus younger adults' CVC productions. J Commun Disord. 1989;22:437–444. doi: 10.1016/0021-9924(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Smith BL, Wasowicz J, Preston J. Temporal characteristics of the speech of normal elderly adults. J Speech Hear Res. 1987;30:522–529. doi: 10.1044/jshr.3004.522. [DOI] [PubMed] [Google Scholar]
- Stach BA. Clinical audiology: an introduction. Clifton Park: Delmar; 2010. [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS) Brain Res. 2009;1268:112–124. doi: 10.1016/j.brainres.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex. 2010;46:15–28. doi: 10.1016/j.cortex.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Shiller DM, Gracco VL. On the time-course and frequency selectivity of the EEG for different modes of response selection: evidence from speech production and keyboard pressing. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2008;119:88–99. doi: 10.1016/j.clinph.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dick AS, Small SL. Functional and structural aging of the speech sensorimotor neural system: functional magnetic resonance imaging evidence. Neurobiol Aging. 2013;34:1935–1951. doi: 10.1016/j.neurobiolaging.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KA, Horii Y. Age and changes in vocal jitter. J Gerontol. 1980;35:194–198. doi: 10.1093/geronj/35.2.194. [DOI] [PubMed] [Google Scholar]
- Wohlert AB. Reflex responses of lip muscles in young and older women. J Speech Hear Res. 1996;39:578–589. doi: 10.1044/jshr.3903.578. [DOI] [PubMed] [Google Scholar]
- Wohlert AB. Tactile perception of spatial stimuli on the lip surface by young and older adults. J Speech Hear Res. 1996;39:1191–1198. doi: 10.1044/jshr.3906.1191. [DOI] [PubMed] [Google Scholar]
- Wohlert AB, Smith A. Spatiotemporal stability of lip movements in older adult speakers. J Speech Lang Hear Res JSLHR. 1998;41:41–50. doi: 10.1044/jslhr.4101.41. [DOI] [PubMed] [Google Scholar]
- Yan JH, Thomas JR, Stelmach GE. Aging and rapid aiming arm movement control. Exp Aging Res. 1998;24:155–168. doi: 10.1080/036107398244292. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]


