Abstract
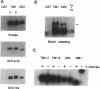
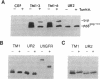
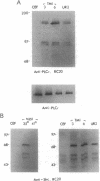
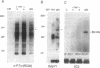
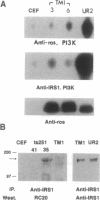
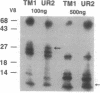
There is a 3-aa insertion in the transmembrane (TM) domain of the p68gag-ros protein-tyrosine kinase encoded by avian sarcoma virus UR2 v-ros as compared with that of the protooncogene c-ros. The effect of this insertion on biological function and biochemical properties of v-Ros protein was investigated by deleting these 3 aa to generate the mutant TM1. This mutant has greatly reduced transforming, mitogenic, and tumorigenic activities despite the fact that the protein-tyrosine kinase activity and cell-surface localization of TM1 protein are unaffected. However, unlike UR2 protein, mutant TM1 protein becomes glycosylated, is differentially phosphorylated, and fails to induce tyrosine phosphorylation of a 88-kDa protein and a major substrate of insulin receptor, insulin receptor substrate 1. The TM1 protein is unable to associate with phosphatidylinositol 3-kinase and fails to promote association of insulin receptor substrate 1 with phosphatidylinositol 3-kinase. By contrast, tyrosine phosphorylation of Shc protein and phospholipase C gamma as well as interaction of Grb2 protein with Shc and SOS protein signaling components are unaltered in the TM1 infected cells. Our results show that the TM-domain sequence of p68gag-ros profoundly affects its function and substrate interaction. The mutant defines a signaling pathway including phosphatidylinositol 3-kinase, insulin receptor substrate 1, and possibly an 88-kDa protein that does not overlap the Ras pathway and is important for full transforming and mitogenic potency of v-ros protein-tyrosine kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Mariuzza R. A., Ermonval M., Acuto O. Lysine 271 in the transmembrane domain of the T-cell antigen receptor beta chain is necessary for its assembly with the CD3 complex but not for alpha/beta dimerization. J Biol Chem. 1990 Mar 5;265(7):4131–4135. [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988 Jul;7(7):2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Ingraham H. A., Cochet C., Walton G. M., Lazar C. S., Sowadski J. M., Rosenfeld M. G., Gill G. N. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem. 1991 Mar 25;266(9):5750–5755. [PubMed] [Google Scholar]
- Chen J. M., Heller D., Poon B., Kang L., Wang L. H. The proto-oncogene c-ros codes for a transmembrane tyrosine protein kinase sharing sequence and structural homology with sevenless protein of Drosophila melanogaster. Oncogene. 1991 Feb;6(2):257–264. [PubMed] [Google Scholar]
- Chen J., Zong C. S., Wang L. H. Tissue and epithelial cell-specific expression of chicken proto-oncogene c-ros in several organs suggests that it may play roles in their development and mature functions. Oncogene. 1994 Mar;9(3):773–780. [PubMed] [Google Scholar]
- Crepaldi T., Prat M., Giordano S., Medico E., Comoglio P. M. Generation of a truncated hepatocyte growth factor receptor in the endoplasmic reticulum. J Biol Chem. 1994 Jan 21;269(3):1750–1755. [PubMed] [Google Scholar]
- Dobashi K., Weiner D. B., Greene M. I. Differential regulation of oncogenic and cellular p185 by serine/threonine kinases. DNA. 1989 Dec;8(10):723–732. doi: 10.1089/dna.1989.8.723. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Frattali A. L., Treadway J. L., Pessin J. E. Evidence supporting a passive role for the insulin receptor transmembrane domain in insulin-dependent signal transduction. J Biol Chem. 1991 May 25;266(15):9829–9834. [PubMed] [Google Scholar]
- Fukui Y., Kornbluth S., Jong S. M., Wang L. H., Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 1989;4(4):283–292. [PubMed] [Google Scholar]
- Garber E. A., Hanafusa T., Hanafusa H. Membrane association of the transforming protein of avian sarcoma virus UR2 and mutants temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1985 Dec;56(3):790–797. doi: 10.1128/jvi.56.3.790-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. S., Koh H. A., Konish Y., Bullock L. D., Huang J. S. Differential processing and turnover of the oncogenically activated neu/erb B2 gene product and its normal cellular counterpart. J Biol Chem. 1990 Feb 25;265(6):3340–3346. [PubMed] [Google Scholar]
- Irurzun A., Pérez L., Carrasco L. Brefeldin A blocks protein glycosylation and RNA replication of vesicular stomatitis virus. FEBS Lett. 1993 Dec 28;336(3):496–500. doi: 10.1016/0014-5793(93)80863-p. [DOI] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Role of gag sequence in the biochemical properties and transforming activity of the avian sarcoma virus UR2-encoded gag-ros fusion protein. J Virol. 1990 Dec;64(12):5997–6009. doi: 10.1128/jvi.64.12.5997-6009.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. The transforming protein P68gag-ros of avian sarcoma virus UR2 is a transmembrane protein with the gag portion protruding extracellularly. Oncogene Res. 1987 Jun;1(1):7–21. [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Two point mutations in the transmembrane domain of P68gag-ros inactive its transforming activity and cause a delay in membrane association. J Virol. 1991 Jan;65(1):180–189. doi: 10.1128/jvi.65.1.180-189.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong S. M., Zong C. S., Dorai T., Wang L. H. Transforming properties and substrate specificities of the protein tyrosine kinase oncogenes ros and src and their recombinants. J Virol. 1992 Aug;66(8):4909–4918. doi: 10.1128/jvi.66.8.4909-4918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Rutter W. J., Wang L. H. Modulating effects of the extracellular sequence of the human insulinlike growth factor I receptor on its transforming and tumorigenic potential. J Virol. 1993 Jan;67(1):9–18. doi: 10.1128/jvi.67.1.9-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Wang L.-H. Oncogenes, Protein Tyrosine Kinases, and Signal Transduction. J Biomed Sci. 1994 Mar;1(2):65–82. doi: 10.1007/BF02257980. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Shibuya M. Tissue-specific expression of rat c-ros-1 gene and partial structural similarity of its predicted products with sev protein of Drosophila melanogaster. J Virol. 1990 May;64(5):2117–2125. doi: 10.1128/jvi.64.5.2117-2125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Jr, Backer J. M., Sun X. J., Shoelson S., Hu P., Schlessinger J., Yoakim M., Schaffhausen B., White M. F. IRS-1 activates phosphatidylinositol 3'-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E., Gödecke A., Walter B., Bladt F., Birchmeier C. Transient and locally restricted expression of the ros1 protooncogene during mouse development. EMBO J. 1991 Dec;10(12):3693–3702. doi: 10.1002/j.1460-2075.1991.tb04937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tessarollo L., Nagarajan L., Parada L. F. c-ros: the vertebrate homolog of the sevenless tyrosine kinase receptor is tightly regulated during organogenesis in mouse embryonic development. Development. 1992 May;115(1):11–20. doi: 10.1242/dev.115.1.11. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D. B., Kokai Y., Wada T., Cohen J. A., Williams W. V., Greene M. I. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989 Oct;4(10):1175–1183. [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Zong C. S., Poon B., Chen J., Wang L. H. Molecular and biochemical bases for activation of the transforming potential of the proto-oncogene c-ros. J Virol. 1993 Nov;67(11):6453–6462. doi: 10.1128/jvi.67.11.6453-6462.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]