Abstract
Prophylactic platelet concentrates transfusion represents a therapeutic choice in patients with chemotherapy-induced thrombocytopenia. This prospective, non-interventional study evaluated the effects of platelet concentrates transfusion on thromboelastometric parameters of platelet function in 36 transfusion occasions for 11 thrombocytopenic children undergoing chemotherapy. Pre- and posttransfusion (1-2 hours) blood samples were analyzed using standard coagulation tests and thromboelastometry (ROTEM) measurements (EXTEM and FIBTEM tests). Platelet component of the clot was calculated based on the EXTEM and FIBTEM maximum clot elasticity (MCE) results. After transfusion, mean platelet count increased from 16.5 × 109/L to 43.0 × 109/L (P < .001) and platelet component increased from 34.1 to 73.0 (P < .001). Statistically significant increases for posttransfusion EXTEM parameters A10, A20, and maximum clot firmness (MCF) were observed compared to pretransfusion values (P < .001). The EXTEM α-angle values increased posttransfusion (P < .05). The FIBTEM measurements were comparable pre- and posttransfusion. The study showed that platelet concentrates transfusion in thrombocytopenic children undergoing chemotherapy improves platelet-related coagulation pattern.
Keywords: thrombocytopenia, platelet function, hemostasis
Introduction
In patients undergoing chemotherapy, transfusion of platelet concentrates is used as a prophylactic or therapeutic means to control bleeding.1 The optimal transfusion policy needs to consider both the risks associated with platelet administration and the efficacy of this intervention. Transmission of pathogens, bacterial in particular, appears to represent the highest risk.2 However, of considerable importance are also the risks due to immune modulation, transfusion-related acute lung injury, and hemolysis due to transfusion errors. In recent years, the more aggressive chemotherapeutic treatments and the occurrence of platelet refractoriness have led to an increase in the demand for platelet concentrates. Changes in the donor population and the increase in perioperative platelet administration for correction of coagulopathy have also contributed to the occasional shortage of supply observed in transfusion centers worldwide.
Children having malignant disorders and thrombocytopenia represent a clinical entity with high exposure to platelet concentrates transfusion. Still, there have been few clinical and epidemiological studies to investigate the efficacy and the consequences of transfusion in this group of patients. To a great extent, many pediatric transfusion criteria remain opinion based, and there is a wide variation in transfusion practices and policies among institutions.
Efficacy of platelet transfusion and platelet viability is generally assessed by measuring immediate and late platelet count increments, as well as bleeding scores and the intertransfusion interval, which reflects the platelet survival.1,3 Several point-of-care methods evaluating the effect of platelet transfusion on coagulation have been investigated. Direct evaluation of platelet function in this setting is complicated due to the fact that the methods available such as “in vitro bleeding time” (Platelet Function Analyzer PFA-100, Siemens Healthcare Diagnostics, Marburg, Germany) and whole blood aggregometry (eg, multiple electrode aggregometry Multiplate Analyzer, Roche Diagnostics International Ltd, Rotkreuz, Switzerland) are not adequate for platelet counts <100 × 109/L.
The immediate effects of the transfusion of platelet concentrates on coagulation may also be analyzed in whole blood by means of thromboelastometry (ROTEM; TEM International GmbH, Munich, Germany).4 This method assesses the speed of initiation of the coagulation process and the contribution of fibrinogen and platelets to the elasticity of the whole blood clot. Clotting may be induced via the extrinsic pathway, generating a clot comprised of fibrin and platelets (EXTEM test). By using a specific platelet inhibitor such as cytochalasin D (FIBTEM test), the quality of the fibrin-based clot may be assessed.5 Furthermore, the platelets contribution to clot elasticity (platelet component parameter) can be calculated from the difference between the maximum clot elasticity (MCE) value obtained with the EXTEM test and the MCE value obtained with the FIBTEM test. Platelet activation in the whole blood test is mainly induced by thrombin, after addition of tissue factor and calcium to the citrated whole blood sample. As thrombin is the strongest platelet activator and is generated in high amounts during the thromboelastometric analysis, it is likely that most platelets available in the sample will be activated and will contribute to the clot firmness. This explains why the effect of mild platelet inhibitors such as aspirin cannot be observed with this analysis. Chemotherapeutic agents can damage bone marrow leading to myelosuppression and decreased platelet counts. In addition, low platelet count can contribute to a decrease in clot firmness, which has been associated with chemotherapy.6 Estcourt et al found that thromboelastometric measurements indicating decreases in clot firmness have been associated with an increased risk of bleeding in patients undergoing chemotherapy with thrombocytopenia.7
Flisberg et al first evaluated in 2010 the effect of platelet transfusion on clot firmness in the ROTEM analysis. Their investigation showed that platelet administration increased clot firmness; however, the study did not investigate whether concomitant changes in the fibrin-based clot also occurred. Hence, it was not clear whether the changes in overall clot firmness were due to an increase in the platelet contribution to clot or also due to changes in the fibrin-based clot firmness, caused by the plasma contained in the platelet concentrates.
The aim of the study was to evaluate the effect of platelet transfusion on thromboelastometric parameters of platelet function in children with severe thrombocytopenia caused by chemotherapy.
Materials and Methods
Patient Population
Following approval from the local Ethics Committee, this prospective, noninterventional study was conducted at St Johann’s Hospital, SALK Salzburg. Parental informed written consent was obtained before inclusion. Thrombocytopenic pediatric patients (<18 years old) undergoing chemotherapy (platelet count <50 × 109/L) who received prophylactic platelets transfusion were identified for the study. Patients received 1 unit of single donor platelet concentrate (34 transfusion occasions) or 1 unit of pooled platelet concentrate (2 transfusion occasions). Each unit of platelet concentrate was ≤6 days old, at a concentration of >3 × 1011. Exclusion criteria were intake of medication influencing coagulation and other coagulation diseases.
Blood collection
Following each transfusion occasion, venous blood samples were drawn into S-Monovette vials (Sarstedt AG, Numbrecht, Germany) containing trisodium citrate 3.13%, 1.06 mol/L, ratio 1:9 with blood). Duplicate blood samples were taken before transfusion and 1 to 2 hours after transfusion.
Coagulation Testing
The following standard coagulation tests were performed: prothrombin time (PT), activated partial thromboplastin time (aPTT), hematocrit, hemoglobin concentration, and platelet count. Prothrombin time (Thromborel S, Siemens Healthcare Diagnostics, Marburg, Germany) and aPTT (Pathromtin SL, Siemens Healthcare Diagnostics) were determined from citrated blood samples using the BCS XP analyzer (Siemens Healthcare Diagnostics). Platelet count and hemoglobin concentration were measured from EDTA samples using the Sysmex XE-2100 analyzer (Sysmex, Norderstedt, Germany).
Citrated whole blood anticoagulated was used for the thromboelastometry (ROTEM) measurements. Recombinant tissue factor was used in the EXTEM tests to activate the extrinsic coagulation pathway, and cytochalasin D was added in the FIBTEM tests to inhibit platelet contribution.
The following measurements were collected:
Clotting time (CT), clot amplitude at 10 and 20 minutes (A10 and A20), MCF, and α-angle (EXTEM test only). Maximum clot elasticity was calculated using the following formula:
The MCE values for EXTEM and FIBTEM tests were used to calculate the platelet component of MCE as follows: EXTEM MCE − FIBTEM MCE.
The calculations presented subsequently were used to determine corrected count increment (CCI) and percentage platelet recovery (PPR) values:
where the blood volume in the PPR formula is assumed to be 75 mL/kg for pediatric patients.
The pediatric blood volume used for the PPR calculation is based on the mid-range value of 70 to 80 mL/kg.8
Statistics
Pre- and posttransfusion values for standard coagulation tests, platelet characteristics, and ROTEM measurements were compared. All outcomes were measured on a continuous scale. When the changes in values over time were found to be normally distributed, the paired t test was used for the analysis. The Wilcoxon matched-pairs test was used for variables where the changes over time were not normally distributed. A P value <.05 was considered significant.
The association between platelet count and a number of other continuous variables was examined. Due to the skewed distribution of the platelet counts, Spearman rank correlation was preferred to analyze the data. Separate analyses were performed for pre- and posttransfusion data.
Results
Demographics
A total of 11 pediatric patients (4 male and 7 female) who received transfusions over 36 occasions were included in this study. The mean age was 12.1 years. The most common diagnosis for patients receiving platelet concentrate transfusion was acute lymphoblastic leukemia. Further demographic data are presented in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics.a,b
| N = 11 | |
|---|---|
| Age, years | 12.1 ± 4.8 |
| Female | 7 (64%) |
| Weight, kg | 41.3 ± 16.7 |
| Height, cm | 147.5 ± 29.3 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 4 (36%) |
| Ewing’s sarcoma | 1 (9%) |
| Neuroblastoma | 1 (9%) |
| Non-Hodgkin’s lymphoma | 1 (9%) |
| Osteosarcoma | 1 (9%) |
| Wilms tumor | 1 (9%) |
| Germ cell tumor | 1 (9%) |
| Myelodysplastic syndrome | 1 (9%) |
Abbreviation: SD, standard deviation.
aData are presented as mean ± SD, as appropriate.
bPercentages do not total to 100 due to rounding.
Standard Coagulation tests
Significant decreases were observed between pretransfusion and posttransfusion values for aPTT, hemoglobin, and hematocrit (P < .001), whereas significant increases in PT values were observed (P < .05; Table 2). Platelet concentrates were transfused in patients with pretransfusion platelet counts of 8 × 109/L to 51 × 109/L (minimum − maximum). Mean platelet count increased posttransfusion to 43.0 × 109/L (interquartile range: 33.8 × 109/L to 55.0 × 109/L).
Table 2.
Standard Coagulation Laboratory Tests and Platelet Characteristics.a
| Pretransfusionb | Posttransfusionb | Change (95% CI) | P Value | |
|---|---|---|---|---|
| Standard coagulation tests | ||||
| PT, % | 87.4 ± 15.2 | 89.8 ± 12.7 | 2.4 (0.4 to 4.5) | .02 |
| aPTT, seconds | 39.0 (34.8-51.5) | 33.0 (30.8-36.5) | −3.0 (−6.0 to −1.0) | <.001 |
| Hemoglobin, g/dL | 9.6 ± 1.4 | 8.8 ± 1.4 | −0.8 (−1.1 to −0.6) | <.001 |
| Hematocrit, % | 26.7 ± 3.9 | 24.6 ± 4.1 | −2.1 (−2.7 to −1.4) | <.001 |
| Platelet characteristics | ||||
| Platelet count, 109/L | 16.5 (13.0-23.0) | 43.0 (33.8-55.0) | 28.0 (20.0 to 31.0) | <.001 |
| Percentage platelet recoveryc | – | 26.6 ± 8.9 | – | |
| Corrected count incrementc, m2/µL | – | 11084.0 ± 3674.9 | – | |
Abbreviations: CI, confidence interval; SD, standard deviation; IQR, interquartile range. Significant P values (<.05) are shown in bold.
aValues are presented as mean ± SD or median (IQR).
bData available for 35 of 36 platelet concentrate units for measurements for pretransfusion standard coagulation tests and 34 of 36 patients for posttransfusion standard coagulation tests.
cData available for 32 of 36 platelet concentrate units, calculations based on 32 units.
Platelet Characteristics
Platelet characteristics information is presented in Table 2. Mean PPR was 26.6% (± 8.9%) and mean CCI was 11084.0 m2/µL (±3674.9 m2/µL). Platelet component increased from 34.1 to 73.0 (P < .001). Median volume of platelets transfused was 233 mL. Most common age of platelets transfused was 4 days (Figure 1).
Figure 1.
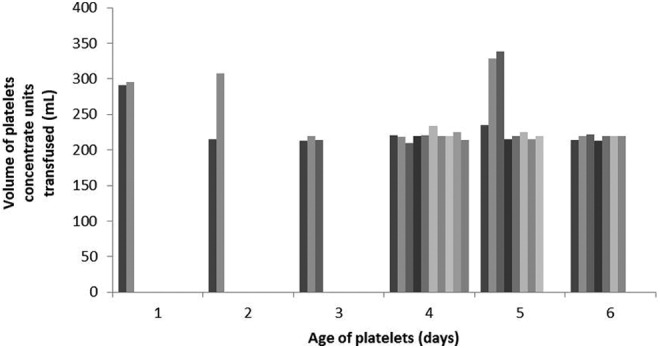
Characteristics of platelet concentrates transfused into thrombocytopenic children undergoing chemotherapy. Data represent 32 platelet concentrate units; data unavailable for 4 patients.
Thromboelastometry Measurements
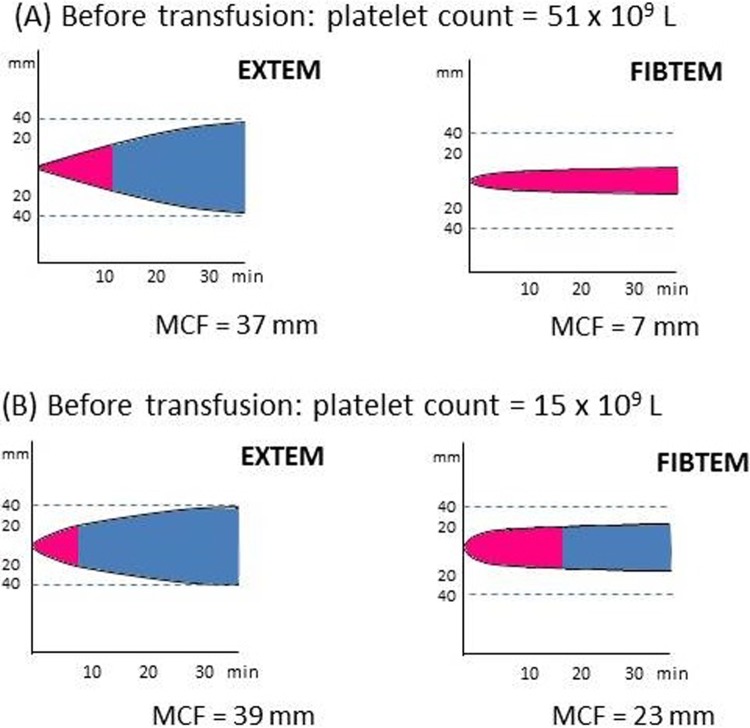
The ROTEM measurements are presented in Table 3. The FIBTEM measurements (A10, A20, and MCF) were comparable pre- and posttransfusion. The EXTEM α-angle values increased posttransfusion (P < .05). Statistically significant increases for posttransfusion EXTEM A10, A20, and MCF values were observed compared to pretransfusion values (P < .001). No significant differences were observed for EXTEM CT measurements. Positive correlations were observed pre- and posttransfusion between platelet count and EXTEM MCE values and platelet count and EXTEM MCF values. No correlations were observed between platelet count and FIBTEM MCE or FIBTEM MCF results. Furthermore, we demonstrate that values for FIBTEM MCF measurements can remain unchanged posttransfusion although those for EXTEM MCF increase (Figure 2).
Table 3.
ROTEM Measurements.a
| Pretransfusionb | Posttransfusionb | Change (95% CI) | P Value | |
|---|---|---|---|---|
| α-angle, ° | 74 (62-77) | 75 (68-78) | 2 (0 to 3) | .008 |
| EXTEM CT, seconds | 53.0 (50.0-57.3) | 52.0 (49.0-57.0) | −1.0 (−3.0 to 1.2) | .27 |
| EXTEM A10, mm | 26.0 ± 9.9 | 37.7 ± 8.2 | 11.7 (9.2 to 14.2) | <.001 |
| EXTEM A20, mm | 32.2 ± 10.8 | 46.9 ±13.5 | 14.8 (10.6 to 18.9) | <.001 |
| EXTEM MCF, mm | 35.0 ± 10.1 | 48.1 ± 8.0 | 13.1 (10.5 to 15.8) | <.001 |
| EXTEM MCE | 57.6 ± 25.2 | 97.1 ± 28.9 | 39.6 (31.8 to 47.3) | <.001 |
| FIBTEM A10, mm | 15.9 ± 7.7 | 16.5 ± 6.9 | 0.6 (−0.1 to 1.3) | .10 |
| FIBTEM A20, mm | 17.3 ± 8.3 | 17.9 ± 7.4 | 0.6 (−0.1 to 1.3) | .08 |
| FIBTEM MCF, mm | 18.6 ± 8.6 | 19.3 ± 7.6 | 0.6 (−0.2 to 1.4) | .11 |
| FIBTEM MCE | 24.3 ± 14.1 | 25.0 ± 12.5 | 0.7 (−0.5 to 1.8) | .26 |
| Platelet component (EXTEM MCE − FIBTEM MCE) | 34.1 ± 18.1 | 73.0 ± 23.0 | 38.9 (30.7 to 47.0) | <.001 |
Abbreviations: CI, confidence interval; SD, standard deviation; IQR, interquartile range. Significant P values (<.05) are shown in bold.
aValues are presented as mean ± SD or median (IQR).
bData available for 35 of 36 platelet concentrate units for all measurements with the exception of pretransfusion EXTEM A20, where data were available for 34 of 36 platelet concentrate units.
Figure 2.
Pretransfusion and posttransfusion assessment of fibrinogen contribution using EXTEM and FIBTEM measurements in a single patient. Although EXTEM maximum clot firmness (MCF) increases posttransfusion (B), FIBTEM values remain the same as pretransfusion (A).
Discussion
The results of this study show that following platelet concentrates transfusion, firmness and elasticity of the whole blood clot, and the speed with which the clot is formed increased significantly. The evaluation of the effect of platelet transfusion on clot strength in patients with thrombocytopenia had previously been investigated using a single test with the ROTEM device and showed that the speed and strength of the clot are both rapidly improved after platelet transfusion.4 These results, however, do not indicate whether the observed improvements were due to an increased contribution of platelets to clot firmness or to a change in the fibrin-based clot firmness, triggered by the plasma contained in the platelet concentrates. To address this point, we expanded on this study using multiple ROTEM tests (EXTEM and FIBTEM) to evaluate how the contribution of platelets and fibrinogen to the whole blood clot is affected by platelet transfusion in thrombocytopenic children undergoing chemotherapy. The increase in platelet component (the difference between EXTEM MCE and FIBTEM MCE values) and the lack of significant differences in FIBTEM measurements indicate that the observed increase in clot firmness parameters (EXTEM MCF, A10, and A20) and EXTEM α-angle values is mainly attributable to platelets. These findings are consistent with the positive correlations observed between platelet count and EXTEM MCF and MCE results.
The ROTEM measurements can provide information on clot quality through parameters such as α-angle and clot firmness. Perioperative transfusion algorithms in various clinical settings have identified α-angle and maximal amplitude (MA) as triggers for fibrinogen supplementation and platelets transfusion, respectively.9–11 However, our data show that α-angle increases significantly following platelet transfusion, demonstrating that it is strongly influenced by the contribution of platelets to clotting. Hence, it does not appear correct to assume, as these algorithms do, that decreased α-angle values strictly reflect fibrinogen deficit, while decreased overall clot firmness (MA or MCF) reflects platelet deficit. Indeed, there is strong evidence that the MA (or MCF) parameter does not represent the sole contribution of platelets to clot firmness, and the assessment of a platelet deficiency is likely to be more appropriate if a “multiple assays” approach is taken. Various studies investigating blood clot’s properties describe the use of the platelet component as parameter reflecting the contribution of platelets to clot strength5,12–14 and a few have described its use during cardiovascular surgery or trauma.15–17 Although guidelines currently recommend the use of multiple viscoelastic assays in order to discriminate between a fibrinogen or platelet deficit and to guide hemostatic therapy,18,19 future investigations would be valuable to determine the clinical benefit of the platelet component as a parameter to guide platelet transfusion.
A comparable EXTEM MCF value can be composed of a low FIBTEM measurement and high platelet component, or a high FIBTEM measurement and low platelet component, as demonstrated in Figure 3. This supports the use of a combination of 2 tests, without platelet inhibitor (EXTEM assay) and with platelet inhibitor (FIBTEM assay) to assess the platelet component and to give an indication of the response to platelet concentrates transfusion.
Figure 3.
Pretransfusion assessment of platelet contribution using EXTEM and FIBTEM measurements in 2 different patients. Although EXTEM maximum clot firmness (MCF) is comparable, (A) shows a high platelet count and a low FIBTEM value, whereas (B) shows a low platelet count and a high FIBTEM value.
It has been demonstrated that in samples with high platelet counts, cytochalasin D alone may not fully inhibit the contribution of platelets in the FIBTEM assay.5 Our data show that significant positive correlations were observed between platelet count and EXTEM MCF as well as MCE values. No significant correlations were observed between platelet count and FIBTEM MCF or MCE values. Our results confirm that platelet inhibition with cytochalasin D was sufficient for the platelet count range in this study. These findings are consistent with a study conducted by Kander et al who found that increasing platelet count improved EXTEM measurements of clot firmness with no change in FIBTEM results.20
Following platelet concentrates transfusion, the aPTT time was significantly shortened unlike what was reported by Flisberg and colleagues who did not observe any change in the aPTT after platelet infusion.4 It should be noted, however, that the increase in platelet count after transfusion was greater in our study (28 × 109/L vs 12 × 109/L in the Flisberg’s study) probably attributable to the pretransfusion platelet count which was twice higher in the patients enrolled in the Flisberg study than in those enrolled in our study. This greater change in platelet count could have impacted on the aPTT, which evaluates the activity of the intrinsic pathway of the coagulation cascade. Indeed, although this test is performed in platelet-poor plasma, the increased platelet count after transfusion might have led to an increase in platelet-derived phospholipids which in turn contributed to a greater activation of the intrinsic coagulation pathway.
Current guidelines recommend a threshold of 10 × 109/L for prophylactic platelet transfusion and as suitable in patients without additional risk factors (eg, sepsis, concurrent use of antibiotics, and other abnormalities of hemostasis).21,22 In a trial investigating the efficacy and safety of therapeutic versus prophylactic platelet transfusion to prevent bleeding in patients with hypoproliferative thrombocytopenia, the authors recommended that in patients with acute myeloid leukemia prophylactic platelet administration should remain the standard of care.23 A similar threshold of 10 × 109/L was recommended by the American Society of Clinical Oncology in their clinical practice guidelines for platelet transfusion in patients with cancer.1 This recommendation was made for adult patients receiving therapy for acute leukemia, and patients with solid tumors during chemotherapy-induced thrombocytopenia. However, the guidelines did note that “a higher threshold may be required in newborns or in patients with signs of hemorrhage, high fever, hyperleukocytosis, rapid fall of platelet count, or coagulation abnormalities and in those undergoing invasive procedures or in circumstances in which platelet transfusions may not be readily available in case of emergencies.” These guidelines also state that it is probably reasonable to use similar guidelines for children and older infants. These reasons directly informed our decision to use a platelet count threshold of 50 × 109/L; indeed, while platelet avoidance might be recommended to prevent the risk of infection, bleeding avoidance is also critical in these thrombocytopenic pediatric patients undergoing chemotherapy. Nevertheless, we agree that further evidence is necessary for this clinical approach, in order to balance the risks of platelet transfusion and the risk of bleeding. Furthermore, the utility of rapidly available coagulation parameters such as platelet component and the potential application of other choices of hemostatic therapy mandate further investigations.
A limitation of the study is that coagulation testing occurred 1 to 2 hours following platelets transfusion. The hemostatic changes observed may have been greater if coagulation testing was performed immediately posttransfusion.
Conclusion
In conclusion, platelet concentrates transfusion in thrombocytopenic pediatric patients undergoing chemotherapy improved platelets contribution to clot firmness. The ROTEM analyses (EXTEM and FIBTEM) allowed for a rapid and specific evaluation of this effect.
Acknowledgments
We would like to thank Simona Perju-Tometschek (from SALK Blood Bank) for her assistance with the data collation. Editorial assistance with manuscript preparation was provided by Meridian HealthComms and funded by CSL Behring.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CS is an employee of CSL Behring and previously received speaker honoraria and research support from Tem International and CSL Behring. The other authors declare no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19 (5):1519–1538. [DOI] [PubMed] [Google Scholar]
- 2. Spiess BD, Royston D, Levy JH, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. [DOI] [PubMed] [Google Scholar]
- 3. Blajchman MA, Slichter SJ, Heddle NM, Murphy MF. New strategies for the optimal use of platelet transfusions. Hematology Am Soc Hematol Educ Program. 2008:198–204. [DOI] [PubMed] [Google Scholar]
- 4. Flisberg P, Rundgren M, Engstrom M. The effects of platelet transfusions evaluated using rotational thromboelastometry. Anesth Analg. 2009;108 (5):1430–1432. [DOI] [PubMed] [Google Scholar]
- 5. Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108 (3):751–758. [DOI] [PubMed] [Google Scholar]
- 6. Larsen OH, Clausen N, Persson E, Ezban M, Ingerslev J, Sørensen B. Whole blood coagulation in children with thrombocytopenia and the response to platelet replacement, recombinant factor VIIa, and a potent factor VIIa analogue. Br J Haematol. 2009;144 (1):99–106. [DOI] [PubMed] [Google Scholar]
- 7. Estcourt LJ, Stanworth SJ, Harrison P, et al. Prospective observational cohort study of the association between thromboelastometry, coagulation and platelet parameters and bleeding in patients with haematological malignancies- the ATHENA study. Br J Haematol. 2014;166 (4):581–591. [DOI] [PubMed] [Google Scholar]
- 8. McLeod BC. Apheresis: Principles and Practice, 3 rd edition Baltimore: AABB Press; 2010. [Google Scholar]
- 9. Gonzalez E, Moore EE, Moore HB, Chapman MP, Silliman CC, Banerjee A. Trauma-induced coagulopathy: an institution's 35 year perspective on practice and research. Scand J Surg. 2014;103 (2):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun W, Jeleniowski K, Zhao X, Shen P, Li D, Hammond JA. Thromboelastography (TEG)-based algorithm reduces blood product utilization in patients undergoing VAD implant. J Card Surg. 2014;29 (2):238–243. [DOI] [PubMed] [Google Scholar]
- 11. Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42 (7):2590–2593. [DOI] [PubMed] [Google Scholar]
- 12. Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75 (5):759–766. [DOI] [PubMed] [Google Scholar]
- 13. Schöchl H, Solomon C, Laux V, Heitmeier S, Bahrami S, Redl H. Similarities in thromboelastometric (ROTEM(R)) findings between humans and baboons. Thromb Res. 2012;130 (3):e107–e1012. [DOI] [PubMed] [Google Scholar]
- 14. Djabir Y, Letson HL, Dobson GP. Adenosine, lidocaine, and Mg2+ (ALM) increases survival and corrects coagulopathy after eight-minute asphyxial cardiac arrest in the rat. Shock. 2013;40 (3):222–232. [DOI] [PubMed] [Google Scholar]
- 15. Solomon C, Hagl C, Rahe-Meyer N. Time course of haemostatic effects of fibrinogen concentrate administration in aortic surgery. Br J Anaesth. 2013;110 (6):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon C, Rahe-Meyer N, Sorensen B. Fibrin formation is more impaired than thrombin generation and platelets immediately following cardiac surgery. Thromb Res. 2011;128 (3):277–282. [DOI] [PubMed] [Google Scholar]
- 17. Solomon C, Traintinger S, Ziegler B, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2011;106 (2):322–330. [DOI] [PubMed] [Google Scholar]
- 18. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30 (6):270–382. [DOI] [PubMed] [Google Scholar]
- 19. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17 (2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kander T, Tanaka KA, Norstrom E, Persson J, Schött U. The Effect and Duration of prophylactic platelet transfusions before insertion of a central venous catheter in patients with bone marrow failure evaluated with point-of-care methods and flow cytometry. Anesth Analg. 2014;119 (4):882–890. [DOI] [PubMed] [Google Scholar]
- 21. Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007:172–178. [DOI] [PubMed] [Google Scholar]
- 22. British Committee for Standards in Haematology BTTF. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122 (1):10–23. [DOI] [PubMed] [Google Scholar]
- 23. Wandt H, Schaefer-Eckart K, Wendelin K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380 (9850):1309–1316. [DOI] [PubMed] [Google Scholar]