Abstract
Background
Low skeletal muscle mass is associated with deterioration of bone mineral density. Because serum creatinine can serve as a marker of muscle mass, we evaluated the relationship between serum creatinine and bone mineral density in an older population with normal renal function.
Methods
Data from a total of 8,648 participants (4,573 men and 4,075 postmenopausal women) aged 45–95 years with an estimated glomerular filtration rate >60 ml/min/1.73 m2 were analyzed from the Fourth Korea National Health and Nutrition Examination Survey (2008–2010). Bone mineral density (BMD) and appendicular muscle mass (ASM) were measured using dual-energy X-ray absorptiometry. Receiver operating characteristic curve analysis revealed that the cut points of serum creatinine for sarcopenia were below 0.88 mg/dl in men and 0.75 mg/dl in women. Subjects were divided into two groups: low creatinine and upper normal creatinine according to the cut point value of serum creatinine for sarcopenia.
Results
In partial correlation analysis adjusted for age, serum creatinine was positively associated with both BMD and ASM. Subjects with low serum creatinine were at a higher risk for low BMD (T-score ≤ –1.0) at the femur neck, total hip and lumbar spine in men, and at the total hip and lumbar spine in women after adjustment for confounding factors. Each standard deviation increase in serum creatinine was significantly associated with reduction in the likelihood of low BMD at the total hip and lumbar spine in both sexes (men: odds ratio (OR) = 0.84 [95% CI = 0.74−0.96] at the total hip, OR = 0.8 [95% CI = 0.68−0.96] at the lumbar spine; women: OR = 0.83 [95% CI = 0.73–0.95] at the total hip, OR=0.81 [95% CI = 0.67–0.99] at the lumbar spine).
Conclusions
Serum creatinine reflected muscle mass, and low serum creatinine was independently associated with low bone mineral density in subjects with normal kidney function.
Introduction
Growing evidence supports cross-talk between bone and muscle because they have common genetic, nutritional, lifestyle, and hormonal determinants [1]. Interactions between muscle and bone can affect bone strength [2], and it has been previously documented that bone functions as a musculoskeletal unit and adapts to the mechanical loads exerted by skeletal muscle [3]. In addition, a progressive decline in bone mineral density (BMD), muscle mass and muscle strength have common key features of the aging process. Accordingly, sarcopenia, the age-related loss of muscle mass, has been suggested as a major risk factor for low BMD and fracture in several epidemiological studies [4–6]. Therefore, identification of sarcopenia is an important factor in older populations suggesting whether individuals have decreased BMD and are therefore at high risk of fragility fracture. Although dual-energy X-ray absorptiometry (DXA) is currently accepted as the gold standard method to measure both muscle mass and BMD, it is expensive and not easily accessible for many populations.
Serum creatinine is primarily a metabolite of creatine phosphate, almost all of which is found in skeletal muscle. Because the amount of creatinine per unit of skeletal muscle mass and the breakdown rate of creatine are both consistent, plasma creatinine concentration is a stable, direct reflection of skeletal muscle mass [7]. In addition, because 24-h urinary creatinine excretion is highly correlated with muscle mass estimates determined using DXA [8], and serum creatinine is highly correlated with 24-h urine excretion in subjects with normal renal function [9], serum creatinine could represent an acceptable and easily measured surrogate marker of muscle mass. Considering that skeletal muscle is a major target tissue of insulin [10], several previous studies have reported lower serum creatinine (reflecting lower skeletal muscle) to be associated with metabolic disorders such as insulin resistance and type 2 diabetes [11, 12]. From those findings and considering the bone-muscle relationship, we speculated that lower serum creatinine might also be associated with deterioration of BMD, especially in subjects without renal insufficiency. However, few studies have reported the association between serum creatinine and BMD.
Therefore, the aim of the present study was to investigate the associations between serum creatinine and BMD in adults with normal kidney function using data from the general Korean population. We also examined whether those associations differ by sex or skeletal sites. We hypothesized that lower serum creatinine, reflecting low muscle mass, might be related to decreased BMD and that serum creatinine could provide information about an individual’s bone health as well as muscle health in subjects without renal insufficiency.
Materials and Methods
Study population and design
The Korea National Health and Nutrition Examination Survey (KNHANES) has been performed periodically since 1998 by the Division of Chronic Disease Surveillance of the Korean Centers for Disease Control and Prevention to assess the health and nutritional status of the civilian, non-institutionalized population of Korea. The KNHANES IV, V was a cross-sectional and nationally representative survey conducted from 2008 to 2010 which are available on the KNHANES website (https://knhanes.cdc.go.kr/knhanes/sub03/sub03_02_02.do; S1 Dataset). The survey was composed of a health interview survey, a nutrition survey, and a health examination survey. The data were collected by household interviews and by direct, standardized physical examinations conducted in mobile examination centers. Nutritional status and medical history were evaluated using a 24-h recall method. Regular exercise was indicated as “yes” when the subject exercised for more than 20 min at a time more than three times per week. Subjects with any pathological disorders (such as cancer, hyperthyroidism, malabsorption, or hepatic failure) or subjects using medications (such as corticosteroids, heparin, or anticonvulsants) known to alter calcium and bone metabolism were excluded from our analysis. We also excluded subjects who used testosterone, anabolic steroids or antiresorptive agents and who have definite renal insufficiency (estimated glomerular filtration rate <60 ml/min/1.73 m2). Among those who participated in the survey and met our inclusion criteria, 8,648 participants were 45 years or older (4,573 men and 4,075 postmenopausal women).
Ethics statement
Because the KNHANES IV survey data are publicly available, ethical approval was not required for this study. Prior to the survey, all participants were informed that they had been randomly chosen to participate in the KNHANES IV survey with the right to refuse to be involved in further analyses, and signed informed consents were obtained. The data we used from the KNHANES database were fully anonymized.
Measurements and definitions of sarcopenia
Total body fat and appendicular skeletal muscle mass (ASM) as well as BMD at the lumbar spine (LS) (L1–4) and hip region were measured using DXA (QDR 4500A; Hologic Inc., Waltham, MA). All men and non-pregnant women aged 20 years and older who received a physical examination in the mobile centers were eligible for bone densitometry analysis unless they had previously fractured both hips. Low BMD was defined as a T-score of –1.0 or less. Relative ASM was calculated as the sum of the mass of skeletal muscle in the arms and legs, divided by the square of the height (ASM/ht2 in kg/m2). A subject was classified as having sarcopenia when he or she had a relative ASM less than one standard deviation (SD) below the sex-specific normal mean for the young reference group (healthy men and women aged 20–39 years) [13]. The cutoff point for sarcopenia was 7.86 kg/m2 for men and 5.71 kg/m2 for women.
Biochemical analysis
Collected blood samples were immediately refrigerated, transported to the Central Testing Institute in Seoul, Korea, and analyzed within 24 h. Serum creatinine levels were determined using a Hitachi 7600 automated chemistry analyzer (Hitachi, Tokyo, Japan) with Creatinine plus (Roche Diagnostics). The estimated glomerular filtration rate derived from the Chronic Kidney Disease Epidemiology Collaboration equation was used to assess renal function; this method is more accurate than previous indices, such as the Modification of Diet in Renal Disease Study equation [14]. Fasting plasma glucose and cholesterol levels were measured with a Hitachi 700–110 chemistry analyzer (Hitachi). Serum 25-hydroxyvitamin D [25(OH)D] concentrations were measured by radioimmunoassay (DiaSorin Inc., Stillwater, MN, USA) using a γ-counter (1470 Wizard; PerkinElmer, Turku, Finland). A chemiluminescence immunoassay (N-tact PTH assay; DiaSorin) was used to measure serum intact parathyroid hormone. Fasting insulin (INS-IRMA; Biosource, Nivelles, Belgium) was measured by an immunoradiometric assay. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: fasting (plasma glucose (mg/dL) x fasting insulin (mIU/mL))/22.5 [15].
Statistical analysis
Statistical analyses were conducted using IBM’s SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). A receiver operating characteristic curve (ROC) was created to find the cut-off point of serum creatinine for predicting sarcopenia. The optimal cut point was 0.88 in men (sensitivity was 57%; specificity was 57% at this level) and 0.75 in women (sensitivity was 70%; specificity was 40% at this level). We classified subjects into two groups according to this cut-off point value of serum creatinine. An independent, two-sample t-test was used to compare differences in the mean values of baseline parameters among the groups. For categorical variables, a chi-square test was used to compare the frequencies among the groups. Partial correlation analyses were used to evaluate the association between serum creatinine and body composition indices with adjustments made for age. Multiple linear regression analysis was then used to determine the association between serum creatinine and the outcome (total hip, femoral neck and lumbar spine BMD) adjusted for confounding factors. Multiple logistic regression analysis was used to examine the association between sarcopenia and low BMD, with the results expressed as odds ratios (OR) and 95% confidence intervals (CI).
Results
Clinical characteristics of the study population
The demographic and clinical characteristics of the patients, who were classified into two groups according to sex-specific cutoff values of creatinine for sarcopenia (Men: serum creatinine ≤ 0.8 mg/dL, Women: ≤ 0.7 mg/dL), are shown in Table 1. The mean age was 60.16±10.06 years (range, 45–93 years) in men and 62.96±9.07 years (range, 45–95 years) in postmenopausal women. The low creatinine group had lower body mass index, weight, waist circumference and ASM in both sexes. BMD at the total hip (TH), femoral neck (FN), and lumbar spine (LS) was significantly lower in the low creatinine group than in the upper normal creatinine group in men, but only BMD at LS was significantly lower in the low creatinine group in postmenopausal women. Fasting insulin and HOMA-IR were higher in the upper normal creatinine group than in the low creatinine group. The frequency of alcohol consumption and the proportion of current smokers were both higher in the low creatinine group in both sexes.
Table 1. Clinical characteristics of the subjects.
| Variable (unit) | Men | Women | ||||
|---|---|---|---|---|---|---|
| Low creatinine (n = 1,189) | Upper normal creatinine (n = 3,384) | P- value | Low creatinine (n = 2,845) | Upper normal creatinine (n = 1,230) | P- value | |
| Serum creatinine (range, mg/dL) | 0.78±0.06 (0.5–0.8) | 1.01±0.09 (0.9–1.3) | <0.001 | 0.65±0.06(0.4–0.7) | 0.83±0.05(0.8–1.0) | <0.001 |
| Age (yr) | 61.1 ± 9.71 | 59.83 ± 10.17 | <0.001 | 62.39 ± 8.87 | 64.27 ± 9.41 | <0.001 |
| Anthropometry | ||||||
| Height (cm) | 166.16 ± 6.31 | 167.3 ± 5.99 | <0.001 | 153.16 ± 5.81 | 153.54 ± 5.92 | 0.054 |
| Weight (kg) | 63.71 ± 9.97 | 67.48 ± 9.75 | <0.001 | 56.54 ± 8.57 | 57.65 ± 8.68 | <0.001 |
| BMI (kg/m2) | 23.02± 2.47 | 24.57 ± 2.42 | <0.001 | 20.51 ± 2.54 | 24.25 ± 3.22 | <0.001 |
| Waist circumference (cm) | 83.64 ± 8.85 | 85.71 ± 8.46 | <0.001 | 82.07 ± 9.27 | 82.92 ± 9.25 | <0.001 |
| Appendicular skeletal muscle (kg) | 20.2 ± 3 | 21.33 ± 3.06 | <0.001 | 13.94 ± 1.94 | 14.28 ± 2.08 | <0.001 |
| RASM a (kg/m2) | 7.29 ± 085 | 7.58 ± 0.87 | <0.001 | 5.93 ± 0.68 | 6.03 ± 0.71 | <0.001 |
| Body fat (%) | 21.12 ± 5.34 | 22.57 ± 4.95 | 0.308 | 33.98 ± 5.51 | 34.41 ± 5.24 | 0.023 |
| Bone mineral density (g/cm2) | ||||||
| Total hip | 0.91 ± 0.13 | 0.944 ± 0.13 | <0.001 | 0.78 ± 0.12 | 0.77 ± 0.13 | 0.005 |
| Femoral neck | 0.74 ± 0.12 | 0.76 ± 0.12 | <0.001 | 0.63 ± 0.11 | 0.61 ± 0.11 | <0.001 |
| Lumbar spine | 0.91 ± 0.15 | 0.96 ± 0.15 | <0.001 | 0.8 ± 0.14 | 0.81 ± 0.14 | 0.013 |
| Hormones and biochemistry | ||||||
| 25(OH)D (ng/mL) | 22.27 ± 7.78 | 21.12 ± 7.2 | <0.001 | 19.1 ± 7.19 | 19.36 ± 7.36 | 0.299 |
| PTH (pg/mL) | 62.99 ± 22.3 | 65.88 ± 25.76 | 0.002 | 67.4 ± 32.07 | 67.93 ± 27.93 | 0.627 |
| Fasting glucose (mg/dL) | 106.22 ± 30.43 | 103.9 ± 27.04 | 0.02 | 100.32 ± 22.86 | 101.95 ± 24.89 | 0.05 |
| Fasting insulin (mIU/mL) | 8.82 ± 4.44 | 9.78 ± 5.69 | <0.001 | 10.02 ± 6.78 | 10.86 ± 5.87 | <0.001 |
| HOMA-IR | 2.37 ± 1.65 | 2.57 ± 2.24 | 0.004 | 2.58 ± 3.27 | 2.82 ± 1.98 | 0.02 |
| eGFR (mL/min/1.73 m2) | 109.33 ± 11.21 | 81.64 ± 9.28 | <0.001 | 99.64 ± 12.9 | 74.4 ± 5.67 | <0.001 |
| eGFR | <0.001 | <0.001 | ||||
| 60–70 mL/min/1.73 m2 (%) | 0 (0%) | 550 (16.3%) | 0 (0%) | 304 (24.8%) | ||
| 71–80 mL/min/1.73 m2 (%) | 0 (0%) | 998 (29.5%) | 181 (6.3%) | 725 (58.9%) | ||
| 81–90 mL/min/1.73 m2 (%) | 4 (0.3%) | 1147 (33.9%) | 707 (24.9%) | 182 (14.8%) | ||
| ≥ 91 mL/min/1.73 m2 (%) | 1185 (99.7%) | 689 (20.3%) | 1957 (68.8%) | 19 (1.5%) | ||
| Daily calcium intake (mg/d) | 536.36±343.52 | 542.24±358.89 | 0.641 | 424.75±306.59 | 395.56±457.68 | 0.017 |
| Alcohol intake ≥ one time/week (%) | 855 (72.6) | 2405 (67.3) | 0.001 | 752 (26.6) | 302 (21.3) | <0.001 |
| Sarcopenia (%) | 816 (75.9) | 1792 (64.5) | <0.001 | 1046 (38.2) | 469 (34.3) | 0.016 |
| Regular exercise b (%) | 216 (18.3) | 655 (18.3) | 0.994 | 354 (12.5) | 188 (13.2) | 0.511 |
| Current smoker (%) | 495 (42) | 186 (33.0) | <0.001 | 495 (42) | 1186 (33.1) | <0.001 |
| Estrogen replacement therapy (%) | - | - | - | 445 (16.5) | 215 (15.8) | 0.56 |
Data presented as n (%) or mean ± standard deviation. BMI, body mass index; RASM, relative appendicular skeletal muscle mass; PTH, parathyroid hormone; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration rate
aAppendicular skeletal muscle mass divided by height squared
bRegular exercise indicated that the subject performed vigorous exercise for more than 20 min at a time more than three times per week
Relationships between serum creatinine, muscle mass and bone density
As shown in S1 Table, we found a positive correlation between serum creatinine and BMD at TH, FN, and LS after adjusting for age (R = 0.162, P < 0.001 at TH; R = 0.221, P < 0.001 at FN; R = 0.274, P < 0.001 at LS). We also found a positive correlation between serum creatinine and total skeletal muscle mass (R = 0.424, P < 0.001), ASM (R = 0.43, P < 0.001) and relative ASM (R = 0.362, P < 0.001). Serum creatinine was also negatively associated with body fat (%) (R = -0.309, P < 0.001). Table 2 presents the independent contribution of serum creatinine to BMD at each site using multiple linear regression analysis. Even after adjustment for all potential confounders, an increase in serum creatinine significantly contributed to an increase of BMD at TH, FN, and LS in both sexes. The association between serum creatinine and BMD was more prominent in men than in women.
Table 2. Multivariate linear regression analysis for bone mineral density in men and women.
| Variables | Total hip | Femoral neck | Lumbar spine | ||||||
| β a | SE | P-value | β a | SE | P-value | β a | SE | P-value | |
| Men | |||||||||
| Serum creatinine (mg/dL) | 0.752 | 0.115 | <0.001 | 0.503 | 0.12 | <0.001 | 1.135 | 0.169 | <0.001 |
| Variables | Total hip | Femoral neck | Lumbar spine | ||||||
| β b | SE | P-value | β b | SE | P-value | β b | SE | P-value | |
| Women | |||||||||
| Serum creatinine (mg/dL) | 0.494 | 0.141 | <0.001 | 0.323 | 0.136 | 0.018 | 1.102 | 0.181 | <0.001 |
aResults expressed as β coefficients. Data adjusted for age, body fat (%), HOMA-IR, current smoking status, alcohol intake, regular exercise, vitamin D and daily calcium intake (mg/day)
bResults expressed as β coefficients. Data adjusted for age, body fat (%), HOMA-IR, current smoking status, alcohol intake, regular exercise, vitamin D and daily calcium intake (mg/day) and estrogen replacement therapy.
Sex-specific prevalence of low BMD according to serum creatinine levels
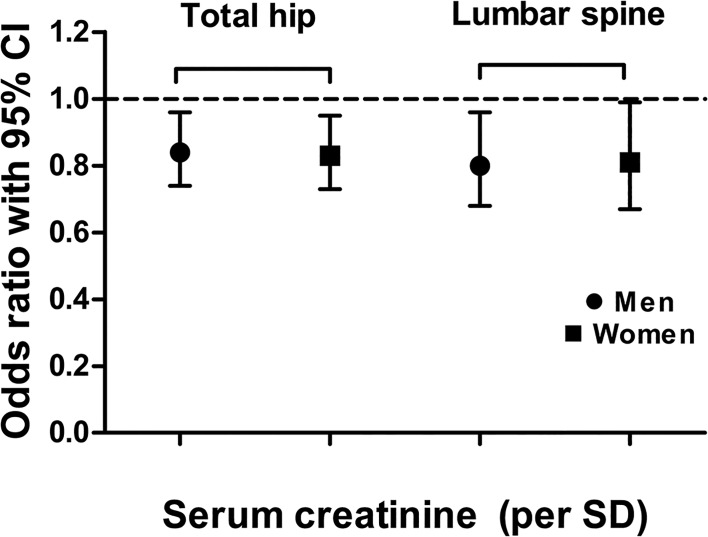
After adjusting for age, the low creatinine group had a significantly elevated OR for low BMD at all sites in both sexes (Table 3). After further adjusting for other conventional confounding covariates (Models 2 and 3), the low creatinine group of men still had a significantly elevated OR for low BMD at all sites, but the low creatinine group of postmenopausal women had an elevated OR for low BMD at only TH and LS. Each standard deviation increase in serum creatinine was significantly associated with a 16% reduction in the likelihood of low BMD at TH and a 20% at LS in men (OR = 0.84, 95% CI = 0.74−0.96 at TH; OR = 0.8, 95% CI = 0.68−0.96 at LS) (Fig 1). In women, each standard deviation increase in serum creatinine was significantly associated with a 17% reduction in the likelihood of low BMD at TH and a 19% reduction at LS (OR = 0.83, 95% CI = 0.73−0.95 at TH, OR = 0.81, 95% CI = 0.67−0.99 at LS). However, each standard deviation increase in serum creatinine was not significantly associated with a low BMD at FN in both sexes (OR = 0.83, 95% CI = 0.69–1 in men and OR = 0.85, 95% CI = 0.68–1 in women).
Table 3. Multivariate odds ratio and 95% confidence interval for low bone mineral density a according to serum creatinine.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Upper normal creatinine | Low creatinine | P-value | Upper normal creatinine | Low creatinine | P-value | |
| Total hip | ||||||
| Incidence b (%) | 392 (13.7%) | 194 (17.6%) | 0.002 | 566 (39.8%) | 995 (35.2%) | 0.004 |
| Model 1 | reference | 1.32 (1.08,1.61) | 0.008 | reference | 1.20(1.02,1.41) | 0.028 |
| Model 2 | reference | 1.32 (1.08,1.62) | 0.005 | reference | 1.23(1.04,1.46) | 0.014 |
| Model 3 | reference | 1.26 (1.01,1.57) | 0.043 | reference | 1.20 (1.01,1.43) | 0.038 |
| Femur neck | ||||||
| Incidence b (%) | 1081 (37.7%) | 501 (45.5%) | <0.001 | 1113 (78.3%) | 2115(74.9%) | 0.016 |
| Model 1 | reference | 1.35 (1.16,1.56) | <0.001 | reference | 1.20 (1.01,1.43) | 0.039 |
| Model 2 | reference | 1.33 (1.14,1.55) | <0.001 | reference | 1.17 (0.98,1.41) | 0.089 |
| Model 3 | reference | 1.28 (1.08,1.52) | 0.004 | reference | 1.11 (0.92,1.34) | 0.27 |
| Lumbar spine | ||||||
| Incidence b (%) | 1050 (37.3%) | 516 (48.1%) | <0.001 | 1011(73%) | 2066 (74.9%) | 0.19 |
| Model 1 | reference | 1.48 (1.28,1.71) | <0.001 | reference | 1.44 (1.22,1.69) | <0.001 |
| Model 2 | reference | 1.48 (1.27,1.71) | <0.001 | reference | 1.39 (1.17,1.65) | <0.001 |
| Model 3 | reference | 1.38 (1.16,1.65) | <0.001 | reference | 1.36 (1.14,1.62) | 0.001 |
aLow bone mineral density: T-score ≤ –1.0
bData are presented using a Chi-square test
Model 1: adjusted for age; Model 2: Model 1+ further adjusted for regular exercise, alcohol intake, current smoking status, 25(OH)D and estrogen replacement therapy (women); Model 3: Model 2+ further adjusted for HOMA-IR, daily calcium intake and body fat (%).
Fig 1. Adjusted odds ratios with 95% confidence interval for the presence of low bone mineral density for each standard deviation (SD) increase in serum creatinine.
*Data were adjusted for age, current smoking status, regular exercise, daily calcium intake (mg/d), HOMA-IR, vitamin D, body fat (%) and estrogen replacement therapy (in women).
Discussion
In this general population-based study of subjects with normal renal function, we found that serum creatinine was closely associated with ASM. Additionally, we demonstrated that significantly decreased BMD was observed in subjects with low serum creatinine (≤ 0.8 mg/dL in men and ≤ 0.7 mg/dL in postmenopausal women). The association between serum creatinine and BMD was more prominent in men than in women. These discoveries provide the first clinical evidence for the notion that subjects with extremely low serum creatinine are at high risk for reduced BMD.
Reduced muscle mass, or sarcopenia, is a well-known risk factor for osteoporosis. Reduced muscle mass affects balance and thereby increases the risk of falls and subsequent fractures [16]. In this way, gradual age-related decline in bone and muscle (i.e., osteoporosis and sarcopenia) can result in increased morbidity and mortality [17]. Considering the close relationship between sarcopenia and osteoporosis and the effects of muscle mass on fracture risk, identification and treatment of those conditions is important in older populations. Therefore, recent studies have suggested a more inclusive name be given to the combination of sarcopenia and osteoporosis, such as ‘dysmobility syndrome,’ which integrates their pathogenesis and unites them as a single therapeutic target [18]. However, although osteoporosis has been clearly defined, the definition of sarcopenia remains unclear [19]. In addition, although DXA is the currently accepted gold standard test for evaluating body composition, including both bone density and muscle mass [20], it is not easily accessible or commonly available to general populations because of time and cost. From this background, we hypothesized that serum creatinine, known to be a stable marker of skeletal muscle mass [21], could be related to bone health status.
Several studies have reported correlations between serum creatinine and lean body mass, estimated anthropometrically using bioimpedence analysis or DXA [22, 23]. Consistent with these findings, we observed that serum creatinine was significantly associated with total and appendicular skeletal muscle mass. Therefore, we further calculated the cut point of serum creatinine for the presence of sarcopenia and it was ≤ 0.8 mg/dL in men and ≤ 0.7 mg/dL in postmenopausal women. Similarly, Harita et al. demonstrated that those who had serum creatinine levels ≤0.6 mg/dl was associated with increased the risk of type 2 diabetes [11]. However, they did not assess the risk of lower creatinine for diabetes considering gender differences although skeletal muscle mass might differ between women and men. Taken together, we can speculate that serum creatinine, a cheap and simple method, can be used as a marker to assess individual’s skeletal health status and it can be also alternative for body composition analysis in subjects with normal renal function.
Chronic kidney disease, especially stage 3 or over, is a well-known risk factor for low BMD [24] and sarcopenia [25]. Our study, which excluded subjects with definite chronic kidney disease, demonstrated that the risk for low BMD remained significantly higher in subjects with low serum creatinine. Furthermore, one SD increase in serum creatinine was associated with a significant reduction in the occurrence of low BMD at TH and LS in both men and postmenopausal women. Those results were valid even after adjusting for confounding factors. Thus, serum creatinine could have a beneficial effect on BMD in subjects with normal renal function. The main pathogenesis for the deleterious effects of low serum creatinine on BMD could be that serum creatinine reflects one’s physical activity status as well as skeletal muscle mass, and both are important for maintaining bone health [26]. In addition, creatinine degradation is stimulated by reactive oxygen species and in particular by the hydroxyl radical. Fernández-Real et al. reported that telomere length of subcutaneous adipose tissue cells was positively associated with serum creatinine but not with GFR. In other words, decreased serum creatinine is associated with a marker of cellular senescence and oxidative stress and consequently decreased serum creatinine may result in deterioration of BMD via oxidative stress [27]. Finally, recent evidences suggest that insulin could stimulate osteoblast differentiation and have some anabolic properties for bone [28]. In our study, a higher serum insulin levels were detected in the upper normal creatinine group than in the low creatinine group. Therefore, lower serum creatinine group may have low potency of anabolic properties for bone due to increased clearance of insulin.
An interesting point of our study is that the relationship between serum creatinine and BMD was more prominent in men aged 45 years or older than in postmenopausal women. Although we could not elucidate why this association was more prominent in men than in women, it might be explained by the fact that females tend to have lower skeletal muscle mass than males. Additionally, increased body fat in postmenopausal women might alter the bone-muscle (creatinine) relationship. Conversion of androgens to estrogens in adipose tissue could have a modest effect on bone, especially in postmenopausal women [29]. However, in men, adipose tissue is not an important sex hormone source and as they have relatively small fat mass, it may not considerably influence on the bone-muscle (creatinine) relationship [30]. Those findings suggest that creatinine can differ by sex, and sex differences should be considered and weighed practically.
The major strength of this study is that the data were collected from a large nationwide survey that included 8,648 participants ages 45 to 95 years throughout Korea. This is the first observational study that extensively investigated the sex-specific association of low serum creatinine with BMD focusing on Korea’s general population. However, this study also has some limitations. First, given that serum creatinine is closely related to skeletal muscle mass, it could be influenced by other factors, such as drugs and other dietary variation. However, we could not collect all that information. Second, to define sarcopenia, we did not measure muscle strength or walk speed, as described by the European Working Group on Sarcopenia in Older People and the European Society for Clinical Nutrition and Metabolism special interest group [31]. Third, because this study used a cross-sectional design and not a longitudinal design, a causal relationship could not be definitively established. Fourth, we could not demonstrate whether this association between serum creatinine and BMD is independent of estimated glomerular filtration rate as there was collinearity in serum creatnine and estimated glomerular filtration rate. Finally, because all participants were single ethnic group, our results may not be representative of the other ethnic population.
In conclusion, our study is the largest population-based study to examine the sex-specific association between serum creatinine and BMD. An excessive decrease of serum creatinine was associated with greater deterioration of BMD in subjects with normal renal function, especially, in men. Our findings suggest that serum creatinine reflects an individual’s skeletal health status as well as muscle mass and that we can indirectly assess both bone and muscle health status from serum creatinine level, which can be easily measured. Monitoring serum creatinine could be helpful to the development of sex-specific strategies for the treatment and prevention of sarcopenia and low BMD.
Supporting Information
(ZIP)
(DOCX)
Acknowledgments
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (MEST) (No. 20110001024).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (MEST) (No. 20110001024).
References
- 1. Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin e2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frost HM. Bone's mechanostat: a 2003 update. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;275(2):1081–101. 10.1002/ar.a.10119 . [DOI] [PubMed] [Google Scholar]
- 3. Schoenau E, Frost HM. The "muscle-bone unit" in children and adolescents. Calcified tissue international. 2002;70(5):405–7. 10.1007/s00223-001-0048-8 . [DOI] [PubMed] [Google Scholar]
- 4. Huh JH, Song MK, Park KH, Kim KJ, Kim JE, Rhee YM, et al. Gender-specific pleiotropic bone-muscle relationship in the elderly from a nationwide survey (KNHANES IV). Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(3):1053–61. 10.1007/s00198-013-2531-2 . [DOI] [PubMed] [Google Scholar]
- 5. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. Journal of the American Medical Directors Association. 2014;15(8):551–8. 10.1016/j.jamda.2014.02.005 . [DOI] [PubMed] [Google Scholar]
- 6. Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Archives of gerontology and geriatrics. 2012;55(2):e48–52. 10.1016/j.archger.2012.05.002 . [DOI] [PubMed] [Google Scholar]
- 7. Kim KM, Lim JS, Kim KJ, Choi HS, Rhee Y, Oh HJ, et al. Dissimilarity of femur aging in men and women from a Nationwide Survey in Korea (KNHANES IV). J Bone Miner Metab. 2013;31(2):144–52. 10.1007/s00774-012-0386-9 . [DOI] [PubMed] [Google Scholar]
- 8. Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. The American journal of physiology. 1999;277(3 Pt 1):E489–95. . [DOI] [PubMed] [Google Scholar]
- 9. Yonemura K, Takahira R, Yonekawa O, Wada N, Hishida A. The diagnostic value of serum concentrations of 2-(alpha-mannopyranosyl)-L-tryptophan for normal renal function. Kidney international. 2004;65(4):1395–9. 10.1111/j.1523-1755.2004.00521.x . [DOI] [PubMed] [Google Scholar]
- 10. Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43(7):821–35. 10.1007/s001250051457 . [DOI] [PubMed] [Google Scholar]
- 11. Harita N, Hayashi T, Sato KK, Nakamura Y, Yoneda T, Endo G, et al. Lower serum creatinine is a new risk factor of type 2 diabetes: the Kansai healthcare study. Diabetes care. 2009;32(3):424–6. 10.2337/dc08-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hjelmesaeth J, Roislien J, Nordstrand N, Hofso D, Hager H, Hartmann A. Low serum creatinine is associated with type 2 diabetes in morbidly obese women and men: a cross-sectional study. BMC endocrine disorders. 2010;10:6 10.1186/1472-6823-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. . [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. . [DOI] [PubMed] [Google Scholar]
- 16. Liu-Ambrose T, Eng JJ, Khan KM, Carter ND, McKay HA. Older women with osteoporosis have increased postural sway and weaker quadriceps strength than counterparts with normal bone mass: overlooked determinants of fracture risk? The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(9):M862–6. . [DOI] [PubMed] [Google Scholar]
- 17. Bonewald LF, Kiel DP, Clemens TL, Esser K, Orwoll ES, O'Keefe RJ, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(9):1857–65. 10.1002/jbmr.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binkley N, Krueger D, Buehring B. What's in a name revisited: should osteoporosis and sarcopenia be considered components of "dysmobility syndrome?". Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(12):2955–9. 10.1007/s00198-013-2427-1 . [DOI] [PubMed] [Google Scholar]
- 19. Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(7):1839–48. 10.1007/s00198-012-1913-1 . [DOI] [PubMed] [Google Scholar]
- 20. Haderslev KV, Haderslev PH, Staun M. Accuracy of body composition measurements by dual energy x-ray absorptiometry in underweight patients with chronic intestinal disease and in lean subjects. Dynamic medicine: DM. 2005;4(1):1 10.1186/1476-5918-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. Journal of cachexia, sarcopenia and muscle. 2013;4(1):19–29. 10.1007/s13539-012-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;51(3):762–6. . [DOI] [PubMed] [Google Scholar]
- 23. Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, et al. Lean body mass estimation by creatinine kinetics. Journal of the American Society of Nephrology: JASN. 1994;4(7):1475–85. . [DOI] [PubMed] [Google Scholar]
- 24. Lee YH, Kim JE, Roh YH, Choi HR, Rhee Y, Kang DR, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008–2011. The Journal of clinical endocrinology and metabolism. 2014;99(10):3879–88. 10.1210/jc.2013-3764 . [DOI] [PubMed] [Google Scholar]
- 25. Kim JE, Lee YH, Huh JH, Kang DR, Rhee Y, Lim SK. Early-stage chronic kidney disease, insulin resistance, and osteoporosis as risk factors of sarcopenia in aged population: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008–2009. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(9):2189–98. 10.1007/s00198-014-2745-y . [DOI] [PubMed] [Google Scholar]
- 26. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clinical journal of the American Society of Nephrology: CJASN. 2008;3(2):348–54. 10.2215/CJN.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews. 2000;80(3):1107–213. . [DOI] [PubMed] [Google Scholar]
- 28. Klein GL. Insulin and bone: Recent developments. World journal of diabetes. 2014;5(1):14–6. 10.4239/wjd.v5.i1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17(9):1563–9. 10.1359/jbmr.2002.17.9.1563 . [DOI] [PubMed] [Google Scholar]
- 30. Mudali S, Dobs AS. Effects of testosterone on body composition of the aging male. Mech Ageing Dev. 2004;125(4):297–304. 10.1016/j.mad.2004.01.004 . [DOI] [PubMed] [Google Scholar]
- 31. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39(4):412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.