Abstract
Background
This study describes the effect of TG4010 vaccine on Health related Quality of Life (HRQOL) in patients with stage IIIb and IV non–small-cell lung cancer (NSCLC).
Methods
148 patients with advanced NSCLC expressing MUC1 were randomly assigned to receive TG4010 plus chemotherapy or chemotherapy alone. HRQOL was assessed with the Functional Assessment of Cancer Therapy-Lung (FACT-L) at baseline and every 6 weeks until disease progression. Time until definitive deterioration (TUDD) of the four well-being dimensions of the FACT-L physical (PWB), functional (FWB), emotional (EWB) and social well-being (SWB) and the Lung Cancer Subscale (LCS) domains were analyzed for a 5-point minimal clinically important difference.
Results
No difference of TUDD of HRQOL has been found between treatment arms. No prognostic factors have been found to have a significant impact on the TUDD of PWB, SWB and LCS domains. The gender, the performance status and the smoking habits seemed to be associated with a shorter TUDD of EWB domain. The smokers and the former smokers seemed to present a shorter TUDD of FWB domain.
Conclusion
This study suggests that adding therapeutic vaccination with TG4010 to standard chemotherapy in patients with advanced NSCLC is associated with a similar evolution in HRQOL compared to chemotherapy alone.
Introduction
Lung cancer is the most common cancer worldwide and the leading cause of cancer death [1].
About 85% to 90% of lung cancers are non-small cell lung cancer (NSCLC) and of these about 75% have locally advanced or disseminated disease at the time of diagnosis. The standard treatment for advanced NSCLC is chemotherapy [2,3]. Traditional chemotherapy regimens have shown real but limited activity in this setting, therefore new strategies are being explored for lung cancer treatment, including targeted active immunotherapies [4,5]. Recent research suggests that the use of therapeutic cancer vaccines may improve overall survival (OS), with reduced toxicity compared with conventional chemotherapy [6]. It is unanimously accepted that the goal of therapy for advanced NSCLC patients is prolongation of OS without negative impact on Health related Quality of Life (HRQOL) and ideally with an improvement of it [7]. Moreover, even when there is no apparent advantage in OS for a new treatment, a positive effect on HRQOL can be seen as a real improvement [8]. The literature also shows the importance of HRQOL as an important prognostic factor of OS in various cancer sites and particularly in lung cancer [9–11]. Therefore, clinical trials including OS in the endpoints are now incorporating symptom scores and HRQOL outcomes in their designs. The potential benefits of palliative chemotherapy on HRQOL have been investigated and demonstrated for several agents in lung cancer trials [12].
A phase IIb multicentric controlled trial was developed to assess TG4010 –an active targeted immunotherapy based on a viral MVA vector which codes for MUC1 tumor-associated antigen and interleukine 2 –in combination with first-line chemotherapy in patients with advanced NSCLC. The primary objective of the study was to show that the addition of TG4010 to chemotherapy improved the progression-free survival (PFS) at 6 months. Both OS and HRQOL were assessed as secondary objectives. The study achieved its primary endpoint on the whole study population and in an exploratory analysis put in evidence a significant benefit on several parameters including OS in a large subgroup of 101 patients defined by pre-treatment normal levels of CD16+CD56+CD69+, a phenotype of activated Natural Killer (aNK) cells also called TrPAL (Triple Positive Activated Lymphocytes) [13]. We report here the results of the HRQOL analyses associated to this clinical trial. To our knowledge this is the first time that the impact of immunotherapy on HRQOL has been studied in the context of a combination with standard chemotherapy for NSCLC patients. The main objective was to describe prospectively HRQOL using the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire by treatment arm. Time until definitive deterioration (TUDD) of the FACT-L domains was defined as a modality of longitudinal HRQOL analysis [14].
Materials and Methods
Study population and treatments
The assessment of HRQOL was a secondary endpoint of a multicentric open label randomized phase IIb trial performed to test the hypothesis that the addition of TG4010 to first line chemotherapy would improve the outcome in patients with advanced NSCLC (NCT00415818). The main eligibility patients’ criteria were patients aged 18 years or older with a confirmed stage IIIb or IV NSCLC, chemotherapy naive for this stage of the disease, expressing MUC1, with at least one lesion measurable by CT-scan according to World Health Organization, a performance status (PS) of 0 or 1 and a life expectancy of at least 4 months. Patients were excluded if they had received prior systemic therapy for advanced stage NSCLC, or if they had concomitant brain metastases (unless successfully treated) or history of another malignancy within the past 5 years, apart from basal-cell carcinoma of the skin or intraepithelial carcinoma of the cervix. The study obtained approval from national health agencies and ethics committees in relevant countries and was done in accordance with the Helsinki Declaration, the International Conference of Harmonization Good Clinical Practice guidelines, and local regulatory requirements. Written informed consent was obtained from each patient. This study started almost 10 years ago and at this time it was not yet so common to register our studies, at least from the beginning. The authors confirm that all ongoing and related trials for this drug/intervention are registered.
The protocol for this trial and supporting CONSORT checklist are available as supporting information (see S1 Checklist and S1 Protocol). List of Ethics Committees is also available in supporting information (see S1 Authorization).
Patients were allocated by a 1:1 randomization (minimization procedure stratified by centre (27 centres of France, Poland, Hungary and Germany), PS (0 vs. 1) and disease stage (IIIb vs. IV)) in one of the two following treatment regimens: in arm 1, patients received chemotherapy (gemcitabine + cisplatin up to 6 cycles) combined with TG4010 subcutaneously at the dose of 108 pfu once per week for 6 weeks then once every 3 weeks up to progression (combination therapy arm), in arm 2, patients received the same chemotherapy alone (control arm).
Sample size calculation
The primary endpoint of this study was 6-month PFS in combination therapy arm. The study was designed as a one-stage, phase IIB trial according to Fleming [15] with an inactivity cut-off chosen as 30% and activity cut-off as 50%. Therefore, the hypotheses of interest were 30% or less patients who were progression free at 6 months (null hypothesis) versus 50% or more patients who were progression free at 6 months. The type I error rate and the type II error rate were both set to 5%, leading to a total sample size of 140 patients. To reject the null hypothesis (6-month PFS ≤30% with combination therapy), at least 40% of patients in the TG4010 group had to be progression free at 6 months.
Health related Quality of life assessment
HRQOL was evaluated using the FACT-L questionnaire at inclusion, every 6 weeks until the end of study (disease progression) and at the end of study visit. The questionnaire had to be fulfilled by the patient at the hospital (during the treatment or the end of treatment visit). The FACT-L is composed by the FACT-General with the specific module of the lung cancer. It contains 36 items in order to measure 4 well-being dimensions: physical well-being (PWB), social well-being (SWB), emotional well-being (EWB) and functional well-being (FWB) and the Lung Cancer Subscale (LCS) [16]. Three global scores are calculated: the FACT-G global score, the FACT-L global score and the Trial Outcome Index (TOI) which is the sum of PWB, FWB and LCS scores. Scores were linearly adjusted to range scores from 0 to 100. A high score corresponded to a high HRQOL level. The HRQOL targeted dimensions were the four well-being domains (PWB, SWB, EWB and FWB) and the LCS.
Statistical analysis
Patient’s socio-demographic and clinical characteristics at baseline were described. The percentage of available questionnaires was reported at each assessment. Patients with HRQOL questionnaire fully completed at baseline and those with at least one missing item at baseline were compared according to baseline characteristics and treatment arm in order to detect non-random missing data profiles. Scores were calculated using the simple imputation method by the mean following the FACT-L scoring guidelines [16]. Scores were described by treatment arm at each measurement time using the mean and standard deviation (SD). Mean difference and 99% confidence interval (99%CI) were calculated at baseline. If the outcome data was normally distributed, Student t test was employed. However, if the outcome data was not normally distributed, Mann Whitney test was used instead.
The TUDD in a HRQOL score is defined as the interval between randomization and the date of the event, i.e. the date where the first significant deterioration in the HRQOL level of the patient was observed as compared to baseline HRQOL score, without any further significant improvement as compared to baseline HRQOL score [14]. Alive patients were censored at the time of the last follow-up if no definitive deterioration was observed before. Only patients with the baseline HRQOL score and at least one follow-up score or death were retained (modified intent to treat analysis). Each dimension was analysed. TUDD curves were calculated using the Kaplan-Meier estimation and were described using medians and 99%CI. As exploratory purpose only, TUDD curves were compared using the log-rank tests. The univariate Cox models were used to calculate the hazard ratio (HR) with a 99%CI. A subgroup analysis was also performed according to the level of aNK cells based on previous observed effect of aNK cells on OS. Variables investigated were gender (women vs. men), age (continuous variable), smoking habits (ceased vs. no vs. yes), PS (1 or more vs. 0), center (dichotomized according to the median of the distribution of patients included by center: ≤3 vs. >3) and the time to toxicity of grade 3–4 (time dependent variable). Variables with a univariate P-value ≤ 0.20 were eligible for the multivariate Cox analyses. Treatment arm and level of aNK cells subgroup (high vs. normal) were forced in multivariate Cox analyses. Their interaction was also introduced and exploratory subgroup analyses were performed according to pre-treatment level of aNK cells based on previous observed effect of aNK cells on OS [13]. Finally, the same variables were introduced in each multivariate model for all HRQoL dimensions.
Data were analysed using software SAS (Version 9.2, SAS Institute Inc, Cary, NC) and R [17]. All tests were two-sided and the type I error was set to 0.01 due to multiple comparisons (Bonferroni adjustment, 5 targeted dimensions of HRQOL).
Results
Study population
One hundred and forty eight patients with advanced stages of NSCLC were randomized: 74 in arm 1 and 74 arm 2. Details of patient’s selection criteria and results of the primary endpoint (i.e. “Progression Free Survival” at 6 months) have been reported elsewhere [13]. The mean age was 60.5 years (SD = 8.1). Fifty-four patients (73.0%) were male, 53 patients (76.8%) had normal level of aNK cells, 68 patients (91.9%) had a stage III cancer, 27 patients (36.5%) were smokers and 35 patients (47.3%) were former smokers (Table 1). Individual-level data are available as supporting information (see S1 Data).
Table 1. Baseline Characteristics of patients.
| All patients (n = 148) | Combination therapy group (n = 74) | Control group (n = 74) | P * | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 59.41 (8.8) | 58.36 (9.4) | 60.48 (8.1) | 0.145 |
| Sex | 0.854 | |||
| Male | 107 (72.3) | 53 (71.6) | 54 (73.0) | |
| Female | 41 (27.7) | 21 (28.4) | 20 (27.0) | |
| ECOG performance status | 0.604 | |||
| 0 | 40 (27.0) | 20 (27.0) | 20 (27.0) | |
| 1 | 107 (72.3) | 53 (71.6) | 54 (73.0) | |
| 2 | 1 (0.7) | 1 (1.4) | 0 (0.0) | |
| Stage at baseline | 0.999 | |||
| IIIB | 12 (8.1) | 6 (8.1) | 6 (8.1) | |
| IV | 136 (91.9) | 68 (91.9) | 68 (91.9) | |
| Level of natural killer cells | 0.337 | |||
| Normal | 101 (73.2) | 21 (30.4) | 16 (23.2) | |
| High | 37 (26.8) | 48 (69.6) | 53 (76.8) | |
| Smoking habits | 0.772 | |||
| No | 22 (14.9) | 10 (13.5) | 12 (8.1) | |
| ceased | 68 (46.0) | 33 (44.6) | 35 (47.3) | |
| Yes | 58 (39.1) | 31 (41.9) | 27 (36.5) |
Data are n (%) unless otherwise specified
* T test for continuous variable and chi-square test or Fisher’s Exact Test for qualitative variables
Significance level set to 0.01 according to Bonferroni adjustment
Missing data
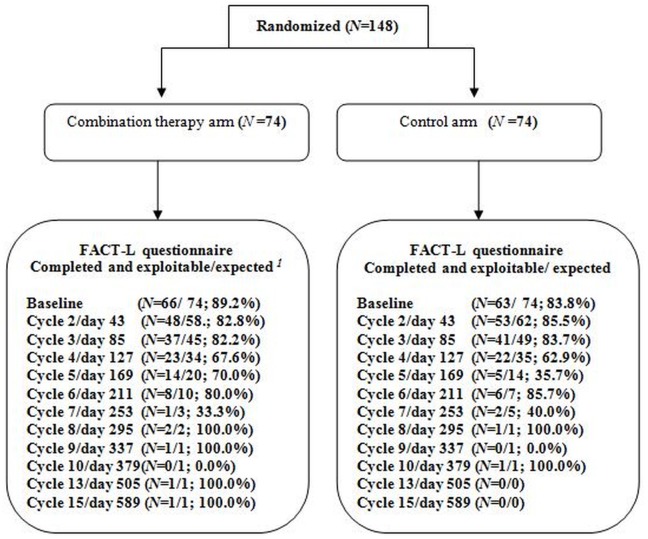
At baseline, 129 patients (87.2%) completed the FACT-L questionnaire: 66 (89.2%) in arm 1 and 63 (83.8%) in arm 2. Fig 1 described the number and percentage of questionnaires completed at each measurement time according to expected completion (patients still in the study at the time of the questionnaire completion).
Fig 1. CONSORT Flow chart showing patients randomised in each group and the number of patients answering FACT-L questionnaire at baseline and follow-up.
Patients still in the study time of the questionnaire completion.
Among the completed and exploitable questionnaires (i.e. questionnaires with less than half of the items missing), less than 2% of the items were missing in all dimensions, at each measurement time, except for the SWB score for which around 10% of the items were missing. Nevertheless, the pattern of missing data was found to be random. Patients with at least one missing item at baseline and those with the entire questionnaire fulfilled were similar for all baseline socio-demographic characteristics.
Description of FACT-L scores
Patients receiving standard treatment presented a better HRQOL at baseline than patients receiving the vaccine (Table 2). These differences were clinically significant with a MCID ≥ 5-point [18] for PWB (-6.86 [99%CI -15.97, 2.25]), EWB (-6.03 [99%CI -15.73, 3.66]) and for TOI (-5.55 [99%CI -13.33, 2.23]), FACT-G (99%CI -5.94 [-12.78, 0.91]) and FACT-L (99%CI -5.46 [-12.02, 1.09]) global scores but not statistically significant.
Table 2. Health Related Quality of Life at baseline according to type of treatment allocated by randomization.
| combination therapy arm (N = 74) | control arm (N = 74) | combination therapy arm—control arm | P (student t test) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference [99%CI] | ||
| PWB score | 65 | 71.56 (21.79) | 62 | 78.42 (17.03) | -6.86 [-15.97, 2.25] | 0.110* |
| SWB score | 66 | 76.28 (15.57) | 62 | 80.75 (19.23) | -4.47 [-12.54, 3.60] | 0.041* |
| EWB score | 66 | 63.62 (23.19) | 63 | 69.66 (18.56) | -6.03 [-15.73, 3.66] | 0.222* |
| FWB score | 66 | 53.92 (23.78) | 62 | 60.45 (24.00) | -6.53 [-17.58, 4.52] | 0.125 |
| LCS score | 67 | 67.16 (16.40) | 63 | 71.03 (17.85) | -3.87 [-11.72, 3.99] | 0.200 |
| TOI index | 65 | 64.26 (17.55) | 61 | 69.81 (15.69) | -5.55 [-13.33, 2.23] | 0.097* |
| FACT-G global score | 65 | 66.37 (15.52) | 61 | 72.31 (13.73) | -5.94 [-12.78, 0.91] | 0.067* |
| FACT-L global score | 65 | 66.48 (14.74) | 61 | 71.95 (13.28) | -5.46 [-12.02, 1.09] | 0.068* |
CI: confidence interval
PWB: Physical Well-Being dimension; SWB: Social Well-Being; EWB: Emotional Well-Being, FWB: Functional Well-Being; LCS: Lung Cancer Subscale; TOI: Trial Outcome Index
Significance level set to 0.01 according to Bonferroni adjustment
*: Mann-Whitney test
Longitudinal analysis of health-related Quality of Life
TUDD of Physical Well-Being score with a 5-point MCID or death
116 patients are retained (58 patients per arm) with at least the baseline PWB score and one follow-up PWB score and/or who died during the study.
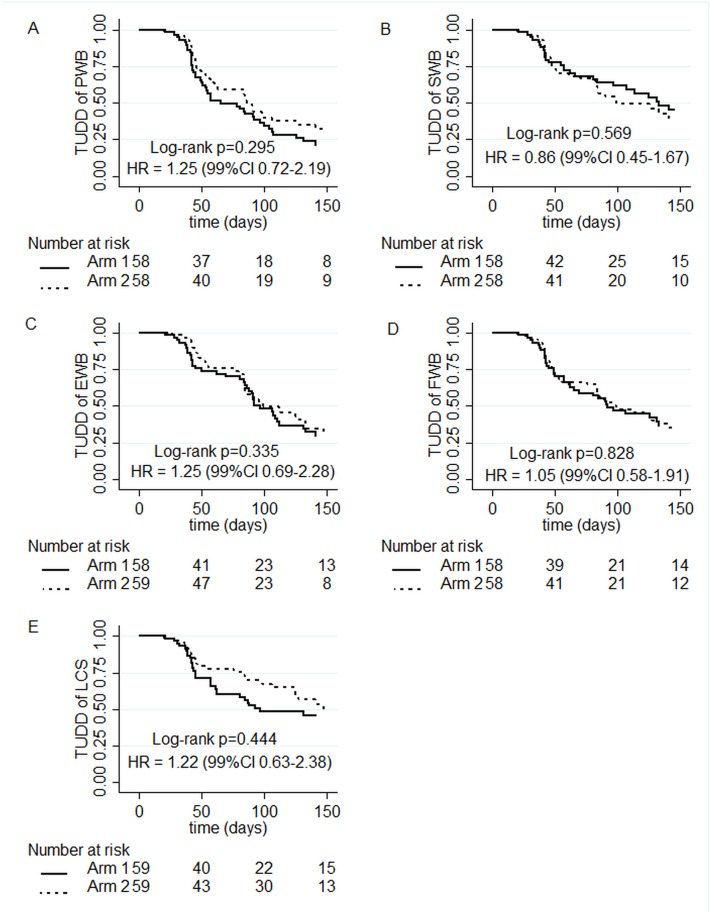
In arm 1 and arm 2 respectively, 46 and 41 patients experienced a definitive deterioration of PWB score of 5 points at least or death (Fig 2, panel A), among the 116 patients retained (58 patients per arm). The median TUDD was 65 days (99%CI 50–107) in arm 1 and 86 days (99%CI 60–201) in arm 2 (log-rank P = 0.295). The Univariate Cox HR of arm 1 vs. arm 2 was 1.25 (99%CI 0.72–2.19).
Fig 2. Time to a five-point definitive deterioration in Health related Quality of Life score or death: (A) Physical Well-Being dimension (PWB); (B) Emotional Well-Being dimension (EWB); (C) Social Well-Being dimension (SWB); (D) Functional Well-Being dimension (FWB); (E) Lung Cancer Subscale (LCS).
Arm 1: combination therapy arm; Arm 2: control arm.
TUDD of Social-Well-Being score of 5-point or death
116 patients are retained (58 patients in each arm) with at least the baseline SWB score and one follow-up SWB score and/or who died during the study.
In arm 1 and arm 2 respectively, 30 and 32 patients experienced a definitive deterioration of SWB score of 5 points at least or death (Fig 2, panel B) among the 116 patients included (58 patients per arm). The median TUDD was 133 days (99%CI 84-NA) in arm 1 and 99 days (99%CI 82-NA) in arm 2 (log-rank P = 0.569). The Univariate Cox HR was 0.86 (99%CI 0.45–1.67).
TUDD of Emotional Well-Being score with a 5-point MCID or death
117 patients are retained (58 in arm 1 and 59 in arm 2) with at least the baseline EWB score and one follow-up EWB score and/or who died during the study.
In arm 1 and arm 2 respectively, 41 and 34 patients experienced a definitive deterioration of EWB score of 5 points at least or death (Fig 2, panel C), among the 117 patients included (58 in arm 1 and 59 in arm 2). The median TUDD was 97 days (99%CI 85–169) in arm 1 and 99 days (99%CI 84-NA) in arm 2 (log-rank P = 0.335). The Univariate Cox HR was 1.25 (99%CI 0.69–2.28).
TUDD of Functional Well-Being score with a 5-point MCID or death
116 patients are retained (58 patients in each arm) with at least the baseline FWB score and one follow-up FWB score and/or who died during the study.
In arm 1 and arm 2 respectively, 38 and 37 patients experienced a definitive deterioration of FWB score of 5 points at least or death (Fig 2, panel D), among the 116 patients included (58 patients per arm). The median TUDD was 92 days (99%CI 62-NA) in arm 1 and 99 days (99%CI 76-NA) in arm 2 (log-rank P = 0.828). The Univariate Cox HR was 1.05 (99%CI 0.58–1.91).
TUDD of Lung Cancer Subscale score with a 5-point MCID or death
118 patients are retained (59 patients per arm) with at least the baseline LCS score and one follow-up LCS score and/or who died during the study.
In arm 1 and arm 2 respectively, 32 and 28 patients experienced a definitive deterioration of LCS score of 5 points at least or death (Fig 2, panel E), among the 118 patients included (59 patients per arm). The median TUDD was 97 days (99%CI 61-NA) for arm 1 and 147 days (99%CI 125-NA) for arm 2 (log-rank P = 0.444). The Univariate Cox HR was 1.22 (99%CI 0.63–2.38).
Multivariate Cox analyses
Table 3 presents univariate and multivariate Cox analyses of TUDD.
Table 3. Univariate and multivariate Cox analyses for a 5-point definitive deterioration in Health Related Quality of Life scores or death.
| Univariate Cox analysis | Multivariate Cox analysis | |||||
|---|---|---|---|---|---|---|
| n (events) | HR (99%CI) | P-value | n (events) | HR (99%CI) | P-value | |
| PWB | 116 (87) | 110 (81) | ||||
| combination therapy arm vs. control arm 1 | 1.25 (0.80–2.19) | 0.295 | 1.36 (0.67–2.76) | 0.260 | ||
| sex: women vs. men | 1.00 (0.54–1.85) | 0.999 | 0.95 (0.49–1.85) | 0.834 | ||
| age 2 | 1.00 (0.97–1.04) | 0.827 | ||||
| smoking habits | 0.450 | 0.581 | ||||
| Yes vs. No | 1.51 (0.64–3.56) | 0.219 | 1.41 (0.58–3.39) | 0.318 | ||
| Ceased vs. No | 1.34 (0.59–3.07) | 0.360 | 1.33 (0.56–3.14) | 0.399 | ||
| aNK cells: high vs. normal 1 | 1.33 (0.71–2.52) | 0.243 | 1.42 (0.56–3.61) | 0.326 | ||
| center: ≤3 vs. >3 | 1.13 (0.88–1.98) | 0.565 | ||||
| performance status: 1 or more vs. 0 | 0.79 (0.42–1.47) | 0.319 | 0.73 (0.37–1.46) | 0.241 | ||
| time to toxicity grade 3–4 | 1.16 (0.74–1.83) | 0.513 | ||||
| treatment arm * aNK cells 1 | 0.95 (0.27–3.56) | 0.912 | 0.82 (0.23–3.03) | 0.704 | ||
| SWB | 116 (62) | 110 (59) | ||||
| combination therapy arm vs. control arm 1 | 0.86 (0.45–1.67) | 0.569 | 0.79 (0.40–1.59) | 0.470 | ||
| sex: women vs. men | 0.85 (0.41–1.77) | 0.580 | 1.03 (0.48–2.22) | 0.926 | ||
| age 2 | 0.99 (0.95–1.03) | 0.379 | ||||
| smoking habits | 0.125 | 0.081 | ||||
| Yes vs. No | 2.01 (0.74–5.48) | 0.071 | 2.13 (0.76–5.98) | 0.062 | ||
| Ceased vs. No | 1.32 (0.49–3.60) | 0.470 | 1.24 (0.43–3.58) | 0.608 | ||
| aNK cells: high vs. normal 1 | 0.87 (0.40–1.85) | 0.624 | 0.90 (0.42–1.92) | 0.793 | ||
| center: ≤3 vs. >3 | 1.45 (0.61–2.29) | 0.170 | ||||
| performance status: 1 or more vs. 0 | 1.66 (0.75–3.65) | 0.098 | 1.75 (0.76–4.03) | 0.088 | ||
| time to toxicity grade 3–4 | 1.06 (0.62–1.80) | 0.848 | ||||
| treatment arm * aNK cells 1 | 1.05 (0.23–4.77) | 0.936 | 1.00 (0.42–1.92) | 0.994 | ||
| EWB | 117 (75) | 111 (70) | ||||
| combination therapy arm vs. control arm 1 | 1.25 (0.69–2.28) | 0.335 | 1.20 (056–2.59) | 0.532 | ||
| sex: women vs. men | 0.47 (0.22–0.99) | 0.009 | 0.43 (0.19–0.97) | 0.008 | ||
| age 2 | 0.98 (0.95–1.02) | 0.161 | ||||
| smoking habits | 0.013 | 0.021 | ||||
| Yes vs. No | 3.19 (1.07–9.47) | 0.006 | 2.88 (0.95–8.71) | 0.014 | ||
| Ceased vs. No | 2.93 (1.01–8.55) | 0.009 | 2.62 (0.87–7.86) | 0.024 | ||
| aNK cells: high vs. normal 1 | 1.47 (0.76–2.85) | 0.129 | 1.79 (0.62–5.15) | 0.158 | ||
| center: ≤3 vs. >3 | 1.02 (0.56–1.87) | 0.621 | ||||
| performance status: 1 or more vs. 0 | 1.96 (0.92–4.13) | 0.021 | 2.17 (0.96–4.93) | 0.010 | ||
| time to toxicity grade 3–4 | 1.05 (0.64–1.71) | 0.856 | ||||
| treatment arm * aNK cells 1 | 1.45 (0.37–5.63) | 0.478 | 1.22 (0.31–4.79) | 0.704 | ||
| FWB | 116 (63) | 110 (69) | ||||
| combination therapy arm vs. control arm 1 | 1.05 (0.58–1.91) | 0.828 | 0.81 (0.37–1.74) | 0.469 | ||
| sex: women vs. men | 0.68 (0.34–1.36) | 0.149 | 0.65 (0.31–1.37) | 0.137 | ||
| age 2 | 1.00 (0.97–1.04) | 0.804 | ||||
| smoking habits | 0.027 | 0.042 | ||||
| Yes vs. No | 2.73 (1.01–7.37) | 0.009 | 2.57 (0.93–7.09) | 0.016 | ||
| Ceased vs. No | 2.34 (0.88–6.24) | 0.025 | 2.14 (0.76–6.05) | 0.059 | ||
| aNK cells cells: high vs. normal 1 | 1.26 (0.65–2.46) | 0.368 | 0.96 (0.35–2.65) | 0.921 | ||
| center: ≤3 vs. >3 | 1.06 (0.58–1.94) | 0.795 | ||||
| performance status: 1 or more vs. 0 | 0.99 (0.51–1.93) | 0.987 | 1.01 (0.47–2.13) | 0.986 | ||
| time to toxicity grade 3–4 | 1.09 (0.66–1.78) | 0.746 | ||||
| treatment arm * aNK cells 1 | 2.27 (0.57–9.00) | 0.124 | 2.03 (0.51–8.10) | 0.189 | ||
| LCS | 118 (60) | 111 (56) | ||||
| combination therapy arm vs. control arm 1 | 1.22 (0.63–2.38) | 0.444 | 1.11 (0.46–2.66) | 0.760 | ||
| sex: women vs. men | 0.71 (0.32–1.55) | 0.258 | 0.76 (0.32–1.77) | 0.401 | ||
| age 2 | 1.01 (0.97–1.05) | 0.535 | ||||
| smoking habits | 0.815 | 0.942 | ||||
| Yes vs. No | 1.22 (0.46–3.26) | 0.601 | 1.05 (0.38–2.94) | 0.897 | ||
| Ceased vs. No | 1.04 (0.40–2.72) | 0.908 | 0.95 (0.34–2.62) | 0.897 | ||
| aNK cells: high vs. normal1 | 1.36 (0.66–2.82) | 0.279 | 1.13 (0.37–3.40) | 0.777 | ||
| center: ≤3 vs. >3 | 1.95 (0.97–3.92) | 0.014 | ||||
| performance status: 1 or more vs. 0 | 1.47 (0.65–3.29) | 0.222 | 1.34 (0.56–3.20) | 0.384 | ||
| time to toxicity grade 3–4 | 1.46 (0.83–2.55) | 0.192 | ||||
| treatment arm * aNK cells 1 | 1.50 (0.34–6.55) | 0.477 | 1.48 (0.33–6.73) | 0.506 | ||
PWB: Physical Well-Being dimension; SWB: Social Well-Being; EWB: Emotional Well-Being, FWB: Functional Well-Being; LCS: Lung Cancer Subscale, aNK cells: activated Natural Killer cells.
1 variable forced in the multivariate analysis
2 continuous variable
*: interaction
Significance level set to 0.01 according to Bonferroni adjustment
Cox multivariate analyses showed that no variables had a significant impact on the TUDD of PWB, SWB and LCS domains. However the gender (male) (P = 0.009) was associated with a shorter TUDD of EWB domain. The smokers and the former smokers tended to present a shorter TUDD of FWB domain, but not statistically significant. The interaction test between treatment arm and the levels of aNK cells was not significant (P = 0.704; 0,994; 0.704; 0.189 and 0.506 respectively for PWB, SWB, EWB, FWB and LCS domains).
TUDD curves estimations according to the level of aNK cells did not reflect a significant effect of the level of aNK cells on the TUDD (S1 and S2 Figs). However, patients with a high level of pre-treatment aNK cells seemed to have a shorter TUDD than those with normal level of aNK cells for PWB (HR arm 1 vs. arm 2: 1.39 (0.50–4.30) for high level of aNK cells vs. 1.29 (0.65–2.57) for normal level of aNK cells), EWB (1.58 (0.52–4.77) vs. 1.15 (0.53–2.47)), FWB (2.23 (0.65–7.65) vs. 0.83 (0.39–1.79)) and LCS domains (1.82 (0.54–6.17) vs. 1.11 (0.47–2.61)), but all these trends were not statistically significant (Table 4).
Table 4. Results of the Kaplan-Meier estimation of the time until definitive deterioration curves according the level of activated Natural Killer (aNK) cells.
| patients with normal level of aNK cells | patients with high level of aNK cells | |||||||
|---|---|---|---|---|---|---|---|---|
| n (events) | median—days (99%CI) | P log-rank | HR combination therapy arm vs. control arm (99%CI) | n (events) | median—days (99%CI) | P log-rank | HR combination therapy arm vs. control arm (99%CI) | |
| PWB | ||||||||
| combination therapy arm | 37 (30) | 81 (54–169) | 0.355 | 1.29 (0.65–2.57) | 18 (13) | 65 (39-NA) | 0.439 | 1.39 (0.50–4.30) |
| control arm | 42 (27) | 91 (62-NA) | 13 (11) | 85 (50-NA) | ||||
| SWB | ||||||||
| combination therapy arm | 37 (20) | 133 (81-NA) | 0.448 | 0.79 (0.35–1.77) | 18 (8) | 131 (45-NA) | 0.715 | 0.83 (0.23–3.03) |
| control arm | 42 (23) | 99 (61-NA) | 13 (8) | 141 (82-NA) | ||||
| EWB | ||||||||
| combination therapy arm | 37 (25) | 107 (90-NA) | 0.644 | 1.15 (0.53–2.47) | 18 (14) | 86 (39-NA) | 0.276 | 1.58 (0.52–4.77) |
| control arm | 43 (22) | 125 (85-NA) | 13 (9) | 96 (84-NA) | ||||
| FWB | ||||||||
| combination therapy arm | 37 (22) | 126 (65-NA) | 0.536 | 0.83 (0.39–1.79) | 18 (13) | 90 (44-NA) | 0.086 | 2.23 (0.65–7.65) |
| control arm | 42 (25) | 98 (76-NA) | 13 (9) | 141 (84-NA) | ||||
| LCS | ||||||||
| combination therapy arm | 37 (19) | 166 (61-NA) | 0.760 | 1.11 (0.47–2.61) | 18 (11) | 88 (39-NA) | 0.202 | 1.82 (0.54–6.17) |
| control arm | 43 (18) | 183 (98-NA) | 13 (8) | 141 (125-NA) | ||||
PWB: Physical Well-Being dimension; SWB: Social Well-Being; EWB: Emotional Well-Being, FWB: Functional Well-Being; LCS: Lung Cancer Subscale
Significance level set to 0.01 according to Bonferroni adjustment
Discussion
The role of therapeutic vaccines in advanced cancer patients would be like with chemotherapy to increase the time to progression and to increase survival while preserving HRQOL. The results previously reported presented the efficacy and safety data of the therapeutic vaccine TG4010 combined with chemotherapy as a first line treatment for patients advanced NSCLC. PFS at 6 months for vaccinated patients was found to be 43% and for the patients on standard chemotherapy was 35% [13]. A significant benefit of survival was observed in the subgroup of patients with normal level of pre-treatment aNK cells, although this finding was based on an unplanned exploratory analysis. According two meta-analyses, a gain in HRQOL has been observed in 50% of trials even if no benefit in term of OS was showed [19,20]. In our study, missing HRQOL data were low except for SWB domain. Concerning this domain, some patients could have chosen to not answer especially questions about sexual activities. Our results showed no statistical difference of TUDD of HRQOL score between the two evaluated therapeutic strategies supporting the good tolerance of TG4010 when added to chemotherapy in this patient population. Only the gender "men" was significantly associated with a shorter TUDD for EWB domain.
Few studies evaluated HRQOL in NSCLC patients treated by therapeutic vaccination and they found benefits to patient HRQOL by additional vaccination [21,22]. Butt et al. showed that HRQOL was maintained longer in patients who received L-BLP25 vaccine and best supportive care (BSC) compared to patients who received BSC alone. HRQOL results obtained in randomized trials using either L-BLP25 or TG4010 suggest that therapeutic vaccine have no negative impact on HRQOL. Further work would be necessary to confirm these findings and incorporation of HRQOL in pivotal phase III trials with therapeutic vaccines is essential. This applies both to trials testing a vaccine in addition to BSC or combined with standard of care.
In the original trial, pre-treatment aNK cells seemed to be a biomarker to target patients appropriate for a treatment with TG4010 [13]. Indeed, an improved clinical outcome for patients with a normal level of aNK cells was shown with a 6-month increase in median OS (17.1 months in the vaccine group versus 11.3 months in the control group) [13]. HRQOL analyses did not differ strikingly according to the level of pre-treatment aNK cells. However, when treated by TG4010 with a normal level of aNK cells tended to present a longer TUDD than patients with a high level of aNK cells. Nevertheless, we failed to demonstrate a statistically significant interaction between treatment arms and level of aNK cells, probably due to a lack of statistical power. Moreover, there were more patients with no follow-up measure in the subgroup of high level of aNK cells: it is suspected that these patients had a lower general health status than those with normal level of aNK cells and were unable to complete HRQOL questionnaires. In subgroup exploratory analyses, a deleterious effect on FWB score was observed in patients with high level of aNK cells, but not statistically significant. A phase IIb–III study taking into account the pre-treatment level of aNK cells has been initiated to prospectively validate these clinical results and further analyze the evolution of HRQOL under chemo-immunotherapy with an acceptable statistical power (NCT01383148).
In conclusion, this study did not show significant differences in HRQOL over the course of the treatment between patients having received or not TG4010 in combination with chemotherapy and this despite the fact that the group of patients who received the vaccine presented a lower HRQOL at baseline. This supports the idea that TG4010 can be combined to first-line chemotherapy without deterioration in patients’ HRQOL. Understanding the relative effects of new treatment on HRQOL is important for effective decision making in this setting [12]. Future research should incorporate HRQOL as a treatment outcome for therapeutic cancer vaccines as well, particularly when the treatment is palliative.
Supporting Information
(DOCX)
(DOC)
(ZIP)
Time to a five-point definitive deterioration in Health-related Quality of Life score or death for patients with a normal level of activated Natural Killer (aNK) cells: (A) Physical Well-Being dimension (PWB); (B) Social Well-Being dimension (SWB); (C) Emotional Well-Being dimension (EWB); (D) Functional Well-Being dimension (FWB); (E) Lung Cancer Subscale (LCS). Arm 1: combination therapy arm; Arm 2: control arm.
(TIF)
Time to a five-point definitive deterioration in Health-related Quality of Life score or death for patients with a high level of activated Natural Killer (aNK) cells: (A) Physical Well-Being dimension (PWB); (B) Social Well-Being dimension (SWB); (C) Emotional Well-Being dimension (EWB); (D) Functional Well-Being dimension (FWB); (E) Lung Cancer Subscale (LCS). Arm 1: combination therapy arm; Arm 2: control arm.
(TIF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Transgene SAS and by ADNA, a French government-funded programme for personalised medicine. GL, BB and JML are full time employees of Transgene SA. Transgene SA provided support in the form of salaries for authors GL, BB and JML, and as the sponsor of the study was involved in the study design, data collection and analysis, decision to publish, or review of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127: 2893–2917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2. American Society of Clinical Oncology. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997; 15: 2996–3018. [DOI] [PubMed] [Google Scholar]
- 3. Gridelli C, Perrone F, Nelli F, Ramponi S, De Marinis F. Quality of life in lung cancer patients. Ann Oncol. 2001; 12 Suppl 3: S21–25. [DOI] [PubMed] [Google Scholar]
- 4. Rossi A, Maione P, Colantuoni G, Ferrara C, Rossi E, Guerriero C, et al. Recent developments of targeted therapies in the treatment of non-small cell lung cancer. Curr Drug Discov Technol. 2009; 6: 91–102. [DOI] [PubMed] [Google Scholar]
- 5. Pallis AG, Serfass L, Dziadziusko R, van Meerbeeck JP, Fennell D, Lacombe D, et al. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer. 2009; 45: 2473–2487. 10.1016/j.ejca.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 6. Decoster L, Wauters I, Vansteenkiste JF Vaccination therapy for non-small-cell lung cancer: review of agents in phase III development. Ann Oncol. 2012; 23: 1387–1393. 10.1093/annonc/mdr564 [DOI] [PubMed] [Google Scholar]
- 7. Belani CP, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Effect of chemotherapy for advanced non-small cell lung cancer on patients' quality of life. A randomized controlled trial. Lung Cancer. 2006; 53: 231–239. [DOI] [PubMed] [Google Scholar]
- 8. Hollen PJ, Gralla RJ Comparison of instruments for measuring quality of life in patients with lung cancer. Semin Oncol. 1996; 23: 31–40. [PubMed] [Google Scholar]
- 9. Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 2001; 31: 233–240. [DOI] [PubMed] [Google Scholar]
- 10. Dharma-Wardene M, Au HJ, Hanson J, Dupere D, Hewitt J, Feeny D. Baseline FACT-G score is a predictor of survival for advanced lung cancer. Qual Life Res. 2004; 13: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 11. Braun DP, Gupta D, Staren ED. Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer. 2011; 11: 353 10.1186/1471-2407-11-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuda A, Yamaoka K, Tango T. Quality of life in advanced non-small cell lung cancer patients receiving palliative chemotherapy: A meta-analysis of randomized controlled trials. Exp Ther Med. 2012; 3: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011; 12: 1125–1133. 10.1016/S1470-2045(11)70259-5 [DOI] [PubMed] [Google Scholar]
- 14. Bonnetain F, Dahan L, Maillard E, Ychou M, Mitry E, Hammel P, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010; 46: 2753–2762. 10.1016/j.ejca.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 15. Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982; 38: 143–151. [PubMed] [Google Scholar]
- 16. Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995; 12: 199–220. [DOI] [PubMed] [Google Scholar]
- 17. Team RDC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0. 2010. Available: http://www.R-project.org/. [Google Scholar]
- 18. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 19. Claassens L, van Meerbeeck J, Coens C, Quinten C, Ghislain I, Sloan EK, et al. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011; 29: 2104–2120. 10.1200/JCO.2010.32.3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saad ED, Adamowicz K, Katz A, Jassem J Assessment of quality of life in advanced non-small-cell lung cancer: an overview of recent randomized trials. Cancer Treat Rev. 2012; 38: 807–814. 10.1016/j.ctrv.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 21. Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005; 23: 6674–6681. [DOI] [PubMed] [Google Scholar]
- 22. O'Brien ME, Anderson H, Kaukel E, O'Byrne K, Pawlicki M, Von Pawel J, et al. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol. 2004; 15: 906–914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(ZIP)
Time to a five-point definitive deterioration in Health-related Quality of Life score or death for patients with a normal level of activated Natural Killer (aNK) cells: (A) Physical Well-Being dimension (PWB); (B) Social Well-Being dimension (SWB); (C) Emotional Well-Being dimension (EWB); (D) Functional Well-Being dimension (FWB); (E) Lung Cancer Subscale (LCS). Arm 1: combination therapy arm; Arm 2: control arm.
(TIF)
Time to a five-point definitive deterioration in Health-related Quality of Life score or death for patients with a high level of activated Natural Killer (aNK) cells: (A) Physical Well-Being dimension (PWB); (B) Social Well-Being dimension (SWB); (C) Emotional Well-Being dimension (EWB); (D) Functional Well-Being dimension (FWB); (E) Lung Cancer Subscale (LCS). Arm 1: combination therapy arm; Arm 2: control arm.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.