Abstract
Background
Randomized, controlled trials have demonstrated that antidepressants are efficacious in the treatment of anxiety disorders in youth. However, there are no recent, systematic analyses of the efficacy, safety or tolerability of these medications in pediatric anxiety disorders. With this in mind, we sought to systematically review and conduct a meta-analysis of double-blind, placebo-controlled-trials of antidepressants in these conditions.
Methods
A systematic review and meta-analysis of prospective, randomized, parallel-group, controlled trials of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SSNRIs) in pediatric patients with non-OCD anxiety disorders was undertaken using a search of PubMed/Medline (1966–2014). The meta-analysis utilized random-effects models to evaluate change in the Pediatric Anxiety Rating Scale or similar anxiety scale, suicidality and adverse events. Additionally, a series of pharmacologic variables (e.g., serotonin binding) were explored with regard to effect size.
Results
Data were included from 9 trials involving 1,673 patients and 6 medications, including 5 SSRIs and 3 SSNRI trials. All SSRI/SSNRIs evaluated demonstrated significant efficacy, and the meta-analytic summary estimate was of moderate magnitude (Cohen's d=0.64, confidence interval [CI]: 0.34–0.96, p=0.0017) and there was evidence of modest heterogenity (I2=0.26, p=0.107). Activation trended towards being more likely with antidepressant treatment (OR: 1.86, CI: 0.98–3.53, p=.054), but no increased risk was observed for nausea/abdominal symptoms (p=0.262) or discontinuation as a result of an adverse event (p=0.132). Treatment-emergent suicidality did not differ between antidepressant-treated youth and those who received placebo (OR: 1.3, CI: 0.53–3.2, p=0.514).
Conclusions
Data for 9 SSRI/SSNRIs suggest superiority to placebo for the treatment of pediatric anxiety disorders with a moderate effect size and a non-significant risk of suicidality.
Keywords: benzodiazepine, buspirone, anxiety disorders
INTRODUCTION
Anxiety disorders are among the most prevalent psychiatric conditions in children and adolescents and, in the United States, may affect 15–20% of youth (Kessler et al., 2012; Marikangas et al., 2010). Moreover, anxiety disorders—when present in youth—increase the risk of suicide attempts (Foley et al., 2006; Jacobson et al., 2008), as well as the risk of secondary mood and anxiety disorders (Pine et al., 1998) and are associated with significant morbidity and mortality. Treatment studies of these disorders, in pediatric patients, largely focus on anxiety symptoms as a homogenous entity and, as such, many psychopharmacologic treatment studies include diagnoses in the “pediatric anxiety disorder triad” (generalized anxiety disorder [GAD], social phobia [SoP] and separation anxiety disorder [SAD]). This approach is based on multiple clinical, phenomenologic, epidemiologic and theoretical factors, including: (1) high co-morbidity among these disorders (Walkup et al., 2008); (2) similar syndromic onset patterns (Beesdo et al., 2010); (3) shared neurophysiology (Blackford et al., 2012; Strawn et al, 2012a) and (4) similar treatment responses to both pharmacotherapy (e.g. selective serotonin reuptake inhibitors [SSRIs]) and cognitive behavioral psychotherapy (Compton et al., 2010; Strawn et al., 2012b; Kendall et al., 2010).
However, despite the abundance of clinical trial data, there are limited meta-analytic evaluations of antidepressants in anxious youth (Bridge et al., 2007; Ipser et al, 2009) and, since the most recent analyses were conducted, the sample of patients from these clinical trials of non-OCD anxiety studies has increased by nearly one third. Moreover, there are no recent, systematic analyses of treatment-related suicidality and common adverse events (e.g., activation, gastrointestinal symptoms) in youth with non-OCD anxiety disorders. By aggregating data from multiple studies, and allowing comparison between studies, meta-analytic techniques allow exploration of the overall magnitude of effect, and relative effect with greater statistical power than can be accomplished in individual trials. With these considerations in mind, we conducted a structured review and meta-analysis of randomized, placebo-controlled trials of SSRIs and SSNRIs for the short-term treatment of GAD, SoP and SAD in children and adolescents.
METHODS
Search Strategy
All meta-analytic methods and sensitivity analyses were specified before conducting the meta-analysis proper. The studies included were obtained through an electronic search of PubMed (1966 through July 2014) and the government clinical trials registry, www.clinicaltrials.gov and the search was completed by two reviewers (AMW) and (JRS) using the search strategy (anxiety OR social phobia OR social anxiety disorder OR SAD OR generalized anxiety disorder OR GAD OR separation anxiety disorder AND selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR votioxetine OR vilazodone). The results of the search were then manually limited to randomized, placebo-controlled trials. The references of all eligible trials and review articles were searched for additional clinical trials.
Criteria for Inclusion of Studies
Studies were included if they were prospective, randomized, parallel-group, placebo-controlled trials that evaluated the efficacy of SSRI or SSNRIs in the treatment of SoP, SAD, GAD or the combination of these three conditions in children or adolescents and used a validated rating scale to measure the severity of the anxiety symptoms.
Outcomes
We focused on the efficacy and tolerability (e.g., activation, gastrointestinal symptoms) and safety outcomes which are considered clinically meaningful and that are reported in at least 5 (>50%) of the included pediatric trials to allow for meaningful comparisons. Consistent with the outcomes used in most trials, we examined the dimensional measures of anxiety symptoms (see below), discontinuation as a result of an adverse event and treatment emergent suicidality.
Statistical Methods
Data were extracted into an Excel (Microsoft, Redmond, WA) spreadsheet in duplicate by two reviewers (JRS and AMW). Additional data related to the methods, demographics, SSRI/SSNRI dosing, duration of the trial, tolerability and adverse events, and other relevant aspects and results of the studies were collected. Missing information was requested from the study authors or investigators when possible. The outcome measurement selected from each included clinical trial was the difference in mean improvement between the antidepressant-treated and placebo-treated groups on a clinical rating scale measuring anxiety symptom severity over the course of the trial, at week 8–12 (or week 16 in the case of 1 trial) as well as treatment-emergent suicidality. Preferred rating scales for assessing anxiety severity were pre-ranked (in order of preference) and included the Pediatric Anxiety Rating Scale (PARS, RUPP, 2002), the Hamilton Anxiety Rating Scale (HAM-A, Hamilton, 1959), Social Anxiety Scale for Children (SASC, La Greca and Stone, 1993) and the Social Phobia and Anxiety Inventory (SPAI). In this regard, we established a hierarchy of preferred anxiety rating scales for our primary outcome a priori so as to decrease the likelihood of inflation and reporting bias (PARS – first; HAM-A-second, SASC-third, SPAI-fourth).
The primary outcome for these analyses was the change in PARS total score (or other dimensional anxiety scale score) from baseline to endpoint, which was typically week 8–12, except in two 16-week trials (March et al., 2007; Wagner et al., 2004), although 12-week data were available for the first of these trials and, to minimize heterogeneity, the 12-week data point was used for this venlafaxine study (March et al., 2007). The difference in change scores between each medication and its corresponding placebo arm was computed. For analysis of binary data representing adverse events and discontinuation, odds ratios were used as the measures of effect. Since all sample sizes were reasonably large and response rates were not close to 0 or 1, the odds ratios were assumed to follow normal distributions with these variances; this approximation allowed us to use similar computational techniques for meta-analysis of mean change scores.
Given the a priori possibility that not all trials would produce exactly equal underlying effect sizes, a random-effects model was considered preferable to a fixed-effects model. In this regard, the fixed-effects model assumes that between-trial variation is completely attributable to sampling error whereas the random-effects model accommodates both within-study and between-study variance and is therefore usually more realistic than the fixed-effects model, as it assumes that factors other than sampling error account for between-trial differences. The model was implemented using restricted maximum likelihood (REML) using SAS PROC MIXED (SAS; Cary, N.C.). The inverse variances of the mean differences were specified as known within-trial parameters in a heterogeneous-variance structure (treating the within-trial variances as known, when they are actually estimated from the data, is standard meta-analytic practice (Sutton, 2000; Cooper, 1994). Statistical heterogeneity was tested using Cochrane's Q test, and its magnitude reported in terms of the I2 statistic (proportion of variability attributable to between-study variation).
Finally, a post-hoc analysis of the relationship between effect size and several psychopharmacologic variables was conducted. We examined the correlation of the individual effect sizes for each study and the potency of the agent being studied with regard to inhibition of norepinephrine reuptake (as reflected by the inhibition constant [Ki]), serotonin (5-hydroxytryptamine, 5-HT) reuptake or the selectivity of the agent for norepinephrine or 5-HT as reflected by the ratio of the Ki for norepinephrine to the Ki for 5-HT (O'Donnell and Shelton, 2011). P-values <0.05 were considered statistically significant and, given the exploratory nature of these analysis, no correction for multiple comparisons was made. Additionally, trials that included concurrent treatment with stimulants (Walkup et al., 2008) (which are pro-dopaminergic and—depending on the class—pro-noradrenergic) were excluded from this analysis (REFERENCE × 3).
Results
Selection of Studies
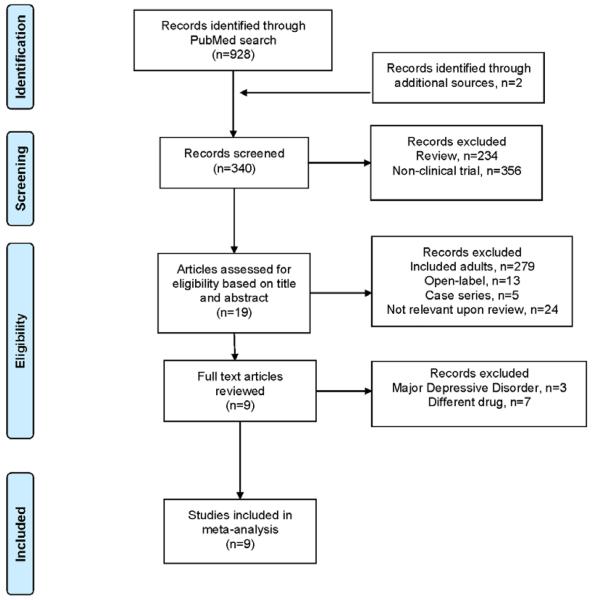
The PubMed search identified 19 articles that were potentially eligible for inclusion in this meta-analysis and an additional study was identified through a search of the 2013 Annual Meeting of the American Academy of Child & Adolescent Psychiatry Meeting abstracts. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, 2009) diagram illustrating the selection procedure—which yielded 9 studies—is shown in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Study Characteristics
We identified 9 double-blind, placebo-controlled studies that included pediatric patients with GAD, SoP and/or SAD and involved treatment with an SSRI or SSNRI. Five of the studies (56%) were federally-funded, with the remaining 4 studies funded by industry and all studies were conducted in the outpatient setting. Trial duration was 8 (RUPP, 2001; Rynn et al., 2007), 9 (Rynn et al., 2001), 10 (Strawn et al., 2013), 12 (Walkup et al., 2008; Birmaher et al., 2003; Beidel et al, 2007), or 16 (March et al., 2007; Wagner et al., 2004) weeks (mean duration: 11.4+3 weeks). Of the 9 trials, one each involved fluvoxamine (N=128, fluvoxamine: n=63, placebo: n=58, flexibly dosed to a maximum of 300 mg per day in adolescents and 250 mg per day in children less than 12 years of age, mean dose: 2.9 mg/kg/day); paroxetine (N=319, paroxetine: n=163, placebo n=156, flexible-dosing, 10–50 mg/day, mean dose 32.6 mg/day) and duloxetine (N=272, duloxetine: n=137, placebo: n=135, flexible-dosing, mean dose 53.6 mg/day). Additionally, there were 2 trials each for sertraline, fluoxetine and venlafaxine. In the first sertraline trial (Rynn et al., 2001), sertraline was initiated at 25 mg daily and increased to 50 mg daily (N=22, sertraline: n=11, placebo: n=58) while in the second (Walkup et al., 2008), sertraline was initiated at 25 mg and titrated to 200 mg daily (N=209, sertraline: n=133, placebo: n=76, mean dose: 133 mg/day). This study, the Child & Adolescent Multimodal Study of Anxiety (CAMS), also included 2 additional groups, one which received cognitive behavioral therapy and another which received sertraline + cognitive behavioral therapy. However, these two arms, because of the presence of an active psychotherapeutic treatment, are not included in this meta-analysis. Two studies evaluated venlafaxine (March et al., 2007; Rynn et al., 2007), one of which involving patients with a primary diagnosis of social phobia (March et al., 2007) (N=285, venlafaxine: n=137, placebo: n=148, flexibly dosed to a maximum of 112.5 mg per day in youth weighing < 33 kg, 150 mg in youth weighing between 33 and 49 kg and 225 mg in youth weighing >50 kg) and a second involving patients with a primary diagnosis of generalized anxiety disorder (Rynn et al., 2007) (N=320, venlafaxine: n=157, placebo: n=163, flexibly dosed to a maximum of 112.5 mg per day in youth weighing < 33 kg, 150 mg in youth weighing between 33 and 49 kg and 225 mg in youth weighing >50 kg). Additionally, two studies evaluated fluoxetine (including one study of youth with SoP). In the first study, a forced-titration of fluoxetine to 20 mg daily (N=74, fluoxetine: n=37, placebo: n=37) was employed (Birmahar et al, 2003). Similarly, Beidel and colleagues (2007) also utilized a forced titration paradigm in patients with SoP and also compared fluoxetine (and placebo) to social effectiveness training for children (SET-C) in children and adolescents (N=122, SET-C: n=57, fluoxetine n=33, placebo: n=32) in which fluoxetine was initiated at 10 mg daily and, during weeks 3–4, was increased to 20 mg daily and then, during weeks 5–6, was increased to 30 mg daily and finally, titrated to 40 mg daily at week 7.
Comorbidities & concurrent treatment
Patients with co-occurring major depressive disorder were excluded in all but one study (n=8) while ADHD was generally allowed, although treatment of ADHD with psychostimulants were excluded in the majority (n=7, 78%) of studies with the exception being CAMS (Walkup et al., 2008) which allowed stable doses of stimulant medications. Concurrent treatment with benzodiazepines was not allowed in any study. Additionally, Beidel and colleagues allowed depressive disorders and other “secondary comorbid diagnoses, with the exception of bipolar disorder, psychosis, conduct disorder, autism spectrum disorders and mental retardation.”
Concomitant psychotherapy was allowed in 3 studies (33%), although this was always accompanied by restrictions. In the study of sertraline for the treatment of GAD (Rynn et al., 2001), psychotherapy was allowed, but could not be cognitive behavioral therapy (CBT), while in the study of venlafaxine ER for the treatment of GAD (Rynn et al., 2007), concomitant psychotherapy was allowed if stable for at least one month. Finally, in the study of duloxetine for GAD (Strawn et al., 2013), patients were permitted to be engaged in psychotherapy, but the modality (e.g., cognitive behavioral therapy, interpersonal psychotherapy, psychodynamic psychotherapy) or intensity could not have changed within 6 weeks of the patient's baseline assessment.
Efficacy outcomes
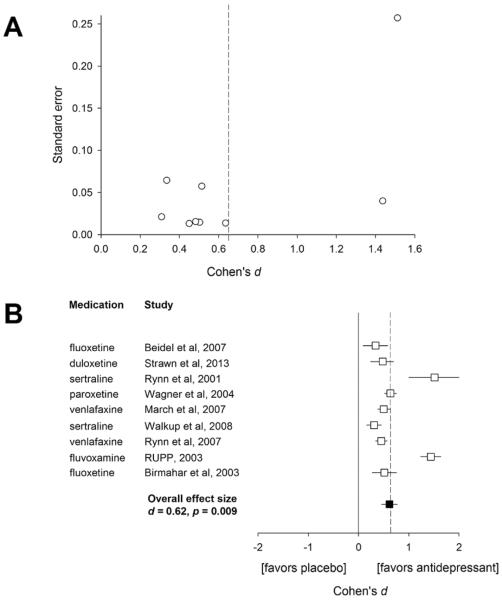
All trials demonstrated significant efficacy compared to placebo. The estimated effect size (Cohen's d) for the reduction in anxiety symptoms was 0.64 (95% confidence interval [CI]: 0.34–0.96, p=0.0017, Figure 2). Additionally, there was modest evidence for heterogeneity (p=0.107 for the heterogeneity test, I2=0.26). Finally, there was no difference in effect sizes between federally-funded (mean effect size: 0.82) and industry funded studies (mean effect size: 0.52, p=0.321).
Figure 2. Funnel plot (A) and forest plot (B) for individual studies of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors in children and adolescents with generalized anxiety disorder, social phobia or separation anxiety disorder.
Effect sizes are based on the change in the dimensional anxiety symptom scale from baseline (see methods) and open symbols represent effect sizes and horizontal lines reflect the 95% confidence intervals for each study. Dashed line represents the estimated effect size (Cohen's d=0.62)
Treatment-emergent suicidality, adverse effects and discontinuation
The meta-analytic estimate of the odds ratio for suicidality (values >1 indicate higher risk with drug than placebo) was 1.30 (CI: 0.53–3.16, p=0.514), failing to suggest a higher risk of suicidality associated with antidepressant treatment in adolescents with non-OCD anxiety disorders. Additionally, there was no evidence of heterogeneity among studies (I2=0, p=0.514).
Antidepressants were not associated with a greater likelihood of nausea or abdominal pain compared placebo (OR: 1.74, CI: 0.54–5.64, p=0.262) nor were they associated with a greater likelihood of all cause discontinuation (OR: 2.11, CI: 0.70–6.3, p=0.132). However, activation strongly trended towards being associated with antidepressant treatment (OR: 1.86, CI: 0.98–3.53, p=.054).
Psychopharmacologic predictors of effect size
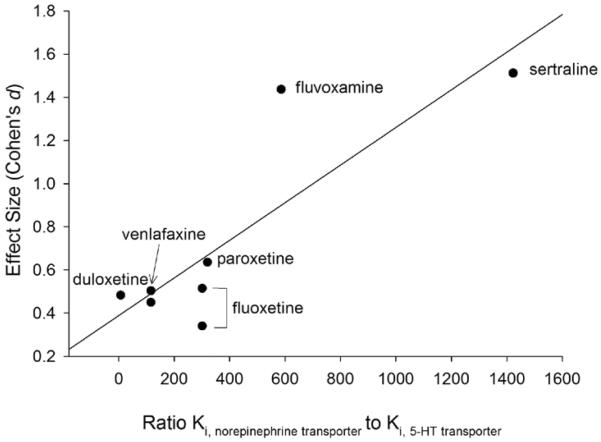
In the post-hoc analyses of the relationships between effect size and psychopharmacologic variables a significant relationship between the specificity for serotonin reuptake relative to norepinephrine reuptake (i.e., the ratio of Ki, norepinephrine to Ki, 5-HT) was observed (Figure 3, R=0.85, p=0.01). Additionally, when this analysis was corrected for sample size, the results remained statistically significant (R=0.79, p=0.021) but no relationships were observed between effect size and potency of 5-HT reuptake inhibition (R=−.17, p=0.68) or norepinephrine reuptake inhibition (R=0.23, p=0.58).
Figure 3. Effect size is predicted by serotonergic selectivity in double-blind, placebo-controlled trials of antidepressants in youth with anxiety disorders.
Serotonergic specificity is reflected by the ratio of the Ki for norepinephrine to the Ki for serotonin, where Ki is the inhibition constant (in nM), based on radioactive ligand transport competition assays using human specific transporter proteins. Note: potency is inversely related to Ki and, given that concurrent treatment with psychostimulants (which affect norepinephrine and dopamine) was allowed in the trial by March et al, 2008, this study was excluded.
DISCUSSION
This meta-analysis of all randomized, double-blind, placebo-controlled trials of SSRIs and SSNRIs in pediatric patients with non-OCD anxiety disorders demonstrates efficacy for all individual agents with an overall modest effect size. Moreover, these antidepressants appear to be well tolerated and all cause discontinuation does not appear to differ between antidepressants and placebo in anxious youth.
It is of interest, although consistent with 1 prior meta-analysis, that we did not observe an increased risk of treatment-emergent suicidality in youth with anxiety disorders (Bridge et al., 2007) who were treated with antidepressants. However, given the rarity of treatment-emergent suicidality, this should be interpreted with caution. Importantly, recent studies of pediatric patients with anxiety disorders have utilized systematic assessments of suicidality (i.e., the Columbia-Suicide Severity Rating Scale, Posner et al., 2011) to assess suicidal ideation and suicidal behavior. Accordingly, the use of this instrument which better captures and prospectively categorizes events may have led to the more accurate characterization of “suicidality” in these trials, thus reflecting a “truer” signal. Moreover, the lower suicidality signal in the pediatric anxiety disorder trials which mirrors the analysis by Bridge and colleagues (2007), who noted that the number needed to harm (NNH) for treatment-emergent suicidality in youth with depressive disorders is 111, while the NNH for treatment-emergent suicidality in youth with anxiety disorders is 143, may also reflect a number of factors related to the design of the anxiety disorder trials compared to clinical trials involving youth with major depressive disorder. These factors include (1) the frequent exclusion of active suicidality and (2) exclusion of patients with MDD, who have higher rates of suicidality and these factors, likely lower the level and risk of suicidal ideation and behavior. Finally, like Bridge and colleagues (2007), but unlike the original FDA meta-analysis (FDA, 2004), we utilized a random effects model to evaluate treatment-emergent suicidality; consequently, our results are more consistent with the analyses of Bridge and colleagues (2007) than with the FDA analysis. Importantly, the fixed-effects model (utilized in the meta-analysis conducted by the USFDA) assumes that between-trial variation is completely attributable to sampling error, whereas the random-effects model, utilized herein, accommodates both within-study and between-study variance and, as a result of the assumption that factors other than sampling error account for between-trial differences yields a more realistic estimate of suicidality risk.
The meta-analytic side effect profile described herein warrants some discussion. In this regard, while studies of SSRIs and SSNRIs in adults with anxiety disorders have generally observed high rates of gastrointestinal symptoms, we failed to observe a significantly increased risk of these symptoms in anxious youth who were treated with antidepressants. This may owe to the heterogeneity in reporting of these symptoms in the trials that were examined (e.g., nausea vs. abdominal pain + nausea vs. vomiting + nausea) and may also relate to the relatively high rates of gastrointestinal symptoms in youth with untreated anxiety (Crawley et al., 2014). Additionally, in this meta-analysis, activation strongly trended towards being more likely with antidepressant treatment compared to placebo treatment. Activation, which occurs at higher rates in pediatric patients, compared to adults, warrants additional discussion. First described more than 2 decades ago in pediatric patients treated with SSRIs, (Lipinski et al., 1989; Riddle et al., 1991) “activation” generally refers to hyperactivity, impulsivity, insomnia or disinhibition and, importantly, is distinct from treatment-emergent mania (Walkup and Labellarte, 2001). Within the pediatric age range, activation appears to be more common in children than in adolescents, and in this regard 10.7% in children compared to 2.1% in adolescents experience activation in randomized, controlled trials of antidepressants (Safer and Zito, 2006), although depending on data collection techniques considerable variability in observed rates of activation exists (Riddle et al., 1991; Reinblatt et al., 2009; Wilens et al., 2003). While these rates of activation are consistent with the rates reported herein (mean: 21.2±19%), a number of developmental factors and pharmacologic factors likely contribute to the variability among these rates in this meta-analysis. As such, activation-related adverse events, in pediatric patients, respond to slower titration of the medication (Riddle et al., 1991; Gualtieri and Johnson, 2006), parallel the pharmacokinetic profile of the medication (Walkup and Labellarte, 2001) and, in one study of the SSRI fluvoxamine, are associated with plasma drug concentrations during the initial phase of treatment (Reinblatt et al., 2009). Thus, medication dose (or plasma drug concentration) appears to influence the likelihood of treatment-emergent activation. More generally, regarding side effects in anxious youth, it is important to emphasize that pediatric patients with anxiety disorders, have high rates of somatic symptoms prior to treatment and that the severity of these symptoms vary directly with the severity of the anxiety symptoms and importantly decrease with treatment (Crawley et al., 2014).
Additionally, differences in antidepressant-treated adult patients with anxiety disorders and those with depressive disorders have been observed, suggesting that either the presence of an anxiety disorder may increase the likelihood of experiencing or perceiving side effects. As an example, adult participants in the large Sequenced Treatment Alternatives to Relieve Depression (STAR*D) who were diagnosed with anxious depression compared to patients with depression without co-occurring anxiety experienced significantly more side effects with SSRI treatment (Fava et al., 2008).
The post-hoc analysis of effect size and serotonergic selectivity in which we noted that larger effect sizes were associated with greater serotonergic selectivity, is of interest. The widely held belief that more serotonergically specific agents are more efficacious for the treatment of anxiety disorders has long been promulgated in psychiatric training and texts, although very few studies (all of which were conducted in adults), have systematically examined this dogma. In this regard, a retrospective study of 9 antidepressant trials baseline anxiety in adults with major depressive disorder who were treated with SSRIs compared with depressed patients who were treated with norepinephrine reuptake inhibitors, mixed norepinephrine-5-HT inhibitors, suggested greater efficacy for more serotonergically-selective agents in anxious depression (Filteau et al., 1995). Additionally, in adults with post-stroke depression, citalopram, which has a high selectivity for serotonin) was associated with greater efficacy in “anxious depressed patients,” compared to the norepinephrine-reuptake inhibitor, reboxetine (Rampello et al., 2004). Additionally, a large meta-analysis of adult patients with anxious depression (N = 1275) revealed a statistically and clinically significant difference between response rates for SSRIs compared to bupropion (Papakastas et al., 2008). However, not all studies corroborate this finding (Rush et al., 2001). Thus, while these findings are too preliminary to necessarily suggest that more serotonergically-selective agents, should be preferentially utilized over agents with more equal effects on 5-HT and norepinephrine reuptake inhibition, they are nonetheless intriguing and it will be of interest to determine if this relationship remains as additional agents, with varying degrees of selectivity, are introduced to the armamentarium.
We note several important limitations of this meta-analysis. First, despite the general similarity of studies, some sources of heterogeneity must be considered. As such, some studies focused on specific disorders within the pediatric anxiety disorders triad (e.g., SoP, GAD) and there are also differences in the continuous outcome measures utilized, titration regimen and duration of the study. Second, the studies differed the severity of baseline anxiety symptoms, raising the possibility of a floor effect in studies that included children with less severity. Third, we cannot exclude the possible effects of study population on placebo response. Fourth, regarding our analyses of suicidality, in contrast to studies of children and adolescents with major depressive disorder (who were universally excluded from these studies), treatment-emergent suicidality was very rare and thus, there is a possibility of type II error with regard to our analyses. Nonetheless, these data are consistent with a prior meta-analysis of suicidality in pediatric patients with non-OCD anxiety disorders and major depressive disorder (Bridge et al., 2007).
In summary, our results suggest similarity among antidepressants in efficacy for the treatment of the non-OCD anxiety disorders in youth; however, in the face of substantial study heterogeneity, we cannot exclude modest differences, although, treatment selection among these mediations may be better governed by factors other than short-term efficacy, such as tolerability, half-life and cost.
Table 1.
Study, Patient and Treatment Characteristics of Included Randomized Controlled Trials of Selective Serotonin Norepinephrine Reuptake Inhibitors (SSNRIs) in Children and Adolescents with Generalized Anxiety Disorder, Social Phobia or Separation Anxiety Disorder.
| Study | Funding | Study design | Group N | Study duration wks | Sex % male | Age yrs | Medication | Outcome measure | Endpoint Dose mg/day | Baseline score ± SD | Endpoint score ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rynn et al, 2001 | Federal | Fixed | 11 | 9 | 67 | 5–17 | Sertraline | HAM-A | 50 | Drug: 20.6±3.6 | 7.8±5.7 |
| 11 | Pbo: 23±4 | 21±7.8 | |||||||||
|
| |||||||||||
| Birmaher et al, 2003 | Federal | Fixed | 37 | 12 | 46 | 7–17 | Fluoxetine | PARS | 20 | Drug: 15.6±3.5 | 7.1±5.9 |
| 37 | Pbo: 14.9±3.5 | 9.3±4.8 | |||||||||
|
| |||||||||||
| RUPP et al, 2001 | Federal | Flexible | 63 | 8 | 51 | 6–17 | Fluvoxamine | PARS | 2.9 ± 1** | Drug: 18.7±2.9 | 7.1±6.1 |
| 65 | Pbo: 19±3.0 | 15.7±5.4 | |||||||||
|
| |||||||||||
| March et al, 2007 | Industry | Flexible | 137 | 12* | 44 | 8–17 | Venlafaxine | SAS-CA | 142 | Drug: 64.8±10.1 | 42.5±1.2 |
| 148 | ER | Pbo: 66.2±10.6 | 49±1.2 | ||||||||
|
| |||||||||||
| Rynn et al, 2007 | Industry | Flexible | 157 | 8 | 58 | 6–17 | Venlafaxine | PARS | NR | Drug: 24.1±3.3 | 14±7 |
| 163 | ER | Pbo: 23.8±3.1 | 16.8±6.5 | ||||||||
|
| |||||||||||
| Walkup et al, 2008 | Federal | Flexible | 133 | 12 | 53 | 7–17 | Sertraline | PARS | 133 | Drug: 18.3±3.9 | 9.8±6.2 |
| 76 | Pbo: 19.6±3.9 | 12.6±6.3 | |||||||||
|
| |||||||||||
| Wagner et al, 2004 | Industry | Flexible | 163 | 16 | 50 | 12–17 | Paroxetine | PARS | 32.6 | Drug: 77.6±28.7 | 29.5±33 |
| 156 | Pbo: 77.1±27.05 | 52.9±33 | |||||||||
|
| |||||||||||
| Strawn et al, 2013 | Industry | Flexible | 135 | 10 | 47 | 7–17 | Duloxetine | PARS | 53.6 | Drug: 17.3±2.2 | 8_±2.5 |
| 137 | Pbo: 17±2.4 | 11±2.7 | |||||||||
DBPCT, double blind, placebo-controlled trial; HAM-A, Hamilton Anxiety Rating Scale; PARS, Pediatric Anxiety Rating Scale, SAS-CA, Social Anxiety Scale for Children & Adolescents; pbo, placebo; NR, not reported
This was a 16 week trial; however, 12 week data were used for the analyses described herein.
mg/kg/day, rather than mg/day.
Acknowledgments
Support was also received from the National Center for Research Resources and the National Center for Advancing Translational Sciences; and from the National Institutes of Health (NIH) through Grant 8 UL1 TR000077-04. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
abbreviations
- (SSRI, SRI)
selective serotonin reuptake inhibitor
- (SSNRI, SNRI)
selective serotonin norepinephrine reuptake inhibitor
- (SAD)
separation anxiety disorder
- (SoP)
social phobia
- (GAD)
generalized anxiety disorder
Footnotes
Disclosures: Dr. Strawn has received research support from Eli Lilly, Shire, Forest, Lundbeck and from the American Academy of Child & Adolescent Psychiatry. Dr. Rynn has received research support from Eli Lilly, Shire, Pfizer and from the NIH and NICHD.
REFERENCES
- 1.Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am. 2012;21(3):501–25. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- 3.Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 4.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;(297):1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 5.Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, Ginsburg GS, Rynn MA, McCracken JT, Waslick BD, Iyengar S, Kendall PC, March JS. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper H. The Handbook of Research Synthesis. Russell Sage Foundation; New York, NY: 1994. [Google Scholar]
- 7.Crawley SA, Caporino NE, Birmaher B, Ginsburg G, Piacentini J, Albano AM, Sherrill J, Sakolsky D, Compton SN, Rynn M, McCracken J, Gosch E, Keeton C, March J, Walkup JT, Kendall PC. Child Psychiatry Hum Dev. 2014. Somatic complaints in anxious youth. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–51. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 9.Filteau MJ, Baruch P, Lapierre YD, Bakish D, Blanchard A. SSRIs in anxious-agitated depression: a post-hoc analysis of 279 patients. Int Clin Psychopharmacol. 1995;10(1):51–4. doi: 10.1097/00004850-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Foley DL, Goldston DB, Costello EJ, Angold A. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017–24. doi: 10.1001/archpsyc.63.9.1017. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 12.Ipser JC, Stein DJ, Hawkridge S, Hoppe L. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev. 2009;3:CD005170. doi: 10.1002/14651858.CD005170.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson CM, Muehlenkamp JJ, Miller AL, Turner JB. Psychiatric impairment among adolescents engaging in different types of deliberate self-harm. J Clin Child Adolesc Psychol. 2008;37(2):363–75. doi: 10.1080/15374410801955771. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell JM, Shelton RC. Drug therapy for depression and anxiety disorders. In: Brunton LL, editor. Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th Edition. McGraw Hill; New York, NY: 2011. pp. 397–416. [Google Scholar]
- 15.Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, He JP, Koretz D, McLaughlin KA, Petukhova M, Sampson NA, Zaslavsky AM, Merikangas KR. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):372–80. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Greca AM, Stone WL. The social anxiety scale for children - revised: factor structure and concurrent validity. J Clinical Child Psychol. 1993;22:17–27. [Google Scholar]
- 17.Lipinski JF, Jr, Mallya G, Zimmerman P, Pope HG., Jr Fluoxetine-induced akathisia: Clinical and theoretical implications. J Clin Psychiatry. 1989;50:339–342. [PubMed] [Google Scholar]
- 18.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149–54. doi: 10.1016/j.biopsych.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR1, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Papakostas GI1, Stahl SM, Krishen A, Seifert CA, Tucker VL, Goodale EP, Fava M. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry. 2008;69(8):1287–92. doi: 10.4088/jcp.v69n0812. [DOI] [PubMed] [Google Scholar]
- 22.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–26. doi: 10.1089/cap.2008.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riddle MA, King R, Hardin MT, Scahill L, Ort S, Chappell P, Rasmusson A, Leckman J. Behavioral side effects of fluoxetine in children and adolescents. J Child Adolesc Psychopharmacol. 1991;1:193–198. [Google Scholar]
- 26.RUPP The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1061–9. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 27.RUPP Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344(17):1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 28.Rush AJ, Trivedi MH, Carmody TJ, Donahue RM, Houser TL, Bolden-Watson C, Batey SR, Ascher JA, Metz A. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131–8. doi: 10.1016/S0893-133X(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 29.Rynn MA, Siqueland L, Rikels K. Placebo-controlled trial of ser- traline in the treatment of children with generalized anxiety dis- order. Am J Psychiatry. 2001;158:2008–2014. doi: 10.1176/appi.ajp.158.12.2008. [DOI] [PubMed] [Google Scholar]
- 30.Rynn MA, Riddle MA, Yeung PP, Kunz N. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am J Psychiatry. 2007;164(2):290–300. doi: 10.1176/ajp.2007.164.2.290. [DOI] [PubMed] [Google Scholar]
- 31.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: Children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16:159–169. doi: 10.1089/cap.2006.16.159. [DOI] [PubMed] [Google Scholar]
- 32.Sutton AJ. Methods for Meta-Analysis in Medical Research. John Wiley & Sons; Chichester, UK: 2000. [Google Scholar]
- 33.Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, Findling RL. A randomized, placebo-controlled study of duloxetine for the treatment of children (7–11 Years) and adolescents (12–17 Years) with generalized anxiety disorder. Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Orlando, FL. October 22–27, 2013; [DOI] [PubMed] [Google Scholar]
- 34.Strawn JR, Wehry AM, DelBello MP, Rynn MA, Strakowski S. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety. 2012a;29(4):328–39. doi: 10.1002/da.21913. [DOI] [PubMed] [Google Scholar]
- 35.Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012b;21(3):527–39. doi: 10.1016/j.chc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration [Accessed April 19, 2014];Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf.
- 37.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61:1153–1162. doi: 10.1001/archpsyc.61.11.1153. [DOI] [PubMed] [Google Scholar]
- 38.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilens TE, Biederman J, Kwon A, Chase R, Greenberg L, Mick E, Spencer TJ. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13:143–152. doi: 10.1089/104454603322163862. [DOI] [PubMed] [Google Scholar]