Abstract
Background
Clinical variables associated with 30-day mortality after lung cancer surgery are well known. However, the effects of non-clinical factors, including insurance coverage, household income, education, type of treatment center, and area of residence, on short term survival are less appreciated. We studied the National Cancer Data Base (NCDB), a joint endeavor of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, to identify disparities in 30-day mortality after lung cancer resection based on these non-clinical factors.
Study Design
We performed a retrospective cohort analysis of patients undergoing lung cancer resection from 2003-2011, using the NCDB. Data were analyzed using a multivariable logistic regression model to identify risk factors for 30-day mortality.
Results
215,645 patients underwent lung cancer resection during our study period. We found that clinical variables such as age, gender, comorbidity, cancer stage, preoperative radiation, extent of resection, positive surgical margins, and tumor size were associated with 30-day mortality after resection. Non-clinical factors including living in lower income neighborhoods with a lesser proportion of high school graduates, and receiving cancer care at a non-academic medical center were also independently associated with increased 30-day postoperative mortality.
Conclusions
This study represents the largest analysis of 30-day mortality for lung cancer resection to date from a generalizable national cohort. Our results demonstrate that, in addition to known clinical risk factors, several non-clinical factors are associated with increased 30-day mortality after lung cancer resection. These disparities require further investigation to improve lung cancer patient outcomes.
Lung cancer is the most common cancer worldwide with highest incidence in the western world (1). For more than fifty years, lung cancer has been the most lethal of all cancer types and is projected to cause 27% of all cancer related deaths in the coming year (1-3). Five year survival with lung cancer is largely dependent on stage at time of diagnosis (3). Although radiation, chemotherapy, and immunotherapy have been explored as therapeutic options, surgical resection is the only treatment to offer definitive cure and is the gold standard for early stage therapy. The possibility of cure, however, is not without risk. Major postoperative complications of lung cancer resection are as high as 32% (4). Early studies have identified older age, significant cardiopulmonary comorbidity, and greater extent of surgical resection as risk factors for these complications (5, 6) However, few groups have studied non-clinical risk factors.
Recent healthcare reforms have refocused national attention on efficient health care delivery and exposed weaknesses in our current medical system (7). After clinical variables are accounted for, disparities in healthcare access and delivery have been increasingly recognized to affect cancer patient outcomes (8). Unmet medical needs in vulnerable demographics including racial and ethnic minorities, as well as lower socioeconomic groups, have recently been reported for lung cancer (9). Significant differences in lung cancer treatment and survival exist among these cohorts (10-12). Therefore, we hypothesized that, in addition to known clinical risk factors, other non-clinical variables were also independently associated with 30-day mortality following lung cancer resection. The aim of this study was to elucidate specific clinical and non-clinical factors that lead to disparities in 30-day survival and identify unique, at-risk patient populations where additional healthcare resources should be focused.
Methods
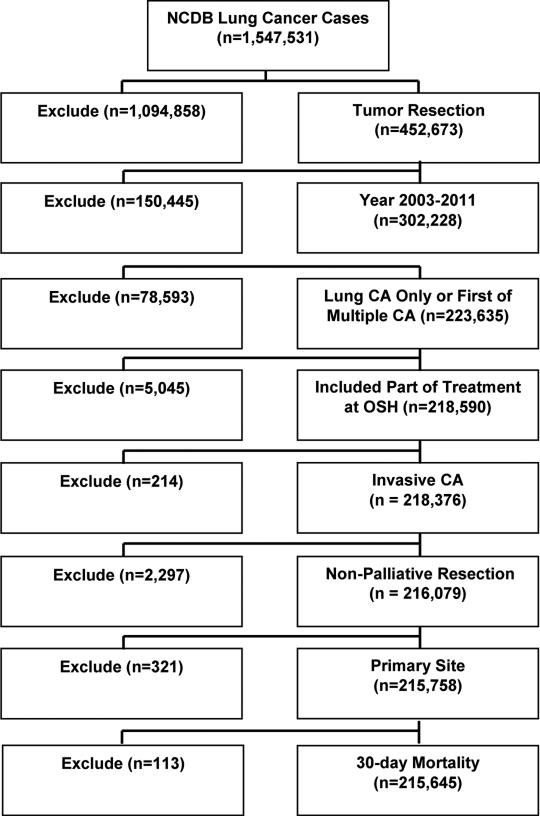
We performed a retrospective cohort study on patients undergoing lung cancer resection between 2003-2011, using the National Cancer Data Base (NCDB), a joint endeavor of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. This registry provides clinical and demographic data on patients treated at 1,500 CoC-approved hospitals across the country. Our patient selection algorithm is depicted in Figure 1. Cases were identified using the non-small cell lung cancer (NSCLC) Participant User File (PUF). Included patients were restricted to those with one lifetime cancer diagnosis or cases where the reported tumor was the first of multiple diagnoses in order to limit confounding effects of prior diagnosis or treatment. Exclusions included cases with cancer in-situ, unknown laterality, unknown 30-day mortality, palliative care patients, and those in which treatment was completed at a center other than the diagnosing and reporting facility. Institutional review board approval was waived by Emory University IRB as NCDB data are de-identified for both patient and facility.
Figure 1.
Patient selection algorithm. NCDB, National Cancer Data Base. CA, cancer. OSH, Outside hospital.
The primary outcome measure was any-cause mortality within 30 days after resection. Clinical factors examined included age, race, gender, Charlson/Deyo comorbidity score (CDS), analytical stage, use of preoperative radiation, the type of surgical procedure performed, the presence of positive surgical margins, tumor size, histology, primary site, grade, scope of regional lymph node (LN) surgery, regional LN positive, number of regional LN examined, and year of diagnosis. Analytical stage included the AJCC pathological stage group if available; otherwise the clinical stage group was used. Histology included adenocarcinoma, adenosquamous carcinoma, squamous carcinoma, large cell carcinoma, other histology, and unknown histology. Other histology was inclusive, but not restricted to spindle cell carcinoma, mucoepidermoid malignancies, neuroendocrine, and mixed malignant tumors.
The non-clinical variables evaluated included insurance coverage, median household income, local education level, treatment facility type, and area of residence. Education level and household income were identified by cross-referencing the patient's zip code to year 2000 US Census data. Education level was defined as the percentage of adults living in a patient's zip code not graduating from high school. The median household income was defined as the median household income for the patient's zip code. Facility type was determined by the Commission on Cancer based on services provided and total annual case number (13). Community cancer programs treat between 100 – 500 cancer patients/year. Comprehensive community cancer programs treat ≥ 500 cancer patients/year and participate in research. Academic programs, including those with NCI designation, treat >500 cancer patients, participate in research, and also provide postgraduate medical education. Area of residence was defined as metropolitan, urban, or rural based on the patient's county of residence and rural-urban continuum codes provided by the United States Department of Agriculture Economic Research Service.
Statistical analysis was conducted using SAS Version 9.3. Descriptive statistics for each variable were reported. The univariate association of each covariate with 30-day mortality was assessed using the chi-square test for categorical covariates and ANOVA for numerical covariates. A multivariable logistic regression model was fit for the outcome of 30-day mortality. All covariates were entered into the model subject to a backward variable selection method using an alpha = .20 criteria for removal from the model. To better understand differences between treatment facilities, the clinical and non-clinical variables were compared across 3 facility types using the chi-square test or ANOVA.
Results
Demographics
Our original NCDB lung database query included 1,547,531 patients. A total of 215,645 patients underwent surgical resection for lung cancer during the study period and met all inclusion criteria. The demographics of our study cohort shown in Table 1 demonstrate that the majority of patients were Caucasian, approximately 65 years old, distributed equally by gender, otherwise healthy, and diagnosed with early stage lung cancer. Most patients did not undergo preoperative radiation as part of their treatment plan. Additionally, more patients underwent lobectomy than any other resection technique. Mean tumor size was 3.34 ± 3.3 cm and histologically cancers were most often adenocarcinoma or squamous cell carcinoma. From the non-clinical variables studied, we found that most patients were covered by government insurance and lived in metropolitan communities with a median household income of < $46,000. Local education level varied markedly and the majority of patients received their cancer care at academic or comprehensive cancer centers.
Table 1.
Descriptive Statistics for Clinical and Non-Clinical Variables
| Variables | n = 215,645 | % |
|---|---|---|
| Clinical variables | ||
| Patient age, y | ||
| ≤60 | 60,285 | 28.0 |
| 61-65 | 34,441 | 16.0 |
| 66-70 | 40,604 | 18.8 |
| 71-75 | 37,416 | 17.4 |
| 76-80 | 27,989 | 13.0 |
| >80 | 14,910 | 6.9 |
| Mean (SD) | 66.14 (10.59) | |
| Race: white | ||
| No | 23,421 | 11.0 |
| Yes | 190,248 | 89.0 |
| Missing | 1,976 | - |
| Sex | ||
| Male | 105,690 | 49.0 |
| Female | 109,955 | 51.0 |
| Charlson/Deyo Score | ||
| 0 | 114,339 | 53.0 |
| 1 | 74,317 | 34.5 |
| 2+ | 26,989 | 12.5 |
| NCDB Analytic Stage Group | ||
| Stage 0-1 | 127,366 | 62.7 |
| Stage II | 35,679 | 17.6 |
| Stage III | 31,090 | 15.3 |
| Stage IV | 8,938 | 4.4 |
| Missing | 12,572 | - |
| Preoperative radiation | ||
| No | 203,956 | 95.8 |
| Yes | 8,967 | 4.2 |
| Missing | 2,722 | - |
| Surgical procedure | ||
| Wedge, < 1 lobe | 32,857 | 15.2 |
| Segmentectomy | 5,918 | 2.7 |
| Lobectomy | 162,405 | 75.3 |
| Pneumonectomy | 14,465 | 6.7 |
| Positive surgical margins | ||
| No | 196,158 | 93.2 |
| Yes | 14,204 | 6.8 |
| Missing | 5,283 | |
| Size of Tumor, cm, mean (SD) | 3.34 (3.3) | |
| Missing | 5,430 | |
| Histology | ||
| Large cell cancer | 8,545 | 4.0 |
| Other histology | 14,360 | 6.7 |
| Unknown histology | 12,184 | 5.7 |
| Squamous cell cancer | 61,061 | 28.3 |
| Adenocarcinomas | 113,744 | 52.7 |
| Adenosquamous cancer | 5,751 | 2.7 |
| Non-Clinical variables | ||
| Insurance coverage | ||
| Not Insured | 4,336 | 2.0 |
| Private Insurance | 76,208 | 35.9 |
| Govt. Insurance | 131,639 | 62.0 |
| Missing | 3,462 | - |
| Median local income | ||
| < $30,000 | 28,661 | 14.1 |
| $30,000 - $34,999 | 39,575 | 19.4 |
| $35,000 - $45,999 | 58,570 | 28.7 |
| > $46,000 | 76,989 | 37.8 |
| Missing | 11,850 | - |
| Local education level | ||
| ≥29%, not high school graduate | 34,578 | 17.0 |
| 20-28.9%, not high school graduate | 50,556 | 24.8 |
| 14-19.9%, not high school graduate | 50,698 | 24.9 |
| < 14%, not high school graduate | 67,945 | 33.3 |
| Missing | 11,868 | - |
| Facility type | ||
| Community | 20,343 | 9.4 |
| Comprehensive | 121,418 | 56.3 |
| Academic/Research | 73,884 | 34.3 |
| Area of residence | ||
| Metropolitan | 161,946 | 80.1 |
| Urban | 35,566 | 17.6 |
| Rural | 4,753 | 2.3 |
| Missing | 13,380 | - |
SD, standard deviation.
Unadjusted Analysis of 30-day Mortality
We first performed a univariate analysis to assess the association of clinical and non-clinical variables with 30-day mortality following lung cancer resection, as shown in Table 2. Clinical variables associated with increased 30-day postoperative mortality included older age, male gender, higher comorbidity score, and greater stage of lung cancer. Other clinical variables including the use of preoperative radiation, pneumonectomy, those with positive surgical margins, and tumors of larger size were also associated with increased 30-day mortality following lung cancer surgery. Race was not associated with 30-day postoperative mortality. The unadjusted association of non-clinical variables with 30-day mortality following lung cancer surgery was also studied. Our data show that patients covered by government insurance, living in non-metropolitan, low median household income neighborhoods, with a lesser proportion of high school graduates, and receiving cancer care at a facility other than an academic treatment center had an association with increased 30-day mortality following lung cancer resection.
Table 2.
Univariate Association with 30-Day Mortality after Lung Cancer Resection
| Covariate | 30-Day mortality | p Value | |
|---|---|---|---|
| Alive at 30 d, n=209,201 | Dead ≤ 30 d, n=6,444 | ||
| Clinical variables | |||
| Patient age, y, n (%) | |||
| ≤60 | 59,336 (98.43) | 949 (1.57) | <0.001 |
| 61-65 | 33,668 (97.76) | 773 (2.24) | |
| 66-70 | 39,528 (97.35) | 1,076 (2.65) | |
| 71-75 | 36,004 (96.23) | 1,412 (3.77) | |
| 76-80 | 26,643 (95.19) | 1,346 (4.81) | |
| >80 | 14,022 (94.04) | 888 (5.96) | |
| Patient age, mean (SD) | 66 (10.59) | 70.63 (9.66) | <0.001 |
| Race, white, n (%) | 0.067 | ||
| No | 22,766 (97.20) | 655 (2.80) | |
| Yes | 184,516 (96.99) | 5,732 (3.01) | |
| Sex, n (%) | <0.001 | ||
| Male | 101,552 (96.08) | 4,138 (3.92) | |
| Female | 107,649 (97.90) | 2,306 (2.10) | |
| Charlson/Deyo Score, n (%) | <0.001 | ||
| 0 | 111,391 (97.42) | 2,948 (2.58) | |
| 1 | 72,020 (96.91) | 2,297 (3.09) | |
| 2+ | 25,790 (95.56) | 1,199 (4.44) | |
| NCDB analytic stage group, n (%) | <0.001 | ||
| 0-1 | 124,377 (97.65) | 2,989 (2.35) | |
| II | 34,464 (96.59) | 1,215 (3.41) | |
| III | 29,855 (96.03) | 1,235 (3.97) | |
| IV | 8,328 (93.18) | 610 (6.82) | |
| Preoperative radiation, n (%) | <0.001 | ||
| No | 198,273 (97.21) | 5,683 (2.79) | |
| Yes | 8,659 (96.57) | 308 (3.43) | |
| Surgical procedure, n (%) | <0.001 | ||
| Wedge (< 1 lobe) | 31,643 (96.31) | 1,214 (3.69) | |
| Segmentectomy | 5,774 (97.57) | 144 (2.43) | |
| Lobectomy | 158,460 (97.57) | 3,945 (2.43) | |
| Pneumonectomy | 13,324 (92.11) | 1,141 (7.89) | |
| Positive surgical margins, n (%) | <0.001 | ||
| No | 190,981 (97.36) | 5,177 (2.64) | |
| Yes | 13,298 (93.62) | 906 (6.38) | |
| Size of tumor, cm, mean (SD) | 3.32 (3.23) | 4.13 (5.11) | <0.001 |
| Histology, n (%) | <0.001 | ||
| Large cell cancer | 8,234 (96.36) | 311 (3.64) | |
| Other histology | 141,62 (98.62) | 198 (1.38) | |
| Unknown histology | 11,751 (96.45) | 433 (3.55) | |
| Squamous cell cancer | 58,460 (95.74) | 2,601 (4.26) | |
| Adenocarcinomas | 111,051 (97.63) | 2,693 (2.37) | |
| Adenosquamous cancer | 5,543 (96.38) | 208 (3.62) | |
| Nonclinical variables | |||
| Insurance coverage, n (%) | <0.001 | ||
| Not insured | 4,216 (97.23) | 120 (2.77) | |
| Private insurance | 74,704 (98.03) | 1,504 (1.97) | |
| Government insurance | 126,944 (96.43) | 4,695 (3.57) | |
| Median local income, n (%) | <0.001 | ||
| < $30,000 | 27,660 (96.51) | 1,001 (3.49) | |
| $30,000 - $34,999 | 38,267 (96.69) | 1,308 (3.31) | |
| $35,000 - $45,999 | 56,780 (96.94) | 1,790 (3.06) | |
| > $46,000 | 74,971 (97.38) | 2,018 (2.62) | |
| Local education level, n (%) | <0.001 | ||
| ≥29% not high school graduate | 33,387 (96.56) | 1,191 (3.44) | |
| 20-28.9% not high school graduate | 48,932 (96.79) | 1,624 (3.21) | |
| 14-19.9% not high school graduate | 49,169 (96.98) | 1,529 (3.02) | |
| < 14% not high school graduate | 66,174 (97.39) | 1,771 (2.61) | |
| Facility type, n (%) | <0.001 | ||
| Community | 19,536 (96.03) | 807 (3.97) | |
| Comprehensive | 117,557 (96.82) | 3,861 (3.18) | |
| Academic/ research | 72,108 (97.60) | 1,776 (2.40) | |
| Area of residence, n (%) | 0.004 | ||
| Metropolitan | 157,169 (97.05) | 4,777 (2.95) | |
| Urban | 34,398 (96.72) | 1,168 (3.28) | |
| Rural | 4,610 (96.99) | 143 (3.01) | |
SD, standard deviation.
Multivariable Analysis of 30-day Mortality
We next performed a multivariable analysis to identify the clinical and non-clinical variables that may be predictive of 30-day mortality following lung cancer resection. Results shown in Table 3 demonstrate that older age, male gender, and patients with a greater number of comorbidities as described by Charlson/Deyo score ≥ 1 had higher odds of 30-day mortality. Additionally, stage II or greater lung cancer, treatment with preoperative radiation, necessitating lobectomy or pneumonectomy, with larger tumor size, and those with positive surgical margins, were also clinical variables associated with greater 30-day postoperative mortality.
Table 3.
Multivariable Regression Analysis of 30-Day Mortality after Lung Cancer Resection.
| Covariate | 30-day mortality | ||
|---|---|---|---|
| Odds Ratio (95% CI) | OR p Value | Type 3 p Value | |
| Clinical variables | |||
| Patient age | 1.06 (1.05-1.06) | <0.001 | <0.001 |
| Sex | <0.001 | ||
| Male | 1.55 (1.45-1.65) | <0.001 | |
| Female | - | - | |
| Charlson/Deyo Score | <0.001 | ||
| 2+ | 1.56 (1.43-1.70) | <0.001 | |
| 1 | 1.12 (1.04-1.20) | 0.002 | |
| 0 | - | - | |
| NCDB Analytic Stage Group | <0.001 | ||
| Stage IV | 1.99 (1.73-2.29) | <0.001 | |
| Stage III | 1.20 (1.10-1.32) | <0.001 | |
| Stage II | 1.14 (1.04-1.23) | 0.003 | |
| Stage 0-1 | - | - | |
| Preoperative radiation | <0.001 | ||
| Yes | 1.30 (1.12-1.52) | <0.001 | |
| No | - | - | |
| Surgical procedure | <0.001 | ||
| Pneumonectomy | 4.19 (3.62-4.84) | <0.001 | |
| Lobectomy | 1.24 (1.11-1.40) | <0.001 | |
| Segmentectomy | 1.02 (0.81-1.27) | 0.882 | |
| Wedge (< 1 lobe) | - | - | |
| Positive surgical margins | <0.001 | ||
| Yes | 1.56 (1.41-1.72) | <0.001 | |
| No | - | - | <0.001 |
| Size of tumor, cm | 1.02 (1.01-1.02) | <0.001 | |
| Histology | <0.001 | ||
| Unknown histology | 1.20 (1.04-1.38) | 0.011 | |
| Squamous cell cancer | 1.44 (1.34-1.54) | <0.001 | |
| Other histology | 0.86 (0.68-1.08) | 0.181 | |
| Large cell cancer | 1.35 (1.13-1.60) | <0.001 | |
| Adenosquamous cancer | 1.30 (1.09-1.54) | 0.004 | |
| Adenocarcinomas | - | - | |
| Non-clinical variables | |||
| Insurance coverage | 0.084 | ||
| Government insurance | 0.98 (0.89-1.08) | 0.696 | |
| Private insurance | 0.91 (0.83-1.00) | 0.05 | |
| Not insured | - | - | |
| Median local income | 0.007 | ||
| < $30,000 | 1.25 (1.10-1.42) | <0.001 | |
| $30,000 - $34,999 | 1.15 (1.03-1.28) | 0.011 | |
| $35,000 - $45,999 | 1.12 (1.02-1.23) | 0.013 | |
| > $46,000 | - | - | |
| Local education level | 0.078 | ||
| ≥29% not high school graduate | 1.16 (1.03-1.31) | 0.016 | |
| 20-28.9% not high school graduate | 1.11 (1.01-1.23) | 0.036 | |
| 14-19.9% not high school graduate | 1.05 (0.95-1.15) | 0.328 | |
| < 14% not high school graduate | - | - | |
| Facility type | <0.001 | ||
| Community | 1.34 (1.20-1.50) | <0.001 | |
| Comprehensive | 1.22 (1.14-1.31) | <0.001 | |
| Academic/Research | - | - | |
| Area of residence | 0.196 | ||
| Metropolitan | 1.07 (0.99-1.16) | 0.071 | |
| Urban | 1.03 (0.95-1.12) | 0.415 | |
| Rural | - | - | |
Variables included in the model, but not shown: grade, scope of regional lymph node surgery, number of regional lymph nodes examined, and year of diagnosis. Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: Regional Lymph Nodes Positive, and Race: White. Number of observations in the original data set, 215,645. Number of observations used, 161,255.
OR, odds ratio.
Regarding the non-clinical variables studied, lower median household income, lower local education level, and treatment at non-academic medical centers were independently associated with increased 30-day mortality. Our data demonstrate an inverse relationship between household income and odds of 30-day mortality following lung cancer resection. We also found that patients living in communities where ≥ 20% of residents did not graduate high school had higher rates of 30-day mortality following lung cancer surgery. Moreover, compared to receiving cancer treatment at academic medical centers, including those with NCI designation, patients treated at comprehensive and community cancer centers had higher odds of 30-day mortality. We found that insurance coverage and area of residence (urban/rural) were not significantly associated with 30-day postoperative mortality.
Unadjusted Analysis of Treatment Facility
To better understand the reduced odds of 30-day mortality for patients treated at academic medical centers, we performed a separate univariate analysis shown in Table 4. Our results show that academic centers treated younger, less comorbid, female patients compared to comprehensive and community cancer centers. Academic centers resected larger tumors with the lowest rates of positive surgical margins and were more likely to utilize preoperative radiation in their treatment plans. Cancer stage, extent of surgical resection, and histology varied markedly between cancer centers. Academic centers tended to treat patients living in more affluent and educated metropolitan communities. Although academic centers treated more uninsured patients as compared to community and comprehensive cancer centers, they were also more likely to treat those with private insurance as well.
Table 4.
Descriptive Statistics by Treatment Facility Type.
| Covariate | Facility Type | p Value | ||
|---|---|---|---|---|
| Community, n=20,343 | Comprehensive, n=121,418 | Academic/research n=73,884, | ||
| Clinical variables | ||||
| Patient age, y, n (%) | ||||
| <=60 | 5,656 (27.80) | 31,967 (26.33) | 22,662 (30.67) | <0.001 |
| 61-65 | 3,221 (15.83) | 19,177 (15.79) | 12,043 (16.30) | |
| 66-70 | 3,832 (18.84) | 23,417 (19.29) | 13,355 (18.08) | |
| 71-75 | 3,620 (17.79) | 21,563 (17.76) | 12,233 (16.56) | |
| 76-80 | 2,610 (12.83) | 16,589 (13.66) | 8,790 (11.9) | |
| >80 | 1,404 (6.90) | 8,705 (7.17) | 4,801 (6.50) | |
| Mean (SD) | 66.23 (10.42) | 66.58 (10.39) | 65.39 (10.92) | <0.001 |
| Race: White, n (%) | <0.001 | |||
| No | 2,278 (11.26) | 10,615 (8.80) | 10,528 (14.45) | |
| Yes | 17,952 (88.74) | 109,954 (91.20) | 62,342 (85.55) | |
| Sex, n (%) | <0.001 | |||
| Male | 10,363 (50.94) | 60,037 (49.45) | 35,290 (47.76) | |
| Female | 9,980 (49.06) | 61,381 (50.55) | 38,594 (52.24) | |
| Charlson/Deyo Score, n (%) | <0.001 | |||
| 0 | 10,562 (51.92) | 61,731 (50.84) | 42,046 (56.91) | |
| 1 | 7,047 (34.64) | 43,653 (35.95) | 23,617 (31.96) | |
| 2+ | 2,734 (13.44) | 16,034 (13.21) | 8,221 (11.13) | |
| NCDB analytic stage group, n (%) | <0.001 | |||
| Stage 0-1 | 11,750 (60.47) | 72,795 (63.61) | 42,821 (61.87) | |
| Stage II | 3,657 (18.82) | 20,174 (17.63) | 11,848 (17.12) | |
| Stage III | 3,050 (15.70) | 16,785 (14.67) | 11,255 (16.26) | |
| Stage IV | 975 (5.02) | 4,678 (4.09) | 3,285 (4.75) | |
| Preoperative radiation, n (%) | <0.001 | |||
| No | 19,208 (96.21) | 115,156 (96.06) | 69,592 (95.23) | |
| Yes | 756 (3.79) | 4,722 (3.94) | 3,489 (4.77) | |
| Surgical procedure, n (%) | <0.001 | |||
| Wedge (< 1 lobe) | 3,488 (17.15) | 18,254 (15.03) | 11,115 (15.04) | |
| Segmentectomy | 473 (2.33) | 3,138 (2.58) | 2,307 (3.12) | |
| Lobectomy | 14,912 (73.30) | 92,147 (75.89) | 55,346 (74.91) | |
| Pneumonectomy | 1,470 (7.23) | 7,879 (6.49) | 5,116 (6.92) | |
| Positive surgical margins, n (%) | <0.001 | |||
| No | 17,765 (91.19) | 110,449 (93.10) | 67,944 (94.05) | |
| Yes | 1,716 (8.81) | 8,190 (6.90) | 4,298 (5.95) | |
| Size of tumor, cm, mean (SD) | 3.41 (3.01) | 3.31 (3.00) | 3.37 (3.81) | <0.001 |
| Histology, n (%) | <0.001 | |||
| Large cell cancer | 791 (3.89) | 4,919 (4.05) | 2,835 (3.84) | |
| Other histology | 961 (4.72) | 7,671 (6.32) | 5,728 (7.75) | |
| Unknown histology | 1,556 (7.65) | 7,077 (5.83) | 3,551 (4.81) | |
| Squamous cell cancer | 6,404 (31.48) | 35,038 (28.86) | 19,619 (26.55) | |
| Adenocarcinomas | 10,070 (49.50) | 63,392 (52.21) | 40,282 (54.52) | |
| Adenosquamous cancer | 561 (2.76) | 3,321 (2.74) | 1,869 (2.53) | |
| Non-clinical variables | ||||
| Insurance coverage, n (%) | <0.001 | |||
| Not Insured | 450 (2.26) | 2,174 (1.81) | 1,712 (2.37) | |
| Private insurance | 6,378 (32.02) | 42,081 (35.08) | 27,749 (38.38) | |
| Government insurance | 13,088 (65.72) | 75,713 (63.11) | 42,838 (59.25) | |
| Median local Income, n (%) | <0.001 | |||
| < $30,000 | 3,058 (15.94) | 15,777 (13.75) | 9,826 (14.06) | |
| $30,000 - $34,999 | 4,615 (24.05) | 22,860 (19.92) | 12,100 (17.32) | |
| $35,000 - $45,999 | 5,930 (30.91) | 34,418 (30.00) | 18,222 (26.08) | |
| > $46,000 | 5,584 (29.10) | 41,677 (36.33) | 29,728 (42.54) | |
| Local education level, n (%) | <0.001 | |||
| ≥ 29% Not high school graduate | 3,654 (19.05) | 19,201 (16.74) | 11,723 (16.78) | |
| 20-28.9% Not high school graduate | 5,461 (28.46) | 28,688 (25.01) | 16,407 (23.48) | |
| 14-19.9% Not high school graduate | 5,308 (27.67) | 28,961 (25.24) | 16,429 (23.51) | |
| < 14% Not high school graduate | 4,762 (24.82) | 37,874 (33.01) | 25,309 (36.22) | |
| Area of residence, n (%) | <0.001 | |||
| Metropolitan | 13,727 (72.61) | 90,598 (79.17) | 57,621 (83.60) | |
| Urban | 4,653 (24.61) | 20,632 (18.03) | 10,281 (14.92) | |
| Rural | 525 (2.78) | 3,204 (2.80) | 1,024 (1.49) | |
p Value is calculated by ANOVA for numerical covariates and chi-square test for categorical covariates.
NCDB, National Cancer Data Base.
Discussion
This study is the largest analysis of 30-day mortality in patients undergoing surgical resection for lung cancer to date derived from a nationally generalizable database. Unlike professional society data, the NCDB is comprised of hospital registry data from more than 1,500 Commission-on-Cancer accredited facilities and reports on 70% of newly diagnosed cancers nationwide (14). Similar to other groups studying multi-institutional data, our data demonstrate that clinical variables including older age, male gender, higher comorbidity score, increased cancer stage, pneumonectomy, positive surgical margins, use of preoperative radiation therapy, and increased tumor size were associated with higher rates of 30-day mortality following lung cancer resection. More importantly, these data show that non-clinical variables, such as living in low income neighborhoods and communities with a lesser proportion of high school graduates, were also factors independently associated with greater 30-day mortality after lung cancer surgery. Additionally, patients treated at academic cancer centers, including those with NCI designation, had lower rates of 30-day postoperative mortality following lung cancer resection.
Few groups have studied non-clinical factors associated with 30-day mortality following lung cancer resection. Previously Johnson and colleagues evaluated the Georgia Comprehensive Cancer Registry and found that lung cancer patients living in areas with higher income disparities and lower levels of education had worse 5-year cancer survival (15). Similarly, Erhunmwunsee et al utilized the Duke Comprehensive Cancer Center Tumor Registry to show that patients living in low income areas and those regions where fewer residents achieved a high school diploma had lesser 6-year cancer-specific survival than patients with a similar disease living in higher socioeconomic status and more educated communities (16). Our data extend these findings to a national level, whereby outcomes for patients undergoing lung cancer resection were similarly impacted by socioeconomic factors including household income and local education. As the median household income in our cohort dropped below $46,000 per year, the association with 30-day mortality increased. Similarly, a lower local education level was associated with greater 30-day postoperative mortality. Surprisingly the association of poor income and education with increased cancer mortality held true for our shorter 30-day postoperative time frame compared to the previous 5 and 6 year studies respectively. Between 2007 and 2012, income disparities and poverty levels increased in the United States from 2.7% to 15%, or 46 million Americans (DeNavas-Walt, US census bureau, 2013). Thus, the pool of Americans with lung cancer at increased risk for worse 30-day postoperative survival based on income and education disparities may still be growing.
Optimally, cancer treatment centers encompass a specialized environment with the proper infrastructure, sufficient volume, and adequate expertise with quality improvement protocols designed for continual evaluation and enhancement (17). High volume hospitals additionally may better provide team-based expertise for complex cases, physicians specialized in the diagnosis and treatment of rare cancer types, and a centralized method for delivering complex medical and surgical care. Disparities in the use of high volume hospitals for cancer care have recently been evaluated. In the 2012 report by Al-Refaie evaluating the National Inpatient Sample during the early 2000s, 66% of patients received their cancer surgery at a high volume hospital. However, most notably, non-white patients, patients with a greater number of comorbidities, and those patients with non-private insurance were more likely to receive their care at low volume hospitals (18). Reports of the Netherlands Cancer Registry have found that higher volume hospitals treating >50 cases/year are more likely to provide surgical resection compared to chemoradiation for later stage lung cancer and these differences in treatment options lead to differences in 30-day mortality (19). Similarly, regionalization of thoracic surgical care to specialized centers in Canada has been associated with decreased in-hospital mortality for pneumonectomy patients (20). Within our study sample, less than 10% of cancer patients received their care at a low volume hospital. Thus, analogous to Al-Refaie's report, the proportion of patients receiving care at low volume institutions may be decreasing. Our study identifies a 30-day mortality advantage for patients treated at higher volume academic or comprehensive community cancer center programs compared to lower volume community programs. We show that academic facilities treat younger, healthier patients from more educated, and affluent communities, which may be factors for such a survival advantage. Additionally, when compared to low volume, community cancer programs, comprehensive cancer programs also treated more affluent, educated, Caucasian patients who were more often covered by private insurance and had earlier stage cancer with fewer positive surgical margins. Collectively these characteristics likely contribute to, however, do not entirely account for the lower rates of 30-day mortality at larger volume academic and comprehensive cancer programs compared to the smaller volume community cancer centers. Community cancer centers still had higher mortality even after controlling for these characteristics.
Of the non-clinical factors evaluated, we found no difference in 30-day mortality following lung cancer resection based on insurance coverage. U.S. Census data published in 2012 showed that 15.4%, or 48 million Americans, went uncovered by health insurance, and Medicaid covered 16.4%, or 51 million Americans (DeNavas-Walt, US census bureau, 2013). Lack of insurance coverage has been associated with worse survival and postoperative complications in critical illness (21), trauma (22), breast cancer (23), uterine cancer (24), colorectal cancer (25, 26), prostate cancer (27), diffuse large cell lymphoma (28), head and neck cancer (29), and lung cancer (30). Attributable causes for worse clinical outcomes in uninsured Americans include more advanced disease at time of diagnosis, fewer diagnostic tests performed, and decreased healthcare literacy (31, 32). Slatore and colleagues have found that uninsured lung cancer patients and those with Medicaid coverage experienced higher incidence rates, more advanced stage at diagnosis, and increased all-cause mortality (30). Our data demonstrated only a marginal association for privately insured patients to have lower 30-day mortality compared to uninsured patients (p = 0.05). However, there was no difference in 30-day mortality between uninsured and government insured patients. Moreover, there were no significant effects of insurance coverage on 30-day mortality overall. With such strong evidence published previously that better insurance coverage is associated with better long term survival and decreased postoperative complications, we hypothesize that our 30-day timeframe of study may be too narrow to identify the impact of this important socioeconomic variable on our study cohort. McMillan and colleagues have recently shown that 30-day mortality rates might significantly underestimate surgically-attributable mortality rates (33). This group has instead proposed that a 90-day postoperative window might better assess mortality attributable to the operation. Their data show that this window captures twice the mortality rate predicted by a 30-day timeframe. Additionally, since establishment of the NCDB in 1998, groups have questioned the validity of insurance reporting (32). Critics have suggested that the reporting of this variable may not take into account supplemental plans and uninsured groups, as Medicaid patients may be retroactively enrolled during the diagnosis and management of lung cancer. The continued analysis of health insurance as a predictor of adverse clinical outcomes in lung cancer is especially important in light of the current policy changes with the Affordable Care Act. Unfortunately, it is difficult to extrapolate our results to predict future outcomes under the new health insurance exchanges due to the paucity of data regarding current patient utilization.
Geography and social environment are important socioeconomic factors that influence individual behaviors, resource access, and healthcare utilization. Although we predicted that these influences could predispose 30-day mortality after lung cancer surgery, our data did not show an association of area of residence with 30-day postoperative mortality. In addition to studying the effects of income and local education, Johnson and colleagues also earlier evaluated non-small cell lung cancer survival at 5 years based upon area of residence (15). They reported that rural residents with lung cancer were most likely to go undiagnosed and less likely to receive treatment. Moreover, Singh et al have recently reported higher annual age-adjusted lung cancer mortality rates for rural residents in the UK (34). Our data do not demonstrate differences in 30-day postoperative mortality based upon area of residence. Similar to insurance coverage the 30-day window may be too narrow to identify the significance of this variable, or more simply, compared to these previous investigations, all patients included in our study received cancer treatment.
Numerous studies have performed large database analyses to determine the effects of clinical variables on 30-day postoperative mortality. Our data corroborate the results of these analyses. Utilizing the Society of Thoracic Surgeons General Thoracic Database (STS GTSD) two separate groups have recently reported risk factors for lung cancer resection (6, 35). Kozower and colleagues evaluated 18,800 patients from 111 medical centers and reported 30-day mortality rates of 2.2% following lung cancer resection. Independent risk factors for mortality in these patients included poor preoperative performance and FEV1, male gender, urgency of procedure, extent of resection, obesity, kidney dysfunction, and chemotherapy/steroid treatment (6). Focusing on patients with extensive disease requiring pneumonectomy, Shapiro and colleagues similarly evaluated risk factors of poor clinical outcomes utilizing 1,267 patients from 80 medical centers reporting to the STS GTSD. In patients requiring pneumonectomy, they found 30-day postoperative mortality rates of 5.6%. Their analysis similarly found that preoperative performance, age >65, male gender, extrapleural resection, and chemoradiation were risk factors for major adverse clinical outcomes following lung cancer resection including the development of pneumonia, acute respiratory distress syndrome, empyema, sepsis, venous thromboembolism, prolonged mechanical ventilation, reintubation, tracheostomy, dysrhythmia, myocardial infarction, reoperation, cerebrovascular event, and 30-day mortality (35). A separate study of the SEER database demonstrated that 90-day mortality following pneumonectomy ranged from 4-16% and predictors of poor outcomes included older age, male sex, unmarried status, cancer stage, tumor size and grade, right sidedness, prior malignancy, number of positive nodes, and few lymph nodes evaluated (36).
Earlier groups reported a few unique differences compared to the above studies with regard to risks factors for early postoperative mortality after lung cancer surgery. In 1994, Deslaurier's group studied 783 patients from 7 centers involved in the Lung Cancer Study Group undergoing lung cancer resection. Their study reported 30-day mortality rates of 3.8% and found that 27% of patients experienced major complications. While major postoperative events were reported for older, male patients with worse preoperative function, this group did not find that the extent of surgical resection was an added risk factor (5). Furthermore, Harpole's study of 3516 Veterans Affairs patients identified other clinical factors imparting increased perioperative risks, including preoperative smoking status, greater duration of procedure, as well as the need for blood and colloid transfusion (37). Our data agree with many of these earlier studies in demonstrating that clinical variables including older age, male gender, higher comorbidity score, increased cancer stage, greater extent of surgical resection, positive surgical margins, use of preoperative radiation therapy, and increased tumor size are associated with increased 30-day mortality following lung cancer resection.
While this study is the largest multi-institutional database analysis of 30-day mortality in patients undergoing lung cancer resection to date, it is not without limitations. The NCDB registry includes 70% of all cancer cases diagnosed in the United States, leaving up to 30% of cancer care unreported in this database. Patients diagnosed and treated in a single physician office not affiliated with a CoC-accredited institution, those undergoing consultation for diagnosis and treatment planning elsewhere, and cases reviewed by pathologists but not entering into a participating hospital for any aspect of care are not reported (38). Our NCDB data request for the years 2003 – 2011 included US Census data from the year 2000. US Census data represents only a snapshot of dynamic variables including income disparity and poverty. Conclusions of this study could be improved by accounting for trends in socioeconomic conditions during the entire course of our study period. Concerns regarding insurance coverage validity, updates and consistency in reporting for changing supplemental health plans, and regional availability of supplemental plans have been raised elsewhere (32). McMillan and colleagues have proposed that a 90-day window might best measure true surgically-related death (33). Our study is also limited by preoperative comorbidity data as quantified by Charlson/Devo score. Derived in 1984 as a predictor of mortality, this scoring system is 30 years old and may be less valid due to changes in the current trends of stage at diagnosis, treatment strategies, and improvements in clinical outcomes (39). Data regarding pretreatment quality of life may better prognosticate outcomes of lung cancer treatment, particularly in aging populations (40). By nature, database registries including this one can provide only a retrospective review of dynamic clinical and socioeconomic variables. A large sample size, such as this, may also overestimate the clinical significance of study variables in spite of statistically significant p values.
The NCDB is the largest U.S. cancer database and is regarded as the largest clinical registry in the world (38). We studied over 215,000 patients in the NCDB undergoing lung cancer resection and found that demographic and clinical factors such as older age, male gender, higher comorbidity score, increased cancer stage, greater extent of surgical resection, positive surgical margins, use of preoperative radiation therapy, and larger tumor size were associated with increased 30-day mortality following lung cancer resection as has been previously described in the literature. However, after controlling for clinical characteristics, our analysis illustrates that non-clinical factors including living in lower income households, less educated communities, as well as receiving cancer care at a facility other than an academic treatment center, were also variables associated with increased 30-day postoperative mortality following lung cancer resection. This information exposes at-risk patient populations for poor outcomes following lung cancer resection and identifies specific patient characteristics where cancer care should be focused to increase efficient healthcare delivery and resource utilization.
Précis.
Both clinical and nonclinical factors are associated with increased 30-day mortality after lung cancer resection. By identifying these nonclinical factors, we find unique patient populations at risk for poor outcomes after lung cancer surgery, and this is where cancer care should be focused.
Acknowledgments
Support: This study was supported by a grant to Emory University from the National Institutes of Health, National Cancer Institute, grant number P30CA138292, and by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University. The data used in the study are derived from a de-identified National Cancer Data Base file awarded to Emory University by the American College of Surgeons and Commission on Cancer. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. This work is also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Dr Nickleach is an employee of IntrinsiQ, Burlington, MA. All other authors have nothing to disclose.
Presented at the American College of Surgeons 100th Annual Clinical Congress, San Francisco, CA, October 2014.
REFERENCES
- 1.Alberg AJ, Ford JG, Samet JM. American College of Chest P. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. In: Society AC, editor. American Cancer Society; Atlanta: 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 3.Ries LAGMD, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. National Cancer Institute; Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/ Posted to the SEER web site. [Google Scholar]
- 4.Duque JL, Ramos G, Castrodeza J, et al. Early complications in surgical treatment of lung cancer: a prospective, multicenter study. Grupo Cooperativo de Carcinoma Broncogenico de la Sociedad Espanola de Neumologia y Cirugia Toracica. Ann Thoracic Surg. 1997;63:944–950. doi: 10.1016/s0003-4975(97)00051-9. [DOI] [PubMed] [Google Scholar]
- 5.Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106:329S–330S. doi: 10.1378/chest.106.6_supplement.329s. [DOI] [PubMed] [Google Scholar]
- 6.Kozower BD, Sheng S, O'Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thoracic Surg. 2010;90:875–881. doi: 10.1016/j.athoracsur.2010.03.115. discussion 881-883. [DOI] [PubMed] [Google Scholar]
- 7.Panzer RJ, Gitomer RS, Greene WH, et al. Increasing demands for quality measurement. JAMA. 2013;310:1971–1980. doi: 10.1001/jama.2013.282047. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Karakiewicz PI, Sammon JD, et al. Disparities in selective referral for cancer surgeries: implications for the current healthcare delivery system. BMJ Open. 2014;4:e003921. doi: 10.1136/bmjopen-2013-003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John DA, Kawachi I, Lathan CS, Ayanian JZ. Disparities in perceived unmet need for supportive services among patients with lung cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Cancer. 2014 doi: 10.1002/cncr.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 11.Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): A California Cancer Registry analysis. Lung Cancer. 2014;85:264–269. doi: 10.1016/j.lungcan.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 13.Surgeons ACo. [June 18, 2014];Commission on Cancer Categories of Accreditation. http://www.facs.org/cancer/coc/categories3.html#.
- 14.Licht PB, Jorgensen OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thoracic Surg. 2013;96:943–949. doi: 10.1016/j.athoracsur.2013.04.011. discussion 949-950. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AM, Hines RB, Johnson JA, 3rd, Bayakly AR. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. 2014;83:401–407. doi: 10.1016/j.lungcan.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH., Jr Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118:5117–5123. doi: 10.1002/cncr.26185. [DOI] [PubMed] [Google Scholar]
- 17.Wouters MW, Jansen-Landheer ML, van de Velde CJ. The Quality of Cancer Care initiative in the Netherlands. Eur J Surg Oncol. 2010;36:S3–S13. doi: 10.1016/j.ejso.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Al-Refaie WB, Muluneh B, Zhong W, et al. Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg. 2012;214:81–87. doi: 10.1016/j.jamcollsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Wouters MW, Siesling S, Jansen-Landheer ML, et al. Variation in treatment and outcome in patients with non-small cell lung cancer by region, hospital type and volume in the Netherlands. Eur J Surg Oncol. 2010;36:S83–92. doi: 10.1016/j.ejso.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Finley CJ, Bendzsak A, Tomlinson G, et al. The effect of regionalization on outcome in pulmonary lobectomy: a Canadian national study. J Thoracic Cardiovasc Surg. 2010;140:757–763. doi: 10.1016/j.jtcvs.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler RA, Noyahr LA, Thornton JD, et al. An official American Thoracic Society systematic review: the association between health insurance status and access, care delivery, and outcomes for patients who are critically ill. Am J Respiratory Crit Care Med. 2010;181:1003–1011. doi: 10.1164/rccm.200902-0281ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haider AH, Chang DC, Efron DT, et al. Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008;143:945–949. doi: 10.1001/archsurg.143.10.945. [DOI] [PubMed] [Google Scholar]
- 23.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N EnglJ Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 24.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecologic Oncol. 2011;122:63–68. doi: 10.1016/j.ygyno.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Robbins AS, Pavluck AL, Fedewa SA, et al. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27:3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 26.Roetzheim RG, Pal N, Gonzalez EC, et al. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90:1746–1754. doi: 10.2105/ajph.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahal BA, Aizer AA, Ziehr DR, et al. The association between insurance status and prostate cancer outcomes: implications for the Affordable Care Act. Prostate Cancer Prostatic Diseases. 2014;17:273–279. doi: 10.1038/pcan.2014.23. [DOI] [PubMed] [Google Scholar]
- 28.Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120:1220–1227. doi: 10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- 29.Kwok J, Langevin SM, Argiris A, et al. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116:476–485. doi: 10.1002/cncr.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slatore CG, Au DH, Gould MK, American Thoracic Society Disparities in Healthcare G An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respiratory Crit Care Med. 2010;182:1195–1205. doi: 10.1164/rccm.2009-038ST. [DOI] [PubMed] [Google Scholar]
- 31.Rosen H, Saleh F, Lipsitz S, et al. Downwardly mobile: the accidental cost of being uninsured. Arch Surg. 2009;144:1006–1011. doi: 10.1001/archsurg.2009.195. [DOI] [PubMed] [Google Scholar]
- 32.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010:16614–621. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 33.McMillan RR, Berger A, Sima CS, et al. Thirty-day mortality underestimates the risk of early death after major resections for thoracic malignancies. Ann Thoracic Surg. 2014 doi: 10.1016/j.athoracsur.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh GK, Siahpush M, Williams SD. Changing urbanization patterns in US lung cancer mortality, 1950-2007. J Community Health. 2012;37:412–420. doi: 10.1007/s10900-011-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thoracic Surg. 2010;90:927–934. doi: 10.1016/j.athoracsur.2010.05.041. discussion 934-935. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez FG, Force SD, Pickens A, et al. Impact of laterality on early and late survival after pneumonectomy. Ann Thoracic Surg. 2011;92:244–249. doi: 10.1016/j.athoracsur.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Harpole DH, Jr, DeCamp MM, Jr, Daley J, et al. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thoracic Cardiovasc Surg. 1999;117:969–979. doi: 10.1016/S0022-5223(99)70378-8. [DOI] [PubMed] [Google Scholar]
- 38.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan H, Li B, Couris CM, Fushimi K, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 40.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]